Abstract
Phenolic compounds are broadly represented in plant kingdom, and their occurrence in easily accessible low-cost sources like wastes from agri-food processing have led in the last decade to an increase of interest in their recovery and further exploitation. Indeed, most of these compounds are endowed with beneficial properties to human health (e.g., in the prevention of cancer and cardiovascular diseases), that may be largely ascribed to their potent antioxidant and scavenging activity against reactive oxygen species generated in settings of oxidative stress and responsible for the onset of several inflammatory and degenerative diseases. Apart from their use as food supplements or as additives in functional foods, natural phenolic compounds have become increasingly attractive also from a technological point of view, due to their possible exploitation in materials science. Several extraction methodologies have been reported for the recovery of phenolic compounds from agri-food wastes mostly based on the use of organic solvents such as methanol, ethanol, or acetone. However, there is an increasing need for green and sustainable approaches leading to phenolic-rich extracts with low environmental impact. This review addresses the most promising and innovative methodologies for the recovery of functional phenolic compounds from waste materials that have appeared in the recent literature. In particular, extraction procedures based on the use of green technologies (supercritical fluid, microwaves, ultrasounds) as well as of green solvents such as deep eutectic solvents (DES) are surveyed.
Keywords: phenolic compounds, agri-food wastes, sustainability, microwave assisted extraction, ultrasound assisted extraction, supercritical fluid extraction, deep eutectic solvents, Naviglio extractor
Introduction
Global food waste approximates 1.3 billion tons per year as the result of primary and secondary processes occurring along the supply chain, which include losses generated during production and postharvest of the food products, that represent about 75% of food losses e.g., in developing African countries, or wastage at the consumption stage as is the case of industrialized countries (North America and Europe) (1, 2). Agri-food industry in particular is responsible for the generation of high volumes of organic wastes (biomasses), reaching up to 140 billion tons per year, although a considerable part of this is not related to food wastage issues (2–4). Disposal of these byproducts represents a cost to the food processor and has a negative impact on the environment. On the other hand these materials can be considered as a largely available, low cost source not only of energy for biofuel production, but also of value-added compounds, whose recovery represents therefore a valuable opportunity (5).
Generally, natural products are considered attractive value-added compounds based on their wide bioactivity spectrum. Among these, a prominent role is occupied by phenolic compounds, which are well-known for their beneficial effects on human health, e.g., in the prevention of cancer and cardiovascular diseases (6–8). These effects have been ascribed in part to their ability to act as potent antioxidants and scavengers of reactive oxygen species, generated under oxidative stress conditions and responsible for the onset of several inflammatory and degenerative diseases (9–11). These properties have therefore prompted the use of natural phenolic compounds not only as food supplements (7, 12–15), but also as additives for functionalization of materials to be used e.g., in biomedicine (16–18), cosmetic (19–22), or food industry (23–27).
In this context of course it is clear that, in order to comply with the principles of the green economy, the recovery of phenolic compounds from agri-food wastes should be achieved using environmentally friendly, sustainable and possibly low-cost procedures. On this basis, this review will provide an overview of the most commonly employed green approaches for the recovery of functional phenolic compounds from agri-food byproducts. In particular microwave assisted extraction (MAE), ultrasound assisted extraction (UAE), and supercritical fluid extraction (SFE) have been considered as well as the use of deep eutectic solvents (DES) as emerging green solvents. A brief description of other promising sustainable methodologies based e.g., on the use of Naviglio extractor®, pulse electric fields (PEF) and steam explosion will also be provided. Patents were excluded since the main aim of this review is an update of those applications that have a potential for further development but may not be ready for a straightforward use in industries.
Phenol-Rich Agri-Food Wastes
Fruit Byproducts
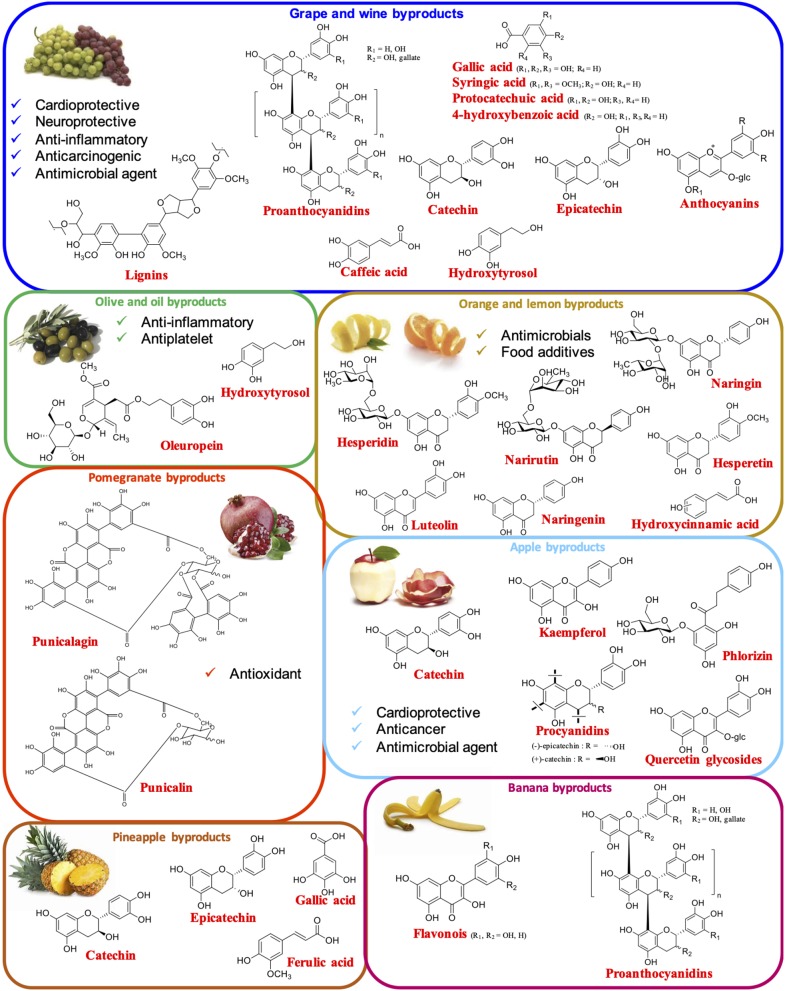
Grape and Wine Byproducts
The main byproduct of the wine industry is known as grape pomace and consists mainly of grape skin, seeds, stems, and remaining pulp (28). Approximately 9 million tons of this waste are produced per year in the world, which represents about 20% w/w of the total grapes used for wine production (29, 30). As to the phenolic composition, an average lignin content of 17–24% w/w has been reported (31). Condensed tannins (proanthocyanidins) represent another main class of polyphenols present in the pomace, together with other small phenolic compounds exhibiting high health beneficial properties, such as cardioprotective, neuroprotective, anti-inflammatory, anticarcinogenic, and antimicrobial activities. Among these, the most abundant are phenolic acids (caffeic, gallic, protocatechuic, 4-hydroxybenzoic, and syringic acid), hydroxytyrosol, and flavonoids, mainly catechin and epicatechin derivatives as well as anthocyanins, which are commonly recovered and used as food colorants (28) (Figure 1).
Figure 1.
Main fruit byproducts and their most prominent phenolic constituents with reported bioactivities.
Olive and Oil Byproducts
The olive oil industry also generates high amounts of byproducts, which are particularly rich in lignans, secoiridoids, and especially hydroxytyrosol, which is one of the most bioactive phenolic compounds present in nature, endowed with anti-inflammatory and antiplatelet properties (12, 28–30, 32) (Figure 1). Soybean (33) and palm oil (34) byproducts have been also described as a valuable source of polyphenols.
Orange and Lemon Byproducts
Citrus peels as well as seeds and pulp deriving from the industrial production of orange and lemon juice, which led to about 15 million tons of waste per year, are an important source of hydroxycinnamic acids and flavonoids, mainly flavanone glycosides (hesperidin, naringin, and narirestin), flavanones (hesperetin and naringenin), and flavone aglycons (luteolin) (28, 30, 35) (Figure 1). Extracts rich in these compounds have been proposed to be used as antimicrobials or as food additives to impart bitter taste to food and beverages (30).
Pomegranate Byproducts
As in the case of citrus fruits, pomegranate juice production leads to the generation of high amounts of wastes (ca. 9 tons for 1 ton of juice) (36), containing very specific compounds such as the ellagitannins punicalagin and punicalin, which are endowed with very high antioxidant potency (17, 30, 37) (Figure 1).
Apple Byproducts
Also apple pomace represents an important source of valuable polyphenols, exhibiting antimicrobial, anticancer, and cardioprotective activities. Among these a prominent role is played by quercetin glycosides, kaempferol, catechin, procyanidins, and especially the dihydrochalcone phlorizin (28, 30, 38–40) (Figure 1).
Other Fruit Byproducts
Banana peels contain high amounts of phenolic compounds, particularly flavonoids and proanthocyanidins (28, 41), whereas pineapple peels are a source of gallic acid, catechin, epicatechin, and ferulic acid (28, 42) (Figure 1). Also different nut shells as well as endocarps and skins of berries (43), apricot (44), acerola (45), xonocostle (46), litchi (47), sea buckthorn (48), pequi (49, 50), juçara (50), and dragon fruit (51) are emerging as valuable sources of phenolic compounds. Tea residues also lead to phenolic-rich extract (52, 53).
Vegetable byproducts
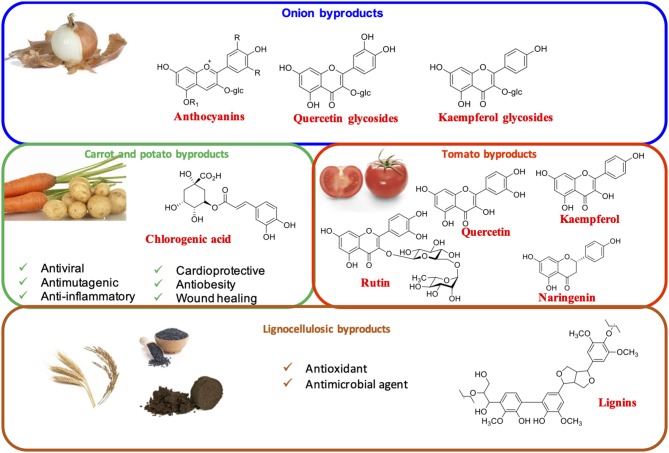
Onion Byproducts
The major byproduct resulting from industrial peeling of onions is represented by the skin, the outer fleshy leaves, and the top and bottom bulbs, which are produced in more than 450,000 tons only in Europe (30, 54). These are particularly rich in flavonoids such as quercetin and kaempferol glycosides. Anthocyanins are also present in red onions (Figure 2) (28, 55).
Figure 2.
Main vegetable and lignocellulosic byproducts. Shown are the most abundant phenolic components and the reported bioactivities.
Carrot Byproducts
The main carrot byproduct is the pomace deriving from carrot juice production. This is rich in hydroxycinnamic acid derivatives, particularly chlorogenic acid, which are known to possess antiviral, antimutagenic, anti-inflammatory, cardioprotective, antiobesity, and wound healing properties (Figure 2) (28, 56).
Potato Byproducts
Potato peels are undoubtedly among the most abundantly produced vegetable byproducts. Their extracts have been proposed for several applications in the food and other sectors. The main phenolic compounds present in potato peels are phenolic acids and derivatives, especially chlorogenic acids (Figure 2) (28).
Tomato Byproducts
Peels and seeds from tomato processing contain mainly flavanones (naringenin glycosylated derivatives) and flavonols, mainly quercetin, rutin, and kaempferol glycoside derivatives (Figure 2) (28, 57).
Other Vegetable Byproducts
Other vegetables that lead to high amounts of byproducts are fennels (58, 59), broccoli (60), cabbages (61), lettuce (62, 63) and artichokes (64, 65).
Lignocellulosic Byproducts
Lignocellulosic agri-food byproducts such as wheat straw (28, 30), wheat bran (66) and distiller's grain (67), spent coffee grounds from the industrial production of soluble coffee (68, 69), sawdust (70, 71) and other wastes from the wood industry (72) have been widely described as a clean source of phenolic compounds, mainly deriving from hydrothermal and/or autohydrolysis processing of lignin, that could be exploited for application in a variety of sectors given their antioxidant and antimicrobial properties (28) (Figure 2).
Green Extraction Techniques
Microwave Assisted Extraction (MAE)
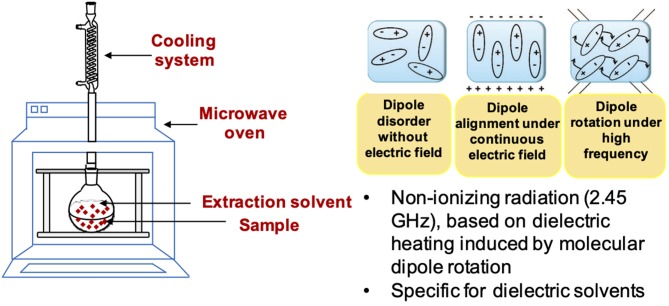
Microwave assisted extraction (MAE) can be classified as a green extraction technique since it shortens the extraction time and reduces the consumption of solvent. The principle on which MAE is based is the dielectric heating, that is the process in which a microwave electromagnetic radiation heats a dielectric material by molecular dipole rotation of the polar components present in the matrix (73) (Figure 3). MAE has been reported to proceed through several distinct steps as the result of heat and mass gradients generated into the matrix: (1) penetration of the solvent into the matrix; (2) solubilization and/or breakdown of the components; (3) transport of the solubilized compounds from the insoluble matrix to the bulk solution; (4) separation of the liquid and residual solid phase (74, 75). Several parameters should be considered to optimize the MAE process, that is solvent, solid to solvent ratio, microwave power and extraction temperature and time. As to the solvent, ethanol, alone or in combination with water, is one of the most commonly used in MAE because it has a good capacity to absorb the microwave energy and exhibits good solubilizing properties toward phenolic compounds. The amount of solvent to be used has to be properly chosen to ensure complete immersion of the sample during the entire irradiation process, avoiding excessive amounts that would require time and energy consumption for removal in the final recovery of the extracted compounds. The choice of the microwave power as well as of extraction temperature and time is dependent on the stability of the compounds to be extracted (75).
Figure 3.
Schematic representation of MAE equipment and characteristics.
Other factors can also affect the efficiency of the extraction, such as the characteristics of the matrix in terms of particle size, the contact surface area and the water content. As an example, higher extraction yields of phenolics can be achieved by milling the sample into smaller particle sizes, although particles smaller than 250 μm can be difficult to separate from the liquid phase at the end of the process (75).
Regarding the instrumental apparatus, MAE can be performed in closed extraction containers, which operate at high pressures and temperatures, allowing for higher extraction yields, or in open vessels operating under milder conditions, at atmospheric pressure. This latter system is particularly suitable for thermolabile compounds and has the advantage of requiring a low-cost instrumentation able to process higher amounts of material. Recently, instruments operating under vacuum or under a nitrogen atmosphere have also been developed (75).
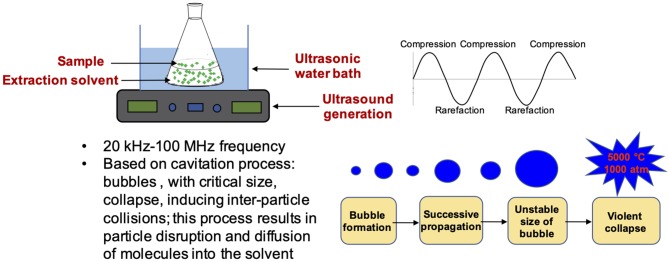
Ultrasound Assisted Extraction (UAE)
As in the case of MAE, ultrasound assisted extraction (UAE) also allows to reduce the time and solvent amount needed to efficiently extract phenolic compounds from agri-food wastes. UAE is considered one of the simplest extraction procedure since it requires common laboratory equipment, that is an ultrasonic bath (76). The technique is based on the cavitation process induced by compression and expansion cycles associated to the passage of ultrasounds (20 kHz-100 MHz frequency) through the sample. The implosion of the cavitation bubbles induces inter-particle collisions which result, among others, in particle disruption and enhanced diffusion of the extractable compounds into the solvent (Figure 4). Sample characteristics such as consistency, rheology and particle mobility can therefore significantly affect the ultrasound energy dispersion and hence the effectiveness of UAE.
Figure 4.
Schematic representation of UAE equipment and characteristics.
UAE is generally performed under static conditions, that is in closed vessels, with no solvent refreshing, or in a dynamic mode, in which fresh solvent is supplied in continuously (75).
