Abstract
Background
An adverse drug reaction (ADR) is a response to a medicine that is not intended and is harmful, and which occurs at normal dose levels for humans. Currently, there are no estimates of the population-based prevalence of ADRs in the Kingdom of Saudi Arabia (KSA).
Objective
The aims of this study were to (1) estimate the population-based prevalence of ADRs in KSA, (2) describe the ADRs experienced by survey respondents, and (3) investigate the level of awareness of the ADR reporting system.
Patients and Methods
This was a cross-sectional survey using stratified, population-based sampling conducted at a chain of community pharmacies.
Results
Analysis was conducted on 5228 surveys; 50.17% of respondents were males, and the mean age was 39 ± 15 years (min = 18, max = 98). The sample prevalence of ADRs was 23.45% (95% CI 22.30–24.60%, P < 0.001). The estimated population prevalence (after weighting) was 28.00% (26.10–30.00%). Gastrointestinal disorders were the most commonly reported ADRs (58.73%), followed by general disorders and administration site conditions (19.74%). The largest drug class that was reported to lead to ADRs was nonsteroidal anti-inflammatory drugs (NSAIDs) (11%). Over 19% of the respondents who experienced an ADR required medical intervention to control the suffering induced by the ADR. Of the respondents who experienced an ADR, 371 (30.26%) were aware of the ADR reporting system but only 53 (14.29%) said that they had filed a report in the system.
Conclusions
Our study estimated that 28% of the population experienced an ADR over a 1-year period in KSA. Risk factors for ADR included certain chronic disease groups and the use of certain classes of medications. Regulatory authorities in KSA intend to conduct more research and deploy educational interventions to reduce ADR rates in KSA. This will hopefully occur in an international context that promotes the standardized measurement of ADRs in the community. A subset of findings from this report was presented in an oral presentation at the Saudi Food and Drug Authority (SFDA) Annual Conference, September 27, 2018. In addition, a subset of findings from this report were presented on a poster at the International Conference of Pharmacoepidemiology and Therapeutic Risk Management (ICPE), August 27, 2019.
Electronic supplementary material
The online version of this article (10.1007/s40801-020-00186-8) contains supplementary material, which is available to authorized users.
Key Points
| Community pharmacists conducted surveys with customers about any ADRs experienced over the last year. |
| After adjusting for population demographics, the yearly prevalence of ADR in KSA was estimated to be 28%, which is in line with studies from other countries. |
| Many of the respondents had risk factors for ADRs, such as chronic disease and multiple medication usage. Future studies will examine the rates of risk factors for ADRs in KSA. |
Introduction
According to the United States (US) Institute of Medicine (IOM), an adverse drug event (ADE) is defined as “an injury resulting from medical intervention related to a drug, including medication errors, adverse drug reactions, allergic reactions and overdoses” [1]. On the other hand, an adverse drug reaction (ADR) is defined by the World Health Organization (WHO) as “a response to a medicine which is noxious and unintended and which occurs at doses normally used in man” [2]. The definitions of ADEs and ADRs overlap and refer to negative medication-related events that take place when an approved drug is used in the community.
ADEs and ADRs are important preventable causes of mortality, morbidity, hospitalization, and increased healthcare costs, and rates of ADEs and ADRs are typically measured in a clinical setting [3]. However, it is difficult to compare the rates and frequencies of ADEs and ADRs between studies due to differences in the study design and data used. One study used national mortality statistics to estimate the annual mortality rate in the US that was attributable to ADRs as 8–12 deaths per year per 10 million [3]. A study of the Hospital Episode Statistics database (collected by the Department of Health in the UK) found that the total number of ADRs in the database was about 53,000 for the period 1998–1999, and that this figure increased to almost 77,000 for the year 2004 [4]. A more rigorous observational study was conducted in two National Health System (NHS) hospitals in the UK, where researchers characterized admissions based on whether they were related to an ADR [5]. The researchers found that among over 18,000 admissions, 6.5% were related to an ADR [5]. Although rates and frequencies are measured in different ways, and different case definitions are used, it is clear that the rates of ADE and ADR in clinical settings are not trivial and that ADEs and ADRs should be regarded as sources of preventable comorbidity.
Rates of ADEs and ADRs in clinical settings differ markedly depending upon the type of clinical data used to measure them. However, there is greater concern over “unmeasured” or community-occurring ADEs and ADRs. The most feasible way to gauge rates of community-occurring ADEs and ADRs is to utilize passive surveillance systems, where the community is asked to report ADRs [6]. Several countries have ADR reporting systems, including Sweden, the US, Australia, Canada, Denmark, the Netherlands, the UK [6], and the Kingdom of Saudi Arabia (KSA) [7]. Studies conducted on these passive surveillance systems have found that there is severe underreporting caused by multiple factors [6, 7].
For this reason, countries have attempted to conduct active surveillance of ADEs and ADRs. One study conducted in Sweden involved mailing a survey gathering data on the prevalence of ADEs and ADRs experienced during the past month to randomly selected members of the population [8]. The survey included definitions of community-occurring ADEs and ADRs that were developed for the study based on the IOM and WHO definitions [8]. The ADEs were defined and then subclassified as ADRs if they achieved a particular level of severity [8]. In their study of 7099 individuals, the researchers found that 19.4% of the respondents had experienced ADEs in the past month [95% confidence interval (CI) 18.5–20.3%] [8]. Of the 2578 ADEs experienced, 847 (32.9%) were classified as ADRs [8].
