Abstract
The aim of this study was to compare the phenolic compound profiles and antioxidant capacities of eight varieties of longan (Dimocarpus longan Lour.) planted in the middle and upper Yangtze River area. The total polyphenols content (TPC) and total flavonoids content (TFC) of dried longan pulp ranged from 1.07 ± 0.05 to 1.22 ± 0.05 mg gallic acid equivalents/g and 0.25 ± 0.07 to 0.87 ± 0.14 mg rutin equivalents/g. UHPLC-QqQ-MS/MS analysis revealed 12 individual polyphenol compounds in longan. The Fuwan8, Dongliang and FD97 varieties showed the strongest DPPH scavenging activity (IC50 of 1.03 g/mL). The highest ABTS+ scavenging activity was found in the Dongliang. In the enzyme assays, the Fuwan8 and Dongliang varieties demonstrated maximum α-amylase and α-glucosidase inhibition activities, with values of 97.56 and 88.58%, respectively. The principal component analysis indicated that the Rongyu8 and Songfengben cultivars have nearly analogous polyphenol compounds.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00708-3) contains supplementary material, which is available to authorized users.
Keywords: Longan, Polyphenol, Antioxidant, α-Amylase, α-Glucosidase
Introduction
Longans (Dimocarpus longan Lour.) belong to the family Sapindaceae, which widely distributed in subtropical zones, such as some southeastern Asian countries and southern China (Wu et al., 2007). The longan is considered to have various polyphenols and nutritional components that are commonly used in Traditional Chinese Medicine (TCM) to treat chronic diseases (Zhang et al., 2018). Li et al. (2015) reported that longans contained high levels of 4-O-methylgallic acid, corilagin, methyl gallate, proanthocyanidins, rutin and ellagic acid, which have been shown possess strong free radical-scavenging activity, inhibit α-glucosidase activity, prevent postprandial hyperglycemia, and enhance glucose tolerance. Hence, polyphenols are the third important index after weight and sugar when evaluating the nutritional value of longan germplasm resources.
Diabetes mellitus (DM) is a chronic disease of disordered carbohydrate metabolism and is characterized by hyperglycaemia, and DM is sensitive to reactive oxygen species (ROS) (Bi et al., 2017). ROS include the superoxide anion (.O2−), hydroxyl radical (.OH) and hydrogen peroxide (H2O2), which can directly damage β-cells, leading to β-cell apoptosis, and affect insulin (Wen et al., 2018). Another important cause of DM is a high consumption of starch, which is converted into monosaccharides and absorbed in the small intestine (De Sales et al., 2017). Therefore, how to effectively inhibiting ROS and monosaccharide absorption is an important way to alleviate DM. There is increasing evidence showing that polyphenols are naturally antioxidants found in high amounts in different fruits, vegetables, and natural products which can scavenge the free radicals as a result of their ability to donate electrons and hydrogen atoms (Plaza et al., 2016). Polyphenols from natural plants also exhibit inhibition of α-glucosidase and α-amylase. α-Glucosidase and α-amylase play significant roles in the regulation of blood glucose levels in the human body. They are the key enzymes of therapeutic approaches for DM to suppress postprandial hyperglycemia by inhibition of carbohydrate-hydrolyzing enzymes (Phan et al., 2013). Currently, the discovery of antioxidants, α-glucosidase inhibitors and α-amylase inhibitors from natural materials, such as food matrices, in the development of physiological functional food or lead compounds for treatment of DM is a promising topic.
Therefore, the aim of the present study was to quantitatively analyze on the phenolics profile, in vitro antioxidant activities, and α-amylase and α-glucosidase inhibitory capacities of eight different species of longans from southern China which were planted in the area of the middle and upper Yangtze River. These findings may provide a basis for evaluating regional longan resources and their nutritive value.
Materials and methods
Materials and reagents
Mature fruits of longan were collected from different areas of southern China and planted in the area of the middle and upper Yangtze River at the harvest stage (September 2018). There were eight varieties, namely, Fuwan8, Baoshi1, Rongyu8, 96–68, Songfengben (Fujian, China), Shuguan (Sichuan, China), Dongliang (Guangdong, China) and FD97 (Guangdong, China).
Folin-Ciocalteu’s phenol reagent, DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)), gallic acid, rutin, TPTZ (2,4,6-tri-(2-pyridyl)-1,3,5-triazine), α-amylase, α-glucosidase, 3,5-dinitrosalicylic acid (DNS), 4-nitrophenyl-α-D-glucopyranoside (pNPG), dibasic anhydrous sodium phosphate, and monobasic anhydrous sodium phosphate were purchased from Beijing Solarbio Science & Technology Co. Ltd. (Beijing, China). Vitamin C, soluble starch, sodium chloride, anhydrous sodium carbonate, ferric chloride and acetate buffer were obtained from Shanghai Yuanye Bio-Technology Co. Ltd. (Shanghai, China). All other reagents were of analytical grade and were commercially available.
Sample extraction
The longan pulp was disrupted and put into a vacuum freeze dryer (Scientz-50ND; Ningbo Scientz Biotechnology Co., LTD, Zhejiang, China) until freeze-dried. Freeze-dried pulp was ground into a fine powder with a muddler. An 80% chilled methanol–water solution was used to prepare the extracts from powered longan pulp. A 1:5 (w/v) ratio of sample powder to solvent underwent high-speed homogenization (FJ200-S; Ningbo Scientz Biotechnology Co., LTD, Zhejiang, China) in an ice-bath for 1 min and then underwent blending with a magnetic stirrer for 5 min. Subsequently, the extract was centrifuged at 6000×g for 10 min. The supernatant was collected in a centrifuge tube, and it was stored in a freezer until further use.