Supercritical Fluid Extraction (SFE)
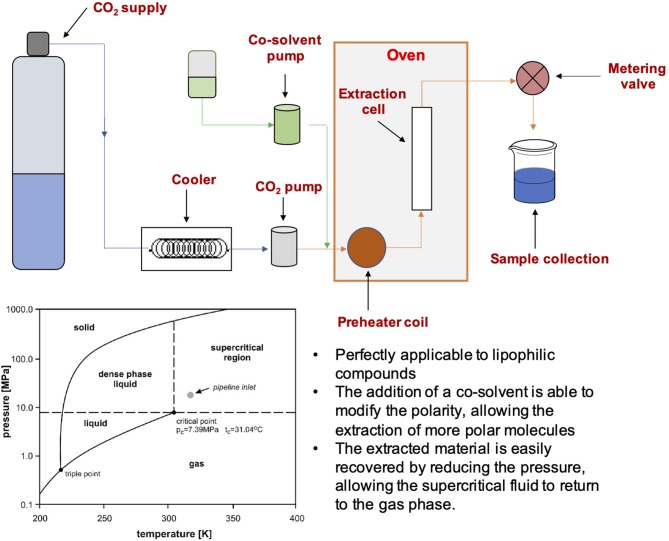
Another green technology, based on supercritical CO2 (scCO2), has been recently considered in order to overcome the environmental concerns related to conventional methods. scCO2 is in fact characterized by immediate advantages over traditional solvent-based methods. It enables the selective extraction of compounds soluble in scCO2, thus perfectly applicable to lipophilic compounds like fats, with no need of concentration steps (77). The addition of a co-solvent (for example ethanol, that is well-tolerated by various industrial sectors) is able to modify the polarity of the scCO2 allowing the extraction of more polar molecules (78) (Figure 5). Moreover, the operative temperatures can be set low enough to avoid the degradation of thermolabile substances. Literature results show a substantial advantage with respect to conventional extraction in terms of easy recovery, selectivity, compounds stability, time, and an overall total energy saving (79).
Figure 5.
Schematic representation of SFE equipment and characteristics.
The high versatility of SFE technique can be extended to industrial scale with the intent to introduce sustainability to large-scale processes. Moreover, the easy removal of CO2 at ambient conditions and its feasible recovery through specific apparatus for its reuse lead to a reduction of reagent-related costs, well-appreciated in the industrial sector.
A relevant aspect in SFE, affecting the extraction rate, is the solubility of target compounds in the scCO2. In this case, temperature and pressure are key thermodynamic parameters that mainly contribute to solubility of target compounds. In details, the increase of pressure enhances the density of supercritical fluid and its solvation power (80). Working on vegetal matrix, high pressure can disrupt plant cell thus facilitating the release and the solubilization of compounds. Temperature has a more complex role: at constant pressure, its increase enhances the vapor pressure of the solute and its solubility in the extractor fluid with a slight but balanced decrease of supercritical fluid density.
Many examples in literature show how the scCO2 has been widely applied to lipophilic molecules extraction (81). Conversely, there are fewer applications on target molecules of higher polarity when the addition of a co-solvent to scCO2 is necessary to enable their extraction. The use of co-solvents affects the physical and chemical intermolecular forces of the system and increases the local density around a solute molecule, achieving specific interactions such as H-bond.
Some works in the literature recently reported the recovery of polyphenols from wastes through scCO2. Bioactive and valuable compounds isolation from agri-food residues by green technologies like scCO2 is of particular interest because it accomplishes the implementation of bio-based-economy policies.
Overall, not only the physico-chemical parameters of scCO2 extraction (temperature, pressure, and amount of co-solvent), but also the biomass nature and processing before extraction (lyophilization, micronization, etc.) deeply affect the final extraction yields and composition, being the diffusivity inside the solid matrix a critical parameter. The process conditions in SFE may differ from one matrix to another, even in the presence of the same compounds (82). The literature in the field is not as wide as in the case of the other green techniques, at least when scCO2 is applied to extract more polar molecules. The optimization of extraction parameters, such as pressure, temperature and the percentage of modifier, together with the understanding of matrix effects, are the key points to yield more polar molecules, but a wide literature sink to be set as background is still missing in this field.
Deep Eutectic Solvent (DES) Extraction
Solid-liquid extraction is one of the most commonly used procedures to extract phenolic compounds e.g., from agri-food wastes (83). However, this methodology typically involves long extraction time periods, high costs, low yields, and the use of organic solvents, which even if exhibit excellent ability in phenolic compound dissolution and extraction, show many intrinsic drawbacks, such as low boiling points, flammability, toxicity, and non-biodegradability (84, 85). On the other hand, water is as an extraction solvent effective only for polar and hydrophilic compounds (86, 87). Therefore, there is a high demand for green solvents exhibiting the same excellent extraction properties of organic solvents, but low-costs and minimal environmental impact (87, 88).
Recently, a new type of eco-friendly and green solvents called deep eutectic solvent (DES) has been developed and applied in the extraction of phenolic compounds (87–90).
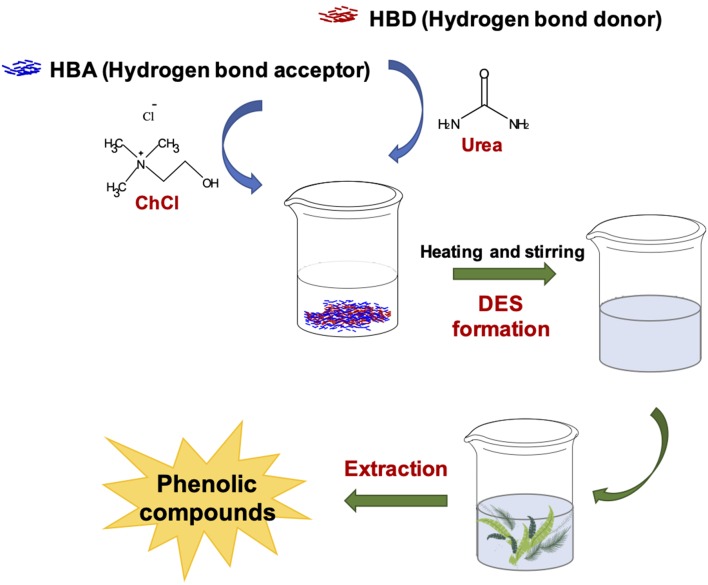
DES preparation was first described by Abbott et al. (91). They are easily prepared by mixing, at a suitable temperature, a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) (91) (Figure 6). Compared to common organic solvents, DES offer many advantages such as low price, easy preparation, and easy availability. Moreover, most of them are biodegradable with very low toxicity (90, 92).
Figure 6.
Schematic representation of extraction of phenolic compounds with DES.
DES can be described by the general formula Cat+X−zY, where Cat+ is typically ammonium, sulfonium, or phosphonium, X− a Lewis base, normally a halide, Y a Lewis or Brønsted acid, that forms a “complex” with X−, and z is the number of Y molecules that interact with the anion (93). These interactions result in the formation of a eutectic mixture, characterized by a melting point lower than that of individual constituents.
The most popular component used for the preparation of DES is choline chloride (ChCl), a cheap and non-toxic salt. The most used HBD are urea, ethylene glycol, glycerol, but also alcohols, amino acids, carboxylic acids and sugars (94, 95). Indeed, very recently, DES have been developed from the combination of primary metabolites and bio-renewable starting materials. These solvents have been called “natural deep eutectic solvents” and have been obtained by combining compounds abundantly present in nature that play important roles for solubilizing, storing or transporting metabolites in living cells and organisms (96, 97).
The physicochemical characteristics of DES, such as freezing point, conductivity, density, viscosity and polarity, normally depend on their composition, therefore it is possible to modulate them by modifying the HBD and HBA components. Generally the densities of DES are higher than water, and higher than the individual components (98). Also the viscosity of most DES is high (> 100 cP) at room temperature (89) as the results of the hydrogen bond network between the components leading to a lower mobility of the species. The large ion size and the electrostatic or van der Waals interactions between the components may also contribute to the high viscosity of DES. The conductivity of DES is generally poor, due to their high viscosity.
The ability of DES of donating and accepting protons and electrons as well as to form hydrogen bonds confers them good dissolution properties toward phenolic compounds, as recently explored also in the case of agri-food wastes.
Application of Green Extraction Techniques to Phenol-Rich Agri-Food Wastes
Grape and Wine Byproducts
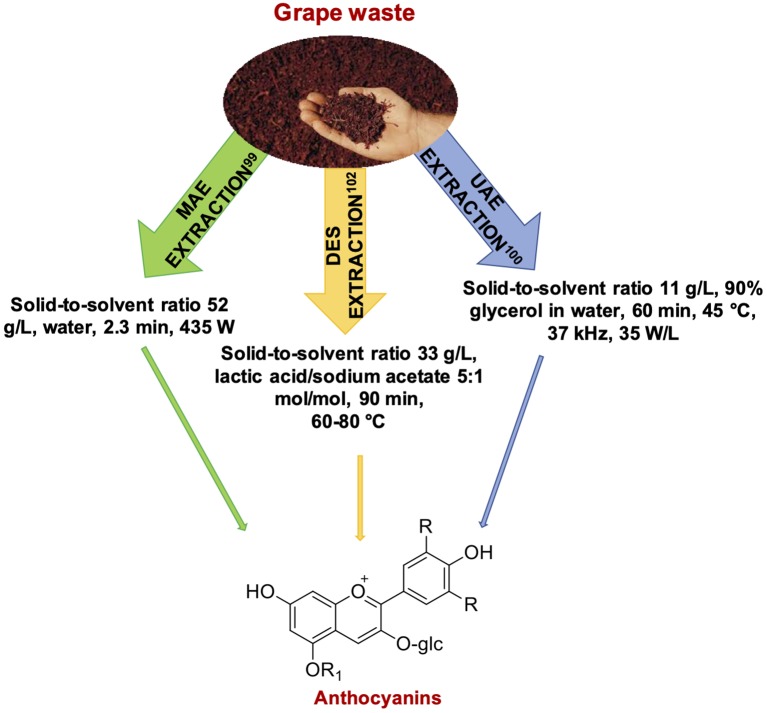
Anthocyanins undoubtedly represent one of the main class of phenolic compounds recovered from grape-processing byproducts. Response surface methodology (RSM) coupled with genetic algorithm allowed to determine the optimal MAE conditions for the recovery of these pigments from grape juice waste. These were microwave power of 435 W, exposure time of 2.3 min and solid to solvent (water) ratio of 52 g/L. Under these conditions an anthocyanin yield of ca. 1.3 mg/g was obtained (99) (Figure 7). Anthocyanins together with other polyphenols have been efficiently extracted from winery byproducts also by UAE, requiring however the use of glycerol (90% w/v in water) as solvent and a lower (11 g/L) solid to solvent ratio (100) (Figure 7). Ten different ChCl-based DES have been also comparatively evaluated as solvents for anthocyanin extraction from grape pomace, and the highest efficiency was found for ChCl-citric acid. On these basis, new citric acid-based DES were prepared, and citric acid/maltose 4:1 molar ratio led to a significantly higher total anthocyanin content (TAC) when compared to reference solvents, particularly when combined with UAE (101). A DES composed of lactic acid-sodium acetate at a molar ratio of 5:1 has also been found efficient for pigment extraction from red grape pomace (Figure 7), whereas a 5:1 glycerol-sodium acetate mixture performed better for flavonoid extraction (102). A significant improvement in anthocyanin extraction yields from wine lees compared to acidified aqueous ethanol has been reported using ChCl-malic acid containing 35% v/v water combined with UAE (extraction time, 30.6 min; ultrasound power, 341.5 W) (103).
Figure 7.
Representative examples of phenolic compounds recovered from grape byproducts.
Resveratrol represents another important bioactive phenolic compounds which has been the focus of several studies directed to the optimization of the better conditions allowing for its efficient extraction from grape and wine byproducts. As an example, orthogonal test indicated a material to ethanol ratio of 50 g/L, an extraction time of 30 min, an extraction temperature of 55°C and a microwave power of 1.0 kW as the best conditions for MAE of resveratrol from grape pomace (104). Yields of about 30 mg per 100 g of dried extract were instead reported from Pinot noir seeds by performing MAE at 60 W for 30 min, using methanol as solvent with a solid to liquid ratio of 200 g/L (105). A more energy-efficient process for resveratrol recovery from red grape wastes has been reported by means of UAE using polyethylene glycol (PEG) as a co-solvent, allowing for lowering the amount of ethanol used in the extraction process. The optimized conditions as determined from RSM-Box-Behnken design involved a combination of 19 min, 54°C, and an ethanol/PEG/water ratio of 48:32:20 v/v/v (106). Likewise, a 1.5% aqueous β-cyclodextrin solution showed to be an excellent UAE medium for grapevine waste (107).
Phenolic compounds have been extracted also from grape skins. Very short extraction times (83 s, with a microwave power of 900 W) have been reported for the MAE of phenolic compounds from grape skins (108). Longer extraction times (50 min, at 65°C, with a solid to liquid ratio of 100 g/L) have been instead reported in the case of UAE using ChCl-oxalic acid as DES in presence of 25% water (109). Promising antioxidant and antiproliferative activity against cancer cells have been described also for a ChCl-malic acid phenolic extract obtained from grape skin (110).
Based on what reported above, it can be concluded that MAE generally requires short extraction times compared to the other techniques, although the use of more innovative methodologies, based e.g., on the use of DES, seem not to have been fully explored yet. Moreover, compared to the other widely exploited green methodology, that is UAE, a survey of the literature revealed MAE to be the first choice for the recovery of phenolic compounds from grape-derived wastes as summarized in the following.
MAE has been reported to be particularly effective in the case of vine shoots from Portuguese grapes. A total phenol content (TPC) of 32 mg gallic acid equivalents (GAE)/g was obtained by extracting dried vine shoots (0.1 g) at solid to solvent ratio of 5 g/L with ethanol: water 6:4 v/v for 20 min at 100°C. The extract thus obtained was significantly more effective than ascorbic acid in protecting erythrocytes against 2,2'-azobis(2-amidinopropane)-induced hemolysis. Moreover, it exhibited quite low IC50 values as inhibitor of acetylcholinesterase (IC50: 17–25 μg/mL) and α-amylase (IC50: 60–74 μg/mL) and presented promising antibacterial and antifungal activity. HPLC analysis indicated gallic acid, catechin, myricetin and kaempferol-3-O-rutinoside as the major contributors to the observed biological activities (111).
Another study also reported ethanol MAE extraction as an effective technique to obtain a polyphenol-rich, antioxidant extract from grapevine shoots. A plant/solvent ratio of 100 g/L was used, at 60°C for 30 min, with a 1.5 kW microwave power, under a 5 bar nitrogen pressure. The same apparatus but using acetone/water 8:2 v/v as the solvent, for 49 min, has been described also for the recovery of polyphenols from hazelnut skins (107).
Grape marc was also found to provide the extract with the highest TPC (143 mg GAE/100 mL) and highest antioxidant properties (239 mmol and 1,145 mmol of Trolox eqs/100 mL from the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) assay, respectively) when MAE was applied to a series of agri-food wastes such as chicory, cabbage, celery, fennel, olive leaf, and grape marc wastes. The extraction was performed at 750 W for 4 min with water, using a solid to liquid ratio of 1,000 g/L. The obtained aqueous extracts were used as water substitute in dough formation to fortify bread, with the grape marc extract conferring food antioxidant activities to both the crust and crumb of bread (112).
Also red wine lees have been described as an important source of polyphenols, using a combination of MAE and membrane-based filtration (113).
Very recently, microwave pretreatment prior to conventional solid-liquid extraction has been found to lead to overall better outcomes for the preparation of polyphenol-rich extracts from winemaking process wastes with cosmeceutical potential (114). High efficiency has been reported also for ultrasound-assisted emulsification-extraction of polyphenols from grape seeds and alperujo, using methanol/water (dispersed phase)-hexane (continuous phase) emulsions formed in the presence of ultrasounds (115). Other sustainable UAE treatments have also been described in the case of grape pomace (116).
The possibility to recover high-value polyphenols by SFE starting from skin and seed fraction of grape pomace has been also investigated (117), comparing the results of conventional and not-conventional extraction. In this work, the temperature and pressure range were 40–60°C and 350–500 bar, respectively. The results confirmed that the final compositions (not reported) of the extract obtained through supercritical and conventional methods were similar, but scCO2 was more selective. In agreement with the literature, results showed that extraction of polyphenols was possible only after the addition of ethanol as co-solvent. In this case, however, co-solvent amounts >5% do not significantly affect the extraction yield. The authors hypothesized that high concentration of ethanol in the scCO2 enhances the formation of strong H-bond between the solvent and the solute. A second speculation concerns the possibility that some polyphenols could be solubilized by the adsorbed-ethanol molecules remaining entrapped in the solid matrix at the end of the SFE process.
Olive and Oil Byproducts
Differently from what reported above for grape-derived wastes, DES has been widely applied to the extraction of phenolic compounds from olive and oil byproducts, combined in some cases with MAE or UAE.
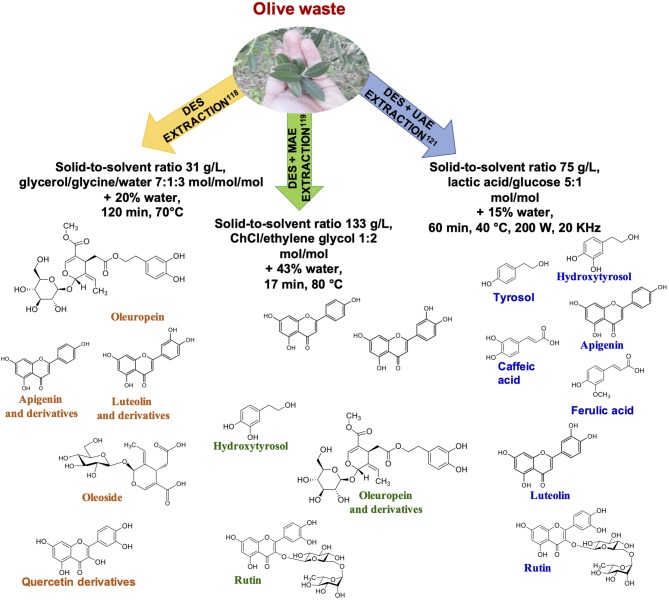
Polyphenol extraction from Olea europaea leaves have been reported using glycerol-glycine-water 7:1:3 molar ratio. Optimized parameters in terms of total polyphenol yield and antioxidant power were 80% in water (w/w) DES concentration and a solid to liquid ratio of 31 g/L, at 70°C. Under these conditions a 18–30% higher total polyphenol yield was obtained compared to 60% aqueous ethanol, aqueous methanol and water, used as reference solvents. Furthermore, the DES extract exhibited significantly higher antiradical activity and reducing power (118) (Figure 8).