Researchers in Italy used a pharmacy-based approach to conduct active surveillance of ADEs and ADRs [9]. They recruited 96 pharmacists in the Campania region of Italy who approached their customers and asked them if they had experienced an ADR [9]. If the customer responded in the affirmative, the pharmacist helped them complete an ADR reporting form [9]. Of the 18,677 patients interviewed, 10.88% reported experiencing an ADR, but a fixed time frame was not given [9].
In KSA, results from a recent qualitative study involving 27 healthcare professionals revealed that there is a need to improve ADR reporting and knowledge about the prevalence of ADRs in KSA [10]. The authors recommended that KSA should improve pharmacovigilance through several means, including conducting research and improving the consistency of ADR reporting [10]. They also listed ongoing challenges to passive surveillance, including a lack of health literacy and medication literacy not only in patients but also in some healthcare professionals [10]. Additionally, healthcare professionals who report ADRs on behalf of their patients face workload-based obstacles to completing reports [10].
To date, no active surveillance of community-occurring ADRs in KSA has been conducted. The aims of this study were therefore to (1) estimate the population-based prevalence of ADRs in the KSA, (2) describe the ADRs experienced by survey respondents, and (3) investigate the level of awareness of KSA’s ADR reporting system.
Methods
Study Design, Population, and Setting
This study was a nationwide cross-sectional survey conducted in KSA of customers at community pharmacies. The survey was delivered face-to-face in Arabic by community pharmacists, who entered the data into an application designed for data collection in this study called QPlatform®. Respondents were included if they were a resident of KSA, aged 18 years or older, a member of an open sampling quota, and visiting a participating community pharmacy between June and August 2018. Interviews took approximately 10 min.
Stratified sampling was facilitated through the QPlatform® application. Quotas were determined based on region, gender, and age (two groups). Target quotas for each cell were programmed into QPlatform®. Once the respondent had been recruited, the first questions they were asked were to do with sampling. Therefore, the app was able to determine their eligibility for inclusion given the stratified sampling approach [11]. All data were coded and stored on the QPlatform database. This study was approved by the Saudi Food and Drug Authority (SFDA) Ethics Committee (#18-0007).
Sampling and Sample Size
Because of budgetary limitations, the lack of a causal hypothesis, and the desire to sample from strata representing all 13 regions, a sampling schedule that was not based on a power calculation was developed. The quota sampling technique was used to get an equal distribution of participants across the 13 regions of Saudi Arabia stratified by age, gender, and region. We used two age groups based on the median age in Saudi Arabia, which is 37. This led to 52 quotas with a total of 100 individuals per quota in this study, for a total sample size of 5200.
Questionnaire Design
No existing validated questionnaire was available, so a new questionnaire was assembled using questions and guidance from the scientific literature (see the Electronic supplementary material, ESM). A structured questionnaire with two main sections was designed. The first section explored sociodemographic characteristics, including age, gender, region, education [12], and chronic conditions [12]. The second section provided the definition of an ADR and asked if the respondent had suffered any ADR incidents during the last 12 months (ADEs were not asked about in the survey). If they responded in the affirmative, they were identified as cases and asked about the nature of their ADR symptoms. The question about having an ADR in the last 12 months was formulated based on the World Health Organization’s (WHO’s) definition of ADRs [2]. Questions about ADR symptoms were guided by the WHO Anatomical Therapeutic Chemical Classification System with Defined Daily Doses [13]. Questions about whether the ADR was life-threatening, required hospitalization, resulted in permanent disability, affected the respondent’s pregnancy, or required medical intervention to prevent further damage were based on the United States Food and Drug Administration’s (US FDA’s) definition of a serious adverse drug experience (ADE) [14]. Respondents were asked to report the drug(s) that caused the ADR, and these were classified according to the Saudi Arabia Ministry of Health (MoH) formulary. Novel questions about the nature of the respondent’s experience with the ADR, including how they identified the drug that caused the effect, the source of that drug, questions on labeling and instructions, care received for the ADR, and the nature of the suffering induced by the ADR were developed and piloted on the survey. Finally, questions were added at the end of the questionnaire to assess knowledge about and the use of the ADR reporting system that enables patients to report ADRs to the Saudi Food and Drug Authority (SFDA).
The questionnaire was reviewed by two focus groups (n = 7 and n = 8) of Saudi residents who were recruited by phone by the SFDA. Feedback from the focus groups was incorporated into the questionnaire and then reviewed by several researchers.
Recruitment Methods
Study participants were recruited from 282 community pharmacies across all 13 regions in KSA by registered pharmacists who were trained to use this study assessment tool. Pharmacists approached pharmacy visitors and obtained verbal consent from the participants. Because of the nature of the public space, it was not possible to develop a denominator and numerator to accurately characterize a response rate. No personally identifiable data were collected. QPlatform® served as a computer-assisted interview (CAI) interface to guide the interviewer (pharmacist) regarding the survey questions [11]. Once the sampling quotas were filled, QPlatform® prevented respondents from ineligible demographic categories from participating.
