Abstract
To determine the physiochemical properties of the 4-α-glucanotransferase from Bifidobacterium sp., the bllj_0114 gene encoding 4-α-glucanotransferase was cloned from Bifidobacterium longum subsp. longum JCM 1217 and expressed in Escherichia coli. The amino acid sequence alignment indicated that the recombinant protein, named BL-αGTase, belongs to the glycoside hydrolase (GH) family 77. BL-αGTase was purified using nickel-nitrilotriacetic acid affinity chromatography and characterized using various substrates. The enzyme catalyzed the disproportionation activity, which transfers a glucosyl unit from oligosaccharides to acceptor molecules, and had the highest activity at 40 °C and pH 6.0. In the presence of 5 mM metal ions, in particular Cu2+, Zn2+, and Fe2+, BL-αGTase activity was reduced. To determine whether BL-αGTase can be used to generate thermoreversible gels, potato starch was treated with BL-αGTase for various reaction times. The BL-αGTase-treated starches showed sol–gel reversibility and melted at 59.6–75.7 °C.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00707-4) contains supplementary material, which is available to authorized users.
Keywords: Bifidobacterium longum, 4-α-glucanotransferase, Thermoreversible gel, Transglycosylation
Introduction
Bifidobacteria are gram-positive, non-gas-producing, anaerobic, non-spore-forming, human gut bacteria (Lee and O’Sullivan, 2010). Members of the genus Bifidobacterium are present in most humans, performing an important role in the gut from birth (Arboleya et al., 2016). Bifidobacteria are widely considered to be probiotics, particularly Bifidobacterium animalis subsp. lactis, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium longum subsp. longum, and Bifidobacterium longum subsp. infantis (Kim et al., 2017). According to recent studies, these bacteria are considered “good” bacteria that inhibit the growth of other bacteria or pathogens and improve the immune system (Wang et al., 2016). Due to the health-promoting effects of probiotics, more dairy products now contain bifidobacteria (Janer et al., 2005). Moreover, bifidobacteria have been used to prevent or treat conditions such as allergic diseases, celiac disease, obesity, and neurological diseases in people of different ages, demonstrating their potential application as probiotics.
4-α-Glucanotransferase (αGTase) belongs to the α-amylase superfamily (Park et al., 2007). αGTase has various activities, such as hydrolysis, disproportionation, and cyclization (Do et al., 2016). αGTase catalyzes the cleavage of glucan, which is transferred to the glucan acceptor to form a new glycosidic bond (van der Maarel and Leemhuis, 2013). Oligosaccharides are efficient donors and oligosaccharides and glucose are acceptors in vitro. Plants can change the molecular structure of starch and grain architecture by transferring maltooligosyl units from one chain to another (Takaha et al., 1996). αGTases are found in glycoside hydrolase (GH) families 13, 57, and 77. Of these, GH77 is composed of αGTase monospecies and about 2000 members are mainly derived from bacteria (Lombard et al., 2014; Takaha et al., 1993). There is a structural difference between GH77 and the members of other GH families. In GH77, the catalytic (β/α)8-barrel TIM-barrel of GH13 is disrupted by several insertions (Godany et al., 2008; Machovič and Janeček, 2003). Three subdomains (B1, B2, and B3) are unique to GH77, while the B3 subdomain serves as GH13 domain C (Imamura et al., 2003; Kuchtová and Janeček, 2015). The GH77 enzymes include α-reactive enzymes that cleave the bonds of the substrate and form new glycosidic bonds or produce cyclic-α-1,4-glucan from a single linear glucan molecule (Godany et al., 2008; van der Maarel and Leemhuis, 2013). Using this activity, αGTase-treated starch has been used to obtain several novel products, such as maltooligosaccharides and cycloamylose. Another application of αGTases is the preparation of thermoreversible starch gels that can be used as gelatin alternatives. These gels dissolve in hot water and form a solid gel after cooling (van der Maarel and Leemhuis, 2013). αGTases also catalyze intermolecular transglycosylation, which is used to increase solubility by binding α-1,4-glucoside to glycoside bonds (van der Maarel and Leemhuis, 2013). Recently, many αGTases have been reported from Escherichia coli, Thermus aquaticus, Aquifex aeolicus, Thermotoga maritima, and Thermotoga thermophilus (Godany et al, 2008; Nguyen et al., 2019). αGTases from E. coli and T. aquaticus can use maltose as a donor at low rates (Bhuiyan et al., 2003; Palmer et al., 1976; Terada et al., 1999). A. aeolicus αGTase is the most thermoactive enzyme reported to date (Lee et al., 2002). αGTases from T. maritima produce isomalto-oligosaccharides from starch when combined with maltogenic amylase from Bacillus stearothermophilus (Lee et al., 2002). Finally, starch treated with T. thermophilus αGTases was free of amylose or amylopectin and had shortened or elongated debranching chains (Kaper et al., 2004; Lee et al., 2002). Moreover, the enzyme reaction product could form a thermoreversible gel under heating and cooling after being dissolved in water (Kaper et al., 2004).