Figure 8.
Representative examples of phenolic compounds recovered from olive byproducts.
The use of different DES prepared from ChCl as HBA combined with MAE has been also reported for the extraction of phenolic compounds from olive leaves. RSM optimized extraction conditions were found to be 80°C and 17 min temperature and irradiation time, respectively, using 43% of water (119) (Figure 8).
Four different DES consisting of ChCl combined with maltose, glycerol, citric, and lactic acid in 1:2 molar ratio, 20% (v/v) of water, at 60°C have been proposed for the MAE of polyphenols from olive kernel and leaves. The best results were obtained with lactic acid based-DES, leading to the highest TPC (120).
Lactic acid-glucose 5:1 mol/mol implemented with 15% of water has also been proposed as a solvent for extraction of phenolic compounds from different byproducts of olive oil industry, combined with 30–60 min UAE at 40°C, using a solid-to-solvent ratio of 75 g/L (121) (Figure 8).
Recently, a blend of lactic acid/ammonium acetate 7:1 molar ratio with β-cyclodextrin (β-CD) has been used to recover polyphenols from olive leaves. The RSM optimized extraction conditions were: stirring speed 300 rpm, DES concentration in water 56% (w/w), solid to liquid ratio 10 g/L and β-CD concentration 0.7% (w/v). Maximum extraction yield was achieved at 80°C, without compromising antioxidant activity. Comparative assessment of the DES/β-CD extraction medium with other green solvents showed that it was a high-performing system providing polyphenol-enriched extract with improved antioxidant characteristics (122).
A relatively few number of papers have reported the UAE of phenols from olive wastes: these include for example recovery of polyphenols from industrial wastes of olive oil production such as olive tree leaves (123), or the obtainment of a phenolic yield of 45 mg/g for a virgin olive oil waste extract under RSM-determined optimum conditions, that is water:methanol 1:1 v/v, 60°C, 21 min (124). Ultrasound assisted enzymatic hydrolysis has also been established for extraction of phenolics from olive waste (125).
Similarly to grape- and wine-derived byproducts, also in this case shorter extraction times and higher efficiencies were obtained by use of MAE compared to conventional extraction methodologies.
Higher amounts of hydroxytyrosol (1.2 g/kg) and higher DNA strand scission inhibition activity compared to conventional extracts were found following MAE of olive pomace using power of 700 W over 10 min in a closed vessel system and 20% ethanol as the solvent (126).
Microwave irradiation has been combined with enzymatic hydrolysis to enhance the recovery of phenolic compounds also from palm oil mill effluents. Ragi tapai, a traditional fermented asian food, was used as the enzyme source, and MAE was performed at a solid to liquid ratio of 50 g/L for 4–5 min, with a microwave power of 180 W, that is low enough to avoid enzyme denaturation. The best results were obtained using 50% ethanol as the solvent, leading to a more than 30% increase in polyphenol extraction yield compared to conventional maceration extraction (127).
The advantages of MAE over conventional extraction techniques in terms of extraction times have been highlighted also for the recovery of isoflavones from soybean processing byproducts. In this case a 187.5 W power was applied for 3 min, using 80% ethanol at a sample to solvent ratio of 40 g/L (128).
Orange and Lemon Byproducts
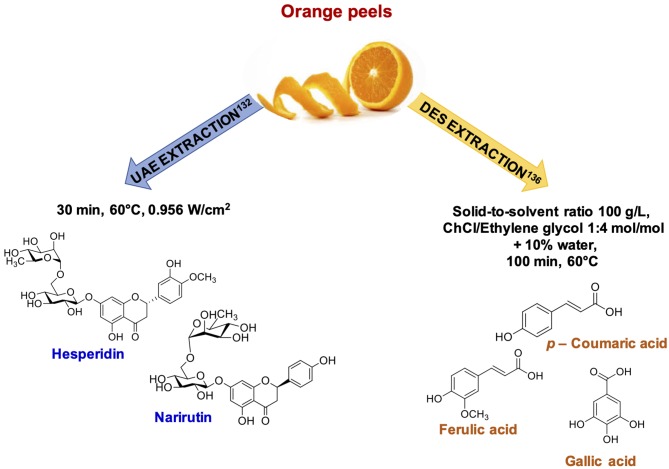
Citrus byproducts seem to represent the most promising agri-food waste for the exploitation of UAE (129, 130). For example, a higher efficiency compared to MAE has been reported for the recovery of phenolic compounds from lime peel waste (131).UAE proved effective also in the case of orange peels, increasing the TPC yield by 30% compared to conventional extraction; statistical analysis revealed that the optimized conditions of ultrasound power and temperature were 0.956 W/cm2 and ca. 60°C, giving a polyphenol yield of ca. 50 mg GAE/100 g of dry matter (132) (Figure 9).
Figure 9.
Representative examples of phenolic compounds recovered from orange byproducts.
In another study a systematic evaluation of UAE parameters, including particle size, extraction time, extraction temperature and ultrasonic power for the recovery of p-coumaric acid, caffeic acid, chlorogenic acids, and hesperidin from citrus waste using pure water as the solvent has been carried out (133).
An economic and environmentally friendly UAE treatment free of organic solvents performed at room temperature for only 3 min was shown to lead to a naringin-rich flavonoid extract from grapefruit wastes, exhibiting a TPC of 75.3 mg GAE/g (134).
UAE combined with the use of ChCl-glycerol-based DES has also been reported in the case of lemon peels and other agri-food wastes (135).
Remaining in the field of DES application, ChCl-based DES prepared using glycerol and ethylene glycol at different molar ratio have been evaluated as potential solvents for the recovery of polyphenols from orange peels. Optimal conditions were found to be: DES containing 10% w/w of water, a temperature of 60°C, a solid to liquid ratio of 100 g/L, and an extraction time of 100 min (136) (Figure 9).
Recently, the effects of physicochemical properties of DES (viscosity, pH and polarity) for extracting flavonoids from citrus peel waste have been also investigated. Based on the strong linear dependence of extraction yield on polarity, a ternary DES composed of ChCl–levulinic acid–N-methyl urea at a molar ratio of 1:1.2:0.8 provided high extraction yields of total flavonoids (137).
Of course, also MAE has been applied as well to citrus processing wastes.
Hesperidin recovery from immature fruit peels of Citrus unshiu has been reported using 70% ethanol at 140°C for 8 min, at a 100 g/L solid to solvent ratio. After 24 h storage at 5°C, ca. 48 mg/g of hesperidin were collected (138).
Microwave hydrodiffusion and gravity (MHG) technique has been instead applied to mandarin leaves, under RSM optimized conditions involving 275 W microwave power, 2 g mandarin leaf and 45 s. TPC and total flavonoid content (TFC) values of ca. 17 mg/g GAE and 1.7 mg/g of catechin equivalents (CE) were determined, which, although lower compared to those obtained by supercritical fluid extraction (SFE), well-correlated with the antioxidant capacity (139).
In a comparative study performed on the residues of industrial processing of fennels, carrots, lemons and tomatoes, MAE has been applied together with maceration and ultrasound assisted extraction (UAE) for the recovery of phenolic compounds. A power of 750 W was used, with a solid to solvent ratio of 40 g/L and a 5 min extraction time; different solvents (methanol, ethanol, water) were used. MAE proved to be particularly effective in the case of carrot wastes, using methanol:water 1:1 v/v as solvent, whereas pure methanol was found to be the best choice for lemon pomace. This latter, in particular, exhibited promising antibacterial activity against Pseudomonas aeruginosa and Clostridium difficile (140).
Pomegranate Byproducts
Apparently, UAE seem to be the only green extraction methodology applied to pomegranate wastes, although a combined ultrasound and microwave assisted extraction methodology has been recently reported to be very efficient for the recovery of ellagic acid from fermented pomegranate wastes (141). Ultrasound pretreatment has been reported as an expedient method to significantly improve punicalagin extraction yield from pomegranate peels using a cellulase-based magnetic nanobiocatalyst. This involved suspension of the solid material in 50 mM phosphate buffer (pH 6) (67 g/L solid to liquid ratio) and 37 kHz ultrasound exposure at 50°C for 20 min (142, 143). Pulsed UAE using water as solvent has been also used for the recovery of polyphenols from pomegranate marc (144).
Apple Byproducts
The superiority of UAE compared to conventional extraction has been proved also in the case of apple pomace. Indeed, in this case, even more efficient than UAE proved to be the ultrasound-assisted micelle-mediated extraction. A 1% water solution of Rokanol B2 was used as solvent, at a 50 g/L solid to solvent ratio. Ultrasound treatment was performed at 50 Hz and 300 W for 30 min. A 7-fold higher TPC was obtained compared to standard UAE with ethanol or water as solvent. Chlorogenic acid, quercetin, and quercetin glyocosides were identified as the main compounds present in the extract (145).
Notably, antioxidant compounds from apple pomace were also efficiently extracted by scCO2 (146). In particular SFE was carried out on fresh, oven- and freeze- dried apple pomace varying pressure (20 or 30 mPa) and temperature (45 or 55°C), in absence and presence of ethanol as co-solvent (5%, v/v). The results were compared to those obtained by Soxhlet extraction with ethanol and boiling water maceration. Results showed that scCO2 was able to extract polyphenols mainly from the oven and freeze-dried apple pomace, suggesting that the pre-treatment affects the scCO2 extract. However, the overall yields were lower when compared to those from conventional solvents methods. The authors justified this unexpected result with the thermal degradation of polyphenols under the working conditions (45–55°C). Concerning the composition, the isolated fractions were rich in quercetin, catechin, myricetin, phlorizin, and phloretin, conferring a high antioxidant activity. Differently, the extract processed by Soxhlet lacked in some polyphenols, accounting for the decrease in the antioxidant activity. Overall, even if the extraction with conventional technique led to higher yields, the SFE process was able to provide an antioxidant enriched fraction.
Onion Byproducts
Onion wastes represent another important source, together with grape-derived byproducts, of anthocyanins, which have been recovered with other polyphenols by UAE or extraction with DES. The first involved the use of 90% aqueous glycerol as the solvent, with a 11 g/L solid to solvent ratio (147), whereas a higher solid to solvent ratio (33 g/L), 90 min, and 40°C were found to be the best conditions when ChCl/1,2-propanediol/water 1:1:1 molar ratio DES was tested (148). The highest total phenol and flavonoid content was instead obtained with a 50 g/L solid to solvent ratio (148).
In another paper UAE of quercetin from onion wastes has been reported: the optimal extraction conditions were determined to be an ethanol percentage of 59% and extraction temperature of 49°C, yielding a total quercetin content of 11 mg per g of dry weight, whereas pH, solid to solvent ratio and extraction time did not significantly affect the extraction yields (149).
As to the use of DES, other authors investigated the use of eutectic mixtures composed of ChCl as hydrogen bond acceptors with sucrose (4:1), urea (1:2), and sorbitol (3:1) implemented with different water contents for phenolic antioxidant extraction from onion peels. The best results were obtained with ChCl-urea-water 1:2:4 mol/mol/mol, at 60°C, for 120 min, at a solid to liquid ratio of 20 g/L, which led to a TPC comparable to that obtained using 70% aqueous methanol. The experiments were carried out also in a modified domestic microwave oven, with a significant reduction in extraction times (5–25 min) (150).
Different DES consisting of sodium propionate as HBA combined with glycerol and lactic acid have also been analyzed for polyphenol extraction from onion wastes. The best results were obtained with 85% w/w aqueous glycerol/sodium propionate at a molar ratio 8:1, 10 g/L solid to liquid ratio, a temperature of 80°C and a stirring speed of 900 rpm. These conditions provided antioxidant power and polyphenols content comparable to other green solvents (151).
Carrot Byproducts
UAE apparently represents the only applied green extraction methodology also in the case of carrot wastes. In particular, chlorogenic acids as well as caffeic acid, catechin and epicatechin have been efficiently recovered by RSM optimized UAE of carrot pomace (152). UAE has been described as a powerful technology also for extraction of anthocyanins from black carrot pomace (153).
Potato Byproducts
Chlorogenic acids are among the main extractable polyphenols from potato byproducts. Both MAE and UAE have been applied to this aim, with the first again allowing for very short extraction times, although requiring higher temperatures and lower sample to solvent ratio. In particular, based on orthogonal array design, MAE was accomplished at 300 W using 60% ethanol as the solvent, at 80°C, for 2 min, with a solid to solvent ratio of 25 g/L, proving to be more efficient than conventional solvent extraction, especially in terms of solvent volumes (154). The RSM-optimized UAE protocol instead involved use of ethanol/water 55/45 v/v in a ultrasound bath (34 kHz frequency) for 35 min at 35°C and a 100 g/L sample to solvent ratio (155).
A DES composed of glycerol and ammonium acetate (molar ratio 3:1) has been also tested for its efficacy for the recovery of phenols from chlorogenic acid rich agri-food solid wastes, including potato peels. The extraction, performed with 80% w/v DES in water, 10 g/L solid to liquid ratio, at 80°C for 3 h and under constant stirring at 600 rpm, demonstrated that the DES was the most efficient in extracting chlorogenic acid derivatives and superior or equally efficient in recovering flavonoids compared to other green solvents (156).
Tomato Byproducts
A number of papers describe tomato byproducts processing with MAE under different conditions using ethanol-water as the solvent. Under the global optimized conditions, that is 20 min, at 180°C, with 47% ethanol, a solid to solvent ratio of 45 g/L, and 200 W microwave power, an extraction yield of 76% was obtained, with a TPC value of 43.9 mg GAE/g and a TFC of 3.5 mg CE/g. Although the antioxidant power as determined by the ABTS assay was found to be lower compared to commonly used food additives, the optimized tomato waste extract was considered as a sustainable alternative to be used in the fortification and functionalization of food (157). MAE was also found to be the more efficient technique for water extraction of tomato wastes. In particular, extraction was performed at 750 W, for 90 s, with a solid to solvent ratio of 100 g/L. Under these conditions an extraction yield of 16% w/w was achieved, which is higher than those obtained by conventional extraction methods (158). The effects of solvents, temperature and times on MAE of polyphenols from tomato peels have also been recently systematically evaluated (159).
Lignocellulosic Byproducts
Ferulic acid and its oligomers were the main phenols identified by HPLC-MS following MAE of brewer's spent grain. 0.75% NaOH was used as the solvent and RSM analysis indicated 15 min extraction time, 100°C extraction temperature and a solid to solvent ratio of 50 g/L as the optimal conditions. A 5-fold higher extraction yield (1.3% w/w) of ferulic acid was obtained with MAE compared to conventional extraction techniques, leading to 0.001–0.27% yields (160).
MAE using 20% ethanol in water as the solvent has been described as an efficient methodology also for the recovery of phenolic compounds from spent coffee grounds (161).
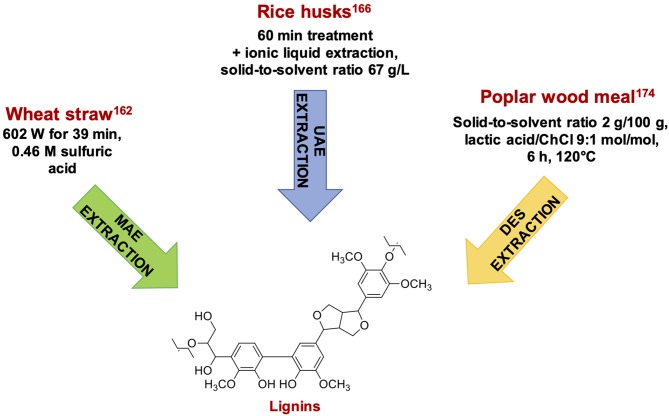
An increase in the wheat straw lignin extraction yield from 3.4 to 11.8% w/w has been also reported, using a microwave radiation power of 602 W for 39 min, and 0.46 M sulfuric acid as the solvent (162) (Figure 10). Another study reported lignin extraction from agri-food wastes by treating the biomass at a 50 g/L solid to liquid ratio in 92% ethanol and 0.32 M sulfuric acid with a microwave power of 250 W for 30 min at 150°C. Under these conditions more than 82% pure lignins were recovered in 35% w/w yield starting from olive kernels (163).
Figure 10.
Representative examples of phenolic compounds recovered from lignocellulosic byproducts.
Although no significant improvement was observed in either extraction yields or antioxidant properties when compared to conventional maceration, the advantages offered by MAE in terms of extraction time have been recognized in particular in the case of eucalyptus (164) and chestnut (165) wood industry wastes.
IC50 values lower compared to the reference antioxidant butylated hydroxytoluene (BHT) were obtained when MAE was applied to maritime pine (P. pinaster) sawdust waste, a byproduct from industry of wood transformation. Both MHG and solvent free microwave extractions were performed, heating the material at 100°C, with a 600 W microwave power, for 40 min. Under these conditions TPC values of ca. 75 mg GAE/g extract were obtained, which were higher than those obtained by applying other extraction methodologies (70). A 40% improvement in polyphenol extraction compared to conventional maceration has been reported also when UAE was applied, which apparently involved milder conditions (0.67 W/cm2 ultrasonic intensity, 40°C, 43 min) compared to MAE (71).
