Abstract
In the present study, we have experimentally and theoretically studied the free-radical quenching property of dihydrocanaric acid (DCA) isolated from seedpods of Holarrhena antidysenterica. A modified method was used to estimate the nitric oxide scavenging effect of the DCA (significant activity of 75.22%) along with methanolic extract of seed pods of Holarrhena antidysenterica (72.80%) compared to the ascorbic acid as standard (40.60%). Studies have also been conducted for superoxide scavenging activity of the DCA (78.82%) and methanolic extract of seed pods (84.28%) compared to quercetin as standard (82.08%). Theoretically, it has been determined by density-functional theory(DFT) calculations using M06-2X hybrid functional and the double-ζ- split-valence 6-31G (d, p) basis set that the nitric oxide scavenging activity of the compound is by the addition of NO radical at double bond position. Predicted biological activity profile of DCA suggests that it has less activity probability (Pa) for toxicity (Pa = 0.730), cytotoxicity (Pa = 0.208), compared to those chemical entities that are already known as anticancer agents indicating that DCA is less toxic and more tolerable for normal cells. Furthermore, molecular docking studies of the DCA with different studied cancer-related receptors [Estrogen receptor (− 60.12 kcal/mol), epidermal growth factor receptor (EGFR) (− 30.33 kcal/mol), estrogen receptor alpha (− 4.82 kcal/mol), uPAR (− 32.55 kcal/mol) and an enzyme having lipid kinase activity phosphoinositide 3-kinase (− 55.94 kcal/mol)] were found to have better binding affinities compared to betulinic acid and doxorubicin. Thus, our findings suggest that the DCA could be a safer and effective alternative in fighting cancer with minimal side effects.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02221-5) contains supplementary material, which is available to authorized users.
Keywords: Radical scavenging, Nitric oxide, Superoxide, DFT, Docking, Receptors
Introduction
Cancer is one of the primary reasons for death today. Globally, one in six deaths is due to cancer. There were 9.6 million deaths in 2018 (WHO) (https://www.who.int/news-room/fact-sheets/detail/cancer). Free radicals have a key role in the initiation and causing cancer (Dizdaroglu et al. 2002). 75% of the anti-cancer drugs present are of natural product origin or semi-synthetic natural compounds (Newman and Cragg 2016; Iqbal et al. 2017; Rayan et al. 2017; Mitra and Dash 2018). Cancer researchers have great hope on natural compounds for their potential to affect multiple cancer cells without side effects. The present communication deals with studies on a significant triterpene, dihydrocanaric acid (DCA) isolated from the seedpods of Holarrhena antidysenterica (Linn.). (Family: Apocynaceae) (Beng. kurchi, Sans. kutaja) which has a reputation for having a cytotoxic effect (Sharma et al. 2014). DCA can serve as a potent molecule to fight cancer.
The compounds which can scavenge or trap nitric oxide radical can be helpful in the treatment of cancer. Free radicals and oxidants can alter the cell membranes and DNA (Droge 2002; Genestra 2007; Pacher et al. 2007) by the formation of conjugated diene compounds, which are cytotoxic and mutagenic. The continuous formation of such conjugated diene compounds and hence DNA damage proceeds fast through a radical chain reaction (Pourahmad et al. 2016). Proteins get damaged by ROS/RNS, leading to structural changes and loss of enzyme activity (Pham-Huy et al. 2008). Oxidative damage to DNA leads to the formation of different oxidative DNA lesions which can cause mutations. Hence, the study of this significant triterpene, DCA, which could act as a potential superoxide (O2·−) and nitric oxide (NO.) inhibitor has been presented in this paper. Presently, we have studied the nitric oxide, superoxide quenching property of DCA and theoretically determined the actual position which is responsible for the radical quenching activity of the compound using DFT calculations. This study helps to understand the structure–activity relationship of DCA in radical-scavenging reactions.
The experimental and theoretical confirmation of the radical scavenging activity of DCA directs us towards finding the anticancer activity of the compound as radical scavenging activity has a key role in treatment of cancer (Khan et al. 2016; Das et al. 2018). Hence, biochemical and bioinformatic approaches have been utilized for the determination of anticancer activity of the studied molecule, DCA. Docking score (kcal/mol) of DCA with receptors responsible for different cancers (breast, lung, prostate, cervical, oral, colon)-[estrogen receptor (Raut et al. 2019), EGFR (Cortiula et al. 2019), androgen receptor (Fujita and Nonomura 2019), estrogen receptor alpha (López-Romero et al. 2019), uPAR (Boonstra et al. 2017)] and phosphoinositide 3-kinase (Yueh et al. 2016), a lipid kinase protein involved in the pathogenesis of cancer, has been compared to that of doxorubicin, well-known chemotherapy drug (Agudelo et al. 2012; Eswaramoorthy et al. 2001) and a natural compound, betulinic acid used in the treatment of cancer.