Data Analysis
Analysis was completed in the R software package [15]. Descriptive analysis was conducted for ADR rates, sample demographic and clinical characteristics, and ADR patterns. Chi-squared tests with alpha set at 0.05 were performed for bivariate analysis. Bivariate analysis was conducted for descriptive purposes, and all results are considered to be post hoc and to have no predefined hypothesis. Weights to use for analysis were calculated from census data provided by the General Authority for Statistics [16]. The survey package in R was used to develop weighted rates with 95% confidence intervals (CIs) [17]. Figures were prepared in Excel.
Results
Between June and August 2018, a total sample of 5228 respondents completed the study. Mean age was 39 ± 15 years (range 18–98 years). Overall, there was an even split of men vs. women (50.17% vs. 49.83%). Median age was 36 years old, which is consistent with the background population, where 69% of the population is under 40 years old [16].
Objective 1: Prevalence of Adverse Drug Reactions in Saudi Arabia
Table 1 provides a bivariate analysis of the sample stratified by those who experienced an ADR vs. those who did not. According to this analysis, there were no significant associations between ADR status and gender (p = 0.8034), cardiovascular disease status (p = 0.1143), arthritis status (p = 0.7610), depression status (p = 0.6251), cancer status (p = 0.8568), or thyroid disease status (p = 0.6548). Those with ADRs were statistically significantly more likely to be in the age range of 37–56 (p < 0.0001), to have a higher level of education (p < 0.0001), and to have high blood pressure (p < 0.0001), high cholesterol (p = < 0.0001), diabetes (p = 0.0037), gastrointestinal diseases (p = 0.0025), and multiple comorbidities (p = 0.0026). Those who reported having chronic lung disease (p = 0.0048), kidney disease (p = 0.0080), or another chronic disease (p = 0.0140), as well as those who reported that they had never been diagnosed with a chronic disease (p = 0.0009) were less likely to have had an ADR in the past year.
Table 1.
Demographics of the sample stratified by adverse drug reaction status
| Category | Level | All | Experienced ADR | Did not experience ADR | Chi square p value |
|---|---|---|---|---|---|
| n, % | n, % | n, % | |||
| All | All | 5228 (100.00) | 1226 (23.45) | 4002 (76.55) | NA |
| Gender | Male | 2623 (50.17) | 593 (48.37) | 2030 (50.72) | 0.8034 |
| Female | 2605 (49.83) | 633 (51.63) | 1972 (49.28) | ||
| Age group | 18–36 | 2626 (50.23) | 590 (48.12) | 2036 (50.87) | < 0.0001 |
| 37–56 | 1961 (37.51) | 508 (41.44) | 1453 (36.31) | ||
| 57+ | 641 (12.26) | 128 (10.44) | 513 (12.82) | ||
| Highest level of education | Less than HS | 1509 (28.86) | 292 (23.82) | 1217 (30.41) | < 0.0001 |
| HS graduate | 946 (18.09) | 250 (20.39) | 696 (17.39) | ||
| College degree | 2003 (38.31) | 562 (45.84) | 1441 (36.01) | ||
| Postgraduate | 252 (4.82) | 60 (4.89) | 192 (4.80) | ||
| Unknown | 518 (9.91) | 62 (5.06) | 456 (11.39) | ||
| Chronic disease | High blood pressure | 749 (14) | 227 (19) | 522 (13) | < 0.0001 |
| High cholesterol | 568 (11) | 173 (14) | 395 (10) | < 0.0001 | |
| Diabetes | 732 (14) | 203 (17) | 529 (13) | 0.0037 | |
| Cardiovascular disease | 288 (6) | 56 (5) | 232 (6) | 0.1143 | |
| Chronic lung disease—asthma and emphysema | 311 (6) | 52 (4) | 259 (6) | 0.0048 | |
| Osteoporosis and rheumatoid arthritis | 422 (8) | 102 (8) | 320 (8) | 0.7610 | |
| Depression or anxiety disorder | 342 (7) | 76 (6) | 266 (7) | 0.6251 | |
| Cancer | 47 (1) | 10 (1) | 37 (1) | 0.8568 | |
| Kidney disease | 165 (3) | 24 (2) | 141 (4) | 0.0080 | |
| Thyroid disease | 236 (5) | 52 (4) | 184 (5) | 0.6548 | |
| Gastrointestinal diseases | 454 (9) | 133 (11) | 321 (8) | 0.0025 | |
| Other chronic disease | 118 (2) | 16 (1) | 102 (3) | 0.0140 | |
| Had never been diagnosed with chronic disease | 1716 (33) | 362 (30) | 1354 (34) | 0.0009 | |
| Number of comorbidities reported | Zero | 1688 (32) | 348 (28) | 1340 (33) | 0.0026 |
| One | 2943 (56) | 711 (58) | 2232 (56) | ||
| Two | 398 (8) | 116 (9) | 282 (7) | ||
| Three | 142 (3) | 35 (3) | 107 (3) | ||
| Four or more | 57 (1) | 16 (1) | 41 (1) |
ADR adverse drug reaction, HS high school. College degree corresponds to a two-year diploma or bachelor’s degree. Postgraduate corresponds to a master’s degree or a doctorate. NA not applicable
Across the entire sample, 33% reported having no chronic disease, while 56% had one, 8% had two, and 4% had three or more. These rates differed significantly between groups in that among those reporting ADR symptoms, 28% had no chronic disease, 58% had one, 9% had two, and 4% had three or more, while those with no ADR symptoms included 33% with no chronic disease, 56% with one, 7% with two, and 4% with three or more. In other words, of the 3540 respondents (68% of sample) who reported at least one chronic disease, 878 (25%) reported experiencing symptoms of an ADR in the last year.
