Abstract
Objective
Stapes surgery is technically challenging, yet its methodology is not standardized. We aim to elucidate preferences in stapes surgery among American Otological Society (AOS) otologists and determine if any common practice patterns exist.
Study design
Cross-sectional study via emailed questionnaire.
Setting
Surgery centers.
Subjects and methods
Members of the AOS were an emailed a survey to quantify variables including surgical volume, anesthetic preference, laser use, type of procedure, footplate sealing technique, antibiotic use, and trainee participation.
Results
Most otologists (71%) performed 2 to 5 stapes surgeries per month under general anesthesia (69%) with stapedotomy (71%) as the preferred procedure. Most (56%) used the rosette method of laser stapedotomy with manual pick debris removal for footplate fenestration. Either the handheld potassium titanyl phosphate (KTP) laser (40%) or handheld carbon dioxide (CO2) laser (33%) was used. The heat-activated memory hook (51%) was the preferred prosthesis. Footplate sealing method was variable, as was antibiotic use among respondents. Trainee participation was limited, as 42% of otologists allowed residents to place the prosthesis, and fewer allowed residents to crimp the prosthesis, and laser or drill the footplate. Surgeons with higher surgical volume (≥ 6 surgeries per month) demonstrated the following statistically significant correlations: footplate fenestration with laser in a rosette pattern and pick for debris removal (rs = −0.365, P = 0.014) and trainee participation with fellows only (rs = 0.341, P = 0.022).
Conclusions
Trends in various surgical decisions showed a lack of consensus in all aspects of stapes surgery.
Keywords: Footplate, Resident training, Surgical training, Laser, Stapes surgery, Stapedotomy, Surgical preference, Otosclerosis, Stapedectomy
Introduction
Surgery of the stapes ranks among one of the most technically demanding of otologic surgeries. Since its inception by Kessel in 1876 and its revival and modernization by Shea in the mid-20th century, it quickly became the standard surgical procedure for the treatment of otosclerosis.1,2 Modern stapes surgery results in closure of the air-bone gaps to less than 10 dB (dB) in over 90% of patients.3 There was a dramatic increase in stapes procedures being performed in the 1960s, followed by a relative plateau and relative decrease in case volume.4 This decrease in case volume has subsequently resulted in fewer opportunities for resident and fellow training.5
Surgical practice is largely based on the apprenticeship model. There are aspects of many, perhaps most, surgical procedures that over time have proven to be fundamental and, thus, have been passed down by mentors and widely adopted. Even these core concepts, however, can evolve as new technologies and better understanding of disease processes develop. In addition, regional differences in core concepts – micro-evolutionary changes – exist. To stay current, systematic reviews and meta-analyses have been used to provide insight into best surgical practice. However, expert opinion is another valuable source of information that can inform both conceptual and procedural surgical knowledge.
Stapes surgery has varied in its technique throughout the history of the procedure, and has evolved over the years to encompass a variety of approaches and reconstructive materials. There is yet no standard protocol for how the procedure should be performed. Variations in techniques include, but are not limited to stapedotomy versus stapedectomy, use of laser versus drill, type of laser used (e.g. carbon-dioxide (CO2), potassium-titanyl-phosphate (KTP), or argon),6, 7, 8, 9, 10, 11 and type of prosthesis used (e.g. piston or bucket-handle).12, 13, 14, 15, 16, 17, 18 Resident and fellow participation in these surgical cases is also variable among institutions and surgeons, depending on the confidence a surgeon has in the apprentice. It has been shown that surgeons with greater experience and case volume in stapes surgery have superior outcomes.19
Due to the tremendous variations in methodology and a lack of standardization, stapes surgery is mainly practiced through individual knowledge and experience. The American Otological Society (AOS) was created in 1868 to promote education and evolution of the field of otology, and is comprised of a membership of elite otologists. This project aims to gain insight and determine if common practice patterns exist among a group of experienced otologic surgeons who perform stapes surgery routinely.
Material and methods
This study did not involve patient data and was deemed to be exempt from institutional review board approval. An online survey consisting of 10 multiple choice questions was sent by email to all AOS members (Appendix). The questions covered the following stapes surgery topics: experience in stapes surgery, anesthesia preference, anatomical approach to the stapes, performance of stapedotomy versus stapedectomy, footplate fenestration technique, type of laser used, type of prosthesis used, material used for sealing the footplate fenestration, perioperative antibiotic use, and degree of resident and/or fellow involvement.