Determination of total polyphenols content
The colorimetric Folin–Ciocalteu method (Karamac et al., 2018) was used to determinate the total polyphenols content (TPC). The TPC of the longan pulp extracts was determined by measuring the absorbance at 760 nm after reaction with Folin-Ciocalteu’s phenol reagent (10 μL), 100 μL of sodium carbonate solution (10%, w/v) and distilled water (80 μL) and incubation in a 96 well cell culture cluster at room temperature for 90 min. The TPC of each sample was expressed as mg of gallic acid equivalents per g of dry weight of longan pulp (mg GAE/g DW).
Determination of the total flavonoids content
The total flavonoids content (TFC) was determined by sodium nitrite-aluminum nitrate colorimetry with rutin as a standard according to Alothman et al. (2009). The sample extract or rutin standard solution (10 μL) was mixed with 10 μL sodium nitrite solution (5%, w/v), 10 μL aluminum nitrate solution (10%, w/v), 100 μL sodium hydroxide solution (4%, w/v) and 120 μL ethanol solution (60%, v/v). After each step, an incubation at room temperature for 15 min occurred, and the absorbance was acquired at 510 nm. The results were expressed as mg of rutin equivalents per g of dry weight of longan pulp (mg RE/g DW).
UHPLC-QqQ-MS/MS analysis
UHPLC-QqQ-MS/MS analysis was performed according to the methodology described by Li et al. (2019). The polyphenol compositions in longans were analyzed using UHPLC (Agilent, Santa Clara, California, USA) and a triple quadruple mass spectrometer (6460QqQ-MS/MS; Agilent) equipped with an electrospray ionization source. The analytical column was a ZORBAX Eclipse Plus C18 column (100 × 2.1 mm i.d., 1.8 μm, Agilent, Waldbronn, Germany). The gradient of the mobile phase was 0.1% aqueous formic acid (A) and acetonitrile (B). The injection volume was equilibrated with 80% A, and the flow rate was 0.4 mL/min. The gradient program was as follows: 0 to 11.5 min from 80% to 10% A, 11.5 to 12.5 min with 10% A, 12.5 to 12.8 min from 10% to 90% A, 12.8 to 13.0 min from 90% to 80% A, and 13 to 15 min with 80% A. The proportion decreased to 10% A in 11.5 min and remained constant until 12.5 min. The sample injection volume was 5 μL at a 30 °C column temperature. Analysis data were collected by multiple reaction monitoring (MRM) in the negative ion mode. After optimization, the source parameters used were as follows: gas temperature of 350 °C and gas flow of 10 L/min. All the compounds were exactly identified by comparing the dynamic MRM information including retention time, collision energies, transitions and fragmentor voltages with reference standards and shown in Table 1. Data acquisition and processing were performed using MassHunter software version B.09.00 (Agilent Technologies Inc., USA).
Table 1.
Identified polyphenols in longans using UPLC-QqQ-MS
| No. | Compounds | RT | MS [M–H]− | MS/MS (m/z) | CE (V) | Fragmentor (V) |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 0.576 | 191 | 108.9 | 35 | 130 |
| 2 | Rutin | 0.586 | 609 | 299.9 | 40 | 160 |
| 3 | Gallic acid | 0.978 | 168.9 | 125,106.8 | 15 | 100 |
| 4 | Narirutin | 1.323 | 579 | 271,295 | 20 | 160 |
| 5 | Naringin | 1.631 | 579 | 271,151 | 35 | 150 |
| 6 | Rhoifolin | 1.733 | 577 | 268.9 | 40 | 150 |
| 7 | Hesperidin | 1.793 | 609 | 301,324.9 | 20 | 160 |
| 8 | Phthalic acid | 1.831 | 137 | 137 | 5 | 135 |
| 9 | Methyl hesperidin | 1.832 | 623 | 315,338.7 | 20 | 135 |
| 10 | Phlorizin | 2.487 | 434.9 | 272.9167 | 20 | 150 |
| 11 | Quercetin | 2.853 | 150.9, 107 | 300.9 | 25 | 130 |
| 12 | Naringenin | 4.212 | 119, 150.8 | 271 | 25 | 100 |
RT retention time (min), CE collision energy, V volt
Determination of antioxidant activity
Antioxidant activity of longan pulp extracts were evaluated using three antioxidant means (DPPH radical scavenging activity, ABTS+ scavenging activity and ferric reducing antioxidant power) as described in our study. The determination was based on the method proposed by Kwak and Ju (2017) with some modifications.
DPPH assay
A volume of 100 μL of four different sample concentrations was mixed with 100 μL of DPPH solution (0.5 mM) and incubated in the dark for 30 min. The absorbance of each reaction mixture was measured at 517 nm. The ability of extracts to scavenge the DPPH was expressed using the half maximal inhibitory concentration (IC50) value.
ABTS+ assay
ABTS+ solution was obtained by reacting 100 mL ABTS solution (7 mM) and 100 mL potassium persulfate solution (2.45 mM) at room temperature in the dark for 12 h. Then, the stock solution was diluted with anhydrous ethanol to stabilize its absorbance value at 734 nm at 0.7 ± 0.02. The absorbance of each mixture was recorded at 734 nm. The antioxidant capacity was expressed as mg vitamin C (VC) equivalents per g of dry weight (DW) of longan pulp.