As shown in Table 1, a total of 1226 respondents (23%) in the sample reported experiencing symptoms of an ADR within the past year. Table 2 shows the weighted and unweighted prevalence of ADR in KSA and by region. As seen in Table 2, the sample prevalence was 23.45% [95% confidence interval (CI) 22.3–24.6%], and the weighted population-based prevalence was 28.00% (95% CI 26.10–30.00%).
Table 2.
Prevalence of adverse drug reactions in Saudi Arabia during 12 months (2017–2018): national and regional estimates
| Category | Level | Sample prevalence of ADR | Estimated population prevalence of ADR | ||||
|---|---|---|---|---|---|---|---|
| Total n | Total n ADR | % (95% CI) | Estimated N | Estimated N of ADRs | % (95% CI) | ||
| All | 5228 | 1226 | 23.45 (22.30–24.60) | 6229,421 | 1743,982 | 28.00 (26.10–30.00) | |
| Region | Al Jouf | 399 | 130 | 32.58 (27.98–37.18) | 101,034 | 31,646 | 31.32 (29.42–32.90) |
| Northern Borders | 405 | 121 | 29.88 (25.42–34.33) | 68,195 | 19,758 | 28.97 (26.94–30.67) | |
| Tabuk | 403 | 90 | 22.33 (18.27–26.40) | 134,576 | 31,017 | 23.05 (20.56–25.15) | |
| Ha’il | 398 | 178 | 44.72 (39.84–49.61) | 195,144 | 80,900 | 41.46 (40.63–42.15) | |
| Al Madinah | 405 | 56 | 13.83 (10.47–17.19) | 235,110 | 38,828 | 16.51 (13.65–18.96) | |
| Al Qasim | 389 | 124 | 31.88 (27.25–36.51) | 307,875 | 97,550 | 31.68 (29.78–33.26) | |
| Makkah | 404 | 107 | 26.49 (22.18–30.79) | 1656,961 | 460,464 | 27.79 (25.48–29.70) | |
| Al Riyadh | 401 | 121 | 30.17 (25.68–34.67) | 1,800,650 | 569,926 | 31.65 (29.68–33.28) | |
| Eastern Province | 410 | 175 | 42.68 (37.90–47.47) | 1,445,400 | 611,725 | 42.32 (41.46–43.03) | |
| Al Baha | 411 | 16 | 3.89 (2.02–5.76) | 12,296 | 455 | 3.70 (1.95–5.32) | |
| Asir | 401 | 34 | 8.48 (5.75–11.21) | 131,302 | 11,849 | 9.02 (6.46–11.31) | |
| Jizan | 400 | 41 | 10.25 (7.28to 13.22) | 108,592 | 11,586 | 10.67 (8.1–12.94) | |
| Najran | 402 | 33 | 8.21 (5.53–10.89) | 32,288 | 2809 | 8.70 (6.22–10.91) | |
ADR adverse drug reactions, CI confidence interval
Objective 2: ADR Symptoms Reported
Table 3 presents the frequency of ADR symptoms reported among the 1226 (23%) respondents who reported experiencing such symptoms within the last year (stratified by gender).
Table 3.
Distribution of ADR symptoms reported within the last year by symptom and gender
| Category | Level | All† n, % |
Gender‡ | Chi square p value | |
|---|---|---|---|---|---|
| Male | Female | ||||
| n, % | n, % | ||||
| All | All | 1226, 100 | 593, 48.37 | 633, 51.63 | NA |
| Symptom category | Gastrointestinal disorders | 720, 58.73 | 312, 43.33 | 408, 56.67 | < 0.0001 |
| General disorders and administration site conditions | 242, 19.74 | 109, 45.04 | 133, 54.96 | 0.2782 | |
| Nervous system disorders | 259, 21.13 | 104, 40.15 | 155, 59.85 | 0.0036 | |
| Psychiatric disorders | 92, 7.50 | 45, 48.91 | 47, 51.09 | 0.9999 | |
| Skin and subcutaneous tissue disorders | 72, 5.87 | 34, 47.22 | 38, 52.78 | 0.9369 | |
| Musculoskeletal and connective tissue disorders | 29, 2.37 | 17, 58.62 | 12, 41.38 | 0.3524 | |
| Cardiac disorders | 49, 4.00 | 24, 48.98 | 25, 51.02 | 1.000 | |
| Respiratory, thoracic, mediastinal disorders | 4, 0.33 | 1, 25.00 | 3, 75.00 | 0.6631 | |
| Reproductive system and breast disorders | 50, 4.08 | 46, 92.00 | 4, 8.00 | < 0.0001 | |
| Vascular disorders | 10, 0.82,00 | 1, 10.00 | 9, 90.00 | 0.0340 | |
| Weight increase | 39, 3.18 | 12, 30.77 | 27, 69.23 | 0.0382 | |
| Renal and urinary disorders | 11, 0.90 | 4, 36.36 | 7, 63.64 | 0.6190 | |
| Eye disorders | 19, 1.55 | 10, 52.63 | 9, 47.37 | 0.8860 | |
| Increased appetite | 30, 2.45 | 13, 43.33 | 17, 56.67 | 0.0007 | |
| Immune system disorders | 9, 0.73 | 5, 55.56 | 4, 44.44 | 0.9217 | |
| Other symptoms | 153, 12.48 | 93, 60.78 | 60, 39.22 | 0.0014 | |
†Column reports column percentages
‡Columns report row percentages
As shown in Table 3, the most common ADRs were gastrointestinal disorders, which were reported by over 58% of the respondents who experienced ADRs within the last year. General disorders and administration site conditions were also relatively high, with over 19% reporting symptoms. Other prevalent ADRs were nervous system disorders (21.13% in the sample) and other symptoms that are not listed (12.48% in the sample).
