Abstract
Squamous cell carcinoma of the oral cavity and oropharynx have been used synonymously and interchangeably in the world literature in the context of head and neck cancers. As the 21st century progresses, divergence between the two have become more evident, particularly due to evidence related to human papillomavirus-associated oropharyngeal squamous cell carcinoma. As such, the American Joint Committee on Cancer recently published the 8th edition Cancer Staging Manual, serving as a continued global resource to clinicians and researchers. Through changes in staging related to T and N clinical and pathologic classifications, the new system is expected to influence current management guidelines of these cancers that have distinct anatomic and etiopathogenic characteristics. This article aims to review such impactful changes in a time of critical transition of the staging of head and neck cancer and how these changes may affect clinicians and researchers worldwide.
Keywords: Oral cancer, Oropharyngeal cancer, Cancer staging, AJCC, Human papillomavirus, Head and neck cancer management
Introduction
Historically, squamous cell carcinoma of the oral cavity (OCSCC) and oropharynx (OPSCC) would be classified together or as separate entities in the literature of head and neck cancer (HNC). Furthermore, oral tongue and base of tongue cancers were often combined and labeled together as “tongue cancers” though they originate from distinct subsites of the tongue. Most notably, emerging evidence related to human papillomavirus (HPV) associated disease has altered the perspective and approach to OPSCC. OCSCC and OPSCC still stand as potentially life-threatening diagnoses particularly in areas with marginalized cancer care resources.1 Nearly 300,000 and 142,000 cases emerge annually for OCSCC (including the lip) and OPSCC, respectively, leading to nearly 145,000 and 96,000 deaths.2 Though research is unraveling novel treatment approaches,3 it is imperative to discern patient prognoses in the settings of novel molecular diagnostics, counseling, treatment planning, and research.
Cancer staging has provided a framework to determine prognosis and design guideline-based treatment for each stage. The modern system took its initial form in the early 1900's by describing cancer as local, regional, or distant disease. Between 1943 and 1952, Pierre Denoix of France built on this idea by classifying cancers by their anatomic location and extensiveness, pioneering the modern TNM (tumor, node, metastasis) system. This system was swiftly adopted by the Union for International Cancer Control (UICC) of Europe in 1953.4 Shortly after in 1959, the American Joint Committee on Cancer (AJCC) was founded, adopting the system in a modified form for its use in the United States (US).5 As both systems gathered wide acceptance, TNM committees of the AJCC and UICC formulated a single system in 1982.2,4,6
The resulting series of AJCC/UICC staging systems along with guideline and evidence-based treatment recommendations including the NCCN (National Comprehensive Cancer Network) of the US have offered continual insight on the optimal management course for HNC. Yet, as the landscape of knowledge undergoes change, categorizations naturally grow antiquated, requiring improvement and adaptation. Particularly, during the emergence of HPV-associated OPSCC and improvement in outcomes were realized, studies suggested that a modification of the staging system may be necessary.7, 8, 9 As such, recent research is indicating clearer separations in the prognostic categorizations of OCSCC and OPSCC. Subsequently, the AJCC recently released the 8th ed. staging manual, effective January 1st 2018.10 The aim of this article is to highlight and comprehensively review the most impactful changes in the new staging system to aid clinicians and researchers across the globe in this time of transition.
Background
Anatomy
Distinguishing the anatomic subsites and borders of the oral cavity and oropharynx are important to the diagnosis and management of OCSCC and OPSCC (Fig. 1). The former begins at the mucocutaneous junction of the lips and extends posteriorly, including the alveolar ridge and gums, the anterior two-thirds of the tongue, floor of the mouth, buccal mucosa, retromolar trigone, and hard palate. The oropharynx begins superiorly at the junction of the soft and hard palate and inferiorly at the circumvallate papilla of the tongue. It is bounded superiorly by the lower surface of the soft palate and inferiorly by the anterior surface of the epiglottis. Subsites of the oropharynx include the soft palate, tonsillar pillar, palatine tonsil, base (posterior 1/3) of tongue (BOT), valleculae, and oropharyngeal walls. These structures collectively represent a significant conduit of the upper aerodigestive tract, including critical lymphoepithelial structures such as the palatine and lingual tonsils, which are common origins of p16-positive OPSCC.
Fig. 1.
Anatomic subsitesof the oral cavity (green) and oropharynx (blue) (used and modified with permission from artist, Lauren Visserman).
Regional neck lymph nodes are classified into six different levels separated by clinical or image-based anatomic landmarks: level Ⅰ (submental); level Ⅱ (upper jugular); level Ⅲ (mid-jugular); level Ⅳ (lower jugular); level Ⅴ (supraclavicular fossa); level Ⅵ (central compartment).
