Abstract
The accumulation of reactive α-dicarbonyl leading to advanced glycation end products (AGEs) have been linked to pathophysiological diseases in many studies, such as atherosclerosis, cataract, cancer, and diabetic nephropathy. Glycation-generated AGEs increase the expression of inflammatory cytokines by transferring signals to the cell by binding them to the receptor for AGEs (RAGE) on their cell surface. The effect of methylglyoxal-derived AGEs (AGE-4) on the induction of matrix metalloproteinases (MMPs) in rat ordinary kidney cells (NRK-52E) was explored in this research, among other AGEs. The cell treated with 100 μg/mL AGE-4 for 24 h showed a substantial rise in MMP-2 and MMP-9 expression relative to BSA control only and other AGEs through ERK, JNK, and NF-B pathways. Our findings therefore suggest that AGE-4 expresses MMPs through the AGE-4-RAGE axis, activating MAPK signals that may contribute to dysfunction of the kidney cell.
Keywords: Advanced glycation end products, Diabetic nephropathy, Matrix metalloproteinase, MAPK, NF-κB
Introduction
Advanced glycation end products (AGEs) are produced by reacting of glycation to reducing sugars and carbonyl compound proteins (Ahmed, 2005; Zhang et al., 2009). Numerous studies have shown that AGEs long-term accumulation of cells in the body causes structural and functional changes in various organs, including kidneys (Linden et al., 2008; Yu et al., 2007 ), in which AGEs affect cellular dysfunction by inducing crosslinking of extracellular matrices, accumulation of kidney proximal epithelial cells, diabetic nephropathy, inflammation, and renal failure (Brownlee, 2001; Tan et al., 2007). In addition, decreased clearance in the kidney causes accumulation of AGEs in the kidney cells in diabetic kidney disease patients (Bhat et al., 2017; Goh and Cooper, 2008; Vlassara and Uribarri, 2014). AGEs bind with a receptor for AGEs (RAGE) that exists on the surface of the cell, and the AGE-RAGE binding promotes reactive oxygen species (ROS) production (Shi et al., 2013), and increases the expression of various cytokines through signaling pathways leading to inflammatory diseases (Del Pozo et al., 2017; Singh et al., 2017). Takeuchi et al. (2000) studied the antigen AGEs antibodies that specifically recognize the five AGE structures (AGE-1, glucose-induced AGEs; AGE-2, glyceraldehyde-induced AGEs; AGE-3, glycolaldehyde-induced AGEs; AGE-4, methylglyoxal (MGO)-induced AGEs; and AGE-5, glyoxal-induced AGEs) and carboxymethyllysine (CML)-AGEs antibodies (Ab) by applying the above-mentioned Ab to a column containing an affinity matrix coupled with five kinds of AGE-BSA or CML-BSA to make non-CML-AGE-Ab-2 to -5. These distinct anti-AGE Ab can detect non-CML-AGEs in the serum from diabetic patients on hemodialysis (Takeuchi et al., 2004). Recent studies by researchers have shown that MGO and AGEs are linked to causing kidney failure (Groener et al., 2017; Singh et al., 2001). However, there are not many studies that examined the effects of AGEs on signal pathways associated with normal kidney epithelial cells, and studies on AGEs signaling mechanisms are needed. Therefore, this study also evaluated whether treatment of kidney NRK-52E cells with AGE-1 to AGE-4 influences the induction of matrix metalloproteinases (MMPs), which might be responsible for the development of kidney fibrotic dysfunction. The signaling pathways that are triggered by AGE-RAGE interaction were also investigated.
Materials and methods
Cell culture of normal rat kidney epithelial cells
NRK-52E cells are renal proximal tubular epithelial cells having patterns of collagen secretion (Fan et al., 1999). NRK-52E cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM, HyClone, Logan, UT, USA) high glucose medium containing 10% (v/v) fetal bovine serum (FBS, GE Healthcare Life Sciences, Chicago, IL, USA) as a supplement and 1% penicillin/streptomycin (HyClone) in a CO2 incubation at 37 °C. The cultural medium has changed three times a week.
Chemicals and antibodies
Fatty acid-free BSA fraction V, PD989052, SB203580, SP600125, BAY 11-7082, phosphatase inhibitor cocktail 2 and All other chemical reagents were used from Sigma Aldrich Company (St. Louis, MO, USA). p38, phosphorylation-p38, JNK, phosphorylation-JNK, ERK, phosphorylation-ERK, NF-κB, RAGE, MMP-2, and MMP-9 antibodies were used from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) and penicillin/streptomycin were obtained from HyClone.