Biostatistics
All analyses and graphs were performed with SPSS 24.0 (SPSS Inc., IBM Corp., Armonk, NY), SigmaPlot 12.5 (Systat Software, Inc., San Jose, CA), and MedCalc 18.2.1 (Ostend, Belgium). Continuous variables were summarized by mean ± SD and range where appropriate. Nominal variables were summarized by frequency and percentage. A Spearman rank correlation model was used to determine associations among variables (number of surgical volume and type of surgical preference). For the purposes of the Spearman rank correlation, surgical volume was stratified into three categories by the number of procedures performed per month: 1 or less (lower volume), 2 to 5, and 6 or more (higher volume). The correlation coefficient (rs value; range, −1.0 to 1.0) is a value that determines the relationship between two variables. Positive values that approach 1.0 signify a positive correlation, and as values approach −1.0, the variables have an inverse correlation. A value of 0 signifies that the two variables are not related. A P value ≤ 0.05 was considered indicative of statistical significance.
Results
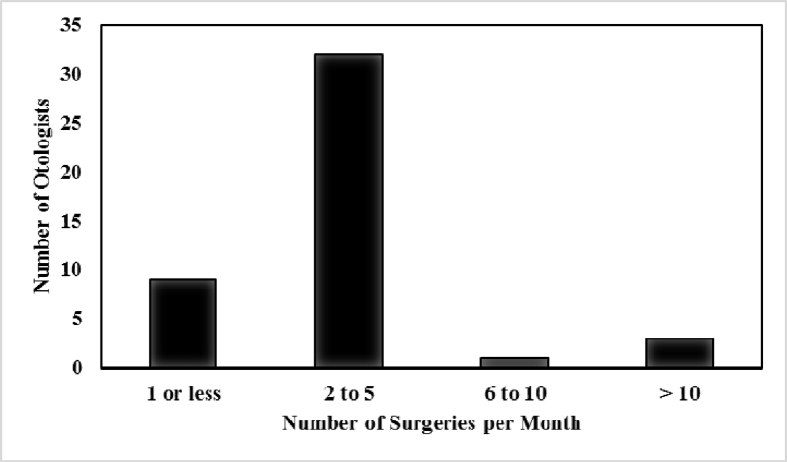
Of the 270 AOS members, 45 (16.7%) responded to the survey. Most respondents (32, 71%) performed 2 to 5 stapes surgeries a month, 9 (20%) performed 1 or fewer per month, 3 (6.7%) performed >10 per month, and 1 (2.2%) performed 6 to 10 per month (Fig. 1). Most (31, 69%) surgeons preferred to perform stapes surgery under general anesthesia, while 6 preferred (13%) local anesthesia, and 8 (18%) used either. The transcanal approach was the predominant approach (41, 91%), while 5 (11%) used an endaural approach, and no-one used a post-auricular approach.
Fig. 1.
Distribution of stapes surgical volume. Four respondents performed 6 or more surgeries per month (Total n = 45).
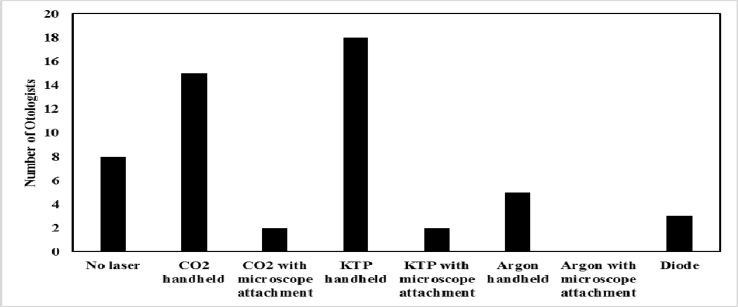
Most surgeons performed stapedotomy (32, 71.1%) instead of stapedectomy (9, 20%), while 4 (8.9%) performed either procedure. The majority (25, 56%) used the rosette method of laser stapedotomy along with manual removal of debris with a pick, while others were distributed among laser in rosette pattern alone (3, 6.7%), laser in rosette pattern along with drill (4, 8.9%), drill alone (4, 8.9%), one-shot laser then manual removal of debris (1, 2.2%), and other methods (8, 18%). Other methods included use of graduated perforators, fenestration picks, other micro-instruments, and removal of half of the stapes footplate. Most surgeons used the handheld KTP laser (18, 40%) or CO2 laser (15, 33%). Others used the handheld argon (Ar) laser (5, 11%), diode laser (3, 6.7%), KTP laser with microscope attachment (2, 4.4%), CO2 laser with microscope attachment (2, 4.4%), or no laser at all (8, 18%) (Fig. 2).