When comparing the symptoms experienced by men to those experienced by women, the only statistically significant differences were seen for gastrointestinal disorders (p < 0.0001), nervous system disorders (p = 0.0036), reproductive system and breast disorders (< 0.0001), vascular disorders (p = 0.0340), weight increase (p = 0.0382), increased appetite (p = 0.0007), and other symptoms not listed above (p = 0.0014). Within each symptom group, women were more likely to report gastrointestinal disorders (56.67% vs. 43.33%), nervous system disorders (59.85% vs. 40.15%), vascular disorders (90.00% vs. 10.00%), and increased appetite (56.67% vs. 43.33%) compared to men, and men were more likely to report reproductive system and breast disorders (92.00% vs. 8.00%) and other unspecified symptoms (50.78% vs. 39.22%) compared to women. Women have menstrual cycles and may become pregnant, which could explain the discrepancy in gastrointestinal disorders and increased appetite. Also, many nervous system disorders such as migraines and multiple sclerosis have higher prevalences in women, which may explain the discrepancy between the sexes in those symptoms. The disparately high reported prevalence of reproductive system symptoms in men compared to women was mainly explained by reports of erectile dysfunction.
Medication Groups that Cause ADRs
The 1226 respondents who reported an ADR listed a total of 822 medications that could have been responsible for their ADRs. The distribution of these medications by class is presented in Fig. 1.
Fig. 1.
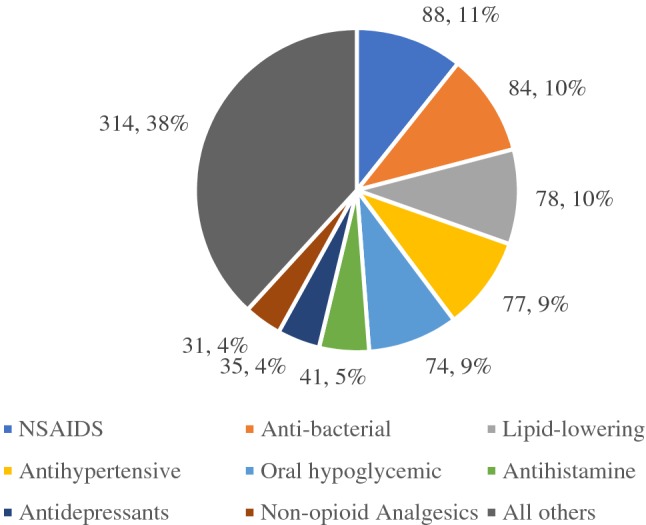
Distribution of medication classes that were reported to cause ADRs. NSAIDS nonsteroidal anti-inflammatory drugs
Per Fig. 1, the largest group of medications that were reported to be associated with ADRs (38%) was a group of 25 drug classes that were grouped together because none of them had many medication reports. The most common members of this large grouping were proton pump inhibitors, respiratory system drugs, thyroid and antithyroid drugs, and cough suppressants. The largest drug classes that were reported to cause ADRs were NSAIDS (11%), antibacterial medications (10%), lipid-lowering medications (10%), antihypertensive medications (9%), and oral hypoglycemics (9%).
ADR Severity and Emotional, Financial, and Physical Suffering
The 1226 respondents who reported that they had experienced ADRs also answered questions on the severity of their ADRs and their ADR-induced suffering. Table 4 shows the results for ADR severity, and Table 5 shows the results for suffering.
Table 4.
Patterns of reported ADR severity
| Category | Level | All† n, % |
Gender‡ | Chi square p value | |
|---|---|---|---|---|---|
| Male | Female | ||||
| n, % | n, % | ||||
| All | All | 1226 (100) | 593 (48.36) | 633 (51.63) | 0.8034 |
| Severity of ADRs reported | Hospitalized only | 37 (3.00) | 14 (37.83) | 23 (62.16) | 0.1800 |
| Required medical intervention | 235 (19.17) | 93 (39.57) | 142 (60.42) | 0.0011 | |
| Hospitalized and required medical intervention | 26 (2.12) | 9 (34.61) | 17 (65.38) | 0.1634 | |
| Did not require medical intervention | 944 (76.99) | 478 (50.63) | 466 (49.36) | 0.7805 | |
†Column reports column percentages
‡Columns report row percentages
Table 5.