Other UAE application to lignocellulosic byproducts include use of ionic liquids to extract lignin from rice husks (166) (Figure 10), whereas a phenolic content of 3.1 mg GAE/g of wheat bran has been obtained by UAE using 64% ethanol as solvent, at 60° C, for 25 min (167).
Ultrasound pretreatment of wheat dried distiller's grain, a coproduct from the ethanol production process, has been reported to increase the phenolic compounds extraction yield by ca. 14%, as a results of increased pore volume and size (168).
UAE of beech bark at 40 kHz frequency for 20 min, at 65°C, using 70% ethanol as solvent led to a phenolic extract containing 72 mg GAE/g beech bark (169). Polyphenols, particularly phlorizin, have been obtained also from UAE of apple bark using 60% acetone (170).
Ultrasound-assisted enzymatic extraction of protein and antioxidant compounds has been described from sesame bran. The RSM optimized parameters were 836 W ultrasound power, 43°C, 98 min, 9.8 pH value and 1.248 enzyme (alcalase) units /100 g of material, with a solid to solvent ratio of 100 g/L (171).
SFE has apparently not been applied to lignocellulosic byproducts for the recovery of phenolic compounds yet, whereas several applications of DES have been reported in particular not only for lignin extraction but also for lignin processing, being in some cases able to efficiently hydrolyze lignin-carbohydrate bonds in hemicellulose.
Four DES mixtures were prepared using ChCl as HBA and four HBD: acetic acid, lactic acid, levulinic acid and glycerol, in order to solubilize lignin from poplar and Douglas fir wood. At 145°C more than 70% lignin present in poplar and more than 50% present in Douglas fir wood was extracted, with ChCl-lactic acid exhibiting the highest extraction yield (172).
The same DES was found to be the best solvent also in the case of lignin extraction from Salix matsudana cv. Zhuliu. After treatment with ChCl-lactic acid 1:10 mol/mol at 120°C for 12 h, the extracted lignin was recovered by precipitation after addition of water and its purity was evaluated, suggesting that the DES not only has a unique capability for the selective extraction of lignin, with a yield of 92%, but is also capable to provide a lignin with high purity degree (95%) (173).
Similar results have been obtained from poplar meal treated with lactic acid/ChCl at 9:1 molar ratio. At 120°C for 6 h an optimal dissolving capacity of 95% has been reached, with a purity of regenerated lignin up to 98.1% (174) (Figure 10).
A facile approach for efficiently cleaving the lignin-carbohydrate bonds using microwave-assisted DES treatment has also been developed. In particular, DES formed by ChCl and oxalic acid dihydrate 1:1 mol/mol was able to solubilize lignin but not microcrystalline cellulose. The extraction was carried out at 80°C, with a microwave power of 800 W and a radiation time of 3 min, which allowed to extract selectively lignin with a high purity (ca. 96%) (175).
Other DES have also been evaluated for wood delignification, based on ChCl as HBA and phenol, α-naphthol, resorcinol or maleic acid as HBD, with the aid of ultrasound irradiation. The results showed that all the DES have good solubility properties toward lignin, leading to more than 48% w/w recovery in the case of resorcinol (176).
In another study lignocellulosic biomass fractionation was carried out using different DES, and mixtures of ChCl with oxalic acid and potassium hydroxide allowed to selectively isolate phenols and cellulose, respectively (177).
Other Fruit and Vegetable Byproducts
A large number of papers report the application of MAE, UAE, SFE, and DES extraction to other agri-food wastes, as summarized in Tables 1–3. Most of these works again highlight the very short extraction times (80 s-37 min) and in some cases the higher yields and antioxidant properties of the extracts obtained with MAE compared to conventional extraction, though sometimes the requirements of higher amounts of solvent has been reported. The higher efficiency compared to traditional extraction also emerges in the case of UAE, which is generally reported to allow the employment of lower temperatures. Also DES generally led to higher extraction yields of polyphenols compared to conventional organic solvents, whereas not much has been reported regarding SFE.
Table 1.
MAE extraction of phenolic compounds from various agri-food wastes.
| Extraction technique | Fruit or vegetable byproduct | Extraction conditions | Polyphenols extraction yields | References |
|---|---|---|---|---|
| Microwave assisted extraction (MAE) | Pineapple waste | solid-to-liquid ratio (S/L) 30 g/L, 15 min, 300 W |
TPC 12.4 mg GAE/g | (178) |
| Banana peel | S/L 28.5 g/L, H2O:ethanol 1:1 v/v, 100 s, 380 W | 2.2% polyphenols | (179) | |
| S/L 20 g/L, pH 1, 6 min, 960 W | TPC 53.8 mg GAE/g | (180) | ||
| Xoconostle | S/L 100 g/L, H2O, 5.5 min, 297 W | TPC 12.9 mg GAE/g TFC 5.6 mg CE/g | (181) | |
| Macadamia tetraphylla | S/L 50 g/L, H2O, 4.5 min, 360 W | TPC 45 mg GAE/g TFC 29 mg rutin equivalents (RE)/g |
(182) | |
| Sterculia nobilis | S/L 30 g/L, 41% ethanol,37 min, 67°C, 700 W. | TPC 3.7 mg GAE/g TFC 0.45 mg quercetin equivalents (QE)/g |
(183) | |
| Peanut shells | Irradiation for 2.6 min, followed by incubation with 0.81% w/w cellulase, pH 5.5, 66°C, 120 min. | 1.8% polyphenols | (184) | |
| Apricot kernel skin | S/L 25 g/L, 43% ethanol, 80°C, 20 min, 400 W | TPC 22 mg GAE/g | (185) | |
| Tobacco waste | S/L 25 g/L, acetone:H2O 3:7 v/v, 4 min, 400 W | 7.8–12.9 mg CA/g | (186) | |
| Pequi and jucara waste | S/L 20 g/L, 94% ethanol, 100 s, 670 W | TPC 3.8 mg GAE/g TFC 1.6 mg QE/g |
(50) | |
| Dragon fruit peel | S/L 24 g/L, H2O, 45°C, 20 min, 400 W | TPC 58 mg GAE/g | (187) | |
| Cabbage outer leaves | S/L 100 g/L, ethanol, 5 min, 100 W | TPC 14.9–19.2 mg GAE/g | (188) | |
| Yarrow dust | S/L 25 g/L, 70% ethanol, 33 s, 170 W. | TPC 238 mg GAE/g TFC 43 mg QE/g | (189) | |
| Horsetail | S/L 22 g/L, 55% ethanol, 80 s, 170 W. | TPC 162 mg GAE/g | (190) | |
| Tea residues | 230°C, H2O, 2 min | 74 % polyphenols | (191) | |
| Camellia oleifera meal | S/L 100 g/L, 80% ethanol, 15 min. | TFC 12.8 mg RE/g | (192) |
Table 3.
UAE, SFE, MHG, and DES extraction of phenolic compounds from various agri-food wastes.
| Extraction technique | Fruit or vegetable byproduct | Extraction conditions | Polyphenols extraction yields | References |
|---|---|---|---|---|
| Ultrasound assisted extraction (UAE) | Artichoke waste | Solid-to-liquid ratio (S/L) 333 g/L in H2O, 60 min, 50W/L |
TPC 0.8–1.4 mg GAE/g | (207) |
| S/L 100 g/L, 50% ethanol, 25°C, 60 min, 240. | 0.02–14.8 mg chlorogenic acid/g | (208) | ||
| Cauliflower waste | S/L 50 g/L, 2 M NaOH, 60°C, 15 min, 37 kHz, 180 W | TPC 7.3 mg GAE/g | (209) | |
| Tobacco waste | S/L 20-100 g/L, ethanol-H2O 60:40–20:80 v/v, 30–70°C, 15–45 min, 37 kHz, 50 W | 3.6–804.2 μg/mL of chlorogenic acid 2.34–10.8 μg/mL of caffeic acid 11.6-93.7 μg/mL of rutin |
(210) | |
| Mustard seed meal | S/L 25 g/L, 70% ethanol, 40°C, 30 min, 60 W | TPC 13.8 mg sinapic acid equivalents/g | (211) | |
| Microwave hydrodiffusion and gravity (MHG) | Broccoli waste | 43 min, 500 W, under atmospheric pressure, in the absence of solvents | 317 μg GAE/mL | (212) |
| Sea buckthorn pomace | 15 min, 400 W | 1147 mg GAE/g | (213) | |
| Supercritical fluid extraction (SFE) | Blueberry waste | Flow rate 0.5 kg/h 5% ethanol + 5% H2O as co-solvents, 20 MPa, 40°C |
TPC 134 mg GAE/g | (214) |
| Deep eutectic solvent (DES) extraction | Ginkgo biloba leaves | S/L 95 g/L, ChCl/malonic acid 1:2 mol/mol + 55% H2O, 65°C, 53 min | 22.2 mg proanthocyanidins/g | (215) |
| Moringa oleifera leaves | S/L 50 g/L, glycerol/sodium acetate 6:1 mol/mol + 20% H2O, 50°C, 180 min | TPC 53.8 mg GAE/g TFC 16.5 mg RE/g |
(216) | |
| Peanut roots | S/L 33 g/L, ChCl/1,4-butanediol 1:3 mol/mol + 40% H2O, 55°C, 40 min | 38.9 mg of resveratrol/kg of sample | (217) | |
| Rue leaves | S/L 50 g/L, ChCl/citric acid 2:1 mol/mol + 20% H2O,30°C, 90 min. | 38.2 mg GAE/g | (218) | |
| Mango waste | S/L 17 g/L, lactic acid/sodium acetate/ H2O 3:1:4 mol/mol/mol, 20 min, 436 W | 56.2 mg GAE/g | (219) |
Table 2.
UAE extraction of phenolic compounds from various agri-food wastes.
| Extraction technique | Fruit or vegetable byproduct | Extractionconditions | Polyphenols extraction yields | References |
|---|---|---|---|---|
| Ultrasound assisted extraction (UAE) | Walnut green husks | solid-to-liquid ratio (S/L) 50 g/L, 60% ethanol, 60°C, 30 min | TPC 6.9 mg GAE/g | (913) |
| Durio zibethinus M. | S/L 77 g/L, n-hexane, 5 min, 261 W/cm2 | TPC 0.7 mg GAE/g | (194) | |
| Lettuce leaves | S/L 20 g/L, 50–75% ethanol, 120 s, 400 W, 24 kHz | 81 μg polyphenols/mL extract | (63) | |
| Acerola residues | S/L 115 g/L, 46% ethanol, 49 min, 50 kHz, 250 W | TPC 10.7 mg GAE/g TFC 5.6 mg QE/g |
(195) | |
| Capsicum and cabbage waste | S/L 50 g/L, 60% methanol, 37°C, 30 min, 40 kHz. | - | (196) | |
| Bamboo leaves | S/L 50–100 g/L, 60–90% ethanol, 30–40 min, 150–250 W | TFC 1.5 mg RE/g | (197) | |
| Ziziphus mauritiana L. | S/L 10 g/L, 60% methanol, 30 min | TPC 12.8 mg GAE/g | (198) | |
| Kudzu roots | S/L 50 g/L, H2O/ethanol 2:8 v/v, 80° C, 6 h | 7.3 g isoflavones/100 g sample | (199) | |
| Coconut shell | S/L 20 g/L, 50% ethanol, 30°C, 15 min, 0.487 W/cm2 | 22.4 mg of phenolics/g of sample | (200) | |
| Aronia melanocarp | S/L 25 g/L, 0–50% ethanol, 20–70°C, 0–240 min, 0–100W | TPC >70 mg GAE/g | (201) | |
| Purple corn cob and husks | S/L 100 g/L, 20 min, 100 W, ethanol/H2O/lactic acid 80:19:1 | TPC 44-47 mg GAE/g | (202) | |
| Euryale ferox | S/L 37 g/L, 62% ethanol, 40°C, 38 min | TAC 2.8 mg/g | (203) | |
| Litchi pericarp | Incubation for 90 min with 0.12 mg/mL 1:1 cellulase/pectinase, S/L 67 g/L, 20% ethanol, 50°C, 80 min, 300 W | 89.6% procyanidin content | (204) | |
| Ginkgo biloba leaves | S/L 100 g/L, phosphate buffer + 68% ethanol, 8.4 mg cellulase, 40°C, 20 min, 218 W | 25.4% flavonoids and 12.4% ginkgolides | (205) | |
| Star anis residues | S/L 49 g/L, 51 % ethanol, pH 5.3, 45°C, 70 mg/g enzyme, 120 min + 60 min sonication time | 14.8% flavonoids | (206) |
Application of Other Sustainable Extraction Methodologies to Agri-food Wastes
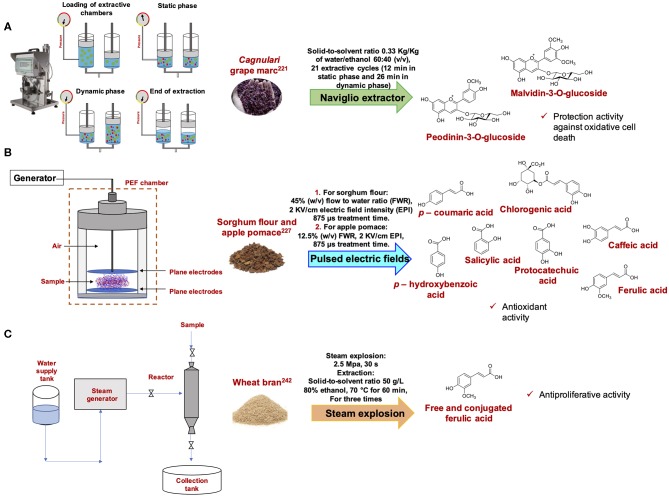
The Naviglio Extractor® is a relatively new solid-liquid extractor that applies the principle that a forced extraction from a solid matrix suspended in a suitable solvent is produced by generating a negative pressure gradient and letting it to go to equilibrium between outside and inside of the solid material (Naviglio's Principle) (Figure 11A). By applying more extractive cycles it is possible to reach the exhaustion of the solid matrix and the extraction of bioactive molecules (220). This new solid–liquid dynamic technology possesses several advantages because it allows to carry out the extraction at room or sub-room temperature thus avoiding thermal stress on thermolabile substances (220). Moreover, the employment of high pressures allows a reduction in the extraction time and a concomitant improvement of the extraction efficacy.
Figure 11.
Schematic representation and examples of extraction of bioactive compounds with (A) Naviglio Extractor®, (B) PEF, and (C) steam explosion.
Naviglio Extractor® has been applied to the recovery of phenolic antioxidants from the Cagnulari grape marc. The extraction, performed using 21 extractive cycles of 1 min and 25 s each for a total of 38 min using water/ethanol (60:40 v/v) as solvent led to recovery of malvidin, peonidin-3-O-glucoside, malvidin-3-(6-acetyl)-glucoside, and malvidin-3-O-glucoside as the main components of the extract exhibiting a TPC of 4.0 g/L. The extract also revealed a significant ability to inhibit the hydrogen peroxide-induced cell death and reactive oxygen species (ROS) generation (221) (Figure 11A). The solid liquid dynamic Naviglio extraction of vine shoot waste from Vitis vinifera Airen variety performed in different conditions provided higher flavonoid and phenolic acid yields in comparison with others solid-liquid extraction methods (222). The vine shoot waste aqueous extract, in particular, stimulated Lactuca sativa radicule elongation (223). Naviglio extraction has also been reported for the recovery of polyphenols from grape peels (224).
Another non-thermal processing sustainable technology is based on the use of pulsed electric fields (PEF). This is a novel extraction method which involves the application of microsecond (μs) pulses of high electric field to a material placed between two electrodes (225) (Figure 11B). A classical system for the treatment of pumpable fluids is composed of a PEF generation unit that consists of a high voltage generator and a pulse generator, a treatment chamber, a proper product process system and a set of monitoring and controlling equipment (225, 226). PEF treatment is able to induce a permeabilization of the cytoplasmatic membranes, facilitating the release of intracellular compounds from the cells. PEF increases the extraction rates and yields of different compounds and does not affect the quality of the extracted products.
Phenolic acids such as protocatechuic, cholorogenic, and salicylic acids and salicylic, ferulic, p-hydroxybenzoic and caffeic acids were found in high concentrations in PEF treated apple pomace and sorghum flour, respectively. The two optimized conditions, 12.5% w/v solid to water ratio, 2 kV/cm electric field intensity and 500 μs treatment time for apple pomace and 45% w/v solid to water ratio, 2 kV/cm electric field intensity and 875 μs for sorghum flour, provided TPC 37% and 25% higher than those obtained by conventional extraction of apple pomace and sorghum flour, respectively (227) (Figure 11B).
PEF-assisted extraction was found to be a suitable technology to maximize total phenolic and flavonoid yields from canola seed cake under optimized conditions (30 V, 30 Hz, 10% ethanol and 10 s exposure time) (228).
The application of PEF improved the recovery of polyphenols also from cocoa bean shell and coffee silver skin (229), Norway spruce bark (230), and blueberry press cake (231).
PEF pretreatment has been also successfully applied to rapeseed stems and leaves (232), fresh tea leaves (233) and borage leaves (234), leading in all cases to an increase in TPC and antioxidant properties of the extracts.
A PEF pretreatment with an energy input of 300 kJ/kg at 20 kV/cm and a subsequent diffusion step in 20% ethanol and 0.3 M sodium hydroxide allowed to obtain high extraction yield of polyphenols from rehydrated flaxseed hulls (235).