Fig. 2.
Distribution of laser utilization in stapes surgery. KTP handheld laser: power of 2 W; CO2 handheld laser: power of 3 W; Argon laser: power of 1.4 W; Diode laser: power of 2 W.
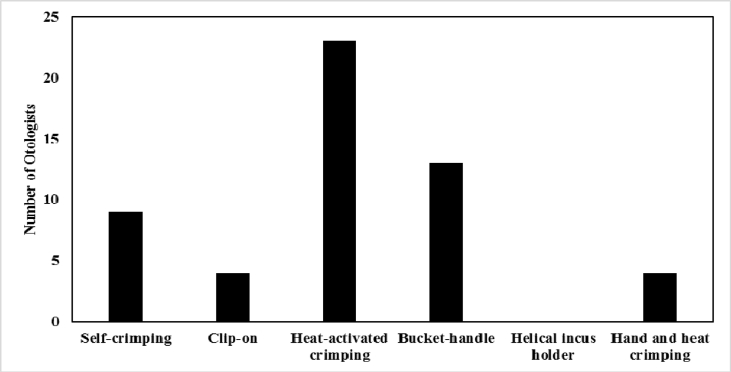
Twenty-three (51%) surgeons preferred the heat-activated memory hook stapes prosthesis, compared to 14 (31%) who preferred the bucket-handle incus prosthesis, 9 (20%) who preferred the self-crimping incus hook, and 4 (8.9%) who preferred the clip-on prosthesis. One respondent used the De La Cruz prosthesis which can be hand or heat crimped (Fig. 3). Sealing of the footplate was performed with blood most often (15, 33%), followed by fat (11, 24%), fascia (9, 20%), nothing (6, 13%), vein (4, 9%), and tragal perichondrium (4, 9%). Fifteen (33%) surgeons used pre-operative antibiotics only, 2 (4%) used post-operative antibiotics only, and 14 (31%) used both. Fourteen (31%) used neither pre nor post-operative antibiotics.
Fig. 3.
Distribution of prosthetic preference in stapes surgery. One respondent used the De La Cruz piston which can be hand or heat crimped.
In terms of resident participation, 19 (42%) otologists would allow residents to place the prosthesis, 13 (29%) would allow residents to crimp the prosthesis, and 12 (27%) would allow residents to laser or drill the footplate. However, 6 (13%) otologists specified that amount of resident participation ultimately depended on resident skill level. Twelve (27%) otologists would only allow fellows to perform the above, while 9 (20%) would only allow residents interested in otology to perform the same. Seven (16%) surgeons did not have residents available.
When stratified by surgical volume (Table 1), lower volume surgeons (one or less surgeries per month) demonstrated the following statistically significant correlations: stapedectomy (rs = 0.294, P = 0.05), fascial footplate sealant (rs = 0.362, P = 0.015), resident participation in laser/drilling of footplate (rs = 0.349, P = 0.019), fellow only participation (rs = −0.302, P = 0.044). Surgeons that performed 2 to 5 procedures per month had a statistically significant preference for only residents interested in otology to participate (rs = 0.341, P = 0.022). Higher surgical volume (≥ 6 surgeries per month) correlated significantly with the following variables: footplate fenestration with laser in a rosette pattern and pick for debris removal (rs = −0.365, P = 0.014) and trainee participation with fellows only (rs = 0.341, P = 0.022). No significant independent predictors were found to be significant using a backwards logistic regression model due to limited sample size.
Table 1.
Spearman rank correlation of preferences stratified by surgical volume.