Many αGTases have been characterized. However, there are few reports of GH77 αGTases from edible bacteria. Therefore, we cloned and expressed the bllj_0114 gene from Bifidobacterium longum. Then, the physiochemical properties of this recombinant enzyme were characterized. Using this novel enzyme, modified starch was prepared and its properties were investigated to determine its applicability for thermoreversible gel production.
Materials and methods
Chemicals and reagents
Bifidobacterium longum subsp. longum JCM 1217 was obtained from the Korean Collection for Type Cultures (KCTC). Escherichia coli MC1061 (F ± , araD139, recA13, D [araABCleu] 7696, galU, galK, ΔlacX74, rpsL, thi, hsdR2, and mcrB) was used as a host for gene cloning and enzyme expression. Luria–Bertani (LB) medium was purchased from BD (Franklin Lakes, NJ, USA). NaCl was purchased from GeorgiaChem (Suwanee, GA, USA). Kanamycin monosulfate was purchased from Duchefa Biochimie (Haarlem, the Netherlands). Glucose (G1), maltose (G2), maltotriose (G3), maltotetraose (G4), maltopentaose (G5), maltohexaose (G6), maltoheptaose (G7), and glycogen from bovine liver, biotechnology-certified dimethyl sulfoxide (DMSO), ZnCl2, and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Amylose was obtained from ICN Biomedicals (Aurora, OH, USA), and amylopectin hydrate, made from waxy corn, was obtained from Tokyo Chemical Industry (Tokyo, Japan). Soluble starch was purchased from Showa Chemical (Tokyo, Japan). Potato starch was purchased from Duksan Pure Chemicals (Ansan, South Korea). CoCl2, CaCl2, and sodium acetate were purchased from Junsei Chemical (Tokyo, Japan). MgCl2 and FeCl2 were purchased from Shinyo Pure Chemicals (Tokyo, Japan). MnCl2 and CuCl2 were purchased from Showa Chemical (Tokyo, Japan). Curry powder was purchased from Ottogi (Anyang, South Korea).
Cloning and sequence analysis
The gene encoding BL-αGTase (bllj_0114) was amplified from the genomic DNA of Bifidobacterium longum (KCTC 3127) by polymerase chain reaction (PCR) using the forward primer bllj_0114F (5′–G GAG CCA TGG CCA CCG ACG CGT ACA GC–3′) and reverse primer bllj_0114R (5′–CGG AAT CTC GAG CCG CTT CTC TCG GCG–3′), which contain NcoI and XhoI restriction enzyme sites (underlined), respectively. The PCR consisted of 1 min at 98 °C for denaturation; 30 cycles of 10 s at 98 °C, 30 s at 57 °C, and 2 min 15 s at 72 °C; and a final 5-min elongation at 72 °C. The amplified PCR products (2.2 kb) were digested with NcoI and XhoI and ligated to the pTKNd119 vector, which contains a kanamycin-resistance gene, BLMA promoter, multiple cloning sites, and hexa-histidine tag sequence (Kim et al., 2013). The final plasmid was transformed into E. coli MC1061 and spread on LB agar plates containing kanamycin.
Protein expression and purification of BL-αGTase
Transformants were cultured in kanamycin-supplemented (50 µg/mL) LB medium (1% (w/v) Bacto-tryptone, 0.5% (w/v) yeast extract, and 0.5% (w/v) NaCl) at 37 °C for 16 h with shaking at 200 rpm. Cells were harvested by centrifugation (6000 × g for 25 min at 4 °C) and resuspended in lysis buffer (900 mM NaCl, 50 mM Tris–HCl buffer (pH 7.5), and 10 mM imidazole). The cells were placed on ice and sonicated at 10 kHz using a Misonix XL-2000 sonicator (Qsonica, Newtown, CT, USA). After cell disruption, the supernatant (crude enzyme) was obtained by centrifugation (6000 × g for 25 min at 4 °C). The supernatant was purified using affinity chromatography with a nickel-nitrilotriacetic acid (Ni–NTA) packed column using washing buffer (900 mM NaCl, 50 mM Tris–HCl (pH 7.5), and 20 mM imidazole) and eluted using elution buffer (900 mM NaCl, 50 mM Tris–HCl (pH 7.5), and 250 mM imidazole). Protein patterns were observed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The SDS-PAGE gel was stained with Coomassie Brilliant Blue R-250 (Sigma–Aldrich) and destained (20% methanol, 10% acetic acid, and water). The concentration of BL-αGTase was measured with a NanoDrop 2000c spectrophotometer (NanoDrop, Thermo Fisher, USA).