Methods
Dihydrocanaric acid (DCA) (Fig. 1) has been isolated from the seedpods of Holarrhena antidysenterica by the usual column chromatographic method (Ghosh et al. 2009). The free-radical quenching properties have been studied experimentally and theoretically.
Fig. 1.
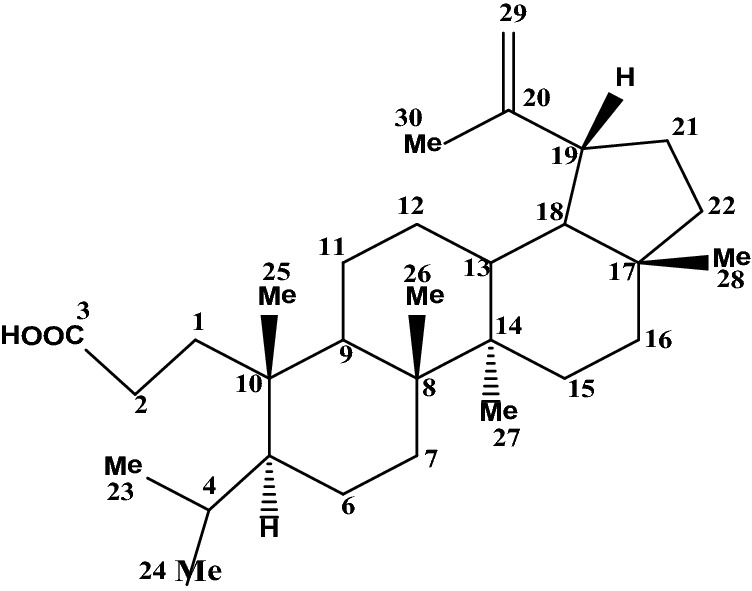
Chemical structure of dihydrocanaric acid (DCA)
Nitric oxide scavenging activity
A high concentration of nitrite has to accumulate for being detected with Griess reagent (Green et al. 1982; Marcocci et al. 1994). To produce a constant flow of nitric oxide over hours, a chemical source of nitric oxide was used, viz., sodium nitroprusside, which spontaneously produces nitric oxide when dissolved in aqueous solution at physiological pH (7.37). Sodium nitroprusside solution was freshly prepared by taking 10 mM sodium nitroprusside, 20 mM phosphate buffer (pH 7.4) previously bubbled with argon. Griess reagent was prepared by adding solution A to solution B. Solution A was prepared by taking 2% w/v sulfanilamide in distilled water and then added to 4% w/v of phosphoric acid. Solution B contained 0.2% w/v N-(1-naphthyl)ethylenediamine dihydrochloride in distilled water. 0.5 mL of the ethanolic extract (1 mg/mL) of the compounds were taken and treated with 0.5 mL of sodium nitroprusside solution and incubated at 25 °C for 150 min. To prepare the control solution, 0.5 mL. of the sample solutions were added to 20 mM phosphate buffer (pH 7.4) and incubated at 25 °C for 150 min. No sodium nitroprusside was added. On completion of the incubation, 1 mL of Griess reagent was added to each sample solution, and the nitric oxide level was estimated spectrophotometrically at 542 nm. The absorbance of the sample solutions was recorded against the absorbance of the control samples (Mandal et al. 2011; Gangwar et al. 2014). Percentage of inhibition of nitric oxide of the methanolic extract of seed pod of the plant and DCA was estimated from the difference of absorbance of the control and test samples.
