Abstract
To develop a beverage with high antioxidant capacity and desirable sensory characteristics, Schisandra chinensis (omija) fruits were added to ale type beer at different time points of the brewing process. The phenolic compounds contents in beer were found to be dependent at the moment of the addition of omija fruit. Addition of omija fruits at the initiation of boiling imparted highest oxidative stability to beer and resulted in highest total phenolic and flavonoid contents in ale beer (606.82 mg GAE/L and 406.75 mg QE/L, respectively). The amounts of schisandrin, gomisin A and gomisin B in beer were 12.10 mg/mL, 3.12 mg/mL and 0.86 mg/mL, respectively. Taken together, it is hypothesized that the addition of omija fruits to traditional brewing process can improve the development of value-added beer products.
Keywords: Antioxidant capacity, Beer, Lignans, Schisandra chinensis, Sensory characteristics
Introduction
Beer is rich in nutrients such as amino acids, carbohydrates, vitamins, phenolic compounds, minerals, etc. (Bamforth, 2002). The main polyphenols present in beer are flavonoids, phenolic acids, tannins, and proanthocyanidins (Piazzon et al., 2010) with origin from malt (70–80%) and hops (20–30%), and substantially contribute to the stability and sensory perception of a given beer (Zhao et al., 2010). As craft beer has gained the position of a measurable force in the beer market, more craft brewers are exploring the manufacture of varying beer styles with new flavors, aromas, and modified manufacturing processes to satisfy the consumers (Sanna and Pretti, 2015). The enrichment of beer with fruits not only adds new flavors but also increases the content of bioactive substances as well as the oxidative stability. Beers fermented with fruits are attracting more are searchers’ attention due to their good biological activity (Ducruet et al., 2017; Nardini and Garaguso, 2020). Cherry, peach, apricot, orange and apple have been used for fruit beers (Nardini and Garaguso, 2020) and improved the beers with desirable sensory characteristics and high antioxidant capacity (Ducruet et al., 2017). There are still many other functional fruits applicable for beer production.
Schisandra chinensis (Turcz.) Baillon is commonly known as “omija” in Korea and “wuweizi” in China. The literal meaning of wuweizi is “berries with five distinctive flavor characteristics: sourness, sweetness, bitterness, saltiness, and astringency”, and its aqueous extract has a pinkish-red color and delightful flavors (Lu and Chen, 2009). The consumption of omija fruits has increased in recent years in many countries, mainly due to the inherent biological effects (Huyke et al., 2007). Recent researches have demonstrated that omija fruits possess various potentially beneficial activities, including antioxidant, antimicrobial, antitumor, and anti-HIV effects (Chen et al., 2012; Qu et al., 2014). To date, more than forty types of bioactive compounds have been identified in omija fruits, such as lignans (Ekiert et al., 2013; Gao et al., 2013; Huang et al., 2013; Kim et al., 2011), volatile oils (Cheng et al., 2014; Lee et al., 2011), polysaccharides (Chen et al., 2012; Qu et al., 2014), and organic acids (Huyke et al., 2007). As the primary bioactive components, lignans such as schisandrin, gomisin A, and gomisin B mainly exist in seeds (4.1–19.2% of whole fruit) (Gao, et al., 2013; Kim et al., 2011). Nowadays, omija fruits are used as a nutritional and functional ingredient in diverse foods, such as tea, yogurt, fruit wine, fruit cake, jam, and other health care products (Ekiert et al., 2013; Kim et al., 2015). However, a few researchers have attempted to employ omija fruits in the process of beer production.
The objective of this work was to develop ale beer enriched with omija fruits with desirable sensory properties, high bioactive compound concentration, as well as high oxidative stability. For this purpose, the dried omija fruits were added to beer at different stages of the brewing process, and the major bioactive compounds present in the beer, and antioxidant activities of the obtained beer were evaluated.
Materials and methods
Materials
Dried omija fruits were purchased from Bongpyeong (Pyeongchang-gun, Gangwon-do, South Korea) and stored in a refrigerator at 4 °C in their original packaging until use. The pale ale malt (two-row spring barley, color 7 EBC) and carared barley malt (color 45 EBC) were purchased from Weyermann Specialty Malting Company (Bamberg, Germany). The dry ale yeasts (Safale US-05) were purchased from Fermentis Ltd. (Marcq-en-Baroeul, France).