FRAP assay
First, the FRAP reagent was prepared using 0.1 mM acetate buffer at pH 3.6, 10 mM TPTZ in 40 mM HCl and 20 mM ferric chloride in a ratio of 10:1:1 (v/v/v). Then, 50 μL of the sample extracts and 100 μL of distilled water were added to the FRAP reagent. After incubation at 37 °C in a water bath for 10 min, the absorbance of each mixture was recorded at 593 nm. FRAP was expressed in terms of mg VC equivalents per g of dry weight (DW) of longan pulp.
α-Amylase inhibitory assay
The assay was performed as described earlier (Shu et al., 2009) with some modifications. Briefly, a soluble starch (5 mg/mL) and α-amylase solution (0.01 Unit) was prepared. Next, the solution in a 96 well plate was mixed and incubated at 37 °C in a water bath for 1 h. Then, the reaction was terminated by the addition of 200 μL DNS color reagent and boiling for 5 min. The absorbance of the resultant solution was measured at 540 nm. The α-amylase inhibitory activity was expressed as % inhibition.
α-Glucosidase inhibitory assay
The α-glucosidase enzyme inhibitory assay was performed in a 96 well plate as described earlier (Wan et al., 2012) and with modifications. Briefly, 20 μL sample solution and 120 μL of 0.1 M phosphate buffer (pH 6.9) containing 29 μL α-glucosidase solution (0.02 Unit) and 50 μL pNPG (3 mM) was incubated in 96 well plates at 37 °C for 40 min. After pre-incubation, 50 μL sodium carbonate solution was added to each well at timed intervals. The absorbance was recorded at 405 nm. The α-glucosidase inhibitory activity was expressed as % inhibition.
Statistical analysis
All analyses were carried out with three replicates (n = 3), and data were reported as the mean ± standard deviation (SD). The data were analysed by a one-way analysis of variance (ANOVA) followed by Tukey’s test, which were performed by SPSS statistical package version 23.0 (SPSS Inc., Chicago, IL, USA), and the significance level of all statistical tests was set at 0.05. The IC50 values of DPPH radical scavenging activity analyses were calculated by GraphPad Prism 6 (GraphPad, La Jolla, CA, USA). The principal component analysis (PCA) was performed by using SIMCA 14.1 (Umeå, Sweden) to detect clustering and grade samples.
Results and discussion
Total polyphenols content of longans
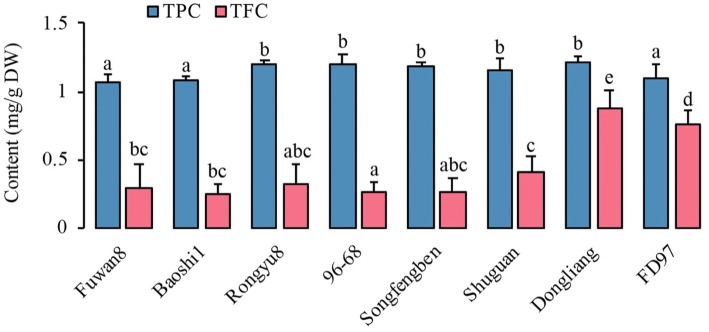
Total polyphenols content (TPC) of longans are shown in Fig. 1. The dried longan pulp contained 1.07 ± 0.06 to 1.22 ± 0.05 mg GAE/g DW. These findings were similar to those obtained in a study by Prasad et al. (2009) that found that TPC values of the type of Shixia, in different solvents, were approximately 17.5 mg GAE/g of DW when the extract solvent was methanol. Among the eight different varieties of longan fruits, the type of Dongliang had the highest value of TPC at 1.22 ± 0.05 mg GAE/g DW, while the fruits of Fuwan8, Baoshi1 and FD97 exhibited the lowest TPC values. Variance analysis of the results showed no significant differences (p > 0.05) among the other five longan fruits (i.e., Rongyu8, 96–68, Songfengben, Shuguan, and Dongliang).
Fig. 1.
The total polyphenols content (TPC) and total flavonoids content (TFC) in different longan fruits of dry weight (DW). Different letters denoted the significant difference at p < 0.05 level
Flavonoids are an important kind of polyphenol compound (Lin et al., 2016). The total flavonoids content (TFC) in the eight dried longan pulp ranged from 0.25 ± 0.07 to 0.87 ± 0.14 mg RE/g of DW, which was close to the TPC. This result suggested that flavonoids were the most main component in polyphenols. In addition, Dongliang had the highest TFC (0.87 ± 0.14 mg RE/g of DW), followed by FD97 (0.76 ± 0.1 mg RE/g of DW), and the extract of 96–68 had the lowest TFC (0.25 ± 0.07 mg RE/g of DW). There was no significant variation (p > 0.05) in the Dongliang and FD97, while significant variation (p < 0.01) between other cultivars was observed.
Concentrations of chemical compounds
The UHPLC-QqQ-MS/MS chromatograms of longan extracts were characterized by the peaks with retention times shown in Fig. S1. There were 12 polyphenol compounds existed in the 8 longan cultivars, including 3 phenolic acids (quinic acid, gallic acid and phthalic acid) and 9 flavonoids (narirutin, naringin, rutin, quercetin, hesperidin, phlorizin, rhoifolin, methyl hesperidin and naringenin, Table 2), in the 8 longan cultivars. Zhang et al. (2018) found 10 polyphenols in 24 Chinese longan fruits that they collected from the zone of the Pearl River Basin. Additionally, they also found gallic acid and quercetin in longan fruits. Therefore, the current study further improved the knowledge of polyphenols fingerprints of longans.