Phylogenetic and sequence analyses
The characterized αGTase sequences of GH77 members were obtained from the Carbohydrate-Active enZymes (CAZy) database (http://www.cazy.org/). The alignment was analyzed using Align X software, a component of Vector NTI Suite 5.5 (InforMax, Bethesda, MD, USA). A tree of the amino sequences was constructed using MEGA6 (http://www.megasoftware.net/) based on the neighbor-joining method (bootstrap: 1000 samples) (Tamura et al., 2013).
Enzyme assay
The transglycosylation reaction activity of BL-αGTase was measured by mixing 125 μL of 0.2% amylose in DMSO, with 25 μL of 1% G2 in 50 mM sodium acetate buffer (pH 6.0) and 300 μL of 50 mM sodium acetate buffer (pH 6.0) and preheating at 40 °C for 5 min. Then, 50 μL of enzyme was added and boiled for 10 min to stop the reaction (Tang et al., 2006). Next, 50 μL of reaction mixture was mixed with 500 μL of Lugol’s solution [0.00,002% (w/v) iodine, 0.0002% (w/v) potassium iodine]), and the absorbance was measured at 620 nm using a spectrophotometer (Multiskan FC; Thermo Fisher Scientific, USA). The standard curve was constructed using 0.3–3 mg/mL amylose solutions in DMSO. One unit of BL-αGTase activity was defined as the amount of enzyme that reduced 0.4 mg/mL of amylose per minute under the analysis conditions (Park et al., 2007).
Effects of temperature and pH on enzyme activity and stability
To determine the optimal temperature, the relative BL-αGTase activity was measured at temperatures of 25–60 °C using amylose and G2 in 50 mM sodium acetate (pH 6.0) as a substrate. Similarly, the relative activities of the enzyme were measured at pH values of 5.0–7.5 using 50 mM sodium acetate (pH 5.0–7.0) and 50 mM KH2PO4–NaOH (pH 7.0–7.5).
Effects of metal ions on enzyme activity
The effects of metal ions on the enzyme activity were examined for various metal ions. The reaction mixture was preheated at 40 °C for 10 min with a 5 mM final concentration of CaCl2, MgCl2, CuCl2, MnCl2, CoCl2, ZnCl2, FeCl2, or EDTA. The enzyme activity was determined under the optimal conditions (40 °C, pH 6.0) using Lugol’s solution and the enzymatic activity in the absence of metal ions was deemed to be 100%.
Thin-layer chromatography (TLC) analysis of the reaction products
TLC was performed by spotting analytical samples onto K5F silica-gel plates (Whatman, Maidstone, UK) and then developing it with solvent system of n-butanol, ethanol, and water (5:5:3, v/v/v). To confirm the reaction products, the plate was dried in an oven and placed in a dipping solution consisting of 0.3% (w/v) N-(1-naphthyl)-ethylenediamine and 5% (v/v) H2SO4 in methanol, and developed at 110 °C for 10 min.
Preparation of BL-αGTase-modified potato starch and thermoreversible gels
The method used to prepare thermoreversible gels was modified from a described method (Kaper et al., 2005). Potato starch was modified using BL-αGTase enzyme. First, 4% (w/v) potato starch was dissolved in 50 mM sodium acetate (pH 6.0) and incubated with 0.24 units of BL-αGTase at 40 °C. Then, the enzyme was inactivated by boiling for 5 min. The modified starch was precipitated by adding nine volumes of ethanol. The precipitate was obtained by centrifugation (6000 × g at 4 °C for 30 min) and dried. The thermoreversible gel (5%, w/v) was prepared by mixing powdered modified starch in demineralized water with 2.44 mM CaCl2 in a tray. The modified starch solution producing the BL-αGTase-treated thermoreversible gel (BTG) was dissolved by incubation at 80 °C and subsequently stored at 4 °C for 16 h (Kaper et al., 2005).
High-performance anion-exchange chromatography (HPAEC)
To analyze the side chain distribution of the modified potato starch sample, it was dissolved in 50 mM sodium acetate (pH 4.0) and reacted with isoamylase (Megazyme; Bray, Wicklow, Ireland) at 50 °C for 72 h. The reaction solution was boiled for 5 min to stop the enzyme reaction. The isoamylase-treated potato starch samples were examined using HPAEC with a CarboPac PA1 guard column (4 × 50 mm; Dionex, Sunnyvale, CA, USA) and CarboPac PA1 column (4 × 250 mm; Dionex) to separate the injected samples with an ED40 electrochemical detector (Dionex). The 20-μL samples were injected and then eluted with 600 mM sodium acetate in 150 mM sodium hydroxide at various gradients at a flow rate of 1 mL/min: from 10 to 30% from 0 to 10 min; from 30 to 40% from 10 to 16 min; from 40 to 50% from 16 to 27 min; from 50 to 60% from 27 to 44 min; from 60 to 65% from 44 to 63 min; from 65 to 66% from 63 to 70 min; and from 66 to 100% from 70 to 71 min (Lee et al., 2008b).