|
Preference |
Correlation coefficient by surgeries per month |
|||||
|---|---|---|---|---|---|---|
| <1 (n = 9) |
2 to 5 (n = 32) |
≥6 (n = 4) |
||||
| rs | P | rs | P | rs | P | |
| Anesthesia | ||||||
| Local | 0.000 | 1.000 | −0.069 | 0.651 | 0.110 | 0.470 |
| General | 0.061 | 0.689 | −0.138 | 0.365 | 0.134 | 0.380 |
| Either | 0.058 | 0.704 | −0.217 | 0.153 | 0.263 | 0.081 |
| Approach | ||||||
| Transcanal | −0.039 | 0.799 | 0.145 | 0.340 | −0.177 | 0.245 |
| Post-Auricular | – | – | – | – | – | – |
| Endaural | 0.000 | 1.000 | −0.087 | 0.571 | 0.138 | 0.366 |
| Other | – | – | – | – | – | – |
| Type of Surgery | ||||||
| Stapedotomy | −0.167 | 0.274 | 0.172 | 0.260 | −0.039 | 0.799 |
| Stapedectomy | 0.294 | 0.050 | −0.243 | 0.108 | −0.027 | 0.861 |
| Either | 0.234 | 0.121 | −0.145 | 0.340 | −0.098 | 0.524 |
| Footplate Fenestration | ||||||
| Laser in Rosette | 0.089 | 0.561 | −0.223 | 0.141 | 0.230 | 0.129 |
| Laser in Rosette then Drill | −0.156 | 0.306 | 0.027 | 0.861 | 0.177 | 0.245 |
| Laser in Rosette then Pick | 0.090 | 0.557 | 0.150 | 0.325 | −0.365 | 0.014 |
| One-Shot Laser | – | – | – | – | – | – |
| One-Shot Laser then Drill | – | – | – | – | – | – |
| One-Shot Laser then Pick | – | – | – | – | – | – |
| Drill Only | 0.039 | 0.799 | 0.027 | 0.861 | −0.098 | 0.524 |
| Other | −0.087 | 0.569 | −0.088 | 0.564 | 0.263 | 0.081 |
| Laser Use | ||||||
| CO2 Handheld | −0.118 | 0.441 | 0.139 | 0.364 | −0.055 | 0.719 |
| CO2 w/Microscope | −0.108 | 0.481 | 0.137 | 0.368 | −0.067 | 0.660 |
| KTP Handheld | 0.224 | 0.140 | −0.121 | 0.430 | −0.122 | 0.424 |
| KTP w/Microscope | 0.162 | 0.288 | −0.100 | 0.511 | −0.067 | 0.660 |
| Ar Handheld | −0.156 | 0.306 | 0.199 | 0.190 | −0.098 | 0.524 |
| Ar w/Microscope | – | – | – | – | – | – |
| None | −0.087 | 0.569 | −0.088 | 0.564 | 0.263 | 0.081 |
| Incus Prosthesis | ||||||
| Crimp-able Hook | −0.111 | 0.467 | −0.049 | 0.749 | 0.234 | 0.121 |
| Clip-on Hook | 0.162 | 0.288 | −0.100 | 0.511 | −0.067 | 0.660 |
| Heat-Activated Memory Hook |
−0.067 | 0.663 | 0.161 | 0.290 | −0.163 | 0.284 |
| Bucket-Handle | 0.172 | 0.260 | −0.135 | 0.378 | −0.027 | 0.861 |
| Helical | – | – | – | – | – | – |
| Other | −0.156 | 0.306 | 0.199 | 0.190 | −0.098 | 0.524 |
| Footplate Sealant | ||||||
| Blood | 0.024 | 0.876 | 0.111 | 0.469 | −0.210 | 0.166 |
| Fascia | 0.362 | 0.015 | −0.208 | 0.171 | −0.178 | 0.243 |
| Fat | −0.111 | 0.467 | −0.049 | 0.749 | 0.234 | 0.121 |
| Other | −0.111 | 0.467 | 0.074 | 0.631 | 0.039 | 0.799 |
| None | −0.033 | 0.831 | −0.038 | 0.802 | 0.107 | 0.483 |
| Perioperative Antibiotics | ||||||
| Pre-op | 0.255 | 0.090 | −0.166 | 0.275 | −0.094 | 0.538 |
| Post-op | 0.093 | 0.544 | −0.244 | 0.107 | 0.257 | 0.088 |
| Pre- and Post-Op | 0.144 | 0.345 | −0.207 | 0.172 | 0.127 | 0.404 |
| None | −0.216 | 0.154 | 0.217 | 0.153 | −0.041 | 0.788 |
| Teaching Participation | ||||||
| Residents Laser/Drill Footplate | 0.349 | 0.019 | −0.217 | 0.153 | −0.145 | 0.341 |
| Residents Place Prosthesis | 0.144 | 0.345 | −0.101 | 0.508 | −0.041 | 0.788 |
| Residents Crimp Prosthesis | 0.203 | 0.180 | −0.217 | 0.153 | 0.059 | 0.700 |
| Residents with Otology Interest | −0.267 | 0.076 | 0.341 | 0.022 | −0.167 | 0.273 |
| Fellows Only | −0.302 | 0.044 | 0.052 | 0.736 | 0.341 | 0.022 |
| Other | 0.144 | 0.345 | 0.005 | 0.976 | −0.210 | 0.166 |
Values achieving statistical significance (P < 0.05) denoted in bold.