Table 2.
Contents of polyphenol in different longan fruits
| Name | Phenolic acids (mg/g DW) | Flavonoids (mg/g DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QA | GA | PA | Narirutin | Naringin | Rutin | Quercetin | Hesperidin | Phlorizin | Rhoifolin | MH | Naringenin | |
| Fuwan8 | 280.27c | NDa | 0.90a | 0.60b | 0.08a | 4.92b | 0.18a | 4.70c | 0.08a | 0.09a | 3.73a | 0.33b |
| Baoshi1 | 23.56a | 0.12ab | 1.13a | 0.10a | 0.06a | 4.92b | 0.16a | 0.65b | 0.11ab | 0.16a | 7.18f | 0.31a |
| Rongyu8 | 77.04b | NDa | 0.50a | 0.11a | 0.05a | 4.11a | 0.18a | 0.39a | 0.09a | 0.08a | 4.15b | 0.31a |
| 96-68 | 24.28a | NDa | 0.36a | 0.70c | 0.06a | 4.17a | 0.19a | 5.60d | 0.13b | 0.08a | 4.16b | 0.33bc |
| Songfengben | 69.42b | 0.56c | 0.48a | 0.07a | 0.07a | 4.45ab | 0.18a | 0.49ab | 0.08a | 0.08a | 4.76d | 0.31a |
| Shuguan | 68.97b | NDa | 2.80b | 0.82d | 0.06a | 8.18c | 0.22a | 6.94f | 0.11ab | 0.10a | 6.89e | 0.33b |
| Dongliang | 22.12a | 0.39b | 0.87a | 1.24f | 0.08a | 12.35d | 1.25b | 8.77 g | 0.19c | 0.47b | 4.42c | 0.34c |
| FD97 | 71.88b | NDa | 0.77a | 0.87e | 0.05a | 15.61e | 1.07b | 6.68e | 0.10ab | 0.29ab | 4.72d | 0.34bc |
Means in the row with different letters indicate significant differences, as determined by ANOVA and Turkey’s post hoc test (p < 0.05)
DW dry weight, ND not detect, QA quinic acid, GA galiic acid, PA phthalic acid, MH methyl hesperidin
Among the three phenolic acids, quinic acid content was the highest followed by phthalic acid, from 22.12 to 280.27 mg/g DW and 0.36 to 2.80 mg/g DW, respectively. Fuwan8 had the highest quinic acid content, followed by Rongyu8, while the quinic acid content of Dongliang was the lowest. Wang et al. (2009) found that quinic acid is derived from chlorogenic acid and demonstrates diverse biological actions, such as antiviral, antioxidant, anti-carcinogenic, and hepatoprotective actions and modulation of signal transduction pathways. For the content of phthalic acid, asides from the Shuguan cultivar, there were no difference at the p > 0.05 level among the longans. However, gallic acid was only found in the three varieties, Baoshi1 (0.12 mg/g DW), Songfengben (0.56 mg/g DW) and Dongliang (0.39 mg/g DW), which may relate to the growth environment of the different varieties. Miao et al. (2018) suggested that varying rainfall, global radiation, temperature and fertilization in different planting areas vary significantly affect the chemical compositions of fruits.
Flavonoids constitute the largest group of food polyphenols and provide health benefits for prevention and treatment of some types of chronic diseases due to their anti-inflammatory and antioxidant properties (Lin et al., 2016). In our study, the highest content among flavonoids was rutin (Table 2). The highest content of rutin appeared in FD97, reaching 15.61 mg/g DW. The content of rutin in Dongliang was ranked the second highest (12.35 mg/g DW) among the 8 longans. Yang et al. (2008) found that rutin metabolites containing vicinyl dihydroxyl groups; it is likely that metal chelation and/or free radical scavenging properties contribute to the inhibition of glucose autoxidation. Furthermore, hesperidin, methyl hesperidin and naringenin, which can inhibit oxidative stress and inflammation by directly reducing free radicals and enhancing endogenous antioxidant systems, have a similar chemical structure (Martini et al., 2017). In this work, ranges of hesperidin, methyl hesperidin and naringenin content were 0.39–8.77 mg/g DW, 3.73–7.18 mg/g DW and 0.31–0.34 mg/g DW, respectively. The highest content of hesperidin was found in Dongliang; the highest content of methyl hesperidin was present in Baoshi1; and the highest content of naringenin appeared in Dongliang. Quercetin, as an inhibitor and free radical scavenger, is another important, unique compound of longan fruits (Nile et al., 2017). Dongliang and FD97 showed the highest quercetin content with no difference at the p > 0.05 level. Similarly, the variety of Dongliang had the highest content of narirutin, phlorizin and rhoifolin. However, the content of these compounds was lower in other varieties. Narirutin, phlorizin and rhoifolin also have good antioxidant activity and anti-hyperglycemic effects in fruits (Karamac et al., 2018). These findings suggested that longan fruits are an excellent resource of polyphenols, which have strong antioxidant and anti-hyperglycemic properties.