Superoxide quenching activity
Superoxide scavenging activity of DCA was determined by monitoring its effects on the reduction of cytochrome c mediated by superoxide. A superoxide generation system was prepared by adding 1 mL of 0.5 M NaOH to 100 mL of dimethyl sulphoxide (DMSO) and incubating it for 30 min at 25 °C. The concentration of 1 mg/mL has been taken for all the studied compounds and control. The reduction has been monitored spectrophotometrically by measuring the increase in absorbance of reduced cytochrome c at 550 nM by adding alcoholic extracts (1 mg/mL) of the compounds to 1 mL oxidized cytochrome c (15 mM) in phosphate buffer. To it, 0.5 mL alkaline DMSO was added and the absorbance measured at 550 nm in the UV spectrophotometer against a blank. A control was prepared under similar conditions without the addition of the extract. To avoid interference of the antioxidants with xanthine oxidase, the enzyme used to generate superoxides, a stable solution was prepared from alkaline sulphoxide. This was done by adding 1 ml of 0.5 M NaOH to 100 ml of dimethyl sulphoxide (DMSO) and incubating it for 30 min at 25 °C. 15 mM oxidized cytochrome c was added to 200 mM potassium phosphate buffer (pH 7.4) and 100 mM of EDTA. The percentage of the superoxide quenching activity was estimated from the difference of absorption of the sample solution from control.
Detection of radical scavenging sites using computational tools
Geometry optimizations are performed using M06-2X hybrid functional and the double-ζ- split-valence 6-31G (d,p) basis set. M06-2X developed by Truhlar (Zhao and Truhlar 2008a, b) is a dispersion inclusive functional and is good enough for this type of reaction studied here. Minima or TSs on the potential energy surface (PES) are verified by vibrational frequency analysis. The solvent effect is computed using the PCM solvent model. All the calculations were carried out on a LINUX-cluster using Gaussian 09 package. Gauss view program was used for the construction of the trial geometries and to analyze the Gaussian output files.
Predicting biological activity of DCA
PASS online tool (https://www.pharmaexpert.ru/passonline/) (Filimonov et al. 2014) was used to predict the pharmacological effects, biological activity, toxic and adverse effects, mechanisms of action, influence on gene expression, etc. of the compound DCA. Canonical SMILES of DCA was searched from PubChem (CC(C)C1CCC2(C(C1(C)CCC(= O)O)CCC3C2(CCC4(C3C(CC4)C(= C)C)C)C)C) and it was used for predicting its biological activities.
Molecular docking
Receptors and ligand
Chemical three-dimensional (3D) conformer of the ligand DCA (PubChem CID, 101449266), doxorubicin (Pubchem CID, 31703) and betulinic acid (PubChem CID, 64971) in SDF format were downloaded from PubChem database (Kim et al. 2015). Protein 3D structures in PDB format of different cancer target proteins estrogen (breast cancer) receptor (ER) (PDB, 2IOK), epidermal growth factor (EGFR) (PDB, 1IVO), human androgen receptor (PDB, 2AO6), phosphoinositide 3-kinase (PI3K) (PDB, 4FLH), estrogen (cervical cancer) receptor (PDB, IXPC) and urokinase-type plasminogen activator receptor (uPAR) (PDB, IYWH) were downloaded from Protein Databank (PDB) (www.rcsb.org) (Berman et al. 2000).
Receptor-ligand docking
Receptor-ligand docking studies were performed using the CLC drug discovery workbench software version 3.0. Following the user manual, optimization of protein and ligand was performed. Molegro Dock algorithm is used by the CLC drug discovery workbench software to study the interaction of protein receptors with chemical ligands. Lower the score higher is the affinity of the ligand for that receptor. Downloaded 3D structure of the receptor and optimized ligand were imported in the software. The potential binding pockets within the protein (receptors) structure were searched and targeted molecular docking between receptor and ligand was conducted. CLC drug discovery visualization tool was used to visualize (https://resources.qiagenbioinformatics.com/manuals/clcdrugdiscoveryworkbench/current/index.php?manual=Introduction_CLC_Drug_Discovery_Workbench.html) the interaction between protein (receptor) and ligand.
Results and discussion
Nitric oxide and superoxide scavenging activity
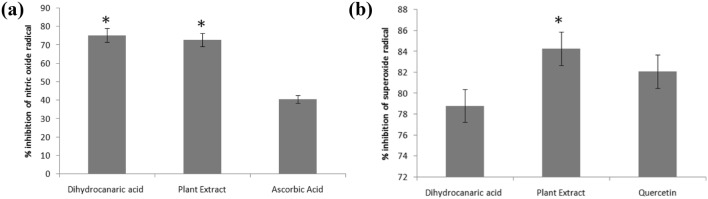
A modified method was used to estimate the nitric oxide scavenging effect of the compound. The nitric oxide scavenging activity indicated that DCA has significant scavenging activity of 75.22%, whereas the methanolic extract of seed pods of Holarrhena antidysenterica has 72.80% compared to the ascorbic acid as standard (40.60%) against nitric oxide (Fig. 2a).