Chemical reagents
Folin and Ciocalteu’s phenol reagent, 2,4,6-tris(2-pyridyl)-s-triazine, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), gallic acid, quercetin, schisandrin, gomisin A, and gomisin B were purchased from Sigma-Aldrich (St Louis, MO, USA). Acetonitrile and formic acid of HPLC grade were purchased from Hanbon Science and Technology Co., Ltd. (Jiangsu, China). All other chemicals and solvents used were of analytical grade or higher and obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Micro-brewing trials
A total of 4.5 kg of grains comprising of pale ale malt and carared malt in a proportion of 9:1 (w/w) was ground using a two-roller grist mill (Winfried Sauer, Frendorf, Germany) at a setting of 0.2 mm, and then mixed with 20 L of water at 50 °C. A commonly used infusion process was chosen as follows: 20 min at 50 °C, 60 min at 64 °C, 20 min at 72 °C, and 10 min at 78 °C (mashing-off) by applying a heating rate of about 1 °C/min. The mash was allowed to settle for a few minutes and the supernatant was transferred to the wort kettle. The sediment was washed twice with hot water (70 °C) to obtain a final volume of 20 L of wort. Subsequently, the wort was boiled for 60 min, followed by the addition of hop pellets (0.8 g/L), whirlpool, and chilling processes. During the wort boiling process, the hop pellets obtained from Lupex GMBH (Hallertau, Germany) were added at three stages: 0.1 g/L of Nugget pellets, 0.5 g/L of Amarillo pellets, and 0.2 g/L of Citra pellets were added after 5 min of boiling, after 30 min of boiling, and after 50 min of boiling, respectively. The crushed dried omija fruits were added to the first lot at a concentration of 2 g/L wort (beer A) at the beginning of boiling.
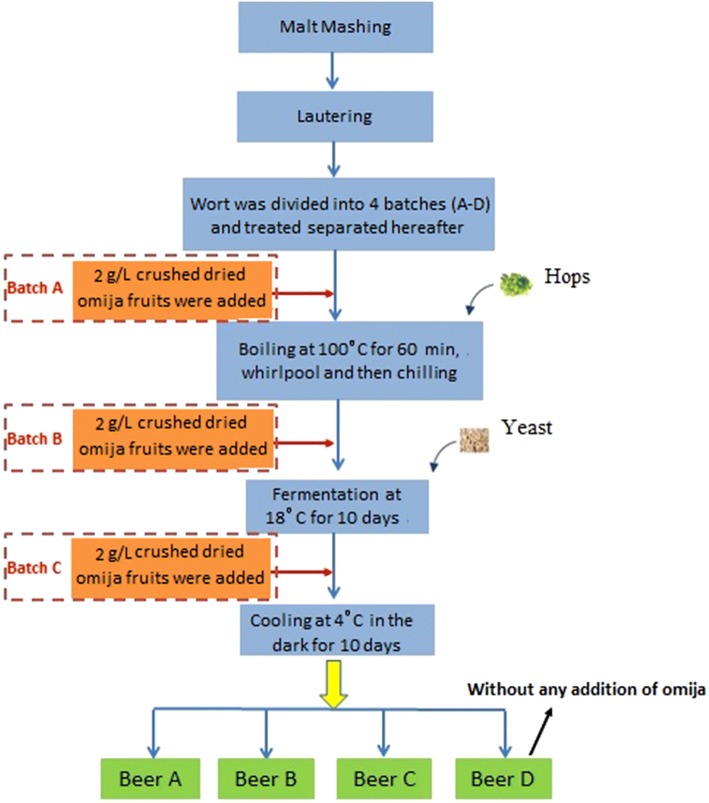
The final hopped worts (around 13°Plato) were immediately transferred directly into 25 L steel tanks (Candirect, Duisburg, Germany) and stored at 20 °C. Prior to fermentation, 16 g of the yeast, Safale US-05, was rehydrated and added into the wort according to the manufacturer’s instructions. The crushed dried omija fruits (2 g/L wort) were also added before the fermentation, which led to the generation of the second lot (beer B). The primary fermentation lasted for 7 days at 18 °C while the secondary fermentation lasted for 3 days at 20 °C. Subsequently, the fermentation fluid was stored at 4 °C for 10 days. Finally, the clarified beer was obtained by centrifugation at 6000×g for 20 min and filled into 500 mL amber glass bottles with flip caps. Before conditioning, the same amount of crushed dried omija fruits was added to the third lot (beer C). The beer made without the addition of dried omija fruits (the fourth lot) was served as a control (beer D). Figure 1 shows the flowchart of the beer production process.
Fig. 1.
Flowchart of the beer production process. In the beers (A–C), the crushed dried omija fruits were added at different stages. The control beer (D) was made without any addition of omija fruits
Forced-aging test and evaluation of the antioxidant capability of beer
Bottled fresh beers were aged in the dark under forced conditions at 40 °C in a thermostatically controlled room. The antioxidant capacity was analyzed by ferric reducing antioxidant power (FRAP) and DPPH radical scavenging activity assays after 0, 3, 6, 9, 12 and 15 days of force-aging, respectively. The DPPH radical scavenging activity of beer samples was determined using the stable 2,2-diphenyl-1-picrylhydrazyl reagent according to a previously described method (Chen et al., 2019; Cho et al., 2019). The determination of FRAP was performed according to the established method (He et al., 2012).
Physicochemical analyses of beer
The original gravity, final alcohol content, pH, and color of the degassed beers were measured using a DMA 4500 density analyzer and Alcolyser Plus (Anton Paar, Austria). The international recommended analyses of bitterness in beer in terms of international bitter units (IBU) were conducted at 275 nm by spectrophotometric measurement, using an acidic solvent extract of beer. Headspace gas chromatography was employed to measure the contents of acetaldehyde, diacetyl, higher alcohols, and esters (Deng et al., 2018). Foam stability was assessed according to the NIBEM value using the NIBEM tester (Haffmans, The Netherlands).