Multivariate analysis of polyphenol compounds
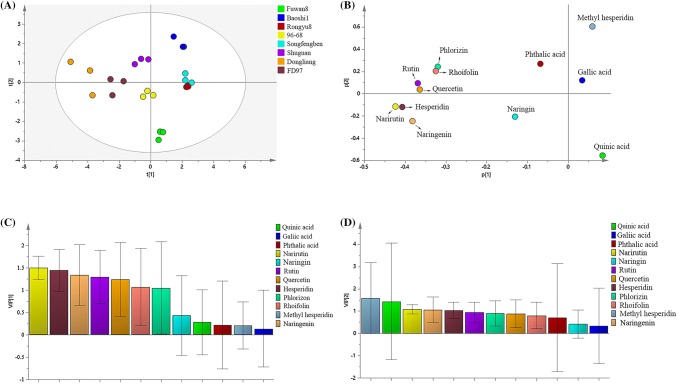
Eight longan samples belonging to the middle and upper Yangtze River area were studied in this work. Due to the diversity of the tested samples in the identification of the biochemical parameters distributions in longans in relation to the varieties, principal component analysis (PCA) was employed. The PCA score plot (Fig. 2A) showed a clear difference between the compositional profiles of the eight longan fruits. The cultivars of Rongyu8 and Songfengben are slightly overlap, this because that they have nearly an analogous polyphenols content. In addition, the varieties of Baoshi1, Songfengben, Rongyu8, Dongliang, FD97 and Shuguan were distinguished by the t [1] axis and t [2] axis in the identified varieties of Baoshi1, Shuguan, 96–68 and Fuwan8 varieties. To find the main compositions that caused the classification, further PLS-DA analysis was performed. As shown in the PLS-DA loading plot (Fig. 2B), narirutin, hesperidin, naringenin, rutin, quercetin, phlorizin and rhoifolin obviously deviated from the origin, indicating that these 7 polyphenol compounds are major contributors to distinguishing the difference in polyphenol composition of the longan varieties. Furthermore, in order to further find which polyphenols components mainly distinguish the first principal component or the second principal component, values higher than 1.0 were selected as markers (Vanesa et al., 2011). The Var ID Primary (VIP) value of the first principal component (Fig. 2C) showed narirutin, hesperidin, naringenin, rutin, quercetin and phlorizin values higher than 1, indicating these 6 polyphenols are the major markers to distinguish Baoshi1, Songfengben, Rongyu8, Dongliang, FD97 and Shuguan. For the second principal component (Fig. 2D), the VIP value of methyl hesperidin was greater than 1. Thus, methyl hesperidin might be a major contributor to the difference in the polyphenol compositions among Baoshi1, Shuguan and Fuwan8, and 96–68. Therefore, multivariate analysis could be helpful in providing valuable information on the classification of the variety and on relationships between polyphenols compositions.
Fig. 2.
(A) PCA score plot for eight longan fruits. (B) PLS-DA loading plot for 12 polyphenol compounds of eight longan fruits. (C) Var ID (Primary) of 12 polyphenol compounds for the first principal component. (D) Var ID (Primary) of 12 polyphenol compounds for the second principal component
Antioxidant capacity of longans
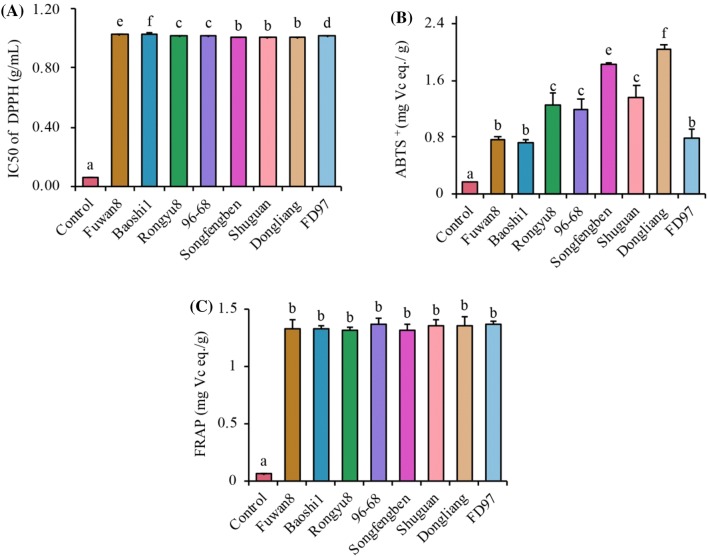
Antioxidant capacity is the most important property of polyphenol compounds (Pang et al., 2018). The antioxidant activities of longan pulp extracts were studied using two stable synthetic radicals (i.e., DPPH and ABTS+) and a model emulsion with FRAP assay. The DPPH scavenging activity of longan extracts are presented in Fig. 3A. The IC50 values of Songfengben, Shuguan and Dongliang extracts were lower than Fuwan8, Baoshi1, Rongyu8, 96–68 and FD97, suggesting that the scavenging activity had no differences at the p > 0.05 level, while the weakest IC50 values was found for the extract and seeds of the Baoshi1 (1.03 g/mL). For the DPPH radical scavenging mechanism, a hydrogen molecule is donated to the radical for its stabilization in the radical form (Kedare and Singh, 2011). Yang et al. (2013) suggested that quercetin and rutin are responsible for radical scavenging activity by donating a hydrogen molecule to the radical for stabilization in the radical form. In this regard, the three cultivars with strong scavenging activity might contain high levels of quercetin and rutin, which could donate a hydrogen to scavenge a DPPH radical.
Fig. 3.