Fig. 2.
Estimation of DCA scavenging activity (a) Nitric Oxide scavenging activity of seed pod (plant) extracts and DCA (b) Superoxide quenching activity of seedpod (plant) extract and DCA
Superoxide (O2·−), an important free radical, is generated in vivo by multiple sources, including xanthine oxidase or oxidative phosphorylation in mitochondria, which can produce superoxide as a result of leakage from the electron transport chain (Zhao et al. 2019; Cadenas and Davies 2000; Brand 2016).
This radical may further be converted into toxic, damaging hydroxyl radicals or peroxynitrite radicals which damage the cellular constituents. It has been found that DCA has comparable superoxide quenching activity (78.82%). However, the methanolic extract of seed pods from Holarrhena antidysenterica showed the maximum inhibition of 84.28%, compared to quercetin (82.08%) as standard (Fig. 2b). Quercetin has a key role in protecting DNA from oxidative damage resulting from the attack of superoxide on DNA oligonucleotides confirming that quercetin is a good scavenger for superoxide free radicals (Nimse and Pal 2015). It has been seen that quercetin has a better free-radical inhibition activity towards superoxide free radicals as compared to ascorbic acid. However, the free-radical inhibition activity for ascorbic acid is more than quercetin in case of NO free radical. Hence, the choice of standard controls has been decided accordingly for determining the respective scavenging activities of DCA (Srimathi Priyanga and Vijayalakshmi 2017). It has also been claimed that quercetin prevents inhibition of NO free radical-induced relaxation in mouse gastric fundus by scavenging superoxide radicals (Ertug et al. 2013; Trinity et al 2016). The radical scavenging activity shows that this molecule can be used in the treatment of cancer, which is a free radical-mediated disease.
Density-functional theory (DFT) analysis
Transition states (TS) of hydrogen abstraction by NO radical at COO–H, C2–H and allylic position at C19-H and addition reactions to the double bond by NO free radical have been calculated both in the gas phase and in ethanol using high-level DFT method. Supplementary Fig. 1 displays the different TS geometries of DCA while scavenging NO from its different positions. DCA has a significant nitric oxide scavenging activity and also comparable superoxide scavenging activity. The position of the molecule which is responsible for its radical quenching activity has been determined by DFT analysis (Supplementary Fig. 1). Transition states (TS) of hydrogen abstraction and addition reactions to the double bond by NO free radical have been calculated both in the gas phase and in ethanol by using a high-level DFT method. The relative energies obtained from this calculation (Supplementary Table 2) show that addition to the double bond is the lowest energy process which makes it the most probable pathway for radical scavenging activity of the isolated molecule. Relative transition state energy is lowest for the addition of NO radicals at unsaturated C–C bond position (0.00 kcal/mol in gas phase); (0.00 kcal/mol in EtOH) in comparison to hydrogen abstraction by NO radical at the allylic position (C19-H) (20.96 kcal/mol in gas phase); (23.57 kcal/mol in EtOH), hydrogen abstraction step by NO radical at C2–H (27.02 kcal/mol in gas phase); (29.15 kcal/mol in EtOH) and hydrogen abstraction step by NO radical at COO–H (39.60 kcal/mol in gas phase); (43.78 kcal/mol in EtOH) (Table 1). The overall result suggests that the DCA has a significant nitric oxide scavenging activity and also comparable superoxide scavenging activity. The position of the molecule which is responsible for its radical quenching activity has been determined by DFT analysis. Transition states (TS) of hydrogen abstraction and addition reactions to the double bond by NO free radical have been calculated both in the gas phase and in ethanol by using a high-level DFT method. The relative energies obtained from this calculation show that addition to the double bond is the lowest energy process which makes it the most probable pathway for radical scavenging activity of the isolated molecule. The radical scavenging activity shows that this molecule can be used in the treatment of cancer, which is a free radical-mediated disease.
Table 1.