Total phenolic and flavonoid contents
Total phenolic content (TPC) was determined using the Folin-Ciocalteu spectrophotometric method (Zhao et al., 2010). The results were expressed as gallic acid equivalents (GAE) mg/L beer. Total flavonoid content (TFC) was measured by the aluminum chloride colorimetric assay (Zhao et al., 2010). The TFC value was expressed as quercetin equivalents (QE) mg/L beer.
Analyses of lignan compounds
Three main active lignans (schisandrin, gomisin A, and gomisin B) present in the degassed beer samples were analyzed by UPLC-MS (Waters H-class equipped with QDa detector, MA, USA) on a 1.7-μm BEH C18 column (2.1 mm × 150 mm) according to Gao et al. (2013) and Kim et al. (2018). The mobile phase consisted of solvent A (distilled water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid) at a flow rate of 0.3 mL/min, with gradient elution as follows: 5–10% solvent A over 0.2 min; 10–55% A over 0.1 min; 55–65% A over 2.7 min; 65–75% A over 1.5 min; 75–80% A over 1.5 min; 80–100% A over 1.0 min; 100% A over 0.5 min; 100–5% A over 0.1 min; and 5% A over 2.4 min.
Sensory analysis
The sensory evaluation was performed by a well-trained panel of ten evaluators as our previous report (Deng et al., 2018). The sensory attributes included the following three aspects: odor (malty aroma, hop aroma and flavor of omija fruit), mouth sensations (freshness and fullness), taste (sweetness, sourness, saltiness, bitterness and astringency) and total acceptability. Each sensory attribute was graded from 1 (the worst grade) to 5 (the best grade) and the average value obtained from the ten panels was used to determine the final score.
Statistical analysis
Data were expressed as mean values ± standard deviation (SD) of three independent replicates. Statistical analysis was performed by one-way analysis of variance, followed by Tukey’s post hoc tests. Results were considered significantly different when p values were < 0.05.
Results and discussion
Physicochemical analyses of beer
The results of standard beer analyses including original gravity, ethanol content, pH, color, bitterness, viscosity, and colloidal haze are shown in Table 1. The original gravity and ethanol content were similar among all the four beer samples (A–D). However, the beer color increased significantly subsequent to the addition of the dried omija fruits during the brewing process compared to control beer D (23.4 EBC). Color values of the three fresh omija beers ranged from 25.8 to 28.6 EBC. The bright red color of omija fruits is known to arise due to high anthocyanin content (Kim et al., 2009; Wu et al., 2011), which may result in the increase in color of the beer. The pigments in omija fruits are highly water-soluble and therefore are of great interest in alcoholic and non-alcoholic beverage-type food processing (Lee et al., 2011). The addition of omija fruits at the beginning of the boiling stage (beer A) resulted in darker beer with deeper red color, which could be due to the formation of colored Maillard reaction products (Ducruet et al., 2017) and the increased extraction of colored compounds from omija fruits due to high temperature.
Table 1.
The major chemical and physical properties of finished beers
| Properties | Beer A | Beer B | Beer C | Beer D |
|---|---|---|---|---|
| Original extract (°Plato) | 13.11 ± 0.02a | 13.09 ± 0.04a | 13.10 ± 0.03a | 13.14 ± 0.03a |
| Ethanol content (% v/v) | 5.20 ± 0.10a | 5.10 ± 0.10a | 5.10a | 5.2 ± 0.10a |
| pH | 4.05 ± 0.03c | 4.22 ± 0.03bc | 4.36 ± 0.02b | 4.77 ± 0.04a |
| Color (EBC) | 28.60 ± 0.10a | 26.6 ± 0.10b | 25.8 ± 0.20bc | 23.4 ± 0.10c |
| Bitterness (IBU) | 29.3 ± 0.20a | 27.9 ± 0.09b | 26.8 ± 0.11b | 24.4 ± 0.10c |
| Colloidal haze (EBC) | 1.18 ± 0.09b | 1.21 ± 0.07b | 7.19 ± 0.11a | 0.24 ± 0.02c |
| Viscosity (cP) | 1.57 ± 0.10a | 1.56 ± 0.10a | 1.52 ± 0.10b | 1.51b |
| Diacetyl (μg/L) | 96.80 ± 1.70a | 89.7 ± 0.80a | 89.6 ± 1.10a | 90.1 ± 1.0a |
| Acetaldehyde (mg/L) | 8.77 ± 0.42a | 8.01 ± 0.26b | 7.94 ± 0.45b | 8.03 ± 0.33b |
| Ethyl acetate (mg/L) | 30.15 ± 0.28a | 27.55 ± 0.30b | 28.02 ± 0.31b | 27.69 ± 0.25b |
| Isoamyl acetate (mg/L) | 2.50 ± 0.17a | 2.21 ± 0.16b | 2.22 ± 0.14b | 2.13 ± 0.11b |
| Ethyl hexanoate (mg/L) | 0.19 ± 0.02a | 0.14 ± 0.03b | 0.13 ± 0.02b | 0.14 ± 0.03b |
| Ethyl caprylate (mg/L) | 0.14 ± 0.03a | 0.15 ± 0.02a | 0.15 ± 0.02a | 0.16 ± 0.01a |
| Propanol (mg/L) | 14.12 ± 0.10a | 14.15 ± 0.13a | 14.04 ± 0.10a | 14.11 ± 0.12a |
| Isobutanol (mg/L) | 23.10 ± 0.14a | 22.99 ± 0.08a | 23.05 ± 0.21a | 23.11 ± 0.14a |
| Isoamyl alcohol (mg/L) | 57.15 ± 0.30a | 57.12 ± 0.21a | 56.62 ± 0.38a | 57.07 ± 0.35a |
Values are the means ± standard deviations of triplicate measurements. Means in a row that does not share the same alphabetical letter represent significant differences at p < 0.05
Addition of dried omija fruits also resulted in significant changes in the pH and bitterness values of beer. A decline in pH and an increase in bitterness were observed (p< 0.05) in the beers produced with the addition of omija fruits during the processing (A–C). The lowest pH value (4.05) and the highest bitterness value (29.3 IBU) were noticed for beer produced with the addition of omija fruits at the beginning of boiling (A). In addition, the pH and bitterness were similar for beers B and C, which could possibly be due to the increase in the extraction of organic acids and bitter compounds from omija fruits due to high temperature. Previously published reports demonstrate similar results stating that addition of omija fruits to other foods, such as cookie dough and wine led to a significant decrease in the pH (Ekiert et al., 2013; Kim et al., 2015).