Trends in epidemiology
OCSCC and OPSCC combined represent 3.1% of all cancers worldwide with annual age-adjusted incidences estimated at 4.0 and 2.1 per 100,000, respectively. The highest rates of OCSCC are in Melanesia with age-adjusted incidences per 100,000 of 22.9 for males and 16.0 for females. While the overall rate of OCSCC is observed to decrease, the total global burden is projected to have a 60% increase by 2030 when accounting for the world's growing population.11,12 On the other hand, OPSCC is most frequent in Western Europe at 7.5 and 1.6 per 100,000 for males and females, respectively. While overall trends of OPSCC vary by region, rates are increasing predominantly among men in more developed countries such as Scotland, Switzerland, Slovakia, and particularly, France.13,14 These increases are attributed to HPV-positive disease even in the face of diminishing rates of tobacco use. Globally, the prevalence of HPV confirmation in these tumors expanded from 32.3% before 1995 to 52.9% in the recent decade.15 Similar to OCSCC, OPSCC is predicted to experience a 60% rise in incidence by 2030.11,12
In the US, HNC as a group is on the general decline. OCSCC is paralleling this pattern while the incidence of OPSCC is accelerating (Fig. 2) and with HPV-positive disease approaching 80% of all OPSCC.16 This dramatic rise is projected to overtake the rate of cervical cancer by 2020.17,18 Concurrently, there is a decline in cancers resulting from tobacco use.18 Of note, by the Surveillance, Epidemiology, and End Results (SEER) database and American Cancer Society, the incidence of ‘tongue’ cancer is inferred to account for both oral tongue and BOT and its rise due to increasing rates of the latter, which is a distinct subsite of the oropharynx (Fig. 2).15,19 Researchers are optimistic that all national databases will eventually categorize the ‘oral tongue’ as separate from the ‘BOT’. Between OCSCC and OPSCC, nearly 50,000 new cases will develop leading to almost 10,000 deaths in the US for 2017 alone.18
Fig. 2.
Age-adjusted SEER incidence rates by subsite in the U.S., all races, both sexes from 1975-2013.19 OCSCC declining overall. The incidence of tongue cancer is inferred to account for the oral tongue and base of tongue, and its rise due to increasing rates of the latter, a distinct subsite of the oropharynx. Rates of OPSCC rising rapidly.
Patient demographics and clinical characteristics
HPV-positive OPSCC truly indicates a unique subset, as HPV-negative OPSCC and all OCSCC demonstrate similarities in demographics.20 These distinctions and other characteristics are summarized in Table 1.16 In addition, studies have indicated HPV-positive OPSCC's predilection for early and large, cystic neck metastasis, resulting in the diagnosis of advanced stage disease (Ⅲ-Ⅳ).7,21,22 Anatomically, OCSCC is most commonly found on the oral tongue, while OPSCC is typically found to affect the tonsil or BOT. When stratifying OPSCC by HPV status, HPV-positive lesions rarely involve the pharyngeal walls or the soft palate when compared to HPV-negative disease.23,24
Table 1.
Demographics and clinical characteristics of OCSCC and OPSCCs.
| Cancer site | Oral cavity | Oropharynx (p16-negative) | Oropharynx (p16-positive) |
|---|---|---|---|
| Demographics | Tobacco (smoking, chewing, betel nut), alcohol Older More African-Americans Lower SES Lower education |
Tobacco (smoking), alcohol Older More African-Americans Lower SES Lower education |
Nonsmoker Male Younger Caucasian Increased sexual partners Higher SES Higher education |
| Common Locations | Oral tongue | Tonsil BOT Pharyngeal wall Soft palate |
Tonsil BOT |
| Common Presentations | Soreness with red or white spots | Sore throat Dysphagia Otalgia Neck mass |
Painless neck mass |
HPV – human papillomavirus; SES – socioeconomic status; BOT – base of tongue.
Diagnosis
Initial workup involves a physical examination, visualizing the primary tumor by either direct or indirect, light-enhanced visualization and/or endoscopy in the outpatient clinic, and sampling the primary tumor with a tissue biopsy or any neck mass with fine needle aspiration biopsy. Although clinical examination alone can establish ‘clinical’ staging, MRI, CT, and/or CT-PET are often utilized to determine the extent of disease, namely, nodal and distant metastases, improving the accuracy of staging.