Antioxidant activity of different longan fruits. (A) DPPH free radical scavenging capacity. (B) ABTS+ eliminating activity. (C) Ferric reducing antioxidant power assay (FRAP). The control group was water. Different letters denoted the significant difference at p < 0.05 level
The radical scavenging activity of longan extracts against ABTS+ is demonstrated as a VC equivalent (Fig. 3B). The extract of Dongliang showed the highest value: 2.03 ± 0.06 mg VC equivalents/g. Fuwan8, Baoshi1 and FD97 extracts showed the lowest values: 0.76 ± 0.04, 0.72 ± 0.05 and 0.79 ± 0.11 mg VC equivalents/g, respectively. Previous research reported that narirutin and quercetin showed a stabilization of an electron donate to ABTS+ for antioxidant effects (Chakraborty and Basu, 2017). Interestingly, Dongliang has higher narirutin and quercetin content than other cultivars, which may contribute to a stronger activity in providing hydrogen ions and transferring electrons and, thereby, scavenging ABTS+.
In the human body, an unpaired electron reacts quickly with peroxides, leading to the formation of alkoxyl radicals. Thus, treating metals with an antioxidant can be considered as an important antioxidant mechanism (Müller et al., 2011). As shown in Fig. 3C, the values of ferric reducing antioxidant power (FRAP) varied from 1.32 ± 0.03 to 1.37 ± 0.03 mg VC equivalents/g, and there were no significant differences among the 8 longans. Prasad et al. (2009) were also reported that polyphenol-rich longan extracts have FRAP capacity. Furthermore, quinic acid, rutin, narirutin and hesperidin that were found in longans but did not possess the ferric reducing antioxidant power and chelated metal ions (Kaiser et al., 2013). Together, the longan polyphenols have considerable antioxidant properties that were closely related to narirutin, hesperidin, naringenin, rutin and quercetin.
α-Amylase inhibitory activity and α-glucosidase inhibitory activity of longans
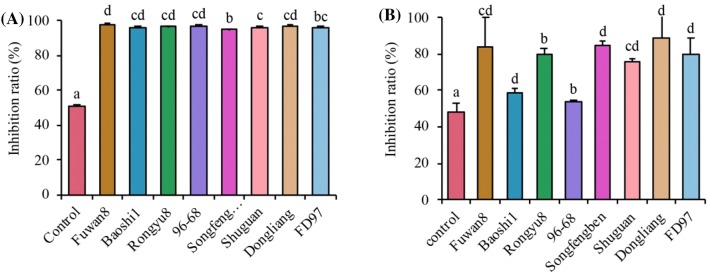
Many recent studies indicate that the polyphenols rich plants have the ability to chelate the enzymes of α-amylase and α-glucosidase, as a result of the number of hydroxyl groups on phenolic and flavonoid compounds with a B ring being closely associated with the inhibitory activity of α-amylase and α-glucosidase enzymes (Liu et al., 2017). The α-amylase and α-glucosidase inhibitory activities of polyphenols extracted from longan fruits are exhibited in Fig. 4. Fuwan8 of the longan fruit had the best α-amylase inhibitory activity (97.56%) compared to other varieties. The α-glucosidase inhibitory activities of Fuwan8, Rongyu8, Songfengben, Dongliang and FD97 were higher than others (p > 0.05). In addition, the lowest α-glucosidase inhibitory activity (53.55%) was found in 96–68. We speculated that the inhibitory activities of glucose metabolic enzymes might be due to the extracts of longan pulp including 12 polyphenols. Among polyphenols of longans, it has already been reported that an OH group in quinic acid may have influenced its inhibitory of properties (Narita and Inouye, 2009). The unsaturated C ring, the 3-OH, the 4-CO, the linkage of the B ring at the 3 position, and the hydroxyl substitution on the B ring enhanced the α-amylase inhibitory activity. In addition, quinic acid also has the capacity to inhibit α-glucosidase due to its hydroxyl groups in quinic acid (Zhang et al., 2016). Furthermore, Li et al. (2009) reported that the similar structures of quercetin and rutin, in which the main driving force was hydrophobic, had a replacement of C3-OH by one glycosyl group. This finding suggested that the possible mechanism by which quercetin and rutin inhibited α-glucosidase. The compound of naringenin, regarding the inhibition of α-glucosidase, exhibited more potent inhibitory activity (IC50: 10.6 ± 0.49 mM) (Jung et al., 2017). Moreover, naringenin in diabetic rats in a post-docking intramolecular hydrogen bonding analysis showed a water molecule mediated hydrogen bonding for N-terminal maltase glucoamylase and N-terminal sucrase iso-maltase, which can inhibit α-glucosidase activity in vivo (Priscilla et al., 2014). Hence, it could be concluded that the polyphenols of the longan in the middle and upper Yangtze River play a significant role in the inhibition of α-amylase and α-glucosidase enzymes, and this effect helps to control the hyperglycemia, which is beneficial for DM.
Fig. 4.