Preparation and identification of AGE-BSA
Fatty acid-free BSA fraction V (20 mg/mL) was incubated with the following sugars: 0.5 mM glucose, 20 mM glyceraldehyde, 20 mM glycolaldehyde, or 20 mM MGO in 0.1 M potassium phosphate buffer containing 0.02% sodium azide and 1 mM DTPA at O2 incubator at 37 °C for 7 days. These glycated AGEs were classified into AGE-1, AGE-2, AGE-3, and AGE-4. The fluorescence of AGEs was measured using a fluoro-intensity multi-sensing microplate reader at 370 nm (excitation) and 440 nm (emission) (HIDEX, Turku, Finland). These AGEs performed dialysis 24 h a day using buffers to remove non-reactive sugars and other low molecular weight reactants. Non-modified BSA as a control underwent the same procedure in the absence of sugars.
mRNA preparation and quantitative reverse transcription-PCR (qRT-PCR)
Total RNA was isolated from NRK-52E cells treated with AGEs for 24 h. In that case of a signal transfer inhibitor experiment, AGEs were treated in cells after 1 h-pretreatment with an inhibitor. Total RNA was extracted using TRIzol reagent from TAKARA Korea (Biomedical Co., Seoul, Republic of Korea), and cDNA was synthesized with the cDNA System Kit (LeGene Biosciences, San Diego, CA, USA). The gene expression of RAGE, MMP-2, MMP-9, and GAPDH were confirmed by qRT-PCR (Bio-Rad Laboratories, Hercules, CA, USA).
Cell extraction and Western blotting
NRK-52E kidney cells were cultured in 6-well plastic culture plates, incubated with 100 μg/mL of AGEs for 24 h, washed with cold PBS, and then suspended in RIPA lysis buffer (Elpis Biotech, Daejeon, Republic of Korea) with protease inhibitors (5 μg/mL of leupeptin and 5 μg/mL aprotinin) for preparation of total cell lysates. In the case of phosphatase inhibitor cell lysate, the inhibitor was added to the same buffer for lysis. To separate the nucleus in a cell, the cell was washed with PBS, and the cytoplasm was separated using a cytoplasmic extraction buffer (10 mM HEPES, pH 7.9 with 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 0.5 mM PMSF, and protease and phosphatase inhibitors). After removing the cytosolic extracts, the nucleus extraction buffer (20 mM HEPES, pH 7.9 with 420 mM KCl, 1 mM DTT, and 25% glycerol, 0.5 mM PMSF, plus protease and phosphatase inhibitors) was used to extract the nucleus (Makita et al., 1992). The protein content in the whole cell and nucleus was determined using the BCA protein assay kit (Pierce Biotechnology, Waltham, MA, USA). Proteins separated from cells were separated by 10% SDS-PAGE polyacrylamide gel electrophoresis and transferred to PVDF membranes (Merck Millipore, Billerica, MA, USA). We used anti-GAPDH antibody as a cytoplasm marker antibody (Santa Cruz Biotechnology Inc.) and the anti-Lamin B1 antibody as a nuclear marker antibody (Santa Cruz Biotechnology Inc.). The PVDF membranes used 5% fat-free dry milk to block the unspecific proteins in 0.1% Tris-saline buffer for 45 min at 25 °C and then, incubate overnight at 4 °C into specific primary antibodies, then incubate specific HRP coupled secondary antibodies for 45 min at 25 °C.
MMP-2 and MMP-9 gelatinase activity with zymography
NRK-52E cells were cultured with 10% FBS in a 6-well plate and seeded at 5 × 105 cells/ml. 100 μg/mL of AGEs were treated to the kidney cell for 24 h, and then conditioned media were collected in a 1.5 mL microcentrifuge tube. After centrifuged (1300 rpm, 20 min, 4 °C), the supernatant was collected. The samples were electrophoresed on an 7.5% acrylamide gel containing 4 mg/mL gelatin from bovine skin (Sigma-Aldrich, St. Louis, Missouri, USA). After electrophoresis, the gel was washed with renaturing buffer for 30 min (2.5% Triton X-100, 50 mM Tris–HCl, pH 7.5), incubated with developing buffer (Tris Base 12.1 g, Tris HCl 63.0 g, NaCl 117 g, CaCl2 27.4 g added distilled water up to 1 L) for overnight at 37 °C, and washed with distilled water, and then stained with Coomassie blue. The presence of MMP-2 and MMP-9 gelatinolytic activity were detected on a blue background after destaining.
Transfection and luciferase reporter assay
NRK-52E cells seeded at 5 × 104 cells/mL in 6well plastic culture plates were grown for 24 h, and Lipopectamine 2000 as transfection reagents (Invitrogen, CA, USA) was used to temporarily infect the intracellular lipopectase reporter Plasmid with NF-κB binding (Invitrogen, Carlsbad, CA, USA). Under the manufacturer’s reagent protocol, NRK-52E kidney cells were treated with AGE for 24 h after infection. Luciferase activity was confirmed 36 h after treatment with AGEs using the Dual-Luciferase Assay (Promega, Madison, WI, USA) (Promega, Madison, WI, USA).