Size-exclusion chromatography (SEC)
The modified starch treated with BL-αGTase was analyzed by SEC–ultra-performance liquid chromatography (Ultimate 3000; Dionex). The method followed Kwak et al. (2016). Samples were separated using a Shodex OHpak SB-806 M HQ SEC column (13 μm, 8.0 × 300 mm; Showa Denko, Kawasaki, Japan) at a flow rate of 0.5 mL/min in 50% DMSO and detected with a RI detector (RI-101; Showa Denko) at 50 °C. The modified starch was diluted to 5 mg/mL with 50% DMSO, filtered through 0.2-μm membranes, and injected. The calculation was performed with dextran (6100 kDa; American Polymer Standards Corporation, Mentor, OH, USA) and the standard was used (Mua, 1997).
Differential scanning calorimetry (DSC)
A DSC (Mettler Toledo, Greifensee, Switzerland) was used to measure the melting point of the gelatinized modified starch samples. To confirm the baseline of the instrument, an empty pan was used. About 10 g of sample was used, and the reference pan was not filled. The gels were heated from 20 to 80 °C at 10 °C/min (Tomsic et al., 2008).
Preparation of curry gel using BTG
Curry powder (10 mg/mL) and BTG-12 h powder (50 mg/mL) were dissolved in demineralized water containing 2.44 mM CaCl2. The mixed suspension was dissolved at 80 °C and then stored at 4 °C for 16 h in a cylindrical frame (Ø 10 × 20 mm). The curry gel (BTG-curry) was put in hot water (70 °C) and the melting point of the BTG-curry was compared with a commercial curry powder.
Preparation of curry gel using BTG
All experiments were conducted at least three times for each sample. The data were analyzed by analysis of variance (ANOVA) and then performed using Duncan’s multiple-range test to compare the means. Significant differences for all results were determined by SPSS (Version 25.0, SPSS Inc., Chicago, Illinois, USA) at p < 0.05.
Results and discussion
Cloning, expression, and purification of BL-αGTase from B. longum subsp. longum JCM 1217
The αGTase gene bllj_0114 was successfully amplified by PCR and the 2.2-kb fragment was ligated into pTKNd119 vector (Fig. S1). The recombinant DNA was transformed into E. coli MC1061. BLLJ_0114 protein was expressed when the transformants were cultured using a shaking incubator at 37 °C and 200 rpm for 16 h. The recombinant protein was purified using Ni–NTA affinity chromatography and a single band of the predicted 82 kDa size was observed on SDS-PAGE (Fig. S2 & Table S1). The purified BLLJ_0114 enzyme was named BL-αGTase.
Characterization of recombinant BL-αGTase
The catalytic activity of BL-αGTase was measured at various temperatures and pH values in a mixture of amylose and G2 as donor and acceptor substrates, respectively. The BL-αGTase enzyme had the highest activity at 40 °C and pH 6.0 (Fig. S3). The enzymatic reaction patterns of BL-αGTase were analyzed using TLC. BL-αGTase effectively transglycosylated an oligosaccharide donor and monosaccharide acceptor. In these reactions, the glucose of the donor molecule was transferred to construct various maltooligosaccharides with α-1,4 linkages (Fig. 1).
Fig. 1.