Discussion
Valsalva was the first to describe otosclerosis due to the fixation of the stapes as a cause of hearing loss in 1704.20 Since then, the management of otosclerosis has evolved from fenestration to mobilization to its current state with stapedectomy and stapedotomy procedures. Shea created the Teflon prosthesis for stapedectomy with successful outcomes and had the support of Howard House, making stapedectomy the mainstay procedure for the otosclerotic stapes in the 1960s.2 However, the procedure has evolved to become more minimally invasive, with more emphasis on stapedotomy. Nevertheless, there is a constant evolution of stapes surgery without consensus on a single superior method.
The literature revealed only one country which has studied its practice patterns in stapes surgery on a nation-wide level. Lancer et al21 performed a survey of 184 British otologists to investigate practices in the United Kingdom. Similar to the United States, there is no standardized guidelines for performing stapes surgery in the UK. In their study, most surgeons (66%) performed fewer than 26 stapes operation per year, or fewer than approximately 2 per month. In our study, 71% performed 2 to 5 operations a month, which may be slightly more than otologists in the UK. Rate of local or general anesthesia preference is similar in the UK study compared to our study. 70% of UK otologists offered training to residents with interest in otology only, and only 19% offered it to all residents, compared to our study population where 47% reserved training for either residents with interest in otology or fellows.
Outcomes by experience
As a technically demanding surgery, there is an associated learning curve with stapes surgery. Hughes demonstrated successful closure of the air-bone gap after approximately 50 surgeries, as he defined to be less than 10 dB in more than 90% of his patients.3 It is known that surgeons with higher volumes produce better outcomes. Respondents in our survey mostly performed 2 to 5 surgeries per month (32, 71%), and most performed more than 2 surgeries per month (36, 80%). Performing more operations allows the surgeon to develop more consistent methods and techniques to achieve success.
Anesthesia preferences
Wegener et al22 performed a systematic review comparing the outcomes of local versus general anesthesia and demonstrated no statistically significant difference in the post-operative air-bone gap. In our study, most (31, 69%) surgeons preferred to perform stapes surgery under general anesthesia, which is consistent with literature results. Otologists in our study did not demonstrate any statistically significant preferences in choice of local or general anesthesia for stapes surgery. This non-significant result suggests that even higher volume surgeons who are known to have superior outcomes did not have a preference in type of anesthesia use, indicating that the choice of local or general anesthesia may be performed on a case by case basis.
Use of lasers
Various lasers have been used for stapes surgery, including the CO2 laser, KTP laser, argon laser, and others. Laser stapedectomy has been shown to have a significantly better odds ratio for air-bone gap closure as compared to non-laser techniques.23 Use of lasers is becoming more common with advances in surgical technology and instrumentation. The majority of otologists in our study who preferred lasers used the handheld KTP (18, 40%) or handheld CO2 laser (15, 33%). The CO2 laser may produce slightly better hearing outcomes, but the clinical relevance of this is currently unclear.8 In our study, there was no significant correlations relating laser preference to surgical volume.
Stapedotomy versus stapedectomy
Stapedotomy involves fenestration of the footplate versus the stapedectomy procedure which involves removal of a portion of the stapes footplate. A review by Cheng et al24 in 2018 suggested that stapedotomy led to better long term hearing results and lower risk of complications compared to stapedectomy. More surgeons are favoring minimally invasive procedures that can be performed as same-day cases. Based on our results, the majority of otologists preferred stapedotomy (32, 71.1%) as compared to stapedectomy (9, 20%), while 4 (8.9%) performed either procedure. This aligns with the current trends and preferences in the UK.21 Interestingly, lower volume surgeons (1 or less surgeries per month) in our study had a positive correlation with choice of the stapedectomy procedure (rs = 0.294, P = 0.05). This result may be dependent on preferences in prior surgical training for these surgeons and conclusions are limited by sample size.
Types of prostheses
There is controversy over the optimal stapes prosthesis. A brief review by Ruckenstein and Nicolli25 did not demonstrate one superior prosthesis. However, a recent meta-analysis by Reis et al26 showed superior hearing outcomes with the nitinol prosthesis versus non-nitinol prosthesis, but only with data available for the short and middle term (<3 years). In our study, most otologists used the heat-activated memory hook prosthesis (23, 51%), supporting the popularity and effectiveness of the nitinol prosthesis. There were no significant correlations demonstrated in our study relating types of prosthesis with regards to surgical volume.