The influence of PEF at different intensity levels (0–7 kV/cm) on pressed orange peels has been also evaluated and the results showed that higher electric field strengths led to an increase in total polyphenol extraction yield and antioxidant activity (236).
Another study proposed a combination of PEF and supplementary aqueous extraction (SAE), which allowed a significant increase of high-added value compound yields and antioxidant capacities of extracts from papaya peels (237). Also in the case of mango peels, the application of two-stage PEF + SAE that included PEF-assisted extraction as the first step and supplementary extraction at 50°C, pH 6, for 3 h as the second step, allowed a noticeable enhancement of TPC (+400%) (238).
Steam explosion is another widely employed and environmentally friendly pretreatment method for vegetable materials. It is based on steam hydrolysis at high temperature (160–280°C), followed by sudden release of high pressure (0.7–4.8 MPa) for relatively short retention time (from several seconds to a few minutes). The treated materials are then discharged through restricted orifices, producing an explosive decompression of biomass (239) (Figure 11C). This results in breakdown of the lignocellulosic structure, hydrolysis of hemicellulose compounds, and depolymerization of the lignin compounds due to rupture of rigid cell wall structure. This technique can therefore be employed as a pretreatment to effectively extract bioactive compounds (240).
Steam explosion and UAE were investigated to develop an effective process for the production of valuable phenolic compounds from sugarcane bagasse lignin. Analysis of the extracts revealed the presence of gallic acid, hydroxybenzoic acid, vanillic acid, p-coumaric acid, ferulic acid, syringic acid, and sinapic acid (241).
Also for wheat bran, the steam explosion treatment at 215°C for 120 s provided free phenolic acid and conjugated phenolic acid yields about 39- and 7-fold higher than those obtained with the untreated sample (242) (Figure 11C).
Finally, high concentrations of hydroxytyrosol and tyrosol were found in olive stones (243) and olive mill solid waste or alperujo (244) after steam explosion pre-treatment.
Conclusions
The main advantages and disadvantages of the extraction methodologies described in this review are briefly summarized in Table 4. Of course, the choice of one methodology over another is dictated not only by consideration of the advantages or drawbacks, but also and above all by the physicochemical characteristics of the materials and the type of compounds to be extracted. As an example, MAE is not recommended for the recovery of thermolabile compounds, but it can be preferable to UAE if the amount of solvent to be used is a critical factor. Compared to MAE and UAE, much less is apparently reported in the literature for other green extraction methodologies, such as extraction with DES and particularly SFE. It is undoubtedly, however, that these emerging techniques will be more and more exploited in the next future to comply with a total respect of the environment and of the green chemistry principles. Indeed, SFE represents a highly clean, no-solvent technology, allowing to operate at very low temperatures, and it can be expected that the current high equipment costs would be significantly reduced as hand when novel perspectives and applications of this technique will appear in the literature. On the other hand, the added value of DES deriving not only from the low price and biodegradability but also from their ability to induce chemical transformations of agri-food materials (e.g., hemicellulose hydrolysis) resulting in higher extraction yields of bioactive polyphenols will certainly contribute to the enlargement of their application fields.
Table 4.
Main advantages and disadvantages of the extraction techniques reviewed in this paper.
| Extraction method | Advantages | Disadvantage |
|---|---|---|
| MAE | • Fast extraction • Low solvent consumption • High extraction yields • Good reproducibility |
• High equipment cost • Filtration required • Very poor efficiency for volatile compounds |
| UAE | • High extraction efficiency • Fast and selective extraction • Low equipment cost • Low operating temperature • Efficient for thermolabile compounds |
• Filtration required • Lack of uniformity in the distribution of ultrasound energy • Potential change in the constitutive molecules • Large amount of solvent |
| SFE | • Fast extraction • Automated system • No filtration required • Possibility to reuse CO2 • No use of toxic solvents • Possibility to tune the polarity of scCO2 • Possibility to extract thermolabile compounds at low temperatures |
• High equipment cost • Elevated pressure required • Risk of volatile compounds losses • Many parameters to optimize |
| DES | • Low price • Biodegradable • Very low toxicity • Possibility to tune polarity, viscosity and density • High extraction yields |
• Filtration is required • High density and/or viscosity |
As a general remark, care should be taken concerning the purity of the extracts obtained, since, given the non-selectivity of the green methodologies described, co-extraction of phenolic compounds with compounds that may be toxic, such as emerging pollutants (EPs), could occur. For example, fruit peels usually contain phytosanitary compounds such as herbicides or fungicides, which although present at low concentrations as the result of post-harvest treatments, could accumulate in the extract thus compromising its safety and limiting its possible uses. On this basis, the development of more selective extraction procedures, particularly in the case of SFE which seems not too much susceptible to extensive modulations of the operative conditions e.g., variation of the co-solvent, represents an important challenge to be faced.
Author Contributions
LP and AN contributed conception and organization of the manuscript. LP, FM, RN, SM, LV, and AN wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank the European Union (FSE, PON Ricerca e Innovazione 2014-2020, Azione I.1 Dottorati Innovativi con caratterizzazione Industriale) for funding a Ph.D. grant to FM.
Glossary
Abbreviations
- ABTS
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)
- β-CD
β-cyclodextrin
- BHT
butylated hydroxytoluene
- CE
catechin equivalents
- ChCl
choline chloride
- DES
deep eutectic solvents
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- EP
emerging pollutants
- GAE
gallic acid equivalents
- FRAP
ferric reducing/antioxidant power
- HBA
hydrogen bond acceptor
- HBD
hydrogen bond donor
- MAE
microwave assisted extraction
- MHG
microwave hydrodiffusion and gravity
- PEF
pulse electric fields
- QE
quercetin equivalents
- RE
rutin equivalents
- ROS
reactive oxygen species
- RSM
response surface methodology
- S/L
solid-to-liquid ratio
- scCO2
supercritical CO2
- SFE
supercritical fluid extraction
- TAC
total anthocyanin content
- TAEC
Trolox equivalent antioxidant capacity
- TFC
total flavonoid content
- TPC
total phenol content
- UAE
ultrasound assisted extraction.
References
- 1.Xia H, Houghton JA, Clark JH, Matharu AS. Potential utilization of unavoidable food supply chain wastes-valorization of pea vine wastes. ACS Sustain Chem Eng. (2016) 4:6002–9. 10.1021/acssuschemeng.6b01297 [DOI] [Google Scholar]
- 2.Zuin VG, Ramin LZ. Green and sustainable separation of natural products from agro-industrial waste: challenges, potentialities, and perspectives on emerging approaches. Top Curr Chem. (2018) 376:3. 10.1007/s41061-017-0182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster-Carneiro T, Berni MD, Dorileo IL, Rostagno MA. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour Conserv Recycl. (2013) 77:78–88. 10.1016/j.resconrec.2013.05.007 [DOI] [Google Scholar]
- 4.Perlatti B, Forim MR, Zuin VG. Green chemistry, sustainable agriculture and processing systems: a Brazilian overview. Chem Biol Technol Agric. (2014) 1:1–9. 10.1186/s40538-014-0005-1 [DOI] [Google Scholar]
- 5.Ayala-Zavala JF, González-Aguilar G, Siddiqui MW. Plant Food byproducts: Industrial Relevance for Food Additives and Nutraceuticals. California, CA: Apple Academic Press; (2018). p. 363. [Google Scholar]
- 6.Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr. (2018) 5:87. 10.3389/fnut.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccolella S, Crescente G, Candela L, Pacifico S. Nutraceutical polyphenols: new analytical challenges and opportunities. J Pharm Biomed Anal. (2019) 175:112774. 10.1016/j.jpba.2019.07.022 [DOI] [PubMed] [Google Scholar]
- 8.Serino A, Salazar G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients. (2019) 11:1–23. 10.3390/nu11010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž, Bren U. Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. (2016) 21:901. 10.3390/molecules21070901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losada-Barreiro S, Bravo-Díaz C. Free radicals and polyphenols: the redox chemistry of neurodegenerative diseases. Eur J Med Chem. (2017) 133:379–402. 10.1016/j.ejmech.2017.03.061 [DOI] [PubMed] [Google Scholar]
- 11.San Miguel-Chávez R. Phenolic antioxidant capacity: a review of the state of the art. In: Soto-Hernández M, Palma-Tenango M, García-Mateos MdR, editors. Phenolic Compounds - Biological Activity. Rijeka: InTech; (2017). p. 59–74. [Google Scholar]
- 12.Vilaplana-Pérez C, Auñón D, García-Flores LA, Gil-Izquierdo A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front Nutr. (2014) 1:18. 10.3389/fnut.2014.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Vizcaino F, Fraga CG. Research trends in flavonoids and health. Arch Biochem Biophys. (2018) 646:107–12. 10.1016/j.abb.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 14.Austermann K, Baecker N, Stehle P, Heer M. Putative effects of nutritive polyphenols on bone metabolism in vivo-evidence from human studies. Nutrients. (2019) 11:1–14. 10.3390/nu11040871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing L, Zhang H, Qi R, Tsao R, Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem. (2019) 67:1029–43. 10.1021/acs.jafc.8b06146 [DOI] [PubMed] [Google Scholar]
- 16.Moulaoui K, Caddeo C, Manca ML, Castangia I, Valenti D, Escribano E, et al. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: in vitro and in vivo wound healing potential. Eur J Med Chem. (2015) 89:179–88. 10.1016/j.ejmech.2014.10.047 [DOI] [PubMed] [Google Scholar]
- 17.Panzella L, Napolitano A. Natural phenol polymers: recent advances in food and health applications. Antioxidants. (2017) 6:30. 10.3390/antiox6020030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Ossa JG, Felice F, Azimi B, Salsano JE, Digiacomo M, Macchia M, et al. Waste autochthonous tuscan olive leaves (Olea europaea var. Olivastra seggianese) as antioxidant source for biomedicine. Int J Mol Sci. (2019) 20:1–15. 10.3390/ijms20235918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zillich O V, Schweiggert-Weisz U, Eisner P, Kerscher M. Polyphenols as active ingredients for cosmetic products. Int J Cosmet Sci. (2015) 37:455–64. 10.1111/ics.12218 [DOI] [PubMed] [Google Scholar]
- 20.Działo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int J Mol Sci. (2016) 17:1–41. 10.3390/ijms17020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanjeewa KKA, Kim EA, Son KT, Jeon YJ. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: a review. J Photochem Photobiol B Biol. (2016) 162:100–5. 10.1016/j.jphotobiol.2016.06.027 [DOI] [PubMed] [Google Scholar]
- 22.Panzella L, Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: recent advances. Cosmetics. (2019) 6:57 10.3390/cosmetics6040057 [DOI] [Google Scholar]
- 23.Ganiari S, Choulitoudi E, Oreopoulou V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci Technol. (2017) 68:70–82. 10.1016/j.tifs.2017.08.009 [DOI] [Google Scholar]
- 24.Martillanes S, Rocha-Pimienta J, Cabrera-Bañegil M, Martín-Vertedor D, Delgado-Adámez J. Application of phenolic compounds for food preservation: food additive and active packaging. In: Soto-Hernández M, Palma-Tenango M, García-Mateos MdR, editors. Phenolic Compounds - Biological Activity. Rijeka: InTech; (2017). p. 39–58. 10.5772/66885 [DOI] [Google Scholar]
- 25.Guillard V, Gaucel S, Fornaciari C, Angellier-Coussy H, Buche P, Gontard N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front Nutr. (2018) 5:121. 10.3389/fnut.2018.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouarab Chibane L, Degraeve P, Ferhout H, Bouajila J, Oulahal N. Plant antimicrobial polyphenols as potential natural food preservatives. J Sci Food Agric. (2019) 99:1457–74. 10.1002/jsfa.9357 [DOI] [PubMed] [Google Scholar]
- 27.Miliň DD, Levi SM, Kosti AŽ. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials. (2019) 9:1629 10.3390/nano9111629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fermoso FG, Serrano A, Alonso-Fariñas B, Fernández-Bolaños J, Borja R, Rodríguez-Gutiérrez G. Valuable compound extraction, anaerobic digestion, and composting: a leading biorefinery approach for agricultural wastes. J Agric Food Chem. (2018) 66:8451–68. 10.1021/acs.jafc.8b02667 [DOI] [PubMed] [Google Scholar]
- 29.Goula AM, Thymiatis K, Kaderides K. Valorization of grape pomace: drying behavior and ultrasound extraction of phenolics. Food Bioprod Process. (2016) 100:132–44. 10.1016/j.fbp.2016.06.016 [DOI] [Google Scholar]
- 30.Mourtzinos I, Goula A. Polyphenols in agricultural byproducts and food waste. In: Watson RR. Polyphenols in Plants: Isolation, Purification And Extract Preparation. London: Academic Press; (2019). p. 23–44. [Google Scholar]
- 31.Teixeira A, Baenas N, Dominguez-Perles R, Barros A, Rosa E, Moreno DA, et al. Natural bioactive compounds from winery byproducts as health promoters: a review. Int J Mol Sci. (2014) 15:15638–78. 10.3390/ijms150915638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Bolanos J, Lopez O, Fernandez-Bolanos J, Rodriguez-Gutierrez G. Hydroxytyrosol and derivatives: isolation, synthesis, and biological properties. Curr Org Chem. (2008) 12:442–63. 10.2174/138527208784083888 [DOI] [Google Scholar]
- 33.Freitas CS, Da Silva GA, Perrone D, Vericimo MA, Dos S, Baião D, et al. Recovery of antimicrobials and bioaccessible isoflavones and phenolics from soybean (glycine max) meal by aqueous extraction. Molecules. (2019) 24:74 10.3390/molecules24010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsouko E, Alexandri M, Fernandes KV, Freire DMG, Mallouchos A, Koutinas AA. Extraction of phenolic compounds from palm oil processing residues and their application as antioxidants. Food Technol Biotechnol. (2019) 57:29–38. 10.17113/ftb.57.01.19.5784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M'hiri N, Ioannou I, Ghoul M, Mihoubi Boudhrioua N. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: a review. Food Rev Int. (2017) 33:587–619. 10.1080/87559129.2016.1196489 [DOI] [Google Scholar]
- 36.Hasnaoui N, Wathelet B, Jiménez-Araujo A. Valorization of pomegranate peel from 12 cultivars: dietary fibre composition, antioxidant capacity and functional properties. Food Chem. (2014) 160:196–203. 10.1016/j.foodchem.2014.03.089 [DOI] [PubMed] [Google Scholar]
- 37.Verotta L, Panzella L, Antenucci S, Calvenzani V, Tomay F, Petroni K, et al. Fermented pomegranate wastes as sustainable source of ellagic acid: antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. (2018) 246:129–36. 10.1016/j.foodchem.2017.10.131 [DOI] [PubMed] [Google Scholar]
- 38.Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature. (2000) 405:903–4. 10.1038/35016151 [DOI] [PubMed] [Google Scholar]
- 39.Kammerer J, Boschet J, Kammerer DR, Carle R. Enrichment and fractionation of major apple flavonoids, phenolic acids and dihydrochalcones using anion exchange resins. LWT - Food Sci Technol. (2011) 44:1079–87. 10.1016/j.lwt.2010.10.008 [DOI] [Google Scholar]
- 40.Panzella L, Petriccione M, Rega P, Scortichini M, Napolitano A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. (2013) 140:672–9. 10.1016/j.foodchem.2013.02.121 [DOI] [PubMed] [Google Scholar]
- 41.Vu HT, Scarlett CJ, Vuong Q V. Optimization of ultrasound-assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J Food Process Preserv. (2017) 41:1–14. 10.1111/jfpp.13148 [DOI] [Google Scholar]