HPV testing
Testing for HPV status is now considered routine for OPSCC in North America.25 However, HPV testing guidelines are yet to be established, and therefore, practices differ by country. Available tests include HPV DNA (E6 oncogene) detection through polymerase chain reaction (PCR), in situ hybridization (ISH) for HPV type-specific DNA or RNA, serum and salivary assays, and immunostaining for p16 protein. While p16 immunostaining is the most popular test due to low cost, high sensitivity, and simple execution, it has a markedly decreased specificity compared to the gold standard PCR for HPV DNA.26 However, standardizing staging by means of p16 may be difficult in developing countries, where access to this prognostic tool and even up-to-date therapies are lacking.27,28 Among viable algorithms, p16-positive immunostaining followed by PCR for HPV DNA is considered the most useful for oropharyngeal tissues.31 In addition, given the ubiquitous expression of p16 in human cells, appropriate recognition of pathologic immunostaining patterns is critical. To qualify as p16-positive, strong nuclear and cytoplasmic staining must be observed diffusely in ≥70% of tumor cells.29,30 illustrating the need for further supplementary testing.
Evolving metrics in staging
Until now, the AJCC staging system has experienced very few major changes related to HNC since its inception, and particularly, changes related to molecular testing that could impact the prediction of prognosis and treatment decisions. Since the implementation of the previous AJCC staging system (7th ed., 2010), treatment standards, patient profiles, HPV and HNC knowledge have evolved, resulting in a new staging system with an implementation date of January 1, 2018. Subsequently, clinicians, researchers, and patients will experience changes in diagnostic methodologies, pre-treatment discussions, and therapy, standing as sources of future challenges when comparing studies on HNC conducted prior to with those occurring after 2018. To follow is a concise summary of the new AJCC staging systems and a worldwide perspective of its implementation and how this will impact patients and clinicians.
Oral cavity cancer
OCSCC staging experienced crucial changes related to the primary site and also cervical lymph nodes that impact sites beyond the oral cavity:
- Primary site
-
•Depth of Invasion (DOI) now included
-
•Local tumor invasiveness now redefined
-
•
- Lymph nodes
-
•Extranodal extension (ENE) now included
-
○Clinical ENE(+) criteria = evidence on physical exam
-
○Pathologic ENE(+) criteria = macroscopic ≥2 mm only
-
○
-
•
Depth of invasion
Historically, the OCSCC staging system depended on estimations by the human senses, such as by size of the visible surface and/or palpability of tumor, or by radiographic measurements. This was particularly challenging in the presence of ulcers, redness, or edema to distinguish malignancy versus secondary findings. There did not exist an objective confirmatory clinical test to accurately determine invasion of extrinsic tongue muscles, size, or depth until obtaining pathologic measurements through biopsy or surgery.
Depth of invasion (DOI) has been found to be an independent prognostic measure for both nodal metastasis and survival in OCSCC.32 Many have studied clinical and pathologic criteria portending its prognostic implication.37 Multiple studies have mentioned DOI in OCSCC within consistent measurements and techniques with respect to points of which the depth measurement vector is drawn.33, 34, 35, 36 Some describe tumor thickness as depth by utilizing the outer(superficial) surface of any exophytic, indurated, or ‘mushrooming’ effects as the reference point; others use the plane corresponding to the outer epithelial layer adjacent to the tumor. According to the new AJCC staging system, DOI is “measured by first finding the ‘horizon’ of the basement membrane of the adjacent squamous mucosa. A perpendicular ‘plumb line’ is established from the horizon to the deepest point of tumor invasion”.10 While it is most accurately determined histologically, it could be estimated in clinic by palpation and minimally aided by imaging. This feature is not novel in the field of oncology, as it has been applied to melanoma and squamous cell carcinoma of the cervix, among other malignancies.
Using DOI in a staging system necessitates separation by categories rather than a continuum of 1 mm increments. Although studies in past decades examined 3, 4, and 5 mm increments to determine lymph node metastasis and survival risk stratification, staging experts were tasked with identifying a system that was simple, confirmed by evidence, and achieved prognostic separation via patient tissue biopsies. For the new OCSCC staging, 5 mm increments were decided upon, categorizing survival more effectively compared to the 7th ed. system that only considered tumor size and local invasiveness.32
However, accurately quantifying DOI is not possible without histologic examination. Ultimately, the most reliable DOI measurement will be obtained at pathologic examination, which may not be available until after definitive treatment. Implications of this include the need for rapidly returned pathologic examination results to educate the patient and allow radiation to begin in a timely fashion.37
Local tumor invasiveness
fdb 9.1.450/W UnicodeHistorically, locally advanced cancers (T3 or T4) considered the extent of tumor rather than size alone. Tumor extent in the distant past utilized terms such as resectable/unresectable, which left significant subjectivity, and also resulted in patients accrued to clinical trials that may have had a wide variety of tumor aggressiveness. More recently, terms were changed to “Moderately advanced local disease”, implying T4a classification, versus “Very advanced local disease”, implying T4b classification. However, some of these criteria remained subjective even with clinical exam and radiographic imaging. This point is can be illustrated by the prior utilization of “extrinsic muscles of the tongue” by cancer indicating T4a classification. However, research shows that the depth to invade the muscles varies along the axis of the lateral tongue, thereby criticizing its role in staging.33 The 8th ed. system no longer considers deep/extrinsic muscle infiltration as T4a classification in OCSCC.