Footplate sealing
There have been various types of footplate sealing materials such as blood, fat, fascia, vein, and tragal perichondrium. Wiet et al27 demonstrated no difference among use of fat, fascia, or vein graft for footplate sealing. Another study by Schmerber et al28 suggested that vein should be used over tragal perichondrium, but there were no significant differences in average pure-tone audiometry. Perkins and Curto29 favored use of vein graft seal over blood seal, but there was no significance in the overall pure-tone audiometry of these patients as well. In our study, otologists performing one or less surgeries per month demonstrated a significant correlation with use of fascia as a sealant (rs = 0.362, P = 0.015). This interesting result should be cautiously interpreted, as there was a limited sample size in this group relative to the survey population. Overall, the use of blood, fat, fascia, vein, and tragal perichondrium was variable in our study, demonstrating a lack of consensus.
Antibiotic use
There is a limited body of evidence directly addressing use of antibiotics in stapes surgery, but a study by Govaerts et al30 demonstrated that stapedotomy operations did not benefit from antibiotic prophylaxis. Recent high-level evidence shows that clean otologic surgery does not require perioperative antibiotic use.31 However, clean-contaminated or contaminated cases should involve intraoperative antibiotics. Intraoperative and postoperative antibiotics were recommended for clean-contaminated skull base cases.31 Since the inner ear is closely related to the intradural space, it is not obvious whether postoperative antibiotics may be beneficial for patients undergoing stapes surgery. Respondents in our study had variable antibiotic use, warranting further investigation of optimal antibiotic use in stapes surgery.
Trainee participation
The American Council of Graduate Medical Education requires residents training in otolaryngology to complete 10 stapedectomy/ossiculoplasty operations during the course of their training.32 However, there is a learning curve associated with stapes operations, and achieving proficiency after 10 cases is simply not realistic. Otologists in our study had variable associations with regards to trainee participation, but the majority of respondents (2–5 surgeries per month) had a statistically significant correlation with a preference for only residents interested in otology to participate in stapes surgery (rs = 0.341, P = 0.022). However, higher volume surgeons (≥ 6 surgeries per month) preferred fellows only to participate (rs = 0.341, P = 0.022). It may be possible that these higher surgeons are more comfortable with fellows as there may be more fellows at these institutions. The use of the mentorship model may be inherently variable among the surveyed otologists and at their respective institutions, contributing to these heterogenous associations.
A recent survey demonstrated that only 25% of residents may plan on performing stapedectomy procedures in their future practice, and the majority of these future otolaryngologists will never perform stapes surgery.33 With the decreasing overall incidence of otosclerosis and opportunities for training of a technically demanding surgery, institutions perhaps should encourage preferential training of residents interested in otology as a subspecialty. Advances in educational tools such as the temporal bone lab and virtual surgical simulation should not be overlooked as possible avenues to encourage the interest of otolaryngology residents to perform stapes surgery and potentially pursue additional otology and neurotology fellowship training.33, 34, 35
Limitations and comments
Limitations to our study include the survey methodology, as there is reporter bias. Also, the response rate to the survey was limited by guidelines allowing for only one email to be sent to AOS members. The overall sample size is not wholly representative of all practicing otologists in the United States and was not adequate on logistic regression analysis to determine independent predictors of preferences.
Howard P. House, who spent most of his life performing stapes surgery, once said that all surgeons should regularly evaluate their surgical outcomes to see if they are operating well; but, if the outcomes are good, then the technique doesn't matter. Although minute differences in outcomes have been shown with different techniques, perhaps whatever is most comfortable for a particular surgeon's hands is the best choice.
Conclusions
Trends in various surgical decisions showed a lack of consensus in all aspects of stapes surgery. Those who performed more surgeries appear to be flexible with the use of either local or general anesthesia, and appeared to disfavor the laser rosette footplate technique. The KTP handheld laser was the most popular laser, and the nitinol self-crimping prosthesis was the most popular prosthesis.
Conflicts of interest and funding
The following authors of this manuscript have no disclosures to report, financial or otherwise. Additionally, there are no conflicts of interest reported by all authors that contributed to the production of this manuscript. There was financial support for this original research project and manuscript. This project was deemed to be exempt from institutional review board as it does not contain any patient information.