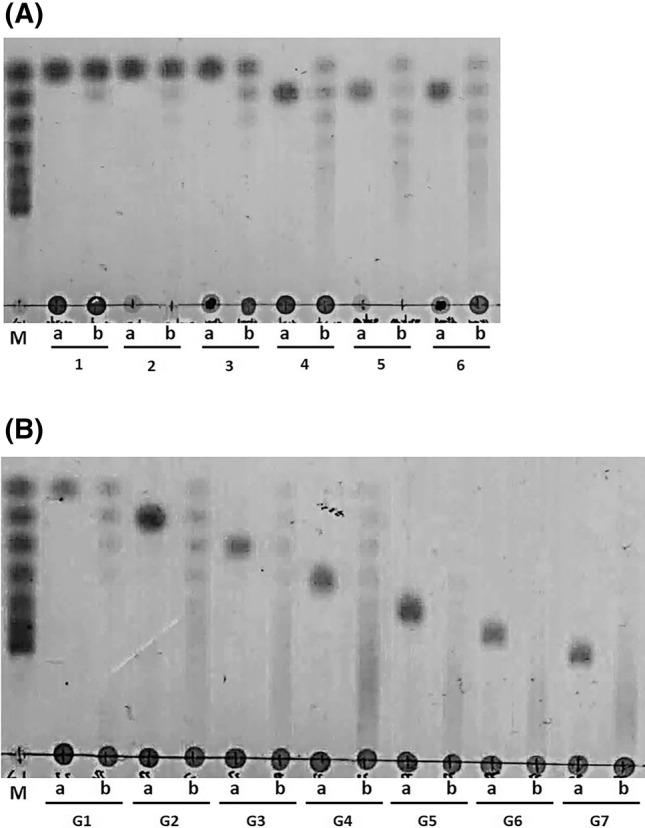
TLC analysis of the transglycosylation products catalyzed by BL-αGTase. (A) BL-αGTase transglycosylation reaction mixtures incubated with G1 (lanes 1–3) or G2 (lanes 4–6) and several oligosaccharide donors were visualized with a dipping solution at 105°C for 10 min. The donors were glycogen (lanes 1, 4), amylose (lanes 2, 5), and amylopectin (lanes 3, 6). (B) BL-αGTase was incubated with soluble starch (donor) and various acceptor molecules (G1–G7). Lane M, standard (G1–G7); a, before reaction; b, after reaction. The enzyme reactions were performed at 40 °C, pH 6.0 for 12 h
Effects of metal ions on enzyme activity
The effect of metal ions on BL-αGTase activity was measured using 5 mM Ca2+, Mg2+, Cu2+, Mn2+, Co2+, Zn2+, Fe2+, and EDTA (Fig. 2). The enzyme activity was reduced in the presence of all ions. BL-αGTase was completely inhibited by 5 mM Fe2+, and the activity with Cu2+ and Zn2+ was 88% and 82% lower than the normal enzyme activity, respectively.
Fig. 2.
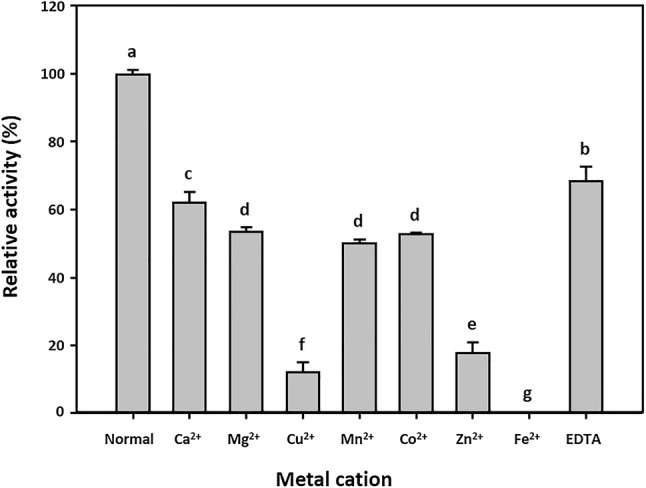
Effects of metal ions on enzyme activity. The purified BL-αGTase activity was measured under standard conditions (pH 6.0 at 40 °C) with amylose and G2 as donor and acceptor in the presence of 5 mM Ca2+, Mg2+, Cu2+, Mn2+, Co2+, Zn2+, Fe2+, or EDTA. Bars with different letters are significantly different at p < 0.05 in Duncan’s multiple range test
Structural analysis of BL-αGTase modified starch
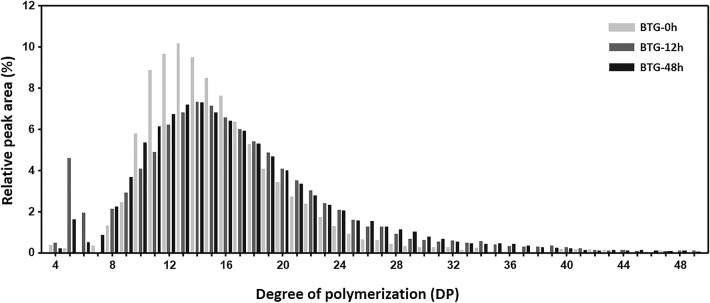
Potato starch was incubated with BL-αGTase and the side chain length distribution of the product was analyzed by HPAEC. As shown in Fig. 3, the side chain length distribution of the starch changed with the BL-αGTase treatment. The ratio of long side chains (degree of polymerization (DP) < 25) was increased by > twofold, due to the transglycosylation of glucans from short side chains (DP < 16). The modified starch was then evaluated using SEC. After a 12-h BL-αGTase treatment, the molecular weight of the modified starch decreased by up to tenfold compared with the control (Table 1).