Oropharyngeal cancer
OPSCC similarly includes crucial changes related to the primary site and cervical lymph nodes:
- Primary site
-
•T0 Staging
-
○Deleted if patient is p16 (HPV) negative
-
○Included if a lymph node in the neck is p16-positive with an unknown primary(UKP)
-
○
-
•p16 (HPV) status now included
-
•
- Lymph nodes
-
•p16 (HPV) Positive
-
○Exranodal extension (ENE) not included
-
○Clinical Node Classification
-
▪cN1 = previous cN1, cN2a and cN2b if ≤6 cm
-
▪cN2 = previous cN2c if ≤6 cm
-
▪cN3 deleted
-
▪
-
○Pathologic Node Classification
-
▪pN1 = ≤4 lymph nodes involved
-
▪pN2 = ≥4 lymph nodes involved
-
▪
-
○
-
•p16 (HPV)Negative
-
○Extranodal extension (ENE) now included
-
▪Clinical ENE(+) criteria = evidence on physical exam
-
▪Pathologic ENE(+) criteria = microscopic ≤2 mm or macroscopic >2 mm
-
▪
-
○Clinical and Pathologic Node Classification
-
▪N0–N2 = previous N0–N2 if ENE(-)
-
▪N3 is now N3a and N3b
-
•N3a = previous N3 and ENE(-)
-
•N3b = now any nodes with ENE(+)
-
•
-
▪
-
○
-
•
p16 positivity
Stark contrasts in disease behaviors based on p16 status proved parts of the 7th ed. staging ineffective in linking stratified stages to prognostic outcomes, particularly as related to nodal metastasis. This is accounted for by the increase in p16-positive cases, which generate smaller primary tumors with greater cervical node involvement.38 Thus, though p16-positive patients were frequently diagnosed as advanced stage due to nodal metastasis, outcomes were much more favorable compared to their p16-negative counterpart irrespective of nodal status.7,21,22
Other findings further the notion that nodal disease and local invasiveness classifications in p16-positive OPSCC must be revised for prognostic and staging purposes.7 These include the notably high rate of an occult primary tumor in the setting of an isolated, p16-positive neck mass. Furthermore, research indicated that the number of pathologic lymph nodes obtained through neck dissection is a valuable metric in the staging of p16-positive OPSCC.41 These observations, in addition to others, led to the proposition for a separate staging system, both clinical and pathological, for HPV-associated OPSCC.7,8,22,39, 40, 41, 42
Extranodal extension
Extranodal extension (ENE), or historically, extracapsular spread/extension, is the extension of malignancy through an affected lymph node capsule. Depending on its severity, clinicians are able to recognize ENE clinically, radiographically, or histologically. It has always been shown to be a poor prognostic marker for regional recurrence and distant metastasis, and thus, was proposed to be incorporated into the new staging system.43,44 Interestingly, ENE is not as negatively predictive in appropriately treated p16-positive OPSCC as it is in p16-negative OPSCC and other HNSCC sites.45,46
While overt, macroscopic ENE is evident on physical exam, imaging, or surgical dissection, the detection of microscopic ENE is significantly more challenging and controversial among pathologists.47 Consequently, inter- and intra-observer variability is high.48 In addition, research describes a more involved histologic technique to detect and distinguish ENE from the rather common neoplastic embolus of the soft tissue.49 The latter consists of a metastatic lesion to soft tissue outside of regional nodes within fat, muscle, or blood vessels, yet some have considered it the product of metastatic tissue replacing/obliterating what was previously a lymph node.50,51
Moreover, without an established standard for ENE classification, the prognostic value of its severity is currently equivocal.43,50,52 Most have quantified ENE by the distance of malignant extension from the outer boundary of the capsule,43,50,52 while others report grading ENE by the relative location of malignancy to the lymph node capsule combined with its distance of extension beyond the capsule.53 One group found that stratifying ENE with a 2 mm extension cutoff did not result in significant differences in survival.52 Interestingly, however, a separate group reported to determine a prognostic cut-off value of 1.7 mm to dichotomize microscopic (≤1.7 mm) from macroscopic ENE (>1.7 mm) in OCSCC.43 While macroscopic ENE-positive tumors had significantly lower disease-specific survival, there was no difference between ENE-negative and microscopic ENE-positive tumors. The 8th ed. system classifies microscopic ENE as ≤2 mm and macroscopic as >2 mm, irrespective of associated stromal reaction, and with respect to staging, consider macroscopic (OCSCC) or macroscopic and microscopic (p16-negative OPSCC) to account for pathologic ENE-positive status.