Edited by Qiuyi Qu and Xin Jin
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wjorl.2019.12.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kessel J. Über die vordere Tenotomie, Mobilisierung und Extraction des Steigbügels; zitiert in Grunert C: Wissenschaftliche Rundschau. Arch Ohrenheilkd. 1897;42:57–58. [Google Scholar]
- 2.Shea J.J., Jr. A personal history of stapedectomy. Am J Otol. 1998;19:S2–S12. [PubMed] [Google Scholar]
- 3.Hughes G.B. The learning curve in stapes surgery. Laryngoscope. 1991;101:1280–1284. doi: 10.1002/lary.5541011205. [DOI] [PubMed] [Google Scholar]
- 4.Vrabec J.T., Coker N.J. Stapes surgery in the United States. Otol Neurotol. 2004;25:465–469. doi: 10.1097/00129492-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Ruckenstein M.J., Staab J.P. Who is performing stapedectomy surgery? Implications for residency and fellowship training. Laryngoscope. 2008;118:1224–1227. doi: 10.1097/MLG.0b013e31816e2ede. [DOI] [PubMed] [Google Scholar]
- 6.Beatty T.W., Haberkamp T.J., Khafagy Y.W., Bresemann J.A. Stapedectomy training with the carbon dioxide laser. Laryngoscope. 1997;107:1441–1444. doi: 10.1097/00005537-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Buchman C.A., Fucci M.J., Roberson J.B., Jr., De La Cruz A. Comparison of argon and CO2 laser stapedotomy in primary otosclerosis surgery. Am J Otolaryngol. 2000;21:227–230. doi: 10.1053/ajot.2000.8380. [DOI] [PubMed] [Google Scholar]
- 8.Kamalski D.M., Wegner I., Tange R.A. Outcomes of different laser types in laser-assisted stapedotomy: a systematic review. Otol Neurotol. 2014;35:1046–1051. doi: 10.1097/MAO.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 9.Sergi B., Lucidi D., De Corso E., Paludetti G. Long-term follow-up after"one-shot" CO(2) laser stapedotomy: is the functional outcome stable during the years? Eur Arch Otorhinolaryngol. 2016;273:3623–3629. doi: 10.1007/s00405-016-3976-7. [DOI] [PubMed] [Google Scholar]
- 10.Young E., Mitchell-Innes A., Jindal M. Lasers in stapes surgery: a review. J Laryngol Otol. 2015;129:627–633. doi: 10.1017/S0022215115001280. [DOI] [PubMed] [Google Scholar]
- 11.Vincent R., Bittermann A.J., Oates J., Sperling N., Grolman W. KTP versus CO2 laser fiber stapedotomy for primary otosclerosis: results of a new comparative series with the otology-neurotology database. Otol Neurotol. 2012;33:928–933. doi: 10.1097/MAO.0b013e31825f24ff. [DOI] [PubMed] [Google Scholar]
- 12.De Bruijn A.J., Tange R.A., Dreschler W.A. Comparison of stapes prostheses: a retrospective analysis of individual audiometric results obtained after stapedotomy by implantation of a gold and a teflon piston. Am J Otol. 1999;20:573–580. [PubMed] [Google Scholar]
- 13.Farrior J.B., Temple A.E. Teflon-wire piston or stainless-steel bucket stapes prosthesis: does it make a difference? Ear Nose Throat J. 1999;78(252–253):257–260. [PubMed] [Google Scholar]
- 14.Kwok P., Fisch U., Strutz J., May J. Stapes surgery: how precisely do different prostheses attach to the long process of the incus with different instruments and different surgeons? Otol Neurotol. 2002;23:289–295. doi: 10.1097/00129492-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Tange R.A., Grolman W., Dreschler W.A. Gold and titanium in the oval window: a comparison of two metal stapes prostheses. Otol Neurotol. 2004;25:102–105. doi: 10.1097/00129492-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Massey B.L., Kennedy R.J., Shelton C. Stapedectomy outcomes: titanium versus teflon wire prosthesis. Laryngoscope. 2005;115:249–252. doi: 10.1097/01.mlg.0000154727.85539.76. [DOI] [PubMed] [Google Scholar]
- 17.Laske R.D., Röösli C., Chatzimichalis M.V., Sim J.H., Huber A.M. The influence of prosthesis diameter in stapes surgery: a meta-analysis and systematic review of the literature. Otol Neurotol. 2011;32:520–528. doi: 10.1097/MAO.0b013e318216795b. [DOI] [PubMed] [Google Scholar]