Among the produced beers, the beer produced with the addition of omija fruits before conditioning (C) had the highest colloidal haze value reaching 7.19 EBC, due to the presence of suspended omija fruits fragments. In other cases, the colloidal haze had positive correlations with the viscosity (p< 0.05), and both the values increased significantly when omija fruits were added within the brewing process compared to the control. It is possible that the polysaccharides, especially pectins released from the omija fruits lead to flocculation (Gancz et al., 2006). In contrast, Ducruet et al. (2017) found that the beers produced with the addition of goji berries before wort boiling and fermentation were less turbid than the control beer.
Moreover, the volatile compounds that contribute to the beer flavor and stability contain diacetyl, acetaldehyde, higher alcohols, and esters (Deng et al., 2015; 2018). It is apparent that the contents of diacetyl and acetaldehyde in the beer produced with the addition of omija fruits at the beginning of boiling (A) were slightly increased, whereas the contents of higher alcohols were scarcely affected compared to the control beer (Table 1). Regarding the esters except for ethyl caprylate, a slight decrease was noted in the omija beer A. No obvious change was observed in the contents of diacetyl, acetaldehyde, higher alcohols, and esters between the other two omija beers (B and C) and the control beer.
Total phenolic and flavonoid contents in beers
The results of TPC and TFC in beer samples are presented in Table 2. Both TPC and TFC increased with the addition of omija fruits to the beers compared to the control. The contents also varied significantly depending on the time of the addition of omija fruits. Among the beer samples, the highest TPC and TFC (606.82 mg GAE/L and 406.75 mg QE/L, respectively) were measured in the beer with omija fruits added at the beginning of boiling (A). The beer produced with the addition of omija fruits prior to fermentation (B) had higher TPC and TFC than the beer C produced with the addition of omija fruits at the latter stage of processing.
Table 2.
Determination of TPC, TFC, and the contents of three lignans in the beers
| Items | Beer A | Beer B | Beer C | Beer D |
|---|---|---|---|---|
| TPC (mg GAE/L) | 606.82 ± 16.64a | 575.00 ± 12.45b | 568.73 ± 13.57b | 519.09 ± 15.78c |
| TFC (mg QE/L) | 406.75 ± 4.05a | 377.19 ± 9.01b | 343.75 ± 7.23c | 303.19 ± 4.91d |
| Schisandrin (mg/L) | 12.10 ± 0.30a | 9.44 ± 0.20b | 8.96 ± 0.20c | UD |
| Gomisin A (mg/L) | 3.12 ± 0.10a | 2.20 ± 0.17b | 2.19 ± 00.11b | UD |
| Gomisin B (mg/L) | 0.86 ± 0.01a | 0.77 ± 0.05b | 0.65 ± 0.07c | UD |
Values are the means ± standard deviations of triplicate measurements. Means in a row that does not share the same alphabetical letter represent significant differences at p < 0.05
TPC total phenolic content, TFC total flavonoid content, UD undetected
Beer rich in phenolic and flavonoid antioxidants exhibits longer shelf life and better sensory properties such as flavor and foam stability compared to the beer with lower antioxidant activity (He et al., 2012; Zhao et al., 2010). Recently, numerous studies were performed with emphasis on total phenolic and flavonoid contents of commercial beers (Mitic et al., 2014; Piazzon et al., 2010; Zhao et al., 2010). Generally, ale beers have much higher TPC and TFC values than lager beers (Piazzon et al., 2010). The TPC values obtained by Folin-Ciocalteu in our study were much higher compared to the ale from Piazzon et al. (2010) (563 mg GAE/L). The TPC value in the beer with omija fruits added at the beginning of boiling (A) was almost as high as that reported for the amber ale beer produced with the addition of goji berries (50 g/L) (Ducruet et al., 2017), which was much higher than those in the commercial apple, peach or apricot beers. There reported also significantly different concentration of TFC in different fruit beers, ranging from 103 to 209 mg QE/L (Mitic et al., 2014) or ranging from 68 mg catechin equivalents/L for a commercial apple beer to 222 mg catechin equivalents/L for a commercial cherry beer (Nardini and Garaguso, 2020), which were lower than the omija beer.