These clinical and pathologic parameters now represent the major changes in OCSCC and OPSCC staging. Additionally, the International Collaboration on Cancer Reporting (ICCR) is in the process of updating the pathologic criteria used in some of these parameters.54
The new staging system (8TH ed.)
A rigorous review of the accumulating data has resulted in the 8th edition of the AJCC staging system (2017; implemented in 2018), major modifications for which are outlined in Table 2.10
Table 2.
Summary comparing 7th and 8th ed. AJCC staging of OCSCC and OPSCC.10
| Change | 7th Ed. (2010) | 8th Ed. (2017) |
||
|---|---|---|---|---|
| Oral Cavity | Oropharynx (p16-negative) | Oropharynx (p16-positive) | ||
| T-Classification |
TX: primary tumor cannot be assessed T0: no primary Tis: carcinoma in situ T1: size ≤2 cm T2: size 2-≤4 cm T3: size >4 cm or extension to lingual surface of epiglottis T4:
|
T1: size ≤2 cm and DOI ≤5 mm T2: size ≤2 cm and DOI 5-≤10 mm or size 2-≤4 cm and DOI ≤10 mm T3: size >4 cm or any tumor>10 mm DOI |
T0 if provenp16+ disease without evidence of primary tumor All locally advanced combined to T4 |
|
| N-Classification |
NX: regional node involvement cannot be assessed N0: no LN involved N1: single ipsi LN ≤3 cm in size N2: N2a: single ipsi LN, 3-≤6 cm in size N2b: multiple ipsi LNs, all ≤6 cm in size N2c: any bi or ctr LNs, all ≤6 cm in size N3: any LN >6 cm in size |
Clinical | ||
|
N0–N2: same as previous and ENE(-) N3 now with subcategories: N3a: previous N3 (size >6 cm) and ENE(-) N3b: any ENE(+), either clinical or radiographic |
|
|||
| Pathologic | ||||
|
Criteria for pathologic ENE(+): Oral cavity: only macroscopic (>2 mm) Oropharynx: micro- (≤2 mm) or macroscopic (>2 mm) N1–N2: same as previous and ENE(-) with exception: N2a includes lymph node ≤3 cm, ENE(+) LN N3 now with subcategories: N3a is previous N3 (size >6 cm) and ENE(-) N3b: ≥3 cm and ENE(+) LN or >1 ENE(+) LNs |
|
|||
| TNM Stage Grouping | Clinical or pathological TNM used for same grouping system | Same as previous | Separate clinical and pathological TNM groupings | |
DOI: depth of invasion; LN: lymph node; ENE(+): extranodal extension present; ENE(-): extranodal extension absent; ipsi: ipsilateral; bi: bilateral; ctr: contralateral.
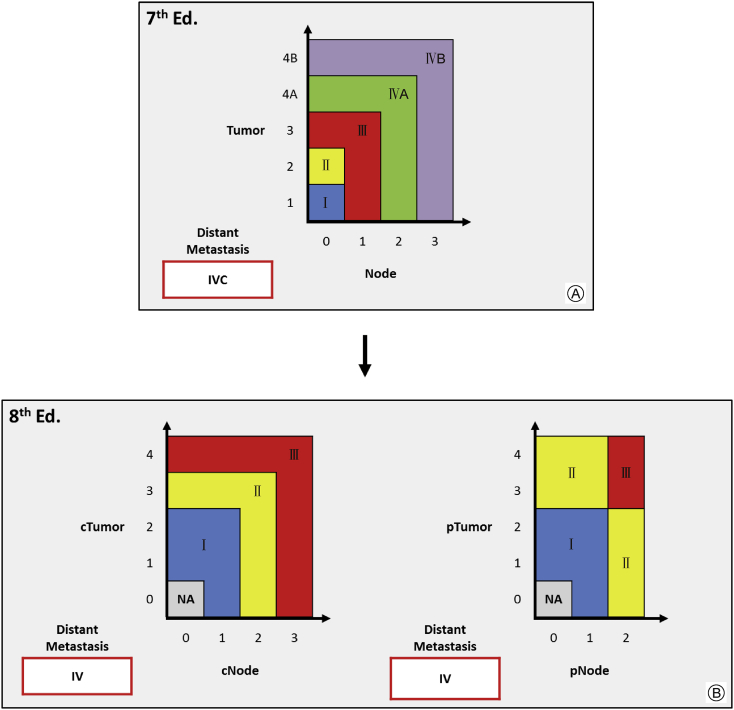
Moreover, it is imperative to note that p16-positive OPSCC staging has separate clinical and pathological TNM stage grouping systems and does not consider ENE (Fig. 3). To summarize these new groupings, clinical Stage I category is expanded to include T0-T2 with cN0-N1 disease, while T3 or cN2 disease would upstage to clinical Stage Ⅱ, and T4 or cN3 disease would upstage to clinical Stage Ⅲ. Similarly, T0-T2 with pN0-N1 disease is considered pathological Stage Ⅰ, however, pathological Stage Ⅱ is rearranged to include T3-T4 with pN0-N1 and T0-T2 with pN2 conditions, and pathological Stage Ⅲ is limited to T3-T4 with pN2 disease. Of note, clinical and pathological Stage Ⅳ disease is only determined by the presence of distant metastasis (M1) and pathological N3 no longer exists.10
Fig. 3.