Stable knockdown of RAGE by small interfering RNA (siRNA)
siRNA targeting RAGE reagents was purchased from Shanghai Gene Pharma (Shanghai, China). NRK-52E cells seeded at 4 × 104 cells/ml in 6-well plastic culture plates were grown until 80% confluence. NRK-52E cells were transfected with siRAGE using Lipofectamine 2000 as transfection reagents, under the manufacturer’s reagents protocols, the final siRAGE concentration for transfection was 100 nM. After treated reagents for 24 h, total mRNA was extracted to confirm the knockdown of RAGE. Cells were cultured with 100 μg/mL of AGEs for 24 h to examine MMP-2 and MMP-9 mRNA expression. The mRNA primer sequences used were as follows: 5′-GACCAACUCUCUCCUAUAUTT-3′ and 5′-AUACAGGAGAGAGUUGGUCTT-3′ for siRAGE; 5′-UCCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′ for negative control siRNA. Data were analyzed based on siRNA using a control group to evaluate mRNA knock-down efficiency.
Statistical analysis
The study data shows using the mean ± standard deviation by at least three experiments. These data are one-way ANOVA analysis after inspection by a t test analysis. A statistically significant difference in a p value of < 0.05.
Result and discussion
MMP-2 and MMP-9 expression in NRK-52E cells treated with AGEs were induced via the ERK/JNK pathways
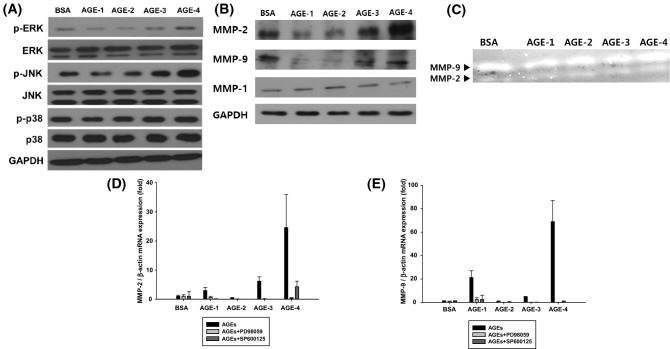
This study measured the viability of NRK-52E cells treated with AGE-1 to -4 (Fig. 1). There was no significant in cell viability when 100 μg/mL AGEs for 24 h. And then when NRK-52E cells were treated by AGEs for 24 h. AGE-4 strongly increased the phosphorylation of ERK and JNK, suggesting that ERK/JNK signaling was activated; however, p-p38 expression was not affected by AGE-4 as well as other AGEs (Fig. 2A). AGEs and RAGE interaction can produce ROS, which enhance MMPs expression via modulation of various transcriptional factors, including NF-κB (Makita et al., 1992). Additionally, AGEs can activate PKC, MAPK, and NF-κB, which regulate TGF-β expression, and subsequently MMP expression (Luo et al., 2014). In our study, NRK-52E cells treated with 100 μg/mL of AGEs for 24 h were examined for MMP-2 and MMP-9 mRNA expression (Fig. 2B). This study also found that MMP-2 and MMP-9 protein levels were the highest with AGE-4 treatment compared to treatment with other AGEs, whereas MMP-1 expression remained unaffected. Also, experiments were conducted to confirm that gelatinolytic activity of MMP-2 and MMP-9 in NRK-52E cells. Gelatinolytic activity is one of the highest when the cell was treated with AGE-4 at 100 μg/mL for 24 h (Fig. 2C). When qRT-PCR checked the expression of MMP-2 and MMP-9 mRNA, the AGE-4-treated cells showed the greatest increase (Fig. 2D, E). Additionally, to examine whether the MAPK pathway is involved in AGEs-induced MMP-2 and MMP-9 expression, NRK-52E cells were pre-treated with specific MAPK inhibitors against ERK (PD98059) and JNK (SP600125) prior to treatment with AGEs. Both PD98059 and SP600125 almost completely suppressed the AGEs-induced MMP-2 and MMP-9 mRNA expression (Fig. 2D, E).
Fig. 1.