Determination of lignan compounds
Lignans are the major and characteristic constituents of omija fruits, especially dibenzocyclooctadiene lignans, which have been found to exhibit various potentially beneficial biological activities (Ekiert et al., 2013). As shown in Table 2, the addition of omija fruits during processing (A–C) increased the contents of three major lignans (schisandrin, gomisin A, and gomisin B) in the finished beers. Similarly, their contents in the obtained omija beers depended strongly at the time of addition of omija fruits during the processing. The lowest contents were found in beer C, whereas the highest contents were noted in beer A, to which omija fruits were added at the beginning of boiling. At this stage, the beers were subjected to the thermal treatment at 100 °C for 60 min. Previous studies (Gerstenmeyer et al., 2013; Lu and Chen, 2009) also pointed out that the moderate heating at 100 °C did not degrade the lignans in foods. In contrast, it resulted in better extractability of the lignans.
Oxidative stability of beers
The changes in the antiradical and reducing the potential of the four beer samples as a result of forced-aging at 40 °C were evaluated using DPPH and FRAP assays (Tables 3 and 4). DPPH radicals have been widely used as model systems to investigate the radical-scavenging abilities of antioxidant compounds (Tafulo et al., 2010). As shown in Table 3, the radical scavenging activity values of the four fresh beer samples (on day 0) ranged from 0.88 to 2.02 mM trolox equivalents (TE). The DPPH radical scavenging activity of each sample declined rapidly within the first 9 days and subsequently stabilized gradually. All the three omija beers exhibited stronger DPPH radical scavenging activities than the control beer D on the same day along the forced-aging period. Significant differences in DPPH radical scavenging activity in the three omija beers were also evident depending on the time of the addition of omija fruits with the highest level in the beer produced with the addition of omija fruits at the beginning of boiling. Reducing power is also believed to be associated with antioxidant activity and serves as an important indicator of beer aging (He et al., 2012; Piazzon et al., 2010). Similar results were obtained in FRAP activity analysis (Table 4). Among the samples, the largest decrease in FRAP value was observed in the control beer D after 15 days of forced-aging. Throughout the entire force-aging period, the beers with added omija fruits exhibited much higher FRAP activities, ranging from 1.86 mM Fe2+ for the beer A to 0.79 mM Fe2+ for the beer C at forced-aging day 15.
Table 3.
Changes in 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity in different beer samples during 15-day force-aging
| Times (day) | Beer A (mM TE) | Beer B (mM TE) | Beer C (mM TE) | Beer D (mM TE) |
|---|---|---|---|---|
| 0 | 2.02 ± 0.13a | 1.68 ± 0.10a | 0.96 ± 0.05a | 0.88 ± 0.03a |
| 3 | 1.70 ± 0.12b | 1.30 ± 0.10b | 0.70 ± 0.06b | 0.69 ± 0.04b |
| 6 | 1.37 ± 0.10c | 1.01 ± 0.07c | 0.44 ± 0.03c | 0.40 ± 0.03c |
| 9 | 0.95 ± 0.09d | 0.69 ± 0.10d | 0.28 ± 0.02d | 0.24 ± 0.01d |
| 12 | 0.80 ± 0.10de | 0.50 ± 0.06e | 0.19 ± 0.07d | 0.20 ± 0.01d |
| 15 | 0.76 ± 0.03e | 0.50 ± 0.05e | 0.21 ± 0.08d | 0.18 ± 0.01d |
Values are the means ± standard deviations of triplicate measurements. Means in a column that does not share the same alphabetical letter represent significant differences at p < 0.05
TE trolox equivalents
Table 4.
Changes in ferric reducing antioxidant power (FRAP) value in different beer samples during 15-day force-aging
| Times (day) | Beer A (mM Fe2+) | Beer B (mM Fe2+) | Beer C (mM Fe2+) | Beer D (mM Fe2+) |
|---|---|---|---|---|
| 0 | 3.01 ± 0.05a | 2.40 ± 0.15a | 1.86 ± 0.08a | 1.79 ± 0.09a |
| 3 | 2.51 ± 0.06b | 1.97 ± 0.16b | 1.50 ± 0.10b | 1.24 ± 0.04b |
| 6 | 2.24 ± 0.06c | 1.64 ± 0.12c | 1.10 ± 0.06c | 0.96 ± 0.03c |
| 9 | 2.02 ± 0.12d | 1.39 ± 0.09d | 0.89 ± 0.05d | 0.70 ± 0.07d |
| 12 | 1.89 ± 0.13e | 1.10 ± 0.10e | 0.90 ± 0.03d | 0.57 ± 0.03e |
| 15 | 1.86 ± 0.14e | 1.01 ± 0.10e | 0.79 ± 0.04d | 0.58 ± 0.02e |
Values are the means ± standard deviations of triplicate measurements. Means in a column that does not share the same alphabetical letter represent significant differences at p < 0.05
Antioxidant activity in beers is attributed mainly to their phenolic compound contents (Ducruet et al., 2017; Zhao et al., 2010). The data showed a good correlation between antioxidant activity and TPC or lignans content in the beer samples. As expected, the beer with the highest TPC exhibited greatest oxidative stability during force-aging. Several studies have evaluated the antioxidant capacities of various beers (Piazzon et al., 2010; Tafulo et al., 2010; Zhao et al., 2010). Piazzon et al. (2010) analyzed the antioxidant activities of different types of beer based on the FRAP assay and reported higher oxidative stability in the polyphenol-enriched beer. Ducruet et al. (2017) mentioned that enriching beer with fruits such as goji berries not only add a new flavor but also increases the content of bioactive compounds and the oxidative stability of the finished beer. Furthermore, Kim et al. (2015) demonstrated that the addition of 1.5% omija fruits extract to a cookie formula increased antioxidant benefits without affecting consumer likings.