Overall Stage Based upon TNM Stage Regrouping for p16-positive OPSCC. A: 7th ed. stage grouping for OCSCC and OPSCC combined B: 8th ed. stage groupings for p16-positive OPSCC are separatefor clinical and pathological staging, showing an expansion of early stage categorization to include traditionally advanced tumor features. NA: not applicable; c: clinical stage; p: pathological stage.
Implications in management
Current therapies for OCSCC and OPSCC include single modality or various combinations of surgery, radiation therapy, chemotherapy, and immunotherapy. Despite changes in the T and N classifications of OCSCC and OPSCC, overall stage-related treatment guidelines have not yet required modification. An example of a well-known evidence based guideline includes the NCCN Guidelines available at www.nccn.org. In general, early-stage OCSCC (Ⅰ or Ⅱ) are treated with definitive surgery (or radiation) unless metastatic, unresectable, or surgery is contraindicated. On the other hand, early-stage OPSCC can be treated with surgery via open or transoral (transoral robotic surgery, transoral laser microsurgery) approach, or radiation therapy alone, while for advanced stage (Ⅲ or IV), combined modality therapy using surgery plus radiation(±chemo) therapy is the standard.40,55 However, recent evidence suggests the need to individually tailor treatment practices as overtreatment has been a topic of concern particularly for early-stage cancers.7,56 Subsequently, prospective studies are motivated to minimize morbidities and de-intensify treatment in patients undergoing aggressive multimodal regimens.40 For advanced, recurrent, unresectable and/or metastatic OCSCC and OPSCC, immunotherapy options are emerging based on recent clinical trials.57, 58, 59
Summary
OCSCC and OPSCC are clinically and pathologically distinct diseases usually arising in separate demographic populations. The rise in p16-positive OPSCCs has become an important issue in the diagnosis, prevention, treatment, and now staging of these cancers. This evolving landscape has culminated in the 8th ed. of the AJCC staging system, which incorporates novel parameters to improve prognostic categorization. Highlights include DOI in OCSCC, separation of OPSCC by p16 status, modification of the N classification in HPV-positive cases, and inclusion of ENE in non-HPV nodal classification. Clinicians worldwide should understand these changes to provide appropriate treatments respective of geographical, cultural, political, financial, and technologic considerations.
Declaration of Competing Interest
The authors declare no conflicts of interest relevant to this report.
Acknowledgement
None.
Edited by Jie Gao
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Hoekstra H.J., Wobbes T., Heineman E., Haryono S., Aryandono T., Balch C.M. Fighting global disparities in cancer care: a surgical oncology view. Ann Surg Oncol. 2016;23:2131–2136. doi: 10.1245/s10434-016-5194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Ervik M. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Internet.http://globocan.iarc.fr [Google Scholar]
- 3.Squamous Cell Carcinoma: Molecular Therapeutic Targets. 1st ed. Springer Netherlands; 2017. [Google Scholar]
- 4.International Union Against Cancer . In: TNM Classification of Malignant Tumours. 3rd ed. Harmer M.H., editor. The Union; Geneva, Switzerland: 1982. [Google Scholar]
- 5.Fleming I.D. AJCC/TNM cancer staging, present and future. J Surg Oncol. 2001;77:233–236. doi: 10.1002/jso.1101. [DOI] [PubMed] [Google Scholar]
- 6.International Union Against Cancer . TNM classification of malignant tumours. In: Sobin L.H., Gospodarowicz M.K., Wittekind C., editors. 7th ed. Wiley-Blackwell; Chichester: 2010. [Google Scholar]
- 7.O'Sullivan B., Huang S.H., Su J. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 8.Keane F.K., Chen Y.H., Tishler R.B. Population-based validation of the recursive partitioning analysis-based staging system for oropharyngeal cancer. Head Neck. 2016;38:1530–1538. doi: 10.1002/hed.24470. [DOI] [PubMed] [Google Scholar]
- 9.Malm I.J., Fan C.J., Yin L.X. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer. 2017;123:1768–1777. doi: 10.1002/cncr.30512. [DOI] [PubMed] [Google Scholar]
- 10.AJCC Cancer Staging Manual . In: 8th ed. Amin M.B., Edge S., Greene F.L., editors. Springer International Publishing; 2017. [Google Scholar]