Patterns of suffering in those who reported experiencing ADRs
| Category | Level | All | Gender | |
|---|---|---|---|---|
| Male | Female | |||
| n, % | n, % | n, % | ||
| All | All | 1226 (100) | 593 (48.37) | 633 (51.63) |
| Patterns of suffering in those reporting ADRs | Suffered physically, emotionally and financially | 163 (13.29) | 88 (53.98) | 75 (46.01) |
| Suffered physically | 528 (43.07) | 248 (46.96) | 280 (53.03) | |
| Suffered emotionally | 332 (27.08) | 149 (44.87) | 183 (55.12) | |
| Suffered financially | 275 (22.43) | 132 (48.00) | 143 (52.00) | |
Approximately 19% of those who suffered from ADRs required medical intervention (monitoring or treatment), but only 26 (2.12%) were hospitalized and required medical intervention, with females more likely to require hospitalization and medical intervention (p = 0.0011). Table 5 shows the patterns of suffering in those reporting ADRs. Among those reporting an ADR, 13% reported suffering physically, emotionally, and financially from the ADR. The most common type of suffering was physical, reported by 43.07%.
Objective 3: Awareness of the ADR Reporting System
The 1226 respondents who reported an ADR were asked if they were aware of the SFDA ADR reporting system; 371 (30.26%) reported that they were aware of it. Those who indicated they were aware of the reporting system were asked if they had ever filed a report in the system; 53 (14.29%) said they had made a report.
Discussion
This study found that in a sample of community pharmacy customers in KSA, over a quarter had experienced symptoms of at least one ADR in the last year. Women were more likely to experience ADR symptoms than men, with the most common symptoms in both men and women being nausea and gastric disorder. This study found that gastrointestinal disorders, nervous system disorders, vascular disorders, and increased appetite were more common ADRs in women, and that reproductive symptoms, specifically erectile dysfunction, were more prevalent in men. Female gender has been shown in multiple studies to be a risk factor for ADRs, but as was pointed out in a recent systematic review, this link is weak and probably confounded by other factors [18]. In our study, higher odds of ADR symptoms were associated with higher education levels and certain patient groups with chronic diseases. Respondents from Eastern Province and Ha’il were more likely to report ADR symptoms in the last year than those from Al Riyadh, while residents of provinces in the southwest corner of KSA were less likely to report ADR symptoms. Approximately one-third of all respondents were aware of the ADR reporting system.
There are no reference data from similar studies in KSA. However, the population-based estimate of the annual rate of ADR in the KSA population, 28%, is not too dissimilar to the monthly estimate of ADEs that occurred in Sweden, which was close to 20%, and of ADRs, which was 7.8% [8]. The estimate is also not inconsistent with the results of a similar pharmacy-based study in Italy, where consumers who were taking at least one medication were asked to report potential ADRs thet had experienced over the previous month, and the rate of reporting was found to be 9.2% [19].
The prevalence of chronic diseases is considered high in this study, due to the fact that this study was conducted in community pharmacies and that pharmacy customers were coming in for prescription refills or consultation. Logically, the more pharmaceuticals an individual consumes, the higher their risk for an ADR. Hence, those with chronic conditions that require medication, such as hypertension and diabetes, would be at higher risk for ADRs. This topic was raised in the systematic review referenced earlier, which noted that polypharmacy and the presence of multiple comorbidities were among the top ten risk factors for an ADR that leads to hospitalization [18]. In the present study, ADRs were found to be more common among those with hypertension, diabetes, high cholesterol, and gastrointestinal diseases. It is likely that this is confounding by indication, as all of these conditions are associated with prescribed medications. Conditions that were not associated with higher ADR rates in the study (such as depression and cardiovascular disease) often go unmedicated, and may be associated with increased symptoms of the condition but not with ADR risk [20, 21].
However, it is clear that the relative prevalence of ADRs in KSA differs markedly with the province. This may partly be due to different distributions of risk factors in different provinces. The primary risk factor was chronic disease status. In our study, Ha’il was found to have a high rate of ADRs; a cross-sectional survey of 5000 Ha’il residents found that the prevalence of diabetes was 31.1% overall, and over 70% of the diabetic patients surveyed were above normal weight [22]. Eastern Province was another region with a high rate of ADR in this study. One study found that the most common diseases in Al Ahsa in Eastern Province were obesity, hypertension, and glucose-6-phosphate dehydrogenase deficiency [23]. Another study in Eastern Province found a high rate of depression among type II diabetics [24]. It is possible that these complex local chronic disease pictures strongly influence not only who is taking medication but also the medications or usage patterns that confer these higher local risks of ADRs. Other risk factors that may differ from province to province include the number of and access to pharmacies and healthcare facilities, the ages of the residents, and the types of industry present. We realized that we need to learn more about the regional differences in KSA.