Fig. 3.
Structural analysis of BTGs using HPAEC. Side chain length distribution based on the HPAEC peak area was normalized. Longer than DP 50 chains of BTG were removed from this figure
Table 1.
Molecular weight of BL-αGTase-treated starches
| Samples | Retention volume (mL) | Molecular weight (Da) |
|---|---|---|
| BTG-0 h | 0.765 | 8.98–7.08E + 07a |
| BTG-12 h | 7.452 | 5.95E + 06 |
| BTG-24 h | 7.624 | 5.02E + 06 |
| BTG-36 h | 7.863 | 3.97E + 06 |
| BTG-48 h | 7.850 | 4.02E + 06 |
aRetention volume and molecular weight of potato starch were adopted from Mua and Jackson (1997)
Preparation and application of BL-αGTase modified starch
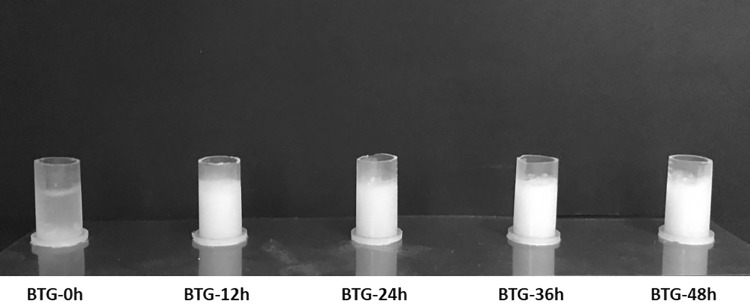
Using the BL-αGTase-treated modified starch powder, thermoreversible gels (BTG) were prepared. These were named BTG-0 h, BTG-12 h, BTG-24 h, BGT-36 h, and BTG-48 h, respectively, for enzymatic reaction times of 0, 12, 24, 36, and 48 h. The starch solution without BL-αGTase treatment (BTG-0 h) did not form a gel when it was stored at 4°C, while the BL-αGTase-treated starches did (Fig. 4). As shown in Fig. 4, a slight increase in opacity was observed with increasing reaction time. Also, the longer period of time BL-αGTase treated, the more solid gel formed (data not shown). This phenomenon was confirmed indirectly by measuring melting temperature of gels using DSC. The melting temperatures of the BTGs tended to increase with the BL-αGTase treatment time (Table 2). To investigate the industrial applicability of BTG, curry powder was mixed with various BTG powders and formed gels. The BTG-48 h with curry powder formed the most stable gel (Fig. S4A). When the BTG-curry was suspended in water at 70 °C, the gel melted completely, while the same amount of curry powder did not (Fig. S4B).
Fig. 4.
Comparison of gelatinized BTGs. After the BL-αGTase enzyme treatment for 0, 12, 24, 36, and 48 h, the obtained modified starches were solubilized and stored at 4 °C. The gel reacted with longer enzyme treatment period tended to be less transparent
Table 2.
Gel-sol conversion temperature analysis using DSC
| Samples | Melting point (°C) |
|---|---|
| BTG-0 h | –a |
| BTG-12 h | 69.2 |
| BTG-24 h | 67.3 |
| BTG-36 h | 70.1 |
| BTG-48 h | 75.7 |
aNot applicable
In this study, we characterized a novel αGTase from B. longum subsp. longum JCM 1217. The amino acid sequence alignment and phylogenetic analysis showed that BL-αGTase is a member of the GH77 αGTases (Fig. S5 and S6). BL-αGTase has the five conserved regions and three catalytic sites that are critical for the transglycosylation activity of GH77 enzymes. αGTase can generate maltooligosaccharides of various lengths using disproportionation activity. Similarly, BL-αGTase produced several maltooligosaccharides using G1 and G2 as acceptor molecules. Compared with G1, G2 was a better acceptor (Fig. 1A). TLC product analysis showed that the longer the BL-αGTase acceptor used, the longer the enzyme products were (Fig. 1B).