- 42.Li T, Shen P, Liu W, Liu C, Liang R, Yan N, et al. Major polyphenolics in pineapple peels and their antioxidant interactions. Int J Food Prop. (2014) 17:1805–17. 10.1080/10942912.2012.732168 [DOI] [Google Scholar]
- 43.Struck S, Plaza M, Turner C, Rohm H. Berry pomace - A review of processing and chemical analysis of its polyphenols. Int J Food Sci Technol. (2016) 51:1305–18. 10.1111/ijfs.13112 [DOI] [Google Scholar]
- 44.Ordoudi SA, Bakirtzi C, Tsimidou MZ. The potential of tree fruit stone and seed wastes in Greece as sources of bioactive ingredients. Recycling. (2018) 3:15–20. 10.3390/recycling3010009 [DOI] [Google Scholar]
- 45.Prakash A, Baskaran R. Acerola, an untapped functional superfruit: a review on latest frontiers. J Food Sci Technol. (2018) 55:3373–84. 10.1007/s13197-018-3309-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzmán-Maldonado SH, Morales-Montelongo AL, Mondragón-Jacobo C, Herrera-Hernández G, Guevara-Lara F, Reynoso-Camacho R. Physicochemical, nutritional, and functional characterization of fruits xoconostle (Opuntia matudae) pears from central-México Region. J Food Sci. (2010) 75:C485–92. 10.1111/j.1750-3841.2010.01679.x [DOI] [PubMed] [Google Scholar]
- 47.Emanuele S, Lauricella M, Calvaruso G, D'Anneo A, Giuliano M. Litchi chinensis as a functional food and a source of antitumor compounds: an overview and a description of biochemical pathways. Nutrients. (2017) 9:992. 10.3390/nu9090992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puganen A, Kallio HP, Schaich KM, Suomela JP, Yang B. Red/green currant and sea buckthorn berry press residues as potential sources of antioxidants for food use. J Agric Food Chem. (2018) 66:3426–34. 10.1021/acs.jafc.8b00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leão DP, Franca AS, Oliveira LS, Bastos R, Coimbra MA. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit byproducts. Food Chem. (2017) 225:146–53. 10.1016/j.foodchem.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 50.Frasao B, Costa M, Silva F, Rodrigues B, Baltar J, Araujo J, et al. Effect of pequi (Caryocar brasiliense) and juçara (Euterpe edulis) waste extract on oxidation process stability in broiler meat treated by UV-C. PLoS ONE. (2018) 13:e0208306. 10.1371/journal.pone.0208306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheok CY, Mohd Adzahan N, Abdul Rahman R, Zainal Abedin NH, Hussain N, Sulaiman R, et al. Current trends of tropical fruit waste utilization. Crit Rev Food Sci Nutr. (2018) 58:335–61. 10.1080/10408398.2016.1176009 [DOI] [PubMed] [Google Scholar]
- 52.Sui W, Xiao Y, Liu R, Wu T, Zhang M. Steam explosion modification on tea waste to enhance bioactive compounds' extractability and antioxidant capacity of extracts. J Food Eng. (2019) 261:51–9. 10.1016/j.jfoodeng.2019.03.015 [DOI] [Google Scholar]
- 53.Gao T, Shi Y, Xue Y, Yan F, Huang D, Wu Y, et al. Polyphenol extract from superheated steam processed tea waste attenuates the oxidative damage in vivo and in vitro. J Food Biochem. (2019) 44:1–11. 10.1111/jfbc.13096 [DOI] [PubMed] [Google Scholar]
- 54.Kiassos E, Mylonaki S, Makris DP, Kefalas P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov Food Sci Emerg Technol. (2009) 10:246–52. 10.1016/j.ifset.2008.10.004 [DOI] [Google Scholar]
- 55.Wiltshire EJ, Eady CC, Collings DA. Induction of anthocyanin in the inner epidermis of red onion leaves by environmental stimuli and transient expression of transcription factors. Plant Cell Rep. (2017) 36:987–1000. 10.1007/s00299-017-2132-1 [DOI] [PubMed] [Google Scholar]
- 56.Akhtar S, Rauf A, Imran M, Qamar M, Riaz M, Mubarak MS. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: a review. Trends Food Sci Technol. (2017) 66:36–47. 10.1016/j.tifs.2017.05.004 [DOI] [Google Scholar]
- 57.Kelebek H, Selli S, Kadiroglu P, Kola O, Kesen S, Uçar B, et al. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. (2017) 220:31–41. 10.1016/j.foodchem.2016.09.190 [DOI] [PubMed] [Google Scholar]
- 58.Parejo I, Viladomat F, Bastida J, Schmeda-Hirschmann G, Burillo J, Codina C. Bioguided isolation and identification of the nonvolatile antioxidant compounds from fennel (Foeniculum vulgare Mill.) waste. J Agric Food Chem. (2004) 52:1890–7. 10.1021/jf030717g [DOI] [PubMed] [Google Scholar]
- 59.Pacifico S, Galasso S, Piccolella S, Kretschmer N, Pan SP, Nocera P, et al. Winter wild fennel leaves as a source of anti-inflammatory and antioxidant polyphenols. Arab J Chem. (2018) 11:513–24. 10.1016/j.arabjc.2015.06.026 [DOI] [Google Scholar]
- 60.Aires A, Carvalho R, Saavedra MJ. Reuse potential of vegetable wastes (broccoli, green bean and tomato) for the recovery of antioxidant phenolic acids and flavonoids. Int J Food Sci Technol. (2017) 52:98–107. 10.1111/ijfs.13256 [DOI] [Google Scholar]
- 61.Gonzales GB, Raes K, Vanhoutte H, Coelus S, Smagghe G, Van Camp J. Liquid chromatography-mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J Chromatogr A. (2015) 1402:60–70. 10.1016/j.chroma.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 62.Llorach R, Tomás-Barberán FA, Ferreres F. Lettuce and chicory byproducts as a source of antioxidant phenolic extracts. J Agric Food Chem. (2004) 52:5109–16. 10.1021/jf040055a [DOI] [PubMed] [Google Scholar]
- 63.Plazzotta S, Manzocco L. Effect of ultrasounds and high pressure homogenization on the extraction of antioxidant polyphenols from lettuce waste. Innov Food Sci Emerg Technol. (2018) 50:11–19. 10.1016/j.ifset.2018.10.004 [DOI] [Google Scholar]
- 64.Lavecchia R, Maffei G, Paccassoni F, Piga L, Zuorro A. Artichoke waste as a source of phenolic antioxidants and bioenergy. Waste Biomass Valoriz. (2019) 10:2975–84. 10.1007/s12649-018-0305-y [DOI] [Google Scholar]
- 65.Jiménez-Moreno N, Cimminelli MJ, Volpe F, Ansó R, Esparza I, Mármol I, et al. Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients. (2019) 11:1723. 10.3390/nu11081723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laddomada B, Caretto S, Mita G. Wheat bran phenolic acids: bioavailability and stability in whole wheat-based foods. Molecules. (2015) 20:15666–85. 10.3390/molecules200915666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Wang S, Huang S, Zhang L, Ge Z, Sun L, et al. Purification of polyphenols from distiller's grains by macroporous resin and analysis of the polyphenolic components. Molecules. (2019) 24:1284. 10.3390/molecules24071284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panusa A, Zuorro A, Lavecchia R, Marrosu G, Petrucci R. Recovery of natural antioxidants from spent coffee grounds. J Agric Food Chem. (2013) 61:4162–68. 10.1021/jf4005719 [DOI] [PubMed] [Google Scholar]
- 69.Conde T, Mussatto SI. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep Biochem Biotechnol. (2016) 46:406–9. 10.1080/10826068.2015.1084514 [DOI] [PubMed] [Google Scholar]
- 70.Meullemiestre A, Kamal I, Maache-Rezzoug Z, Chemat F, Rezzoug SA. Antioxidant activity and total phenolic content of oils extracted from Pinus pinaster sawdust waste. screening of different innovative isolation techniques. Waste Biomass Valoriz. (2014) 5:283–92. 10.1007/s12649-013-9237-8 [DOI] [Google Scholar]
- 71.Meullemiestre A, Petitcolas E, Maache-Rezzoug Z, Chemat F, Rezzoug SA. Impact of ultrasound on solid-liquid extraction of phenolic compounds from maritime pine sawdust waste. kinetics, optimization and large scale experiments. Ultrason Sonochem. (2016) 28:230–9. 10.1016/j.ultsonch.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 72.Panzella L, Moccia F, Toscanesi M, Trifuoggi M, Giovando S, Napolitano A. Exhausted woods from tannin extraction as an unexplored waste biomass: evaluation of the antioxidant and pollutant adsorption properties and activating e ects of hydrolytic treatments. Antioxidants. (2019) 8:10–14. 10.3390/antiox8040084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ran X li, Zhang M, Wang Y, Adhikari B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: a review. Crit Rev Food Sci Nutr. (2019) 59:450–61. 10.1080/10408398.2017.1377149 [DOI] [PubMed] [Google Scholar]
- 74.Veggi PC, Martinez J, Meireles MAA. Fundamentals of microwave extraction. In: Chemat F, Gravotto G. editors. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice. New York, NY: Springer; (2013) p. 15–52. [Google Scholar]
- 75.Talmaciu AI, Volf I, Popa VI. A comparative analysis of the green techniques applied for polyphenols extraction from bioresources. Chem Biodivers. (2015) 12:1635–51. 10.1002/cbdv.201400415 [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Salas P, Morales-Soto A, Segura-Carretero A, Fernández-Gutiérrez A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules. (2010) 15:8813–26. 10.3390/molecules15128813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.da Silva RPFF, Rocha-Santos TAP, Duarte AC. Supercritical fluid extraction of bioactive compounds. Trends Anal Chem. (2016) 76:40–51. 10.1016/j.trac.2015.11.013 [DOI] [Google Scholar]
- 78.De Melo MMR, Silvestre AJD, Silva CM. Supercritical fluid extraction of vegetable matrices: applications, trends and future perspectives of a convincing green technology. J Supercrit Fluids. (2014) 92:115–76. 10.1016/j.supflu.2014.04.007 [DOI] [Google Scholar]
- 79.Pereira CG, Meireles MAA. Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food Bioprocess Technol. (2010) 3:340–72. 10.1007/s11947-009-0263-2 [DOI] [Google Scholar]
- 80.Brunner G. Gas Extraction - An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes. Steinkopff: Springer; (1996). p. 400. [Google Scholar]
- 81.Martínez J, Aguiar A. Extraction of triacylglycerols and fatty acids using supercritical fluids - review. Curr Anal Chem. (2013) 10:67–77. 10.2174/1573411011410010006 [DOI] [Google Scholar]
- 82.Pourmortazavi SM, Hajimirsadeghi SS. Supercritical fluid extraction in plant essential and volatile oil analysis. J Chromatogr A. (2007) 1163:2–24. 10.1016/j.chroma.2007.06.021 [DOI] [PubMed] [Google Scholar]
- 83.Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. (2007) 30:3268–95. 10.1002/jssc.200700261 [DOI] [PubMed] [Google Scholar]
- 84.Ikeda M. Public health problems of organic solvents. Toxicol Lett. (1992) 64–65:191–201. 10.1016/0378-4274(92)90189-Q [DOI] [PubMed] [Google Scholar]
- 85.Welton T. Solvents and sustainable chemistry. Proc R Soc A Math Phys Eng Sci. (2015) 471:20150502. 10.1098/rspa.2015.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shanab K, Neudorfer C, Schirmer E, Spreitzer H. Green solvents in organic synthesis: an overview. Curr Org Chem. (2013) 17:1179–87. 10.2174/1385272811317110005 [DOI] [Google Scholar]
- 87.Zainal-Abidin MH, Hayyan M, Hayyan A, Jayakumar NS. New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Anal Chim Acta. (2017) 979:1–23. 10.1016/j.aca.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 88.Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte ARC. Natural deep eutectic solvents - Solvents for the 21st century. ACS Sustain Chem Eng. (2014) 2:1063–71. 10.1021/sc500096j [DOI] [Google Scholar]
- 89.Tang B, Zhang H, Row KH. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J Sep Sci. (2015) 38:1053–64. 10.1002/jssc.201401347 [DOI] [PubMed] [Google Scholar]
- 90.Ruesgas-Ramón M, Figueroa-Espinoza MC, Durand E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: overview, challenges, and opportunities. J Agric Food Chem. (2017) 65:3591–601. 10.1021/acs.jafc.7b01054 [DOI] [PubMed] [Google Scholar]
- 91.Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. (2003) 9:70–1. 10.1039/b210714g [DOI] [PubMed] [Google Scholar]
- 92.Radošević K, Bubalo CM, Srček VG, Grgas D, Dragičević TL, Redovniković RI. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol Environ Saf . (2015) 112:46–53. 10.1016/j.ecoenv.2014.09.034 [DOI] [PubMed] [Google Scholar]
- 93.Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem Rev. (2014) 114:11060–82. 10.1021/cr300162p [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. (2012) 41:7108–46. 10.1039/c2cs35178a [DOI] [PubMed] [Google Scholar]
- 95.Francisco M, Van Den Bruinhorst A, Kroon MC. Low-transition-temperature mixtures (LTTMs): a new generation of designer solvents. Angew Chemie Int Ed. (2013) 52:3074–85. 10.1002/anie.201207548 [DOI] [PubMed] [Google Scholar]
- 96.Choi YH, van Spronsen J, Dai Y, Verberne M, Hollmann F, Arends IWCE, et al. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. (2011) 156:1701–5. 10.1104/pp.111.178426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta. (2013) 766:61–8. 10.1016/j.aca.2012.12.019 [DOI] [PubMed] [Google Scholar]
- 98.Abbott AP, Barron JC, Ryder KS, Wilson D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem Eur J. (2007) 13:6495–501. 10.1002/chem.200601738 [DOI] [PubMed] [Google Scholar]
- 99.Varadharajan V, Shanmugam S, Ramaswamy A. Model generation and process optimization of microwave-assisted aqueous extraction of anthocyanins from grape juice waste. J Food Process Eng. (2017) 40:1–9. 10.1111/jfpe.12486 [DOI] [Google Scholar]
- 100.Trasanidou D, Apostolakis A, Makris DP. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem Eng Commun. (2016) 203:1317–25. 10.1080/00986445.2016.1189416 [DOI] [Google Scholar]
- 101.Jeong KM, Zhao J, Jin Y, Heo SR, Han SY, Yoo DE, et al. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch Pharm Res. (2015) 38:2143–52. 10.1007/s12272-015-0678-4 [DOI] [PubMed] [Google Scholar]
- 102.Patsea M, Stefou I, Grigorakis S, Makris DP. Screening of natural sodium acetate-based low-transition temperature mixtures (LTTMs) for enhanced extraction of antioxidants and pigments from red vinification solid wastes. Environ Process. (2017) 4:123–35. 10.1007/s40710-016-0205-8 [DOI] [Google Scholar]
- 103.Bosiljkov T, Dujmić F, Bubalo CM, Hribar J, Vidrih R, Brnčić M, et al. Natural deep eutectic solvents and ultrasound-assisted extraction: green approaches for extraction of wine lees anthocyanins. Food Bioprod Process. (2017) 102:195–203. 10.1016/j.fbp.2016.12.005 [DOI] [Google Scholar]
- 104.Wang B. Orthogonal test design for optimisation of extraction of trans-resveratrol from pinot noir-grape pomace. Nat Prod Res. (2012) 26:821–9. 10.1080/14786419.2011.559638 [DOI] [PubMed] [Google Scholar]
- 105.Casazza AA, Aliakbarian B, Mantegna S, Cravotto G, Perego P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J Food Eng. (2010) 100:50–5. 10.1016/j.jfoodeng.2010.03.026 [DOI] [Google Scholar]
- 106.Babazadeh A, Taghvimi A, Hamishehkar H, Tabibiazar M. Development of new ultrasonic–solvent assisted method for determination of trans-resveratrol from red grapes: optimization, characterization, and antioxidant activity (ORAC assay). Food Biosci. (2017) 20:36–42. 10.1016/j.fbio.2017.08.003 [DOI] [Google Scholar]
- 107.Alexandru L, Binello A, Mantegna S, Boffa L, Chemat F, Cravotto G. Efficient green extraction of polyphenols from post-harvested agro-industry vegetal sources in Piedmont. Comptes Rendus Chim. (2014) 17:212–7. 10.1016/j.crci.2013.09.012 [DOI] [Google Scholar]
- 108.Pedroza MA, Amendola D, Maggi L, Zalacain A, De Faveri DM, Spigno G. Microwave-assisted extraction of phenolic compounds from dried waste grape skins. Int J Food Eng. (2015) 11:359–70. 10.1515/ijfe-2015-0009 [DOI] [Google Scholar]
- 109.Bubalo CM, Curko N, Tomašević M, Ganić KK, Redovnikovic IR. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. (2016) 200:159–66. 10.1016/j.foodchem.2016.01.040 [DOI] [PubMed] [Google Scholar]
- 110.Radošević K, Curko N, Srček VG, Bubalo CM, Tomašević M, Ganić KK, et al. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT - Food Sci Technol. (2016) 73:45–51. 10.1016/j.lwt.2016.05.037 [DOI] [Google Scholar]
- 111.Moreira MM, Barroso MF, Porto JV, Ramalhosa MJ, Švarc-Gajić J, Estevinho L, et al. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci Total Environ. (2018) 634:831–42. 10.1016/j.scitotenv.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 112.Baiano A, Viggiani I, Terracone C, Romaniello R, Del Nobile MA. Physical and sensory properties of bread enriched with phenolic aqueous extracts from vegetable wastes. Czech J Food Sci. (2015) 33:247–53. 10.17221/528/2014-CJFS [DOI] [PubMed] [Google Scholar]