Studies of pharmacological utilization in KSA have yielded similar results to those given by corresponding studies in other countries: they indicate that the largest proportion of prescriptions are written for NSAIDs, and the next largest proportion is written for antibacterial agents [25]. This prescribing pattern can be related to the most prevalent symptoms in the population. These findings are consistent with a systematic review of risk factors for ADR in older individuals, which also found patterns of higher risk in certain areas [26].
In this study, higher education was associated with higher rates of ADR. This paradoxical finding may be explained by information bias, as those with higher health literacy were more likely to understand what an ADR is and report it. Even within provinces, education levels, health literacy, and literacy itself can vary widely. As an example, a case–control study of colorectal cancer patients in Makkah found that low cancer awareness (especially among individuals who were illiterate) was strongly associated with case status [27].
In terms of awareness of the ADR reporting system, the results in this study reflect those found in a systematic review of patient awareness of ADR reporting systems [28]. It is important to point out that the SFDA system is aimed at facilitating the filing of patient reports of ADRs directly onto the system either online or by phone; clinicians are not expected to be involved with the reports. On the other hand, some systems are aimed mainly at clinicians, so patient awareness of those systems tends to be lower [28]. In this study, approximately 70% of respondents were not aware of the reporting system, and the systematic review found that rates in other countries of poor awareness of ADR reporting systems ranged from a low of 44% in Portugal to a high of 93.8% in the United Kingdom (UK) [28].
To better understand the regional differences seen in this study, we realized that we need a better understanding of the prevalence of chronic diseases in the different provinces, and this will be a subject for future research. We also feel that we need a better understanding of the levels of health literacy, especially medication literacy, in the KSA population. These features will help us improve patient medication adherence and compliance, which was put forth as a risk factor for ADRs in the referenced systematic review [18]. Therefore, studies on the levels of health literacy should be conducted, and future research should focus on medication literacy in the KSA population. In addition, it would be helpful if there was an international effort to generate consensus surveillance case definitions for community-occurring ADEs and ADRs. This would allow researchers to synchronize their definitions in their research and would make results easier to compare.
The main strength of this study was that the results provide the first population-based estimate of ADR rates during 1 year in KSA. This study will enable regulatory authorities to develop an infrastructure to monitor this metric as part of public health and to craft interventions that could be targeted to populations with low literacy or chronic diseases in order to reduce rates of ADR. A limitation of the study is that the ADRs were self-reported rather than observed, making them more at risk of measurement error and recall bias. However, we believe that the collection of the data in interviews performed by registered pharmacists reduced such effects in this study, as the pharmacist read every question to the respondent and tried to get the respondent to remember if they had experienced such an event. Methodologic research supports this contention [29]. Another limitation of the study is that recruiting participants from community pharmacies may have introduced a selection bias toward healthier respondents as well as respondents with particular shopping habits (e.g., those filling prescriptions for themselves or family members). However, this does not necessarily invalidate the study, as research has shown that selection bias rates are different at different locations, so selection bias may have been mitigated by including many pharmacies and locations in this study [30].
Conclusion
In conclusion, our study calculated a 1-year population-based rate of ADR in KSA of 28%, and identified risk factors for ADRs, such as certain age and education groups and certain chronic disease groups. We also observed that low health literacy and low medical literacy in KSA may lead to measurement and reporting challenges. We recommend conducting more research and deploying educational interventions to reduce ADR rates in KSA. Hopefully this can take place in an international context that promotes standardized measurement of ADEs and ADRs in the community.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
RA: designed the study, supervised data collection, conducted data analysis, and wrote the manuscript. RA: edited the manuscript. ASA: edited the manuscript. TA: designed the data collection tool and revised the data analysis. NFB: designed the study and supervised its progress. All authors made substantial contributions to the editing of the manuscript.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Compliance with Ethical Standards
Funding
This study was funded by way of a cooperation agreement between Al-Dawaa Pharmacies and the Saudi Food and Drug Authority (SFDA). The SFDA would like to thank Al-Dawaa Pharmacies for helping with the data collection in this study.
Conflict of interest
Rasha Almubark, Rawabi Aljadani, Amani S. Alqahtani, Thamir Alshammri, and Nasser F. BinDhim declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
References
- 1.Committee on Quality of Health Care in America. To err is human: building a safer health system. Washington, DC: National Academies Press; 2000. http://www.ncbi.nlm.nih.gov/books/NBK225182/. Accessed 22 Oct 2018. [PubMed]
- 2.World Health Organization. Safety of medicines—a guide to detecting and reporting adverse drug reactions—why health professionals need to take action. Introduction. Geneva: WHO; 2002. http://apps.who.int/medicinedocs/en/d/Jh2992e/1.html. Accessed 31 Oct 2018.