The structural analysis of the BL-αGTase-modified starch showed that BL-αGTase elongated the side chains and reduced the molecular weight of the starch from 8.9 × 107 to 4 × 106 Da, indicating that BL-αGTase hydrolyzed the α-1,4 glucosidic linkage of the fragment between the amylopectin clusters (Fig. 3, Table 1). αGTases have been used to modify the structure of starches and αGTase-modified starches have novel rheological and nutritional properties, such as hyperglycemic effects, fat-replacer properties, and thermoreversible gelation (Bhuiyan et al., 2003; Kaper et al., 2005; Kim et al., 2013; Lee et al., 2008a). Likewise, the BL-αGTase-modified starch also formed gels. The properties of the BTG gels were strongly related to the degree of enzyme reaction (Table 2). The DSC results indicated that the melting temperature of the gel could be adjusted by controlling the BL-αGTase reaction time, which is very advantageous in industrial use. For the application of the gel-forming property, BTG-curry was prepared as an alternative to instant soup powder. As shown in Fig. S4, BTG powder is a potential gel-making agent. To make an extract powder, a drying step is required. Often, the drying step consumes energy and can cause a loss of flavor. Therefore, a gel-type extract could be advantageous for maintaining the quality of some foods. A gel-type extract could also be advantageous for ready-to-cook food because it melts easily.
In conclusion, the novel enzyme BL-αGTase was identified and applied to starch modification. To our knowledge, BL-αGTase is the first αGTase from an edible bacterium, suggesting that BL-αGTase could potentially be applied in the food industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Hallym Research Fund (HRF-201905-005).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Da-Woon Jeong, Email: jeongdawoon@hallym.ac.kr.
Hyun-Mo Jeong, Email: Pomme@hallym.ac.kr.
Yu-Jeong Shin, Email: yujeong0115@hallym.ac.kr.
Seung-Hye Woo, Email: shye94@hallym.ac.kr.
Jae-Hoon Shim, Email: jhshim@hallym.ac.kr.
References
- Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria populations in human health and aging. Front. Microbiol. 2016;7:1–9. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan S, Kitaoka M, Hayashi K. A cycloamylose-forming hyperthermostable 4-α-glucanotransferase of Aquifex aeolicus expressed in Escherichia coli. J. Mol. Catal. B-Enzym. 2003;22:45–53. doi: 10.1016/S1381-1177(03)00005-5. [DOI] [Google Scholar]
- Do VH, Mun S, Kim YL, Rho SJ, Park KH, Kim YR. Novel formulation of low-fat spread using rice starch modified by 4-alpha-glucanotransferase. Food Chem. 2016;208:132–141. doi: 10.1016/j.foodchem.2016.03.101. [DOI] [PubMed] [Google Scholar]
- Godany A, Vidova B, Janecek S. The unique glycoside hydrolase family 77 amylomaltase from Borrelia burgdorferi with only catalytic triad conserved. FEMS Microbiol. Lett. 2008;284:84–91. doi: 10.1111/j.1574-6968.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- Imamura H, Fushinobu S, Yamamoto M, Kumasaka T, Jeon BS, Wakagi T, Matsuzawa H. Crystal structures of 4-alpha-glucanotransferase from Thermococcus litoralis and its complex with an inhibitor. J. Biol. Chem. 2003;278:19378–19386. doi: 10.1074/jbc.M213134200. [DOI] [PubMed] [Google Scholar]
- Janer C, Arigoni F, Lee BH, Pelaez C, Requena T. Enzymatic ability of Bifidobacterium animalis subsp. lactis to hydrolyze milk proteins: identification and characterization of endopeptidase O. Appl. Environ. Microb. 71: 8460-8465 (2005) [DOI] [PMC free article] [PubMed]
- Kaper T, Talik B, Ettema TJ, Bos H, van der Maarel MJ, Dijkhuizen L. Amylomaltase of Pyrobaculum aerophilum IM2 produces thermoreversible starch gels. Appl. Environ. Microb. 2005;71:5098–5106. doi: 10.1128/AEM.71.9.5098-5106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper T, van der Maarel MJ, Euverink GJ, Dijkhuizen L. Exploring and exploiting starch-modifying amylomaltases from thermophiles. Biochem. Soc. Trans. 2004;32:279–282. doi: 10.1042/bst0320279. [DOI] [PubMed] [Google Scholar]
- Kim MS, Jang JH, Kim YW. Overproduction of a thermostable 4-alpha-glucanotransferase by codon optimization at N-terminus region. J. Sci. Food Agr. 2013;93:2683–2690. doi: 10.1002/jsfa.6084. [DOI] [PubMed] [Google Scholar]