- 11.Gupta B., Johnson N.W., Kumar N. Global Epidemiology of head and neck cancers: a continuing challenge. Oncology. 2016;91:13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- 12.Shield K.D., Ferlay J., Jemal A. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67:51–64. doi: 10.3322/caac.21384. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi A.K., Anderson W.F., Lortet-Tieulent J. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diz P., Meleti M., Diniz-Freitas M. Oral and pharyngeal cancer in Europe: incidence, mortality and trends as presented to the global oral cancer forum. Translational Res Oral Oncol. 2017;2 2057178X17701517. [Google Scholar]
- 15.Stein A.P., Saha S., Kraninger J.L. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21:138–146. doi: 10.1097/PPO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehanna H., Beech T., Nicholson T. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi A.K., Engels E.A., Pfeiffer R.M. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Cancer Society . 2017. Cancer Facts & Figures 2017. [Google Scholar]
- 19.National Cancer Institute . National Institutes of Health; 2017. Site Recode ICD-O-3/WHO 2008 Definition.https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html [Google Scholar]
- 20.Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 21.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S.H., Xu W., Waldron J. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33:836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 23.McIlwain W.R., Sood A.J., Nguyen S.A., Day T.A. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:441–447. doi: 10.1001/jamaoto.2014.141. [DOI] [PubMed] [Google Scholar]
- 24.Amsbaugh M.J., Yusuf M., Cash E. Distribution of cervical lymph node metastases from squamous cell carcinoma of the oropharynx in the era of risk stratification using human papillomavirus and smoking status. Int J Radiat Oncol Biol Phys. 2016;96:349–353. doi: 10.1016/j.ijrobp.2016.06.2450. [DOI] [PubMed] [Google Scholar]
- 25.Maniakas A., Moubayed S.P., Ayad T. North-American survey on HPV-DNA and p16 testing for head and neck squamous cell carcinoma. Oral Oncol. 2014;50:942–946. doi: 10.1016/j.oraloncology.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Qureishi A., Mawby T., Fraser L., Shah K.A., Møller H., Winter S. Current and future techniques for human papilloma virus (HPV) testing in oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2017;274:2675–2683. doi: 10.1007/s00405-017-4503-1. [DOI] [PubMed] [Google Scholar]
- 27.Adeyi A., Olugbenga S. The challenges of managing malignant head and neck tumors in a tropical tertiary health center in Nigeria. Pan Afr Med J. 2011;10:31. [PMC free article] [PubMed] [Google Scholar]
- 28.Sanabria A., Domenge C., D'cruz A., Kowalski L.P. Organ preservation protocols in developing countries. Curr Opin Otolaryngol Head Neck Surg. 2010;18:83–88. doi: 10.1097/MOO.0b013e3283378f40. [DOI] [PubMed] [Google Scholar]
- 29.El-Naggar A.K., Westra W.H. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 30.Grønhøj Larsen C., Gyldenløve M., Jensen D.H. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer. 2014;110:1587–1594. doi: 10.1038/bjc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smeets S.J., Hesselink A.T., Speel E.J. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 32.Byers R.M., El-Naggar A.K., Lee Y.Y. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue. Head Neck. 1998;20:138–144. doi: 10.1002/(sici)1097-0347(199803)20:2<138::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Boland P.W., Pataridis K., Eley K.A., Golding S.J., Watt-Smith S.R. A detailed anatomical assessment of the lateral tongue extrinsic musculature, and proximity to the tongue mucosal surface. Does this confirm the current TNM T4a muscular subclassification. Surg Radiol Anat. 2013;35:559–564. doi: 10.1007/s00276-013-1076-6. [DOI] [PubMed] [Google Scholar]
- 34.International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Ebrahimi A., Gil Z. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140:1138–1148. doi: 10.1001/jamaoto.2014.1548. [DOI] [PubMed] [Google Scholar]
- 35.Hubert Low T.H., Gao K., Elliott M., Clark J.R. Tumor classification for early oral cancer: re-evaluate the current TNM classification. Head Neck. 2015;37:223–228. doi: 10.1002/hed.23581. [DOI] [PubMed] [Google Scholar]
- 36.Kane S.V., Gupta M., Kakade A.C., D' Cruz A. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32:795–803. doi: 10.1016/j.ejso.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Graboyes E.M., Garrett-Mayer E., Ellis M.A. Effect of time to initiation of postoperative radiation therapy on survival in surgically managed head and neck cancer. Cancer. 2017;123:4841–4850. doi: 10.1002/cncr.30939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marur S., D'Souza G., Westra W.H., Forastiere A.A. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keane F.K., Chen Y.H., Neville B.A. Changing prognostic significance of tumor stage and nodal stage in patients with squamous cell carcinoma of the oropharynx in the human papillomavirus era. Cancer. 2015;121:2594–2602. doi: 10.1002/cncr.29402. [DOI] [PubMed] [Google Scholar]