- 3.Shepherd G, Mohorn P, Yacoub K, et al. Adverse drug reaction deaths reported in United States: vital statistics, 1999–2006. Ann Pharmacother. 2012;46:169–75. 10.1345/aph.1P592. [DOI] [PubMed]
- 4.Patel H, Bell D, Molokhia M, et al. Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005. BMC Clin Pharmacol. 2007;7:9. 10.1186/1472-6904-7-9. [DOI] [PMC free article] [PubMed]
- 5.Pirmohamed M. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aagaard L, Hansen EH. Consumers’ reports of suspected adverse drug reactions volunteered to a consumer magazine. Br J Clin Pharmacol. 2010;69:317–318. doi: 10.1111/j.1365-2125.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alshammari TM, Al-Kathiri WH, Louet HL, et al. Completeness of adverse drug reactions reports of the Saudi adverse event reporting system. Saudi Med J. 2015;36:821–828. doi: 10.15537/smj.2015.7.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakkarainen KM, Sundell KA, Petzold M, et al. Prevalence and perceived preventability of self-reported adverse drug events—a population-based survey of 7099 adults. PLoS One. 2013;8:e73166. doi: 10.1371/journal.pone.0073166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parretta E, Rafaniello C, Magro L, et al. Improvement of patient adverse drug reaction reporting through a community pharmacist-based intervention in the Campania region of Italy. Exp Opin Drug Saf. 2014;13:21–29. doi: 10.1517/14740338.2014.939582. [DOI] [PubMed] [Google Scholar]
- 10.Aljadhey H, Mahmoud MA, Alshammari TM, et al. A qualitative exploration of the major challenges facing pharmacovigilance in Saudi Arabia. Saudi Med J. 2015;36:1097–1102. doi: 10.15537/smj.2015.9.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BinDhim NF. QPlatform. 2012. http://shproject.net. Accessed 30 Oct 2018.
- 12.National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Atlanta: US CDC; 2015. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed 25 Feb 2016.
- 13.World Health Organization. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). Washington, DC: WHO; 2018.https://www.who.int/classifications/atcddd/en/. Accessed 31 Oct 2018.
- 14.United States Food and Drug Administration. Code of federal regulations title 21. Silver Spring: US FDA; 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=314.80. Accessed 10 Jan 2020.
- 15.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. http://www.R-project.org.
- 16.Saudi Arabia General Authority for Statistics. Homepage. https://www.stats.gov.sa/en. Accessed 6 Jan 2020.
- 17.Lumley T. Survey: analysis of complex survey samples. 2018. https://CRAN.R-project.org/package=survey. Accessed 21 Oct 2018.
- 18.Suggett E, Marriott J. Risk factors associated with the requirement for pharmaceutical intervention in the hospital setting: a systematic review of the literature. Drugs Real World Outcomes. 2016;3:241–263. doi: 10.1007/s40801-016-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leone R, Moretti U, D’Incau P, et al. Effect of pharmacist involvement on patient reporting of adverse drug reactions: first Italian study. Drug Saf. 2013;36:267–276. doi: 10.1007/s40264-013-0028-8. [DOI] [PubMed] [Google Scholar]
- 20.Durand H, Hayes P, Morrissey EC, et al. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35:2346–2357. doi: 10.1097/HJH.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein CM, Gathright EC, Garcia S. Relationship between depression and medication adherence in cardiovascular disease: the perfect challenge for the integrated care team. Patient Prefer Adherence. 2017;11:547–559. doi: 10.2147/PPA.S127277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed H, Ginawi I, Alshammari F, et al. Current burden of diabetes in Kingdom of Saudi Arabia in an epidemiological survey. Egypt Acad J Biol Sci C Physiol Mol Biol. 2014 doi: 10.21608/eajbsc.2014.16034. [DOI] [Google Scholar]
- 23.Sayegh HAA, Qurini AAA, Khan AS, et al. Patterns of eating associated with the chronic diseases in Al-Ahsa, Saudi Arabia. Int J Commun Med Public Health. 2017;4:3517–3523. doi: 10.18203/2394-6040.ijcmph20174213. [DOI] [Google Scholar]
- 24.El Mahalli A. Prevalence and predictors of depression among type 2 diabetes mellitus outpatients in Eastern Province, Saudi Arabia. Int J Health Sci (Qassim) 2015;9:119–126. [PMC free article] [PubMed] [Google Scholar]
- 25.Irshaid YM, Al-Homrany MA, Hamdi AA, et al. A pharmacoepidemiological study of prescription pattern in outpatient clinics in Southwestern Saudi Arabia. Saudi Med J. 2004;25:1864–1870. [PubMed] [Google Scholar]
- 26.Alhawassi TM, Krass I, Bajorek BV, et al. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–2086. doi: 10.2147/CIA.S71178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alazzeh AY, Azzeh FS. Active lifestyle patterns reduce the risk of colorectal cancer in the Mecca region, Saudi Arabia: a case-control study. Eur J Cancer Prev. 2018;27:438–442. doi: 10.1097/CEJ.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 28.Al Dweik R, Stacey D, Kohen D, et al. Factors affecting patient reporting of adverse drug reactions: a systematic review. Br J Clin Pharmacol. 2017;83:875–883. doi: 10.1111/bcp.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brüderl J, Castiglioni L, Ludwig V, et al. Collecting event history data with a panel survey: combining an electronic event history calendar and dependent interviewing. Methods Data Anal. 2017;11:22. doi: 10.12758/mda.2016.013. [DOI] [Google Scholar]
- 30.Edwards LA, Campbell P, Taylor DJ, et al. Healthy shopper? Blood pressure testing in a shopping centre pop-up in England. BMC Public Health. 2019;19:42. 10.1186/s12889-018-6370-0. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