(A) α-Amylase and (B) α-glucosidase inhibit activities of different varieties of longan fruits. The control group was water. Different letters denoted the significant difference at p < 0.05 level
Correlation analysis
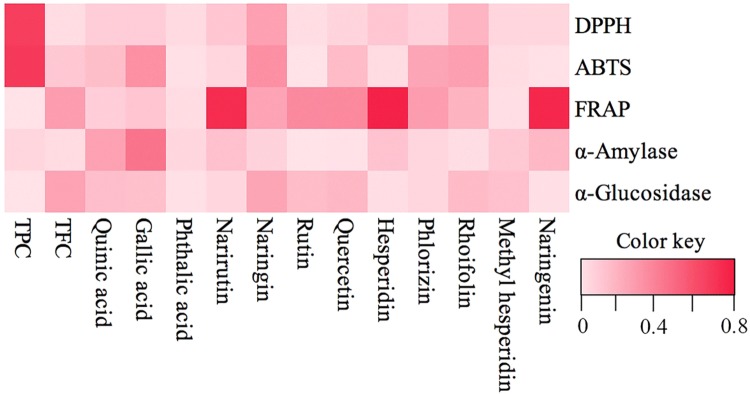
Correlation analysis was conducted to reveal the correlation between polyphenol compositions and bioactivities (antioxidant capacity and α-amylase and α-glucosidase inhibitory activities) of longans. The TPC of dried longan pulp extracts exhibited strong correlations with DPPH (r2 = 0.649) and ABTS (r2 = 0.672). This finding verified that the antioxidant activity of dried longan pulp extracts was associated with their phenolic compounds. The narirutin, hesperidin and naringenin of longans showed a strong correlation with FRAP (r2 = 0.722, r2 = 0.762 and r2 = 0.737, respectively). Figure 5 also presents that gallic acid has a strong correlation with α-amylase and α-glucosidase inhibitory activities. The correlation analysis indicated that the polyphenols of longan fruits are important active compounds, and different constituents mainly contribute to different activity.
Fig. 5.
Heat map showing the results of the correlation analysis
In conclusion, we explored the nutrition and functional compounds of eight different longan fruits planted in the middle and upper Yangtze River that have potential antioxidant and anti-diabetes effects. The UHPLC-QqQ-MS/MS analysis and PCA revealed that narirutin, hesperidin, naringenin, rutin, quercetin, phlorizin and rhoifolin were the main polyphenols in longan fruits. The results suggested that longan fruits can potentially be used as an ingredient in functional foods with antioxidant activity and as a means of hyperglycemia management. These results suggested that the polyphenol compositions and antioxidant capacity are considered as vital indexes for longan germplasm sources. Moreover, these results suggested that the polyphenol compositions and antioxidant capacity are considered as vital indexes for longan germplasm sources.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Scientific Research Foundation of Yangtze Normal University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhuo Wang, Email: 549346357@qq.com.
Xiaoxv Gao, Email: 122807783@qq.com.
Wenfeng Li, Email: DrNaruto@snnu.edu.cn, Email: shanxiliwenfeng@163.com.
Si Tan, Email: tsok1990715@163.com.
Qiaoran Zheng, Email: zheng_qr@163.com.
References
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Bi XY, Lim J, Henry CJ. Spices in the management of diabetes mellitus. Food Chem. 2017;217:281–293. doi: 10.1016/j.foodchem.2016.08.111. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Basu S. Multi-functional activities of citrus flavonoid narirutin in Alzheimer’s disease therapeutics: An integrated screening approach and in vitro validation. Int. J. Biol. Macromol. 2017;103:733–743. doi: 10.1016/j.ijbiomac.2017.05.110. [DOI] [PubMed] [Google Scholar]
- De Sales PM, De Souza PM, Dartora M, Resck IS, Simeoni LA, Fonseca-Bazzo YM, De Oliveira Magalhães P, Silveira D. Pouteria torta epicarp as a useful source of α-amylase inhibitor in the control of type 2 diabetes. Food Chem. Toxicol. 2017;109:962–969. doi: 10.1016/j.fct.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Jung HA, Paudel P, Seong SH, Min BS, Choi JS. Structure-related protein tyrosine phosphatase 1B inhibition by naringenin derivatives. Bioorg. Med. Chem. Lett. 2017;27:2274–2280. doi: 10.1016/j.bmcl.2017.04.054. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Hartmann KI, Kammerer DR, Carle R. Evaluation of the effects of thermal treatments on color, polyphenol stability, enzyme activities and antioxidant capacities of innovative pasty celeriac (Apium graveolens L. var. rapaceum (Mill.) DC.) products. Eur. Food Res. Technol. 2013;237:353–365. doi: 10.1007/s00217-013-1998-6. [DOI] [Google Scholar]
- Karamac M, Orak HH, Amarowicz R, Orak A, Piekoszewski W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018;258:1–7. doi: 10.1016/j.foodchem.2018.03.041. [DOI] [PubMed] [Google Scholar]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Ju J. Glycine max Merr. leaf extract possesses anti-oxidant properties, decreases inflammatory mediator production in murine macrophages, and inhibits growth, migration, and adhesion in human cancer cells. Food Sci. Biotechnil. 2017;26:245–253. doi: 10.1007/s10068-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Xu JL, Mu Y, Han L, Liu RH, Cai YP, Huang XS. Chemical characterization and anti-hyperglycaemic effects of polyphenol enriched longan (Dimocarpus longan Lour.) pericarp extracts. J. Funct. Foods. 2015;13:314–322. doi: 10.1016/j.jff.2015.01.006. [DOI] [Google Scholar]
- Li WF, Wang X, Zhang J, Zhao X, Wu Y, Tan S, Zheng QR, Gao XX. Multivariate analysis illuminates the effects of vacuum drying on the extractable and nonextractable polyphenols profile of loquat fruit. J. Food Sci. 2019;4:726–737. doi: 10.1111/1750-3841.14500. [DOI] [PubMed] [Google Scholar]