- 40.Horne Z.D., Glaser S.M., Vargo J.A. Confirmation of proposed human papillomavirus risk-adapted staging according to AJCC/UICC TNM criteria for positive oropharyngeal carcinomas. Cancer. 2016;122:2021–2030. doi: 10.1002/cncr.30021. [DOI] [PubMed] [Google Scholar]
- 41.Roberts T.J., Colevas A.D., Hara W., Holsinger F.C., Oakley-Girvan I., Divi V. Number of positive nodes is superior to the lymph node ratio and American Joint Committee on Cancer N staging for the prognosis of surgically treated head and neck squamous cell carcinomas. Cancer. 2016;122:1388–1397. doi: 10.1002/cncr.29932. [DOI] [PubMed] [Google Scholar]
- 42.Klozar J., Koslabova E., Kratochvil V., Salakova M., Tachezy R. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. 2013;107:625–633. doi: 10.1002/jso.23292. [DOI] [PubMed] [Google Scholar]
- 43.Wreesmann V.B., Katabi N., Palmer F.L. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1192–E1199. doi: 10.1002/hed.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzel S., Sagowski C., Kehrl W., Metternich F.U. The prognostic impact of metastatic pattern of lymph nodes in patients with oral and oropharyngeal squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2004;261:270–275. doi: 10.1007/s00405-003-0678-8. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P., Lewis J.S., Jr., Piccirillo J.F., Kallogjeri D., Haughey B.H. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118:3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 46.Amini A., Jasem J., Jones B.L. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncol. 2016;56:1–7. doi: 10.1016/j.oraloncology.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 47.van den Brekel M.W., Lodder W.L., Stel H.V., Bloemena E., Leemans C.R., van der Waal I. Observer variation in the histopathologic assessment of extranodal tumor spread in lymph node metastases in the neck. Head Neck. 2012;34:840–845. doi: 10.1002/hed.21823. [DOI] [PubMed] [Google Scholar]
- 48.Lewis J.S., Jr., Tarabishy Y., Luo J. Inter- and intra-observer variability in the classification of extracapsular extension in p16 positive oropharyngeal squamous cell carcinoma nodal metastases. Oral Oncol. 2015;51:985–990. doi: 10.1016/j.oraloncology.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coatesworth A.P., MacLennan K. Squamous cell carcinoma of the upper aerodigestive tract: the prevalence of microscopic extracapsular spread and soft tissue deposits in the clinically N0 neck. Head Neck. 2002;24:258–261. doi: 10.1002/hed.10020. [DOI] [PubMed] [Google Scholar]
- 50.Jose J., Coatesworth A.P., Johnston C., MacLennan K. Cervical node metastases in squamous cell carcinoma of the upper aerodigestive tract: the significance of extracapsular spread and soft tissue deposits. Head Neck. 2003;25:451–456. doi: 10.1002/hed.10214. [DOI] [PubMed] [Google Scholar]
- 51.Woolgar J.A., Rogers S.N., Lowe D., Brown J.S., Vaughan E.D. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39:130–137. doi: 10.1016/s1368-8375(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 52.Greenberg J.S., Fowler R., Gomez J. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97:1464–1470. doi: 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 53.Lewis J.S., Jr., Carpenter D.H., Thorstad W.L., Zhang Q., Haughey B.H. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24:1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.International Collaboration on Cancer Reporting http://www.iccr-cancer.org/ Available from: URL:
- 55.Sher D.J., Rusthoven C.G., Khan S.A., Fidler M.J., Zhu H., Koshy M. National patterns of care and predictors of neoadjuvant and concurrent chemotherapy use with definitive radiotherapy in the treatment of patients with oropharyngeal squamous cell carcinoma. Cancer. 2017;123:273–282. doi: 10.1002/cncr.30255. [DOI] [PubMed] [Google Scholar]
- 56.Cracchiolo J.R., Baxi S.S., Morris L.G. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer. 2016;122:1523–1532. doi: 10.1002/cncr.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauml J., Seiwert T.Y., Pfister D.G. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seiwert T.Y., Burtness B., Mehra R. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 59.Ferris R.L., Blumenschein G., Jr., Fayette J. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]