Sensory evaluation
For further analysis of the additional effects of omija fruits on beer quality, the organoleptic characteristics of the beer samples were assessed (Table 5). Based on the sensory evaluation, it was apparent that the four fresh beers had little differentiation in the mouthfeel including fullness and freshness. However, significant differences (p< 0.05) were observed for sourness and bitterness as the scores decreased in the omija beers (A–C) compared to control beer D, suggesting that the decline in pH and increase in bitterness intensity of omija beers. This finding was consistent with the aforementioned data of the chemical analyses of beer. Compared to control beer D, sweetness, saltiness and astringency exhibited no subsequent changes by the addition of omija fruits during the brewing (A–C). Beer bitterness is produced primarily by the hops and by a few other bitter-tasting molecules such as polyphenols and proteins (Deng et al., 2018). Since the same hopping regime used in our work, it is possible that the bitterness difference was originated from the polyphenols and proteins released from the omija fruits. Another observation of the omija beers (A–C) was a very distinct and palatable omija berry-fruit flavor. The volatile oils which are largely made up of monoterpenes and sesquiterpenes are the main components of omija fruits and responsible for imparting the characteristic fruity flavor (Lee et al., 2011). Overall, the most preferred product among the three omija beers was the beer A produced with the addition of omija fruits at the initiation of boiling (Table 5). The enrichment of beer with omija fruits led considerably high amounts of phenolic compounds especially lignans in the beer and can be considered as a valid advantage. Other researches were also conducted to increase the quality of beer by adding goji berry, green tea, black tea or oolong tea (Ducruet et al., 2017; Rong et al., 2016; Xu et al., 2018) having a profound impact on beer flavor due to the volatile changes connected with the yeast fermentation.
Table 5.
The sensory profiles of different beer samples
| Sensory attributes | Beer A | Beer B | Beer C | Beer D |
|---|---|---|---|---|
| Total acceptability | 4.0a | 3.0b | 3.0 ± 0.5b | 4.0a |
| Malty aroma | 3.0b | 2.5b | 3.0b | 4.0a |
| Hop aroma | 3.0b | 3.0b | 3.0b | 4.0a |
| Omija fruit flavor | 3.0a | 3.5 ± 0.5a | 3.5a | 1.0b |
| Sweetness | 3.0a | 3.5a | 3.0a | 3.0a |
| Sourness | 3.5 ± 0.5a | 2.5ab | 3.0a | 2.0b |
| Saltiness | 2.0a | 2.0a | 2.0a | 1.5a |
| Bitterness | 4.0a | 3.5a | 3.5a | 3.5a |
| Astringency | 1.5a | 1.5a | 1.5a | 1.5a |
| Freshness | 4.0a | 4.0a | 4.0a | 3.5a |
| Fullness | 3.5a | 3.5a | 3.5a | 3.5a |
Values are the means ± standard deviations of triplicate measurements. Means in a row that does not share the same alphabetical letter represent significant differences at p < 0.05
To conclude, the observed results clearly exhibit that addition of omija fruits during brewing could increase the level of bioactive compounds such as lignans in beer. The contents of lignans and total phenolic compounds in the omija beers strongly depended on the moment of the addition of omija fruits in the brewing process. The addition of omija fruits at the initiation of boiling led to the extraction of the phenolic compounds and development of ale beer with the highest oxidative stability and the best sensorial attributes. However, the colloidal haze of beer significantly increased in the beer produced with the omija addition. Consequently, further research is necessary to unravel the methods to improve the clarity of omija beers.
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (31801517), Shandong Provincial Natural Science Foundation (ZR2019BC010) and the Advanced Talents Foundation of Qingdao Agricultural University (No. 6631118039). This project was also partially supported by the generous financial support of the Youlchon Foundation Nongshim Corporation and its affiliated companies (D. Kim), by the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the Agriculture, Food and Rural Affairs Research Center Support Program, funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (D. Kim, 710012-03-1-HD220), Republic of Korea. The present study has been also conducted by the research grants (2018R1D1A1A09083366, D. Kim, 2018R1D1A1B07049569, T.T.H. Nguyen, 2018R1C1B6006348, I. Mok) of NRF, Republic of Korea and by the OTTOGI Corporation through the Research and Publication Project.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yang Deng, Email: dengyang719@hotmail.com.