- 113.Arboleda Meija JA, Parpinello GP, Versari A, Conidi C, Cassano A. Microwave-assisted extraction and membrane-based separation of biophenols from red wine lees. Food Bioprod Process. (2019) 117:74–83. 10.1016/j.fbp.2019.06.020 [DOI] [Google Scholar]
- 114.Matos MS, Romero-Díez R, Álvarez A, Bronze MR, Rodríguez-Rojo S, Mato RB, et al. Polyphenol-rich extracts obtained from winemakingwaste streams as natural ingredients with cosmeceutical potential. Antioxidants. (2019) 8:355. 10.3390/antiox8090355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pérez-Serradilla JA, Priego-Capote F, Luque De Castro MD. Simultaneous ultrasound-assisted emulsification-extraction of polar and nonpolar compounds from solid plant samples. Anal Chem. (2007) 79:6767–74. 10.1021/ac0708801 [DOI] [PubMed] [Google Scholar]
- 116.González-Centeno MR, Knoerzer K, Sabarez H, Simal S, Rosselló C, Femenia A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.) - A response surface approach. Ultrason Sonochem. (2014) 21:2176–84. 10.1016/j.ultsonch.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 117.Manna L, Bugnone CA, Banchero M. Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. J Supercrit Fluids. (2015) 104:204–11. 10.1016/j.supflu.2015.06.012 [DOI] [Google Scholar]
- 118.Athanasiadis V, Grigorakis S, Lalas S, Makris DP. Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valoriz. (2018) 9:1985–92. 10.1007/s12649-017-9997-7 [DOI] [Google Scholar]
- 119.Alañón ME, Ivanović M, Gómez-Caravaca AM, Arráez-Román D, Segura-Carretero A. Choline chloride derivative-based deep eutectic liquids as novel green alternative solvents for extraction of phenolic compounds from olive leaf. Arab J Chem. (2018) 13:1685–701. 10.1016/j.arabjc.2018.01.003 [DOI] [Google Scholar]
- 120.Chanioti S, Tzia C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov Food Sci Emerg Technol. (2018) 48:228–239. [Google Scholar]
- 121.Fernández M de los Á, Espino M, Gomez FJV, Silva MF. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial byproducts. Food Chem. (2018) 239:671–8. 10.1016/j.foodchem.2017.06.150 [DOI] [PubMed] [Google Scholar]
- 122.Chakroun D, Grigorakis S, Loupassaki S, Makris DP. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: screening, optimisation, temperature effects and stability. Biomass Convers Biorefinery. (2019) 1–12. 10.1007/s13399-019-00521-2 [DOI] [Google Scholar]
- 123.Sahin S, Samli R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason Sonochem. (2013) 20:595–602. 10.1016/j.ultsonch.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 124.Icyer NC, Toker OS, Karasu S, Tornuk F, Bozkurt F, Arici M, et al. Combined design as a useful statistical approach to extract maximum amount of phenolic compounds from virgin olive oil waste. LWT - Food Sci Technol. (2016) 70:24–32. 10.1016/j.lwt.2016.02.029 [DOI] [Google Scholar]
- 125.Wang Z, Wang C, Zhang C, Li W. Ultrasound-assisted enzyme catalyzed hydrolysis of olive waste and recovery of antioxidant phenolic compounds. Innov Food Sci Emerg Technol. (2017) 44:224–34. 10.1016/j.ifset.2017.02.013 [DOI] [Google Scholar]
- 126.Jurmanović S, Jug M, Safner T, Radić K, Domijan AM, Pedisić S, et al. Utilization of olive pomace as a source of polyphenols: optimization of microwave-assisted extraction and characterization of spray-dried extract. J Food Nutr Res. (2019) 58:51–62. [Google Scholar]
- 127.Transactions CS, Saifuddin N, Saltanat A, Refal H. Enhancing the removal of phenolic compounds from palm oil mill effluent by enzymatic pre-treatment and microwave-assisted extraction. Chem Sci Trans. (2014) 3:1083–93. 10.7598/cst2014.797 [DOI] [Google Scholar]
- 128.Hua L, Guoqin H, Dan L. Application of the microwave-assisted process to the fast extraction of isoflavone from the waste residue of the soybeans. Bull Korean Chem Soc. (2009) 30:2687–90. 10.5012/bkcs.2009.30.11.2687 [DOI] [Google Scholar]
- 129.Min LJ, Zhao MX. Studies on ultrasonic assisted extraction technology of flavonoid from orange peel. Adv Mater Res. (2013) 815:317–20. 10.4028/www.scientific.net/AMR.815.317 [DOI] [Google Scholar]
- 130.Dar NG, Hussain A, Paracha GM, Akhter S. Evaluation of different techniques for extraction of antioxidants as bioactive compounds from citrus peels (industrial by products). Am J Agric Environ Sci. (2015) 15:676–82. [Google Scholar]
- 131.Rodsamran P, Sothornvit R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. (2019) 28:66–73. 10.1016/j.fbio.2019.01.017 [DOI] [Google Scholar]
- 132.Boukroufa M, Boutekedjiret C, Petigny L, Rakotomanomana N, Chemat F. Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason Sonochem. (2015) 24:72–9. 10.1016/j.ultsonch.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 133.Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Bowyer MC, Scarlett CJ, et al. Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. (2018) 21:20–6. 10.1016/j.fbio.2017.11.001 [DOI] [Google Scholar]
- 134.Garcia-Castello EM, Rodriguez-Lopez AD, Mayor L, Ballesteros R, Conidi C, Cassano A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT - Food Sci Technol. (2015) 64:1114–22. 10.1016/j.lwt.2015.07.024 [DOI] [Google Scholar]
- 135.Mouratoglou E, Malliou V, Makris DP. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valoriz. (2016) 7:1377–87. 10.1007/s12649-016-9539-8 [DOI] [Google Scholar]
- 136.Ozturk B, Parkinson C, Gonzalez-Miquel M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep Purif Technol. (2018) 206:1–13. 10.1016/j.seppur.2018.05.052 [DOI] [Google Scholar]
- 137.Xu M, Ran L, Chen N, Fan X, Ren D, Yi L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. (2019) 297:124970. 10.1016/j.foodchem.2019.124970 [DOI] [PubMed] [Google Scholar]
- 138.Inoue T, Tsubaki S, Ogawa K, Onishi K, Azuma J. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. (2010) 123:542–7. 10.1016/j.foodchem.2010.04.051 [DOI] [Google Scholar]
- 139.Ateş F, Sahin S, Ilbay Z, Kirbaşlar I. A Green valorisation approach using microwaves and supercritical CO2 for high-added value ingredients from mandarin (Citrus deliciosa Tenore) leaf waste. Waste Biomass Valoriz. (2019) 10:533–46. 10.1007/s12649-017-0074-z [DOI] [Google Scholar]
- 140.Di Donato P, Taurisano V, Tommonaro G, Pasquale V, Jiménez JMS, de Pascual-Teresa S, et al. Biological properties of polyphenols extracts from agro industry's wastes. Waste Biomass Valoriz. (2018) 9:1567–78. 10.1007/s12649-017-9939-4 [DOI] [Google Scholar]
- 141.Moccia F, Flores-Gallegos AC, Chávez-González ML, Sepúlveda L, Marzorati S, Verotta L, et al. Ellagic acid recovery by solid state fermentation of pomegranate wastes by Aspergillus niger and Saccharomyces cerevisiae: a comparison. Molecules. (2019) 24:1–11. 10.3390/molecules24203689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Talekar S, Patti AF, Vijayraghavan R, Arora A. Complete utilization of waste pomegranate peels to produce a hydrocolloid, punicalagin rich phenolics, and a hard carbon electrode. ACS Sustain Chem Eng. (2018) 6:16363–74. 10.1021/acssuschemeng.8b03452 [DOI] [Google Scholar]
- 143.Talekar S, Patti AF, Vijayraghavan R, Arora A. Recyclable enzymatic recovery of pectin and punicalagin rich phenolics from waste pomegranate peels using magnetic nanobiocatalyst. Food Hydrocoll. (2019) 89:468–80. 10.1016/j.foodhyd.2018.11.009 [DOI] [Google Scholar]
- 144.Turrini F, Boggia R, Donno D, Parodi B, Beccaro G, Baldassari S, et al. From pomegranate marcs to a potential bioactive ingredient: a recycling proposal for pomegranate-squeezed marcs. Eur Food Res Technol. (2020) 246:273–285. 10.1007/s00217-019-03339-4 [DOI] [Google Scholar]
- 145.Malinowska M, Sliwa K, Sikora E, Ogonowski J, Oszmianski J, Kolniak-Ostek J. Ultrasound-assisted and micelle-mediated extraction as a method to isolate valuable active compounds from apple pomace. J Food Process Preserv. (2018) 42:1–9. 10.1111/jfpp.13720 [DOI] [Google Scholar]
- 146.Ferrentino G, Morozova K, Mosibo OK, Ramezani M, Scampicchio M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J Clean Prod. (2018) 186:253–61. 10.1016/j.jclepro.2018.03.165 [DOI] [Google Scholar]
- 147.Katsampa P, Valsamedou E, Grigorakis S, Makris DP. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box-Behnken experimental design and kinetics. Ind Crops Prod. (2015) 77:535–43. 10.1016/j.indcrop.2015.09.039 [DOI] [Google Scholar]
- 148.Oancea S, Radu M. Phenolic composition and antioxidant activity of green-solvent-based extracts of red onion wastes. AGROFOR Int J. (2018) 3:106–13. 10.7251/AGRENG1803106O [DOI] [Google Scholar]
- 149.Jang M, Asnin L, Nile SH, Keum YS, Kim HY, Park SW. Ultrasound-assisted extraction of quercetin from onion solid wastes. Int J Food Sci Technol. (2013) 48:246–52. 10.1111/j.1365-2621.2012.03180.x [DOI] [Google Scholar]
- 150.Pal CBT, Jadeja GC. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J Sci Food Agric. (2019) 99:1969–79. 10.1002/jsfa.9395 [DOI] [PubMed] [Google Scholar]
- 151.Stefou I, Grigorakis S, Loupassaki S, Makris DP. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol Environ Policy. (2019) 21:1563–74. 10.1007/s10098-019-01727-8 [DOI] [Google Scholar]
- 152.Jabbar S, Abid M, Wu T, Hashim MM, Saeeduddin M, Hu B, et al. Ultrasound-assisted extraction of bioactive compounds and antioxidants from carrot pomace: a response surface approach. J Food Process Preserv. (2015) 39:1878–88. 10.1111/jfpp.12425 [DOI] [Google Scholar]
- 153.Agcam E, Akyildiz A, Balasubramaniam VM. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. (2017) 237:461–70. 10.1016/j.foodchem.2017.05.098 [DOI] [PubMed] [Google Scholar]
- 154.Wu T, Yan J, Liu R, Marcone MF, Aisa HA, Tsao R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. (2012) 133:1292–8. 10.1016/j.foodchem.2011.08.002 [DOI] [Google Scholar]
- 155.Riciputi Y, Diaz-de-Cerio E, Akyol H, Capanoglu E, Cerretani L, Caboni MF, et al. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato byproducts using response surface methodology. Food Chem. (2018) 269:258–63. 10.1016/j.foodchem.2018.06.154 [DOI] [PubMed] [Google Scholar]
- 156.Manousaki A, Jancheva M, Grigorakis S, Makris D. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: a comparison with conventional eco-friendly solvents. Recycling. (2016) 1:194–204. 10.3390/recycling1010194 [DOI] [Google Scholar]
- 157.Pinela J, Prieto MA, Barreiro MF, Carvalho AM, Oliveira MBPP, Curran TP, et al. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: a sustainable approach towards the needs of the today's society. Innov Food Sci Emerg Technol. (2017) 41:160–71. 10.1016/j.ifset.2017.02.004 [DOI] [Google Scholar]
- 158.El-Malah MH, Hassanein MMM, Areif MH, Al-Amrousi EF. Utilization of Egyptian Tomato waste as a potential source of natural antioxidants using solvents, microwave and ultrasound extraction methods. Am J Food Technol. (2015) 10:14–25. 10.3923/ajft.2015.14.25 [DOI] [Google Scholar]
- 159.Bakić MT, Pedisić S, Zorić Z, Dragović-Uzelac V, Grassino AN. Effect of microwave-assisted extraction on polyphenols recovery from tomato peel waste. Acta Chim Slov. (2019) 66:367–77. 10.17344/acsi.2018.4866 [DOI] [PubMed] [Google Scholar]
- 160.Moreira MM, Morais S, Barros AA, Delerue-Matos C, Guido LF. A novel application of microwave-assisted extraction of polyphenols from brewer's spent grain with HPLC-DAD-MS analysis. Anal Bioanal Chem. (2012) 403:1019–29. 10.1007/s00216-011-5703-y [DOI] [PubMed] [Google Scholar]
- 161.Ranic M, Nikolic M, Pavlovic M, Buntic A, Siler-Marinkovic S, Dimitrijevic-Brankovic S. Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J Clean Prod. (2014) 80:69–79. 10.1016/j.jclepro.2014.05.060 [DOI] [Google Scholar]
- 162.Demirhan H, Fauzi A, Skoulou VK, Haywood SH, Zein SH. Wheat straw bio-refining. part I: optimization of the microwave radiation process with sulphuric acid pre-treatment. Curr Microw Chem. (2017) 4:205–18. 10.2174/2213335604666170719113659 [DOI] [Google Scholar]
- 163.Manara P, Zabaniotou A, Vanderghem C, Richel A. Lignin extraction from Mediterranean agro-wastes: impact of pretreatment conditions on lignin chemical structure and thermal degradation behavior. Catal Today. (2014) 223:25–34. 10.1016/j.cattod.2013.10.065 [DOI] [Google Scholar]
- 164.Fernández-Agulló A, Freire MS, González-Álvarez J. Effect of the extraction technique on the recovery of bioactive compounds from eucalyptus (Eucalyptus globulus) wood industrial wastes. Ind Crops Prod. (2015) 64:105–13. 10.1016/j.indcrop.2014.11.031 [DOI] [Google Scholar]
- 165.Fernández-Agulló A, Freire MS, Antorrena G, Pereira JA, González-Álvarez J. Effect of the extraction technique and operational conditions on the recovery of bioactive compounds from chestnut (Castanea sativa) bur and shell. Sep Sci Technol. (2014) 49:267–77. 10.1080/01496395.2013.838264 [DOI] [Google Scholar]
- 166.Ullah Z, Man Z, Khan AS, Muhammad N, Mahmood H, Ben Ghanem O, et al. Extraction of valuable chemicals from sustainable rice husk waste using ultrasonic assisted ionic liquids technology. J Clean Prod. (2019) 220:620–9. 10.1016/j.jclepro.2019.02.041 [DOI] [Google Scholar]
- 167.Wang J, Sun B, Cao Y, Tian Y, Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. (2008) 106:804–10. 10.1016/j.foodchem.2007.06.062 [DOI] [Google Scholar]
- 168.Izadifar Z. Ultrasound pretreatment of wheat dried distiller's grain (DDG) for extraction of phenolic compounds. Ultrason Sonochem. (2013) 20:1359–69. 10.1016/j.ultsonch.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 169.Tanase C, Domokos E, Coşarcă S, Miklos A, Imre S, Domokos J, et al. Study of the ultrasound-assisted extraction of polyphenols from beech (Fagus sylvatica L.) bark. BioResources. (2018) 13:2247–67. 10.15376/biores.13.2.2247-2267 [DOI] [Google Scholar]
- 170.Withouck H, Boeykens A, Vanden Broucke M, Moreira MM, Delerue-Matos C, De Cooman L. Evaluation of the impact of pre-treatment and extraction conditions on the polyphenolic profile and antioxidant activity of Belgium apple wood. Eur Food Res Technol. (2019) 245:2565–78. 10.1007/s00217-019-03373-2 [DOI] [Google Scholar]
- 171.Görgüç A, Bircan C, Yilmaz FM. Sesame bran as an unexploited by-product: effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. (2019) 283:637–45. 10.1016/j.foodchem.2019.01.077 [DOI] [PubMed] [Google Scholar]
- 172.Alvarez-Vasco C, Ma R, Quintero M, Guo M, Geleynse S, Ramasamy KK, et al. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): a source of lignin for valorization. Green Chem. (2016) 18:5133–41. 10.1039/C6GC01007E [DOI] [Google Scholar]
- 173.Li T, Lyu G, Liu Y, Lou R, Lucia LA, Yang G, et al. Deep eutectic solvents (DESs) for the isolation of willow lignin (Salix matsudana cv. zhuliu). Int J Mol Sci. (2017) 18:2266. 10.3390/ijms18112266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen Y, Zhang L, Yu J, Lu Y, Jiang B, Fan Y, et al. High-purity lignin isolated from poplar wood meal through dissolving treatment with deep eutectic solvents. R Soc Open Sci. (2019) 6:181757. 10.1098/rsos.181757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y, Chen W, Xia Q, Guo B, Wang Q, Liu S, et al. Efficient cleavage of lignin–carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. Chem Sus Chem. (2017) 10:1692–1700. 10.1002/cssc.201601795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Malaeke H, Housaindokht MR, Monhemi H, Izadyar M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J Mol Liq. (2018) 263:193–9. 10.1016/j.molliq.2018.05.001 [DOI] [Google Scholar]
- 177.Mamilla JLK, Novak U, Grilc M, Likozar B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioenergy. (2019) 120:417–25. 10.1016/j.biombioe.2018.12.002 [DOI] [Google Scholar]
- 178.Kaavya R, Kalpana L, Kumar AA. Microwave methods for the extraction of bioactive components and enzymes from pineapple waste and its application in meat tenderization. Int J Agric Sci. (2017) 9:4612–20. [Google Scholar]
- 179.Shengjiu G, Kaimei Z, Caizhen L, Yunsheng J, Yourui X. Microwaves-assisted extraction of polyphenols from banana peel. Med plant. (2014) 5:21–4. [Google Scholar]