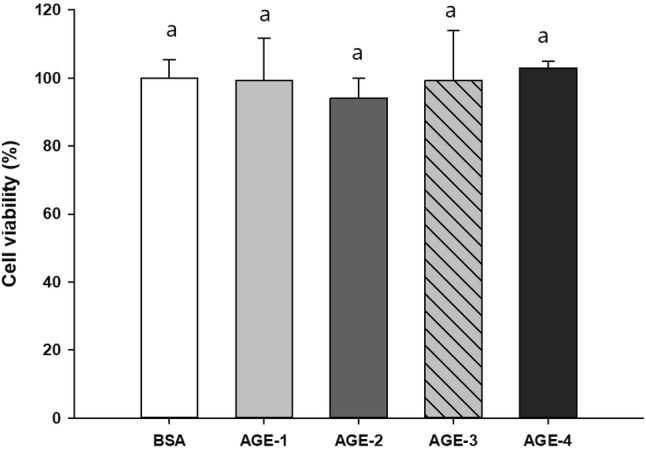
NRK-52E cells were incubated in DMEM high media containing 4.5 g/L of glucose in the absence and presence of 100 μg/mL of advanced glycation end products (AGEs) for 24 h. NRK-52E cells viability (mean ± SEM, n = 4) was measured by using MTT assay and expressed as percent cell survival relative to bovine serum albumin control. BSA, bovine serum albumin; AGE-1, glucose-induced AGEs; AGE-2, glyceraldehyde-induced AGEs; AGE-3, glycolaldehyde-induced AGEs; AGE-4, methylglyoxal (MGO)-induced AGEs; and AGE-5, glyoxal-induced AGEs
Fig. 2.
Enhanced metalloproteinases (MMP)-2 and MMP-9 protein expression were mediated by the ERK/JNK pathways in AGEs-treated NRK-52E cells. The activation levels of MMP-2 and MMP-9 in cells treated with AGEs and MAPK inhibitors (A) Immunodetection of phospolylation-ERK and phospolylation -JNK in untreated and AGEs-treated NRK-52E cells. (B) Protein expression of MMP-2 (72 kDa) and MMP-9 (92 kDa) were analyzed by Western blotting. MMP-2 and MMP-9 protein expression were determined in the NRK-52E cells treated with AGEs (100 μg/mL) for 24 h. (C) Twenty 24 h after the cells were treated with 100 μg/mL of AGEs, MMP-2 and MMP-9 gelatinolytic activity in NRK-52E cells was evaluated by zymography. Effect of treatment with ERK inhibitor (PD98059) and JNK inhibitor (SP600125) on the activation of (D) MMP-2 and (E) MMP-9 were evaluated using quantitative reverse transcription-PCR (qRT-PCR)
Treatment with AGE-4 upregulates MMP-2 and MMP-9 activation through activation of the NF-κB translocation
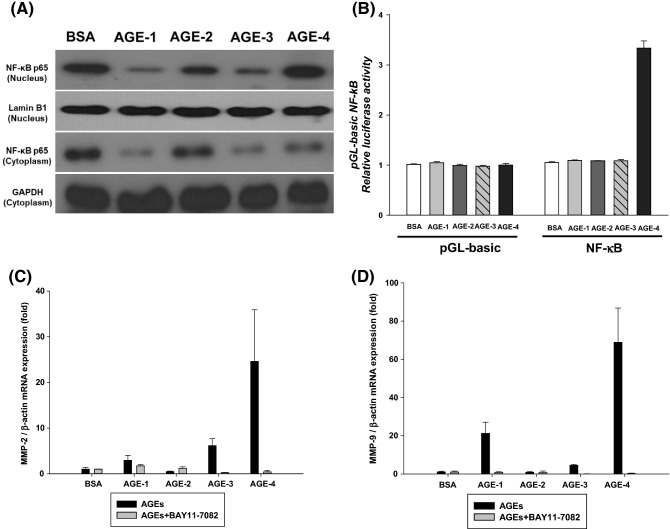
Next, it was investigated to confirm that AGEs causing the expression of MMP-2 and MMP-9 in NRK-52E cells transfer NF-κB from the cytoplasmic to the nucleus as a transcription factor. The expression of MMP-2 and MMP-9 in kidney cells was found to be controlled by moving NF-κB into the nucleus, which has been demonstrated to be regulated by AGE-4. Genetic activation of MMP-2 and MMP-9 is induced by the NF-κB gene-binding site located at the start of the transcription (Kanwar et al., 2011). Protein expression analysis using Western blots also showed an increased expression of NF-κB in the nucleus of the cell when AGE-4 was treated (Fig. 3A). As shown in Fig. 3B, we found that NF-κB has the highest luciferase activity when treating kidney cells with AGE-4 than when treating other AGEs. Pretreatment with BAY 11–7082 as NF-κB inhibitor, suppressed the mRNA expression of MMP-2 and MMP-9 mediated by AGE-4 (Fig. 3C, D). Overall, these data strongly suggest that AGE-4 enhances MMP-2 and MMP-9 through translocation of the NF-κB.
Fig. 3.