- 18.Wegner I., Verhagen J.J., Stegeman I., Vincent R., Grolman W. A systematic review of the effect of piston diameter in stapes surgery for otosclerosis on hearing results. Laryngoscope. 2016;126:182–190. doi: 10.1002/lary.25408. [DOI] [PubMed] [Google Scholar]
- 19.Yung M.W., Oates J. The learning curve in stapes surgery and its implication for training. Adv Otorhinolaryngol. 2007;65:361–369. doi: 10.1159/000098861. [DOI] [PubMed] [Google Scholar]
- 20.Valsalva A.M. Pitteri; Venice, Italy: 1741. Pera, Hoc Est, Tractatus de Aure Humana. [Google Scholar]
- 21.Lancer H., Manickavasagam J., Zaman A., Lancer J. Stapes surgery: a national survey of British otologists. Eur Arch Otorhinolaryngol. 2016;273:371–379. doi: 10.1007/s00405-015-3560-6. [DOI] [PubMed] [Google Scholar]
- 22.Wegner I., Bittermann A.J., Zinsmeester M.M., van der Heijden G.J., Grolman W. Local versus general anesthesia in stapes surgery for otosclerosis: a systematic review of the evidence. Otolaryngol Head Neck Surg. 2013;149:360–365. doi: 10.1177/0194599813493393. [DOI] [PubMed] [Google Scholar]
- 23.Fang L., Lin H., Zhang T.Y., Tan J. Laser versus non-laser stapedotomy in otosclerosis: a systematic review and meta-analysis. Auris Nasus Larynx. 2014;41:337–342. doi: 10.1016/j.anl.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Vasama J.P., Kujala J., Hirvonen T.P. Is small-fenestra stapedotomy a safer outpatient procedure than total stapedectomy? ORL J Otorhinolaryngol Relat Spec. 2006;68:99–102. doi: 10.1159/000091211. [DOI] [PubMed] [Google Scholar]
- 25.Ruckenstein M.J., Nicolli E.A. Is there a “best” stapes prosthesis? Laryngoscope. 2012;122:2123–2124. doi: 10.1002/lary.23353. [DOI] [PubMed] [Google Scholar]
- 26.Reis L.R., Donato M., Almeida G., Castelhano L., Escada P. Nitinol versus non-Nitinol prostheses in otosclerosis surgery: a meta-analysis. Acta Otorhinolaryngol Ital. 2018;38:279–285. doi: 10.14639/0392-100X-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiet R.J., Battista R.A., Wiet R.M., Sabin A.T. Hearing outcomes in stapes surgery: a comparison of fat, fascia, and vein tissue seals. Otolaryngol Head Neck Surg. 2013;148:115–120. doi: 10.1177/0194599812463184. [DOI] [PubMed] [Google Scholar]
- 28.Schmerber S., Cuisnier O., Charachon R., Lavieille J.-P. Vein versus tragal perichondrium in stapedotomy. Otol Neurotol. 2004;25:694–698. doi: 10.1097/00129492-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Perkins R., Curto F.S. Laser stapedotomy: a comparative study of prostheses and seals. Laryngoscope. 1992;102:1321–1327. doi: 10.1288/00005537-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Govaerts P.J., Raemaekers J., Verlinden A., Kalai M., Somers T., Offeciers F.E. Use of antibiotic prophylaxis in ear surgery. Laryngoscope. 1998;108:107–110. doi: 10.1097/00005537-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Patel P.N., Jayawardena A.D.L., Walden R.L., Penn E.B., Francis D.O. Evidence-based use of perioperative antibiotics in otolaryngology. Otolaryngol Head Neck Surg. 2018;158:783–800. doi: 10.1177/0194599817753610. [DOI] [PubMed] [Google Scholar]
- 32.Education AC for G.M. ACGME; 2018. Required Minimum Number of Key Indicator Procedures for Graduating Residents. [Google Scholar]
- 33.Montague P., Bennett D., Kellermeyer B. How was your otology training? A survey of recent otolaryngology residents. Otol Neurotol. 2017;38:1535–1539. doi: 10.1097/MAO.0000000000001601. [DOI] [PubMed] [Google Scholar]
- 34.Bhutta M.F. A review of simulation platforms in surgery of the temporal bone. Clin Otolaryngol. 2016;41:539–545. doi: 10.1111/coa.12560. [DOI] [PubMed] [Google Scholar]
- 35.Lui J.T., Hoy M.Y. Evaluating the effect of virtual reality temporal bone simulation on mastoidectomy performance: a meta-analysis. Otolaryngol Head Neck Surg. 2017;156:1018–1024. doi: 10.1177/0194599817698440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.