- 180.Vu HT, Scarlett CJ, Vuong QV. Maximising recovery of phenolic compounds and antioxidant properties from banana peel using microwave assisted extraction and water. J Food Sci Technol. (2019) 56:1360–70. 10.1007/s13197-019-03610-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dávila-Hernández G, Sánchez-Pardo ME, Gutiérrez-López GF, Necoechea-Mondragon H, Ortiz-Moreno A. Effect of microwave pretreatment on bioactive compounds extraction from xoconostle (Opuntia joconostle) byproducts. Rev Mex Ing Quim. (2019) 18:191–204. 10.24275/uam/izt/dcbi/revmexingquim/2019v18n1/Davila [DOI] [Google Scholar]
- 182.Dailey A, Vuong QV. Optimum conditions for microwave assisted extraction for recovery of phenolic compounds and antioxidant capacity from macadamia (Macadamia tetraphylla) skin waste using water. Processes. (2016) 4:1–15. 10.3390/pr4010002 [DOI] [Google Scholar]
- 183.Zhang JJ, Li Y, Lin SJ, Li HB. Green extraction of natural antioxidants from the Sterculia nobilis fruit waste and analysis of phenolic profile. Molecules. (2018) 23:1059. 10.3390/molecules23051059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang G, Hu M, He L, Fu P, Wang L, Zhou J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod Process. (2013) 91:158–68. 10.1016/j.fbp.2012.09.003 [DOI] [Google Scholar]
- 185.Han ZP, Liu RL, Cui HY, Zhang ZQ. Microwave-assisted extraction and LC/MS analysis of phenolic antioxidants in sweet apricot (Prunus armeniaca L.) kernel skins. J Liq Chromatogr Relat Technol. (2013) 36:2182–95. 10.1080/10826076.2012.717057 [DOI] [Google Scholar]
- 186.Li Z, Huang D, Tang Z, Deng C, Zhang X. Fast determination of chlorogenic acid in tobacco residues using microwave-assisted extraction and capillary zone electrophoresis technique. Talanta. (2010) 82:1181–5. 10.1016/j.talanta.2010.06.037 [DOI] [PubMed] [Google Scholar]
- 187.Zain MN, Nazeri MA. Antioxidant and mineral content of pitaya peel extract obtained using microwave assisted extraction (MAE). Aust J Basic Appl Sci. (2016) 10:63–8. [Google Scholar]
- 188.Chaisamlitpol S, Hiranvarachat B, Srichumpoung J, Devahastin S, Chiewchan N. Bioactive compositions of extracts from cabbage outer leaves as affected by drying pretreatment prior to microwave-assisted extraction. Sep Purif Technol. (2014) 136:177–83. 10.1016/j.seppur.2014.09.002 [DOI] [Google Scholar]
- 189.Milutinović M, Radovanović N, Corović M, Šiler-Marinković S, Rajilić-Stojanović M, Dimitrijević-Branković S. Optimisation of microwave-assisted extraction parameters for antioxidants from waste Achillea millefolium dust. Ind Crops Prod. (2015) 77:333–41. 10.1016/j.indcrop.2015.09.007 [DOI] [Google Scholar]
- 190.Milutinović M, Radovanović N, Rajilić-Stojanović M, Šiler-Marinković S, Dimitrijević S, Dimitrijević-Branković S. Microwave-assisted extraction for the recovery of antioxidants from waste Equisetum arvense. Ind Crops Prod. (2014) 61:388–97. 10.1016/j.indcrop.2014.07.039 [DOI] [Google Scholar]
- 191.Tsubaki S, Sakamoto M, Azuma J. Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions. Food Chem. (2010) 123:1255–8. 10.1016/j.foodchem.2010.05.088 [DOI] [Google Scholar]
- 192.Zheng L, Chen L, Li J, Liang L, Fan Y, Qiu L, et al. Two kaempferol glycosides separated from Camellia oleifera meal by high-speed countercurrent chromatography and their possible application for antioxidation. J Food Sci. (2019) 84:2805–11. 10.1111/1750-3841.14765 [DOI] [PubMed] [Google Scholar]
- 193.Tabaraki R, Rastgoo S. Comparison between conventional and ultrasound-assisted extractions of natural antioxidants from walnut green husk. Korean J Chem Eng. (2014) 31:676–83. 10.1007/s11814-013-0279-1 [DOI] [Google Scholar]
- 194.Kam WYJ, Abas F, Hussain N, Mirhosseini H. Comparison of crude extract from Durio zibethinus M. (durian) leaf waste via ultrasound-assisted extraction and accelerated solvent extraction: antioxidant activity and cytotoxicity. Nat Prod Res. (2019) 1–5. 10.1080/14786419.2018.1564296 [DOI] [PubMed] [Google Scholar]
- 195.Rezende YRRS, Nogueira JP, Narain N. Comparison and optimization of conventional and ultrasound assisted extraction for bioactive compounds and antioxidant activity from agro-industrial acerola (Malpighia emarginata DC) residue. LWT Food Sci Technol. (2017) 85:158–69. 10.1016/j.lwt.2017.07.020 [DOI] [Google Scholar]
- 196.Liang JL, Yeow CC, Teo KC, Gnanaraj C, Chang YP. Valorizing cabbage (Brassica oleracea L. var. capitata) and capsicum (Capsicum annuum L.) wastes: in vitro health-promoting activities. J Food Sci Technol. (2019) 56:4696–704. 10.1007/s13197-019-03912-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li H, Li Y, Li G, Tan X, Chen G, Zhang Y. Ultrasonically assisted simultaneous extraction of isoorientin, orientin, and vitexin from leaves of Neosinocalamus affinis (Rendle) Keng f. (N. affinis). Sep Sci Technol. (2013) 48:1987–97. 10.1080/01496395.2013.779280 [DOI] [Google Scholar]
- 198.Memon AA, Memon N, Luthria DL, Pitafi AA, Bhanger MI. Phenolic compounds and seed oil composition of Ziziphus mauritiana L. fruit. Polish J Food Nutr Sci. (2012) 62:15–21. 10.2478/v10222-011-0035-3 [DOI] [Google Scholar]
- 199.Kwun KH, Kim GJ, Shin HJ. Ultrasonication assistance increases the efficiency of isoflavones extraction from kudzu (Pueraria lobata Ohwi) roots waste. Biotechnol Bioprocess Eng. (2009) 14:345–8. 10.1007/s12257-008-0199-9 [DOI] [Google Scholar]
- 200.Rodrigues S, Pinto GAS, Fernandes FAN. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason Sonochem. (2008) 15:95–100. 10.1016/j.ultsonch.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 201.D'Alessandro LG, Dimitrov K, Vauchel P, Nikov I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem Eng Res Des. (2014) 92:1818–26. 10.1016/j.cherd.2013.11.020 [DOI] [Google Scholar]
- 202.Fernandez-Aulis F, Hernandez-Vazquez L, Aguilar-Osorio G, Arrieta-Baez D, Navarro-Ocana A. Extraction and identification of anthocyanins in corn cob and corn husk from Cacahuacintle maize. J Food Sci. (2019) 84:954–62. 10.1111/1750-3841.14589 [DOI] [PubMed] [Google Scholar]
- 203.Wu CY, Wang H, Fan XH, Yue W, Wu QN. Waste Euryale ferox salisb. leaves as a potential source of anthocyanins: extraction optimization, identification and antioxidant activities evaluation. Waste Biomass Valoriz. (2019) 46 10.1007/s12649-019-00762-2 [DOI] [Google Scholar]
- 204.Li S, Yang Y, Li J, Zhu Z, Lorenzo JM, Barba FJ. Increasing yield and antioxidative performance of Litchi pericarp procyanidins in baked food by ultrasound-assisted extraction coupled with enzymatic treatment. Molecules. (2018) 23:1–13. 10.3390/molecules23092089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou G, Ma J, Tang Y, Wang X, Zhang J, Duan JA. Multi-response optimization of ultrasonic assisted enzymatic extraction followed by macroporous resin purification for maximal recovery of flavonoids and ginkgolides from waste Ginkgo biloba fallen leaves. Molecules. (2018) 23:1029. 10.3390/molecules23051029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huang D, Zhou X, Si J, Gong X, Wang S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem Cent J. (2016) 10:1–9. 10.1186/s13065-016-0202-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Punzi R, Paradiso A, Fasciano C, Trani A, Faccia M, De Pinto MC, et al. Phenols and antioxidant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke byproducts. Nat Prod Commun. (2014) 9:1315–8. 10.1177/1934578X1400900924 [DOI] [PubMed] [Google Scholar]
- 208.Rabelo RS, MacHado MTC, Martínez J, Hubinger MD. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J Food Eng. (2016) 178:170–80. 10.1016/j.jfoodeng.2016.01.018 [DOI] [Google Scholar]
- 209.Gonzales GB, Smagghe G, Raes K, Van Camp J. Combined alkaline hydrolysis and ultrasound-assisted extraction for the release of nonextractable phenolics from cauliflower (Brassica oleracea var. botrytis) waste. J Agric Food Chem. (2014) 62:3371–6. 10.1021/jf500835q [DOI] [PubMed] [Google Scholar]
- 210.BanoŽić M, Banjari I, Jakovljević M, Šubarić D, Tomas S, Babić J, et al. Optimization of ultrasound-assisted extraction of some bioactive compounds from tobacco waste. Molecules. (2019) 24:1611. 10.3390/molecules24081611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dubie J, Stancik A, Morra M, Nindo C. Antioxidant extraction from mustard (Brassica juncea) seed meal using high-intensity ultrasound. J Food Sci. (2013) 78:542–8. 10.1111/1750-3841.12085 [DOI] [PubMed] [Google Scholar]
- 212.Ferreira SS, Passos CP, Cardoso SM, Wessel DF, Coimbra MA. Microwave assisted dehydration of broccoli byproducts and simultaneous extraction of bioactive compounds. Food Chem. (2018) 246:386–93. 10.1016/j.foodchem.2017.11.053 [DOI] [PubMed] [Google Scholar]
- 213.Périno-Issartier S, Zill-e-Huma, Abert-Vian M, Chemat F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food byproducts. Food Bioprocess Technol. (2011) 4:1020–8. 10.1007/s11947-010-0438-x [DOI] [Google Scholar]
- 214.Paes J, Dotta R, Barbero GF, Martínez J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J Supercrit Fluids. (2014) 95:8–16. 10.1016/j.supflu.2014.07.025 [DOI] [Google Scholar]
- 215.Cao J, Chen L, Li M, Cao F, Zhao L, Su E. Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J Pharm Biomed Anal. (2018) 158:317–26. 10.1016/j.jpba.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 216.Karageorgou I, Grigorakis S, Lalas S, Makris DP. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur Food Res Technol. (2017) 243:1839–48. 10.1007/s00217-017-2887-1 [DOI] [Google Scholar]
- 217.Chen J, Jiang X, Yang G, Bi Y, Liu W. Green and efficient extraction of resveratrol from peanut roots using deep eutectic solvents. J Chem. (2018) 2018:1–9. 10.1155/2018/4091930 [DOI] [Google Scholar]
- 218.Pavić V, Flačer D, Jakovljević M, Molnar M, Jokić S. Assessment of total phenolic content, in vitro antioxidant and antibacterial activity of Ruta graveolens L. extracts obtained by choline chloride based natural deep eutectic solvents. Plants. (2019) 8:1–69. 10.3390/plants8030069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pal CBT, Jadeja GC. Microwave-assisted extraction for recovery of polyphenolic antioxidants from ripe mango (Mangifera indica L.) peel using lactic acid/sodium acetate deep eutectic mixtures. Food Sci Technol Int. (2019) 26:78–92. 10.1177/1082013219870010 [DOI] [PubMed] [Google Scholar]
- 220.Naviglio D. Naviglio's principle and presentation of an innovative solid-liquid extraction technology: Extractor Naviglio®. Anal Lett. (2003) 36:1647–59. 10.1081/AL-120021555 [DOI] [Google Scholar]
- 221.Posadino AM, Biosa G, Zayed H, Abou-Saleh H, Cossu A, Nasrallah GK, et al. Protective effect of cyclically pressurized solid–liquid extraction polyphenols from Cagnulari grape pomace on oxidative endothelial cell death. Molecules. (2018) 23:1–12. 10.3390/molecules23092105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sánchez-Gómez R, Zalacain A, Alonso GL, Salinas MR. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J Agric Food Chem. (2014) 62:10861–72. 10.1021/jf503929v [DOI] [PubMed] [Google Scholar]
- 223.Sánchez-Gómez R, Sánchez-Vioque R, Santana-Méridas O, Martín-Bejerano M, Alonso GL, Salinas MR, et al. A potential use of vine-shoot wastes: the antioxidant, antifeedant and phytotoxic activities of their aqueous extracts. Ind Crops Prod. (2017) 97:120–7. 10.1016/j.indcrop.2016.12.009 [DOI] [Google Scholar]
- 224.Gallo M, Formato A, Giacco R, Riccardi G, Lungo D, Formato G, et al. Mathematical optimization of the green extraction of polyphenols from grape peels through a cyclic pressurization process. Heliyon. (2019) 5:e01526 10.1016/j.heliyon.2019.e01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Soliva-Fortuny R, Balasa A, Knorr D, Martín-Belloso O. Effects of pulsed electric fields on bioactive compounds in foods: a review. Trends Food Sci Technol. (2009) 20:544–56. 10.1016/j.tifs.2009.07.003 [DOI] [Google Scholar]
- 226.Barba FJ, Parniakov O, Pereira SA, Wiktor A, Grimi N, Boussetta N, et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res Int. (2015) 77:773–98. 10.1016/j.foodres.2015.09.015 [DOI] [Google Scholar]
- 227.Lohani UC, Muthukumarappan K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov Food Sci Emerg Technol. (2016) 35:29–35. 10.1016/j.ifset.2016.03.012 [DOI] [Google Scholar]
- 228.Teh SS, Niven BE, Bekhit AEDA, Carne A, Birch EJ. Microwave and pulsed electric field assisted extractions of polyphenols from defatted canola seed cake. Int J Food Sci Technol. (2015) 50:1109–15. 10.1111/ijfs.12749 [DOI] [Google Scholar]
- 229.Barbosa-Pereira L, Guglielmetti A, Zeppa G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. (2018) 11:818–35. 10.1007/s11947-017-2045-6 [DOI] [Google Scholar]
- 230.Bouras M, Grimi N, Bals O, Vorobiev E. Impact of pulsed electric fields on polyphenols extraction from Norway spruce bark. Ind Crops Prod. (2016) 80:50–8. 10.1016/j.indcrop.2015.10.051 [DOI] [Google Scholar]
- 231.Bobinaite R, Pataro G, Lamanauskas N, Šatkauskas S, Viškelis P, Ferrari G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their byproducts. J Food Sci Technol. (2015) 52:5898–905. 10.1007/s13197-014-1668-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yu X, Bals O, Grimi N, Vorobiev E. A new way for the oil plant biomass valorization: polyphenols and proteins extraction from rapeseed stems and leaves assisted by pulsed electric fields. Ind Crops Prod. (2015) 74:309–18. 10.1016/j.indcrop.2015.03.045 [DOI] [Google Scholar]
- 233.Zderic A, Zondervan E, Meuldijk J. Breakage of cellular tissue by pulsed electric field: extraction of polyphenols from fresh tea leaves. Chem Eng Trans. (2013) 32:1795–800. 10.3303/CET1332300 [DOI] [Google Scholar]
- 234.Segovia FJ, Luengo E, Corral-Pérez JJ, Raso J, Almajano MP. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: pulsed electric fields (PEF) applications. Ind Crops Prod. (2015) 65:390–6. 10.1016/j.indcrop.2014.11.010 [DOI] [Google Scholar]
- 235.Boussetta N, Soichi E, Lanoisellé JL, Vorobiev E. Valorization of oilseed residues: extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind Crops Prod. (2014) 52:347–53. 10.1016/j.indcrop.2013.10.048 [DOI] [Google Scholar]
- 236.Luengo E, Álvarez I, Raso J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov Food Sci Emerg Technol. (2013) 17:79–84. 10.1016/j.ifset.2012.10.005 [DOI] [Google Scholar]
- 237.Parniakov O, Barba FJ, Grimi N, Lebovka N, Vorobiev E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res Int. (2014) 65:337–43. 10.1016/j.foodres.2014.09.015 [DOI] [Google Scholar]
- 238.Parniakov O, Barba FJ, Grimi N, Lebovka N, Vorobiev E. Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem. (2016) 192:842–8. 10.1016/j.foodchem.2015.07.096 [DOI] [PubMed] [Google Scholar]
- 239.Wang K, Chen J, Sun SN, Sun RC. Steam explosion. In: Pandey A, Negi S, Binod P, Larroche C, editors. Pretreatment of Biomass: Processes and Technologies. Amsterdam: Elsevier B.V; (2015). p. 75–104. 10.1016/B978-0-12-800080-9.00006-2 [DOI] [Google Scholar]
- 240.Fu X, Chen H. Air-steam explosion enhancing the extraction efficiency of chlorogenic acid from leaves of Eucommia ulmoides Oliver. Sep Purif Technol. (2015) 146:317–25. 10.1016/j.seppur.2015.03.054 [DOI] [Google Scholar]
- 241.Juttuporn W, Thiengkaew P, Rodklongtan A, Rodprapakorn M, Chitprasert P. Ultrasound-assisted extraction of antioxidant and antibacterial phenolic compounds from steam-exploded sugarcane bagasse. Sugar Tech. (2018) 20:599–608. 10.1007/s12355-017-0582-y [DOI] [Google Scholar]
- 242.Liu L, Zhao M, Liu X, Zhong K, Tong L, Zhou X, et al. Effect of steam explosion-assisted extraction on phenolic acid profiles and antioxidant properties of wheat bran. J Sci Food Agric. (2016) 96:3484–91. 10.1002/jsfa.7532 [DOI] [PubMed] [Google Scholar]
- 243.Fernández-Bolaños J, Felizón B, Brenes M, Guillén R, Heredia A. Hydroxytyrosol and tyrosol as the main compounds found in the phenolic fraction of steam-exploded olive stones. J Am Oil Chem Soc. (1998) 75:1643–49. 10.1007/s11746-998-0106-8 [DOI] [Google Scholar]
- 244.Rincón B, Rodríguez-Gutiérrez G, Bujalance L, Fernández-Bolaños J, Borja R. Influence of a steam-explosion pre-treatment on the methane yield and kinetics of anaerobic digestion of two-phase olive mil solid waste or alperujo. Process Saf Environ Prot. (2016) 102:361–9. 10.1016/j.psep.2016.04.010 [DOI] [Google Scholar]