Enhanced MMP-2 and MMP-9 activity is mediated by the NF-κB pathway in the AGEs-treated NRK-52E cells. (A) Immunodetection of the NF-κB subunit p65 in the nucleus fraction of AGEs-treated NRK-52E cells followed by separation on 10% SDS–polyacrylamide gels. (B) Activation of the NF-κB promoter at the binding site in AGEs-treated and untreated NRK-52E cells was analyzed using the luciferase reporter assay. Translocation of NF-κB as a transcription factor from the cytoplasm to the nucleus in NRK-52E cells. Effect of treatment with NF-κB inhibitor (BAY11-7082) on the expression of (C) MMP-2 and (D) MMP-9 were evaluated using qRT-PCR
Activation of MMP-2 and MMP-9 via AGE-RAGE interaction
Finally, we investigated the role of RAGE in the induction of MMP-2 and MMP-9 in cells treated with AGEs. mRNA expression of RAGE was knocked down in siRAGE-transfected NRK-52E cells (Fig. 4A). MMP-2 (Fig. 4B) and MMP-9 expression (Fig. 4C) in siRAGE-transfected AGE-4-treated cells was compared with one in siControl cells. These results demonstrate that AGE-4 binds to RAGE and upregulates the activation of MMP-2 and MMP-9 (Fig. 5).
Fig. 4.
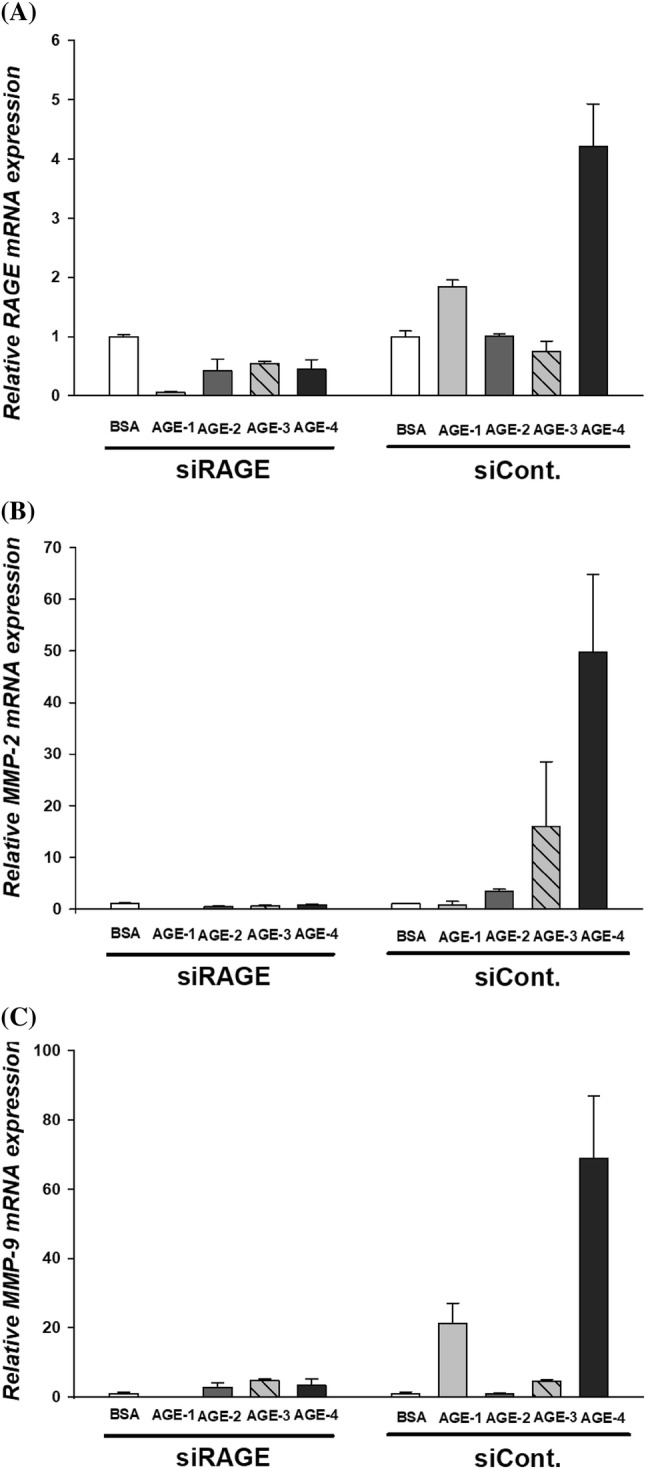
Detection of MMP-2 and MMP-9 expression after siRAGE transfection. Effects of RAGE knockdown by siRNA transfection in NRK-52E cells. NRK-52E were transfected with siRAGE (100 nM) using Lipofectamine 2000 transfection reagents and then treated with AGEs (100 µg/mL) for 24 h. Total mRNA was extracted and MMP-2, MMP-9, and β-actin expression was determined using qRT-PCR. (A) Downregulation of RAGE mRNA expression. (B) MMP-2 and (C) MMP-9 expression was enhanced by siRAGE-transfection of AGEs-treated NRK-52E cells
Fig. 5.