Relative free energy for H- abstraction at different position of DCA by NO radical
| Reactions | kcal mol−1 (gas phase) | kcal mol−1 (ethanol phase) |
|---|---|---|
| DCA-C–C unsaturated + NO | 0.00 | 0.00 |
| DCA-C2-H + NO | 27.02 | 29.15 |
| DCA-C19H + NO | 20.96 | 23.57 |
| DCA-COO-H + NO | 39.60 | 43.78 |
Predicted biological activity profile of DCA
PASS online predicted results suggest that DCA is likely to have antineoplastic activity (Pa range 0.5–0.8) for lung, breast, colon, and cervical cancer (Table 2). Results also indicate that DCA could also be active in Prostate cancer treatment. Pa and Pi represent the probability of a compound to be active or inactive for a particular biological activity. Compound with Pa > 0.5 is more likely to be effective in action and treatment. Here, results suggest that DCA could be a promising compound in treating different types of cancer. Results suggest that DCA has comparable Pa values (Supplementary Table 1) to the known cancer drugs (lupeol and betulinic acid) suggesting DCA is also an active compound and could be used for treating different cancer types (especially for breast and colon). High Pa (probability “to be active”) value suggests that the studied compound is more likely to an active compound and its activity should be determined through an experimental assay. Compounds with Pa values < 0.5 could be considered non-active for the particular studied biological activity. Data in Supplementary Table 1 suggest that DCA has less activity probability (Pa) for toxicity (Pa = 0.730), cytotoxicity (Pa = 0.822), weakness (Pa = 0.356) and inflammation (Pa = 0.729) compared to lupeol and betulinic acid with higher Pa values. Predicted Pa value of all the tested chemical compounds for different biological activities was found to be higher than their corresponding Pi values. Overall data show that DCA is an active compound and could be further studied for molecular docking studies to predict its binding affinity with the different types of cancer-related receptors. In the future, the activity of DCA could be tested experimentally on the selected biological activity assays.
Table 2.
Biological activity predictions of Dihydrocanaric acid (DCA) using PASS online
| Pa (active) | Pi (inactive) | Activity |
|---|---|---|
| 0.889 | 0.005 | Antineoplastic |
| 0.879 | 0.006 | Mucomembranous protector |
| 0.778 | 0.004 | Antineoplastic (lung cancer) |
| 0.704 | 0.005 | Antineoplastic (breast cancer) |
| 0.667 | 0.006 | Antineoplastic (colon cancer) |
| 0.533 | 0.005 | Antineoplastic (cervical cancer) |
| 0.504 | 0.009 | Prostate cancer treatment |
| 0.486 | 0.022 | Prostate disorders treatment |
Molecular docking
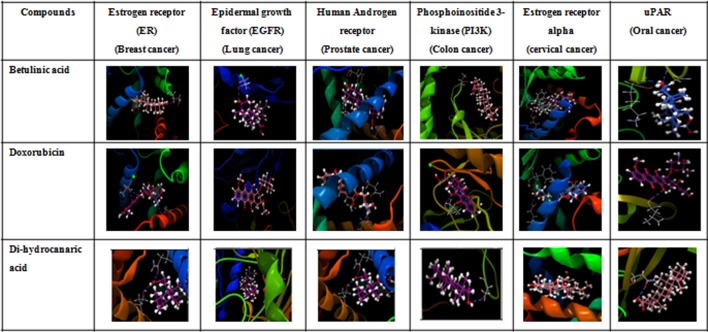
In the present work, different types of cancer (breast, lung, prostate, colon, cervical, oral) receptors (estrogen receptor, epidermal growth factor, androgen receptor, estrogen receptor alpha, and uPAR) and phosphoinositide 3-kinase protein were docked with DCA along with betulinic acid and doxorubicin. Protein docking interactions among receptors and ligand are shown in (Fig. 3). Molecular docking results suggest that DCA shows a higher binding affinity with most of the cancer-related receptors studied (Table 3). Doxorubicin showed the highest binding (− 140.21 kcal/mol) affinity for androgen receptor related to prostate cancer, while DCA (− 22.93 kcal/mol) showed better binding energy compared to betulinic acid (− 8.48 kcal/mol). For oral cancer-related receptor, uPAR-binding energy of DCA (− 32.55 kcal/mol) is comparable to doxorubicin (− 37.84 kcal/mol) (Table 3). Overall results indicate DCA seems to be a promising natural compound for the treatment of cancer.
Fig. 3.
Molecular docking of dihydrocanaric acid, betulinic acid, doxorubicin with estrogen receptor (ER) (PDB, 2IOK), epidermal growth factor (EGFR) (PDB, 1IVO), human androgen receptor (PDB, 2AO6), Phosphoinositide 3-kinase (PI3K) (PDB, 4FLH), Estrogen receptor alpha (PDB, IXPC) and uPAR (PDB, IYWH)
Table 3.