- Kim NR, Jeong DW, Ko DS, Shim JH. Characterization of novel thermophilic alpha-glucosidase from Bifidobacterium longum. Int. J. Biol. Macromol. 2017;99:594–599. doi: 10.1016/j.ijbiomac.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Kuchtová A, Janeček Š. In silico analysis of family GH77 with focus on amylomaltases from Borreliae and disproportionating enzymes DPE2 from plants and bacteria. Biochim. Biophys. Acta. 2015;1854:1260–1268. doi: 10.1016/j.bbapap.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Kim MG, Kim YW, Ban HS, Won MS, Park JT, Park KH. Properties of a glycogen like polysaccharide produced by a mutant of Escherichia coli lacking glycogen synthase and maltodextrin phosphorylase. Carbohyd. Polym. 2016;136:649–655. doi: 10.1016/j.carbpol.2015.09.091. [DOI] [PubMed] [Google Scholar]
- Lee HS, Auh JH, Yoon HG, Kim MJ, Park JH, Hong SS, Kang MH, Kim TJ, Moon TW, Kim JW, Park KH. Cooperative action of alpha-glucanotransferase and maltogenic amylase for an improved process of isomaltooligosaccharide (IMO) production. J. Agric. Food Chem. 2002;50:2812–2817. doi: 10.1021/jf011529y. [DOI] [PubMed] [Google Scholar]
- Lee JH, O’Sullivan DJ. Genomic insights into bifidobacteria. MMBR. 2010;74:378–416. doi: 10.1128/MMBR.00004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Kim YR, Park KH, Lee HG. Rheological and gelation properties of rice starch modified with 4-alpha-glucanotransferase. Int. J. Biol. Macromol. 2008;42:298–304. doi: 10.1016/j.ijbiomac.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi SJ, Shin SI, Park KH. Structural and rheological properties of sweet potato starch modified with 4-α-Glucanotransferase from Thermus aquaticus. Food Sci. Biotechnol. 2008;17:705–712. [Google Scholar]
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovič M, Janeček S. The invariant residues in the α-amylase family: just the catalytic triad. Biologia. 2003;58:1127–1132. [Google Scholar]
- Mua J, Jackson D. Relationships between functional attributes andmolecular structures of amylose and amylopectin fractions from corn starch. J. Agr. Food Chem. 1997;45:3848–3854. doi: 10.1021/jf9608783. [DOI] [Google Scholar]
- Nguyen DHD, Park SH, Tran PL, Kim JW, Le QT, Boos W, Park JT. Characterization of the transglycosylation reaction of 4-alpha-glucanotransferase (MalQ) and its role in glycogen breakdown in Escherichia coli. J. Microbiol. Biotechnol. 2019;29:357–366. doi: 10.4014/jmb.1811.11051. [DOI] [PubMed] [Google Scholar]
- Palmer TN, Ryman BE, Whelan WJ. The action pattern of amylomaltase from Escherichia coli. Eur. J. Biochem. 1976;69:105–115. doi: 10.1111/j.1432-1033.1976.tb10863.x. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim HJ, Kim YH, Cha HJ, Kim YW, Kim TJ, Kim YR, Park KH. The action mode of Thermus aquaticus YT-1 4-α-glucanotransferase and its chimeric enzymes introduced with starch-binding domain on amylose and amylopectin. Carbohydr. polym. 2007;67:164–173. doi: 10.1016/j.carbpol.2006.05.018. [DOI] [Google Scholar]
- Takaha T, Yanase M, Okada S, Smith SM. Disproportionating enzyme (4-alpha-glucanotransferase; EC 2.4.1.25) of potato. purification, molecular cloning, and potential role in starch metabolism. J. Biol. Chem. 268: 1391-1396 (1993) [PubMed]
- Takaha T, Yanase M, Takata H, Okada S, Smith SM. Potato D-enzyme catalyzes the cyclization of amylose to produce cycloamylose, a novel cyclic glucan. J. Biol. Chem. 1996;271:2902–2908. doi: 10.1074/jbc.271.6.2902. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 30: 2725-2729 (2013) [DOI] [PMC free article] [PubMed]
- Tang SY, Yang SJ, Cha H, Woo EJ, Park C, Park KH. Contribution of W229 to the transglycosylation activity of 4-alpha-glucanotransferase from Pyrococcus furiosus. Biochim. Biophys. Acta. 2006;1764:1633–1638. doi: 10.1016/j.bbapap.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Terada Y, Fujii K, Takaha T, Okada S. Thermus aquaticus ATCC 33923 amylomaltase gene cloning and expression and enzyme characterization: Production of cycloamylose. Appl. Environ. Microbiol. 1999;65:910–915. doi: 10.1128/AEM.65.3.910-915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsic M, Prossnigg F, Glatter O. A thermoreversible double gel: characterization of a methylcellulose and kappa-carrageenan mixed system in water by SAXS, DSC and rheology. J. Colloid Interf. Sci. 2008;322:41–50. doi: 10.1016/j.jcis.2008.03.013. [DOI] [PubMed] [Google Scholar]
- van der Maarel MJ, Leemhuis H. Starch modification with microbial alpha-glucanotransferase enzymes. Carbohydr. Polym. 2013;93:116–121. doi: 10.1016/j.carbpol.2012.01.065. [DOI] [PubMed] [Google Scholar]
- Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016;22:589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.