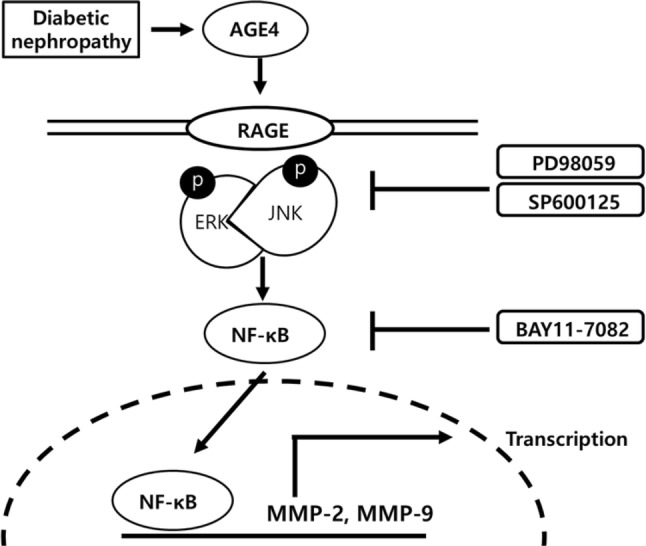
Proposed mechanism by which AGE-4 induced with fibrosis marker expression. Exposure to AGE-4 activate RAGE. Its activation increases NF-κB activation, which affects the protein levels of the MMP-2 and MMP-9. Finally, AGE-4 binding RAGE induced MAPK/ERK, JNK signal and NF-κB expression
Diabetic neuropathy in diabetes worldwide has been studied as a major cause of chronic kidney failure (Mason and Wahab, 2003; Xu et al., 2014). The main cause of kidney inflammation in diabetic patients was the accumulation of AGE in the kidneys, causing intraperitoneal inflammation (Kanwar et al., 2011; Stitt, 2001; Xu et al., 2014). The binding of AGEs to RAGE triggers pro-inflammatory intracellular signaling cascades (Rojas et al., 2018). A major cause for inflammation is the accumulation of AGEs in the kidney of diabetic nephropathy patients (Ahmed, 2005). Binding of AGEs to RAGE activates multiple cellular signaling MAPK cascades, including ERK activation in different cell types, such as smooth muscle cells, tubular myofibroblasts, and monocytes and SAPK/JNK, p38 activation in monocytes/macrophages and tumor cell (Mason and Wahab, 2003). Our previous studies revealed the effect of AGEs on various cell types. Glycated whey protein conjugated with glucose activates ERK and JNK in HepG2 cells (Pyo et al., 2016), while another conjugate of lysozyme and galactomannan enhances the activation of JNK, ERK, and NF-κB pathways (Ha et al., 2013). RAGE-mediated cellular signaling cascades indicate that different AGEs as well as cell types might involve different pathways. In addition to the five types of AGEs, Takeuchi et al. (2000) reported two more (AGE-6; 3-deoxyglucosone-derived AGEs and AA-AGE; acetaldehyde-derived AGEs) in the serum of a diabetic patient who makes hemodialysis using immunochemical specific antibodies (Makita et al., 1992). Among the different types of AGE specific structures that can occur in vivo, (AGE-2; glyceraldehyde-induced AGEs) and AA-AGE are proposed to cause neuronal toxicity (Stitt, 2001). The concentrations of AGE-2 and AA-AGE to induce neuronal cell death are 250–2000 μg/mL, as determined by MTT assay. In our experiment, we treated NRK-52E cells with 100 μg/mL of AGEs to achieve more than 85% cell viability and avoid causing cellular toxicity (Fig. 1). As a major precursor of AGEs, MGO was given in drinking water to normal rats for 14 weeks until 6 months of age (Rodrigues et al., 2014). The MGO-treated group had a phenotype similar to that of the same age-paired type 2 diabetic rats, including oxidative stress and structural kidney lesions. MGO treatment caused AGEs-related injuries and progression of diabetic nephropathy (Bourajjaj et al., 2003). AGE-4, MGO-induced AGE appears to be involved in causing diabetes and vascular complications (Rodrigues et al., 2014). Also, AGE-human serum albumin-induced RAGE expression has been shown to be mediated by MAPK/ERK and p38 dependent pathways, and not by MAPK/JNK independent pathway (Sun et al., 2009). The present study first reported that in NRK-52E kidney cells, compared to treatment with AGE-1 to -3. AGE-4 was activated by the phosphorylation action of ERK and JNK through binding with receptors, moving the transcription control factor, NF-κB, into the nucleus of the cell to activate it. In addition, this research showed the possibility of inducing MMPs to cause diabetic