Molecular docking (binding) score of studied ligands with different cancer related receptors
| Cancer type | Receptors/protein | Dihydrocanaric acid (kcal/mol) | Betulinic acid (kcal/mol) | Doxorubicin (kcal/mol) |
|---|---|---|---|---|
| Breast | Estrogen receptor | − 60.12 | − 44.64 | − 51.67 |
| Lung | EGFR | − 30.33 | − 30.00 | − 29.62 |
| Prostate | Androgen receptor | − 22.93 | − 8.48 | − 140.21 |
| Colon | Phosphoinositide 3-kinase | − 55.94 | − 44.89 | − 54.53 |
| Cervical | Beta estrogen receptor | − 4.82 | 11.09 | 90.77 |
| Oral | uPAR | − 32.55 | − 27.66 | − 37.84 |
Conclusion
DCA showed highly significant nitric oxide scavenging and superoxide scavenging activity. DFT calculations further confirmed that DCA has a potent radical scavenging activity due to the addition reaction of the radical in the unsaturated C–C bond of the molecule. DCA was also predicted to be biologically active and less toxic to normal cells compared to other anticancer chemical entities. Molecular docking results further indicated that DCA has high affinity or binding with multiple cancer-related receptors and protein. Therefore, the molecule could serve as a potent anticancer agent. In the future, experimental studies are needed to confirm the activity, cytotoxicity of DCA on related cancer cell lines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Sincere thanks to the Department of Science and Technology-WOS (A) for financial assistance (Project No. SR/WOS(A)/CS-157/2016). Dr.Kuheli Chakrabarty, Department of Chemistry, Visva Bharati for her support. Professor Julie Banerji Ex-professor of Calcutta University, India, Professor Avijit Banerji, Department of Chemistry, National Research Institute for Ayurvedic Drug Development, Kolkata, India, and Dr.Manoj Kar, Nil Ratan Sircar Medical College and Hospital, Kolkata, India for their initial help. Sincere thanks to the Director, Chittaranjan National Cancer Institute, Kolkata, India.
Author contributions
AG: Experimental work and manuscript writing. GJT: Bioinformatics analysis and manuscript writing. CKP: Helped in analysis and manuscript writing.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Contributor Information
Anindita Ghosh, Email: anins30@rediffmail.com.
Gopal Ji Tiwari, Email: gopal.jt@gmail.com.
Chinmay Kumar Panda, Email: chinmaykumar.panda@cnci.org.in.
References
- Agudelo D, Bourassa P, Bruneau J, Berube G, Asselin É, Tajmir-Riahi H-A. Probing the binding sites of antibiotic drugs doxorubicin and N-(trifluoroacetyl) doxorubicin with human and bovine serum albumins. PLoS ONE. 2012;7(8):e43814. doi: 10.1371/journal.pone.0043814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28(1) [DOI] [PMC free article] [PubMed]
- Boonstra MC, Van Driel PBAA, Keereweer S, Prevoo HAJM, Stammes MA, Baart VM, Sier FM. Preclinical uPAR-targeted multimodal imaging of locoregional oral cancer. Oral Oncol. 2017;66:1–8. doi: 10.1016/j.oraloncology.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cortiula F, De Maglio G, Cangi MG, Gerratana L, Lisanti C, Bonura S, Fasola G, Follador A (2019) Third-generation tyrosine kinase inhibitor in the treatment of epidermal growth factor receptor mutated squamous cell lung cancer: a tailored therapy approach. Ann Transl Med 7(1) [DOI] [PMC free article] [PubMed]
- Das S, da Silva CJ, Silva MDM, Dantas MDDA, de Fátima Â, Ruiz ALTG, da Silva-Júnior EF (2018) Highly functionalized piperidines: Free radical scavenging, anticancer activity, DNA interaction and correlation with biological activity. Journal of advanced research, 9, 51–61 [DOI] [PMC free article] [PubMed]
- Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32(11):1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Ertuğ PU, Aydinoglu F, Ozturk OG, Singirik E, Ögülener N. Comparative study of the quercetin, ascorbic acid, glutathione and superoxide dismutase for nitric oxide protecting effects in mouse gastric fundus. Eur J Pharmacol. 2013;698(1–3):379–387. doi: 10.1016/j.ejphar.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Eswaramoorthy S, Kumaran D, Swaminathan S. Crystallographic evidence for doxorubicin binding to the receptor-binding site in Clostridium botulinum neurotoxin B. Acta Crystallogr D Biol Crystallogr. 2001;57(11):1743–1746. doi: 10.1107/s0907444901013531. [DOI] [PubMed] [Google Scholar]