Juho Lim, Email: juholim@snu.ac.kr.
Thi Thanh Hanh Nguyen, Email: hara2910@snu.ac.kr.
Il-Kyoon Mok, Email: mokpodong@snu.ac.kr.
Meizi Piao, Email: piaomeizi2009@126.com.
Doman Kim, Email: kimdm@snu.ac.kr.
References
- Bamforth CW. Nutritional aspects of beer—a review. Nutr. Res. 2002;22:227–237. doi: 10.1016/S0271-5317(01)00360-8. [DOI] [Google Scholar]
- Chen Y, Tang JB, Wang XK, Sun FX, Liang SJ. An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz.) Baill. Int. J. Biol. Macromol. 2012;50:844–848. doi: 10.1016/j.ijbiomac.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Chen MF, Zhang YY, He MD, Li CY, Zhou CX, Hong PZ, Qian ZJ. Antioxidant peptide purified from enzymatic hydrolysates of Isochrysis Zhanjiangensis and its protective effect against ethanol induced oxidative stress of HepG2 cells. Biotechnol. Bioproc. Eng. 2019;24:308–317. doi: 10.1007/s12257-018-0391-5. [DOI] [Google Scholar]
- Cheng ZY, Yang YJ, Liu Y, Liu ZG, Zhou HL, Hu HB. Two-steps extraction of essential oil, polysaccharides and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Pharmaceut. Biomed. 2014;96:162–169. doi: 10.1016/j.jpba.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Cho UM, Choi DH, Yoo DS, Park SJ, Hwang HS. Inhibitory effect of ficin derived from fig latex on inflammation and melanin production in skin cells. Biotechnol. Bioproc. Eng. 2019;24:288–297. doi: 10.1007/s12257-019-0010-0. [DOI] [Google Scholar]
- Deng Y, Liu JY, Li L, Fang HJ, Tu JX, Li B, Liu J, Li HP, Xu ZB. Reduction and restoration of culturability of beer-stressed and low-temperature-stressed Lactobacillus acetotolerans strain 2011-8. Int. J. Food Microbiol. 2015;206:96–101. doi: 10.1016/j.ijfoodmicro.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Deng Y, Bi H, Yin H, Yu JH, Dong JJ, Yang M, Ma YL. Influence of ultrasound assisted thermal processing on the physicochemical and sensorial properties of beer. Ultrason. Sonochem. 2018;40:166–173. doi: 10.1016/j.ultsonch.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Ducruet J, Rebenaque P, Diserens S, Kosinska-Cagnazzo A, Heritier I, Andlauer W. Amber ale beer enriched with goji berries—the effect on bioactive compound content and sensorial properties. Food Chem. 2017;226:109–118. doi: 10.1016/j.foodchem.2017.01.047. [DOI] [PubMed] [Google Scholar]
- Ekiert RJ, Szopa A, Ekiert H, Krzek J, Dzik E. Analysis of lignans in Schisandra chinensis fruits, leaves, biomasses from in vitro cultures and food supplements. J. Funct. Foods. 2013;5:1576–1581. doi: 10.1016/j.jff.2013.06.008. [DOI] [Google Scholar]
- Gancz K, Alexander M, Corredig M. In situ study of flocculation of whey protein-stabilized emulsions caused by addition of high methoxyl pectin. Food Hydrocoll. 2006;20:293–298. doi: 10.1016/j.foodhyd.2005.02.022. [DOI] [Google Scholar]
- Gao XM, Wang RR, Niu DY, Meng CY, Yang LM, Zheng YT, Yang GY, Hu QF, Sun HD, Xiao WL. Bioactive dibenzocyclooctadiene lignans from the stems of Schisandra neglecta. J. Nat. Prod. 2013;76:1052–1057. doi: 10.1021/np400070x. [DOI] [PubMed] [Google Scholar]
- Gerstenmeyer E, Reimer S, Berghofer E, Schwartz H, Sontag G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013;138:1847–1855. doi: 10.1016/j.foodchem.2012.11.117. [DOI] [PubMed] [Google Scholar]
- Giridharan VV, Thandavarayan RA, Sato S, Ko KM, Konishi T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radical Res. 2011;45:950–958. doi: 10.3109/10715762.2011.571682. [DOI] [PubMed] [Google Scholar]
- Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008;591:293–299. doi: 10.1016/j.ejphar.2008.06.074. [DOI] [PubMed] [Google Scholar]
- He G, Du J, Zhang K, Wei G, Wang W. Antioxidant capability and potableness of fresh cloudy wheat beer stored at different temperatures. J. Inst. Brewing. 2012;118:386–392. doi: 10.1002/jib.54. [DOI] [Google Scholar]
- Huang TL, Lin JCT, Chyau CC, Lin KL, Chang CMJ. Purification of lignans from Schisandra chinensis fruit by using column fractionation and supercritical antisolvent precipitation. J. Chromatogr. A. 2013;1282:27–37. doi: 10.1016/j.chroma.2013.01.091. [DOI] [PubMed] [Google Scholar]
- Huyke C, Engel K, Simon-Haarhaus B, Quirin KW, Schempp CM. Composition and biological activity of different extracts from Schisandra sphenanthera and Schisandra chinensis. Planta. Med. 2007;73:1116–1126. doi: 10.1055/s-2007-981559. [DOI] [PubMed] [Google Scholar]