nephropathy. Although we did not investigate the chemical structure of AGE-4, glycation by MGO has been reported to affect mainly arginine. The formation of hydroimidazolones (MG-Hs) leads to the loss of positive charges leading to a loss of positive charge (Bourajjaj et al., 2003). MG-Hs are known to contribute to renal failure by interacting with RAGE (Xue et al., 2014). Due to formation of a positive feedback mechanism, AGEs increase RAGE expression in various cell types, which propagates an AGE-RAGE response (Bierhaus et al., 2005). In this study, we also observed that exposure of NRK-52E cells to AGE-4 induced the expression of RAGE. siRAGE transfection of kidney cells entirely knocked down RAGE expression induced by AGE-4. In recent years, investigators have reported that AGE-RAGE interaction activates signaling pathways, resulting in the activation of MMPs (Zhu et al., 2012). MMPs family is known to cause chronic kidney disease (CKD), and kidney fibrosis represents a failed wound healing in progressive CKD (Klein et al., 2004). MMP-2 and MMP-9 are known to promote cancer cell growth, including the metastasis of cancer cells (Deng et al., 2017) and are profibrotic through induction of renal tubular cell epithelial-mesenchymal transition (Zhu et al., 2012). In our study, MMP-2 and MMP-9 expression in NRK-52E cells increased after treatment with AGE-4. It has been confirmed that the combination of RAGE and AGEs regulates the signaling system and that downstream system regulates NF-κB as a transcription factor. These results are thought to help the basic research on kidney fibrosis by AGEs. In this study, our findings demonstrated that the treatment of NRK-52E kidney cells with AGE-4, compared with AGE-1 to -3 combined with RAGE, then stimulated signal transmission, activated ERK and JNK MAPK pathways through phosphorylation and increased gene expression of MMP-2 and-9 through NF-κB transcription. The AGE-4 induced by methylglyoxal plays an important role in causing diabetic nephrosis. AGE-4 initiated signal transductions including phosphorylation of ERK, JNK, and NF-κB leading to the production of inflammatory cytokines. RAGE plays an important role in the reaction of AGE, causing various signal delivery systems in the cell. We investigated the effect of different AGEs on kidney damage through signaling pathways involving MMPs family activation by RAGE, ERK, JNK, and NF-κB pathways in kidney cells. However, the in vivo experiments performed to verify the implication of this study remain to confirm whether AGE-4 exerts excessive proteolytic activity in the context of kidney fibrotic dysfunction.
Acknowledgements
This study was supported by Main Research Program (E0164400-04) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science, ICT and Future Planning, the National Research Foundation of Korea grant funded by the Korea government (MEST) (No. 2017R1A2B4012182), Korea University Research Grant No. K1516071, and School of Life Sciences and Biotechnology of Korea University for BK21 PLUS. The authors are grateful to the Korea University-CJ Food Safety Center (Seoul, Republic of Korea) for allowing access to their equipment and facilities.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
So-Ra Jeong, Email: wjdxmfhf@naver.com.
Ho-Young Park, Email: hypark@kfri.re.kr.
Yoonsook Kim, Email: kimyus@kfri.re.kr.
Kwang-Won Lee, Email: kwangwon@korea.ac.kr.