- Li YQ, Zhou FC, Gao F, Bian JS, Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009;57:11463–11468. doi: 10.1021/jf903083h. [DOI] [PubMed] [Google Scholar]
- Lin WQ, Wang WT, Yang H, Wang DL, Ling WH. Influence of intestinal microbiota on the catabolism of flavonoids in mice. J. Food Sci. 2016;81:3026–3034. doi: 10.1111/1750-3841.13544. [DOI] [PubMed] [Google Scholar]
- Liu SY, Ai ZY, Qu FF, Chen YQ, Ni DJ. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against α-amylase, α-glucosidase and intestinal glucose uptake. Food Chem. 2017;234:168–173. doi: 10.1016/j.foodchem.2017.04.151. [DOI] [PubMed] [Google Scholar]
- Martini S, Conte A, Tagliazucchi D. Phenolic compounds profile and antioxidant properties of six sweetcherry (Prunus avium) cultivars. Food Res. Int. 2017;97:15–26. doi: 10.1016/j.foodres.2017.03.030. [DOI] [PubMed] [Google Scholar]
- Miao J, Li X, Zhao C, Gao X, Wang Y, Gao W. Active compounds, antioxidant activity and α -glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018;248:330–339. doi: 10.1016/j.foodchem.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129:139–148. doi: 10.1016/j.foodchem.2011.04.045. [DOI] [Google Scholar]
- Narita Y, Inouye K. Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas α-amylase isozymes I and II. J. Agric. Food Chem. 2009;57:9218–9225. doi: 10.1021/jf9017383. [DOI] [PubMed] [Google Scholar]
- Nile SH, Nile AS, Keum YS, Sharma K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017;235:119–126. doi: 10.1016/j.foodchem.2017.05.043. [DOI] [PubMed] [Google Scholar]
- Pang YH, Ahmed S, Xu YJ, Beta T, Zhu Z, Shao YF, Bao J. Bound phenolic compounds and antioxidant properties of whole grain andbran of white, red and black rice. Food Chem. 2018;240:212–221. doi: 10.1016/j.foodchem.2017.07.095. [DOI] [PubMed] [Google Scholar]
- Phan MAT, Wang J, Tang J, Lee YZ, Ng K. Evaluation of α-glucosidase inhibition potential of some flavonoids from Epimedium brevicornum. LWT Food Sci. Technol. 2013;53:492–498. doi: 10.1016/j.lwt.2013.04.002. [DOI] [Google Scholar]
- Plaza M, Batista ÂG, Cazarin CBB, Sandahl M, Turner C, Östman E, Júnior MRM. Characterization of antioxidant polyphenols from Myrciaria jaboticab a peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem. 2016;211:185–197. doi: 10.1016/j.foodchem.2016.04.142. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Yang E, Yi C, Zhao M, Jiang Y. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innovative Food Sci. Emerging Technol. 2009;10:155–159. doi: 10.1016/j.ifset.2008.11.007. [DOI] [Google Scholar]
- Priscilla DH, Roy D, Suresh A, Kumar V, Thirumurugan K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem Biol Interact. 2014;210:77–85. doi: 10.1016/j.cbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Shu XS, Lv JH, Tao J, Li GM, Li HD, Ma N. Antihyperglycemic effects of total flavonoids from Polygonatum odoratum in STZ and alloxan-induced diabetic rats. J. Ethnopharmacol. 2009;124:539–543. doi: 10.1016/j.jep.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Vanesa GC, Noelia RC, María Eugenia LG, Luis Vicente PV, Luis María PD. Principal component analysis (PCA) and multiple linear regression (MLR) statistical tools to evaluate the effect of E-beam irradiation on ready-to-eat food. J. Food Compos. Anal. 2011;24:456–464. doi: 10.1016/j.jfca.2010.11.010. [DOI] [Google Scholar]
- Wan C, Yuan T, Cirello AL, Seeram NP. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012;135:1929–1937. doi: 10.1016/j.foodchem.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Wang GF, Shi LP, Ren YD, Liu QF, Liu HF, Zhang RJ, Li Z, Zhu FH, He PL, Tang W, Tao PZ, Li C, Zhao WM, Zuo JP. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res. 2009;83:186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Wen H, Tang B, Stewart AJ, Tao Y, Shao Y, Cui Y, Yue H, Pei J, Liu Z, Mei L, Yu R, Jiang L. Erythritol attenuates postprandial blood glucose by inhibiting α-glucosidase. J. Agric. Food Chem. 2018;66:1401–1407. doi: 10.1021/acs.jafc.7b05033. [DOI] [PubMed] [Google Scholar]
- Wu YL, Yi GY, Zhou B, Zeng J, Huang YH. The advancement of research on litchi and longan germplasm resources in China. Sci. Hortic. 2007;114:143–150. doi: 10.1016/j.scienta.2007.07.016. [DOI] [Google Scholar]
- Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008;41:1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- Yang Y, Liu X, Wu HR, He XF, Bi YR, Zhu Y, Liu ZL. Radical scavenging activity and cytotoxicity of active quinic acid derivatives from Scorzonera divaricata roots. Food Chem. 2013;138:2057–2063. doi: 10.1016/j.foodchem.2012.10.122. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tu ZC, Yuan T, Wang H, Xie X, Fu ZF. Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016;208:61–67. doi: 10.1016/j.foodchem.2016.03.079. [DOI] [PubMed] [Google Scholar]
- Zhang R, Khan SA, Lin Y, Guo D, Pan X, Liu L, Wei Z, Zhang Y, Deng Y, Zhang M. Phenolic profiles and cellular antioxidant activity of longan pulp of 24 representative Chinese cultivars. Int. J. Food Prop. 2018;21:746–759. doi: 10.1080/10942912.2018.1425705. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.