- Kaplan NM, Palmer BF. Nutritional and health benefits of beer. Am. J. Med. Sci. 2000;320:320–326. doi: 10.1097/00000441-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Kim SR, Lee MK, Koo KA, Kim SH, Sung SH, Lee NG, Markelonis GJ, Oh TH, Yang JH, Kim YC. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J. Neurosci. Res. 2004;76:397–405. doi: 10.1002/jnr.20089. [DOI] [PubMed] [Google Scholar]
- Kim SH, Joo MH, Yoo SH. Structural identification and antioxidant properties of major anthocyanin extracted from Omija (Schizandra chinensis) fruit. J. Food Sci. 2009;74:C134–C140. doi: 10.1111/j.1750-3841.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh MS, Hong J, Jang YP. Quantitative analysis of major dibenzocyclooctane lignans in Schisandrae fructus by online TLC-DART-MS. Phytochem. Anal. 2011;22:258–262. doi: 10.1002/pca.1273. [DOI] [PubMed] [Google Scholar]
- Kim MK, Lee JM, Do JS, Bang WS. Antioxidant activities and quality characteristics of omija (Schizandra chinesis Baillon) cookies. Food Sci. Biotechnol. 2015;24:931–937. doi: 10.1007/s10068-015-0120-1. [DOI] [Google Scholar]
- Kim SM, Lim HS, Lee SB. Discovery of a RuBisCO-like protein that functions as an oxygenase in the novel d-hamamelose pathway. Biotechnol. Bioproc. Eng. 2018;23:490–499. doi: 10.1007/s12257-018-0305-6. [DOI] [Google Scholar]
- Lee HJ, Cho IH, Lee KE, Kim YS. The compositions of volatiles and aroma-active compounds in dried omija fruits (Schisandra chinensis Baillon) according to the cultivation areas. J. Agric. Food Chem. 2011;59:8338–8346. doi: 10.1021/jf200762h. [DOI] [PubMed] [Google Scholar]
- Lu Y, Chen DF. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A. 2009;1216:1980–1990. doi: 10.1016/j.chroma.2008.09.070. [DOI] [PubMed] [Google Scholar]
- Mitic SS, Paunovic DD, Pavlovic AN, Tosic SB, Stojkovic MB, Mitic MN. Phenolic profiles and total antioxidant capacity of marketed beers in Serbia. Int. J. Food Prop. 2014;17:908–922. doi: 10.1080/10942912.2012.680223. [DOI] [Google Scholar]
- Nardini M, Garaguso I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020;305:125437. doi: 10.1016/j.foodchem.2019.125437. [DOI] [PubMed] [Google Scholar]
- Piazzon A, Forte M, Nardini M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010;58:10677–10683. doi: 10.1021/jf101975q. [DOI] [PubMed] [Google Scholar]
- Qu HM, Liu SJ, Zhang CY. Antitumor and antiangiogenic activity of Schisandra chinensis polysaccharide in a renal cell carcinoma model. Int. J. Biol. Macromol. 2014;66:52–56. doi: 10.1016/j.ijbiomac.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Rong L, Peng LJ, Ho CT, Yan SH, Meurens M, Zhang ZZ, Li DX, Wan XC, Bao GH, Gao XL, Ling TJ. Brewing and volatiles analysis of three tea beers indicate a potential interaction between tea components and lager yeast. Food Chem. 2016;197:161–167. doi: 10.1016/j.foodchem.2015.10.088. [DOI] [PubMed] [Google Scholar]
- Sanna V, Pretti L. Effect of wine barrel ageing or sapa addition on total polyphenol content and antioxidant activities of some Italian craft beers. Int. J. Food Sci. 2015;50:700–707. doi: 10.1111/ijfs.12666. [DOI] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Tafulo PAR, Queiros RB, Delerue-Matos CM, Sales MGF. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010;43:1702–1709. doi: 10.1016/j.foodres.2010.05.014. [DOI] [Google Scholar]
- Wu X, Yu X, Jing H. Optimization of phenolic antioxidant extraction from Wuweizi (Schisandra chinensis) pulp using random-centroid optimazation methodology. Int. J. Mol. Sci. 2011;12:6255–6266. doi: 10.3390/ijms12096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Guo MM, Du JH, Zhang ZA. Cloudy wheat beer enriched with okra [Abelmoschus esculentus (L.) Moench]: Effects on volatile compound and sensorial attributes. Int. J. Food Prop. 2018;21:304–315. [Google Scholar]
- Yan F, Zhang QY, Jiao L, Han T, Zhang H, Qin LP, Khalid R. Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomedicine. 2009;16:805–813. doi: 10.1016/j.phymed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zhao HF, Chen WF, Lu J, Zhao MM. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010;119:1150–1158. doi: 10.1016/j.foodchem.2009.08.028. [DOI] [Google Scholar]