References
- Ahmed N. Advanced glycation end products-role in pathology of diabetic complications. Diabetes. Res. Clin. Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat S, Mary S, Giri AP, Kulkarni MJ. Advanced glycation end products (AGEs) in diabetic complications. In: Mechanisms of Vascular Defects in Diabetes Mellitus, vol 17, pp 423–449. Springer, Charm (2017)
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Bourajjaj M, Stehouwer CDA, van Hinsbergh VWM, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem. Soc. Trans. 2003;31:1400–1402. doi: 10.1042/bst0311400. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Del Pozo CH, Shekhtman A, Ramasamy R, Schmidt AM. The receptor for advanced glycation final product: mechanism and treatment opportunity for obesity and diabetes. Enzym. Metab. J. 2017;2:1–10. [Google Scholar]
- Deng R, Mo F, Chang B, Zhang Q, Ran H, Yang S, Zhu Z, Hu L, Su Q. Glucose-derived AGEs enhance human gastric cancer metastasis through RAGE/ERK/Sp1/MMP2 cascade. Oncotarget. 2017;8:104216–104226. doi: 10.18632/oncotarget.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast trans differentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Goh SY, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- Groener JB, Oikonomou D, Cheko R, Kender Z, Zemva J, Kihm L, Muckenthaler M, Peters V, Fleming T, Kopf S, Nawroth PP. Methylglyoxal and advanced glycation end products in patients with diabetes – what we know so far and the missing links. Exp. Clin. Endocrinol. Diabetes. 2017;1:5–67. doi: 10.1055/s-0043-106443. [DOI] [PubMed] [Google Scholar]
- Ha YM, Chun SH, Hong ST, Koo YC, Choi HD, Lee KW. Immune enhancing effect of a Maillard-type lysozyme-galactomannan conjugate via signaling pathways. Int. J. Biol. Macromol. 2013;60:399–404. doi: 10.1016/j.ijbiomac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. Mech. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Vellenga E, Fraaije MW, Kampsa WA, de Bont ESJM. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit. Rev. Oncol. Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Linden E, Cai W, He JC, Xue C, Li Z, Winston J, Vlassara H, Uribarri J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin. J. Am. Soc. Nephrol. 2008;3:691–698. doi: 10.2215/CJN.04291007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Hara T, Ishido Y, Yoshihara A, Oda K, Makino M, Ishii N, Hiroi N, Suzuki K. Rapid preparation of high-purity nuclear proteins from a small number of cultured cells for use in electrophoretic mobility shift assays. BMC Immunol. 2014;15:586. doi: 10.1186/s12865-014-0062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Z, Vlassara H, Ceramai A, Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J. Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1358–1373. doi: 10.1097/01.ASN.0000065640.77499.D7. [DOI] [PubMed] [Google Scholar]
- Pyo MC, Yang SY, Chun SH, Oh NS, Lee KW. Protective effects of Maillard reaction products of whey protein concentrate against oxidative stress through an Nrf2-dependent pathway in HepG2 cells. Biol. Pharm. Bull. 2016;39:1437–1447. doi: 10.1248/bpb.b16-00029. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Matafome P, Crisóstomo J, Silva DS, Sena C, Pereira P, Seiça R. Advanced glycation end products and diabetic nephropathy:a comparative study using diabetic and normal rats with methylglyoxal-induced glycation. J. Physiol. Biochem. 2014;70:173–184. doi: 10.1007/s13105-013-0291-2. [DOI] [PubMed] [Google Scholar]
- Rojas A, Anazco C, Gonzalez I, Araya P. Extracellular matrix glycation and receptor for advanced glycation end-products activation: a missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis. 2018;39:515–521. doi: 10.1093/carcin/bgy012. [DOI] [PubMed] [Google Scholar]
- Shi L, Yu X, Yang H, Wu X. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. Plos One. 2013;8:e66781. doi: 10.1371/journal.pone.0066781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Chaudhari BP, Kakkar P. Baicalin and chrysin mixture imparts cyto-protection against methylglyoxal induced cytotoxicity and diabetic tubular injury by modulating RAGE, oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2017;50:67–75. doi: 10.1016/j.etap.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br. J. Ophthalmol. 2001;85:746–753. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Liang C, Ren Y, Zhen Y, He Z, Wang H, Tan H, Pan X, Wu Z. Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic. Res. Cardiol. 2009;104:42–49. doi: 10.1007/s00395-008-0738-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol. Med. 2000;6:114–125. doi: 10.1007/BF03401779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Kikuchi S, Sasaki N, Suzuki T, Watai T, Iwaki M, Bucala R, Yamagishi SI. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr. Alzheimer. Res. 2004;1:39–46. doi: 10.2174/1567205043480582. [DOI] [PubMed] [Google Scholar]
- Tan ALY, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin. Nephrol. 2007;27:130–143. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr. Diab. Rep. 2014;14:453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Xiao L, Xiao P, Yang S, Chen G, Liu F, Kanwar YS, Sun L. A glimpse of matrix metalloproteinases in diabetic nephropathy. Curr. Med. Chem. 2014;21:3244–3260. doi: 10.2174/0929867321666140716092052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Ray R, Singer D, Böhme D, Burz DS, Rai V, Hoffmann R, Shekhtman A. The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry. 2014;53:3327–3335. doi: 10.1021/bi500046t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhang Y, Zhang H, Li Y. SOCS3 overexpression inhibits advanced glycation end product-induced EMT in proximal tubule epithelial cells. Exp. Ther. Med. 2007;13:3109–3115. doi: 10.3892/etm.2017.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Ames JM, Smith RD, Baynes JW, Metz TO. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J. Proteome. Res. 2009;8:754–769. doi: 10.1021/pr800858h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012;21:123–129. doi: 10.1111/j.1600-0625.2011.01408.x. [DOI] [PubMed] [Google Scholar]