- Filimonov D, Lagunin A, Gloriozova T, Rudik A, Druzhilovskii D, Pogodin P, Poroikov V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem Heterocycl Compd. 2014;50(3):444–457. [Google Scholar]
- Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: a review. World J Men's Health. 2019;37(3):288–295. doi: 10.5534/wjmh.180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwar M, Gautam MK, Sharma AK, Tripathi YB, Goel RK, Nath G. Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensis fruit extract on human erythrocytes: an in vitro study. Sci World J. 2014;2014:1–12. doi: 10.1155/2014/279451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19(9):1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Banerji A, Mandal S, Banerji J (2009) A new sesquiterpenoid coumarin from Ferula assafoetida. Nat Product Commun 4(8):1934578X0900400801 [PubMed]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA, Khalil AT. Plant-derived anticancer agents: a green anticancer approach. Asian Pac J Tropical Biomed. 2017;7(12):1129–1150. [Google Scholar]
- Khan MA, Rahman MM, Sardar MN, Arman MSI, Islam MB, Khandakar MJA, Alam AK. Comparative investigation of the free radical scavenging potential and anticancer property of Diospyros blancoi (Ebenaceae) Asian Pac J Tropical Biomed. 2016;6(5):410–417. [Google Scholar]
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Shoemaker BA. PubChem substance and compound databases. Nucleic Acids Res. 2015;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Romero R, Rodríguez-Esquivel M, Romero-Morelos P, García-Avilés JE, Serafín-Castillo A, Huerta Padilla VM, Martínez-Castillo MA. The expression of transcription factor BORIS and its association with the estrogen receptor beta (ER-β) in cervical carcinogenesis. Int J Clin Exp Pathol. 2019;12(9):3208. [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Hazra B, Sarkar R, Biswas S, Mandal N. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evid-Based Complement Alternat Med. 2011;2011:1–11. doi: 10.1093/ecam/nep072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci L, Packer L, Droy-Lefaix M-T, Sekaki A, Gardès-Albert M. Methods in enzymology. Amsterdam: Elsevier; 1994. [46] Antioxidant action of Ginkgo biloba extract EGb 761; pp. 462–475. [DOI] [PubMed] [Google Scholar]
- Mitra S, Dash R. Natural products for the management and prevention of breast cancer. Evid-Based Complement Alternat Med. 2018;2018:1–23. doi: 10.1155/2018/8324696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015;5(35):27986–28006. [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89. [PMC free article] [PubMed] [Google Scholar]
- Pourahmad J, Salimi A, Seydi E (2016) Role of oxygen free radicals in cancer development and treatment. In Free radicals and diseases, vol 16. IntechOpen, pp 315–330
- Raut PK, Kim SH, Choi DY, Jeong GS, Park PH. Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem Pharmacol. 2019;161:73–88. doi: 10.1016/j.bcp.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE. 2017;12(11):e0187925. doi: 10.1371/journal.pone.0187925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Hussain S, Bakshi M, Bhat N, Saxena AK. In vitro cytotoxic activity of leaves extracts of Holarrhena antidysenterica against some human cancer cell lines. Indian J Biochem Biophys. 2014;51:46–51. [PubMed] [Google Scholar]
- Srimathi PK, Vijayalakshmi K. Investigation of antioxidant potential of quercetin and hesperidin: an in vitro approach. Asian J Pharma Clin Res. 2017 [Google Scholar]
- Trinity JD, Broxterman RM, Richardson RS. Regulation of exercise blood flow: role of free radicals. Free Radic Biol Med. 2016;98:90–102. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh AE, Payne SN, Leystra AA, Van De Hey DR, Foley TM, Pasch CA, Deming DA (2016) Colon cancer tumorigenesis initiated by the H1047R mutant PI3K. PloS one 11(2) [DOI] [PMC free article] [PubMed]
- Zhao Y, Truhlar DG. Density functionals with broad applicability in chemistry. Acc Chem Res. 2008;41(2):157–167. doi: 10.1021/ar700111a. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoret Chem Acc. 2008;120(1–3):215–241. [Google Scholar]
- Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling. Int J Mol Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.