Abstract
Objective
To investigate the expressions of MAPK10, c-Jun and Itga6 in laryngeal carcinoma and its influence on the sensitivity to docetaxel, cisplatin and 5-fluorouracil (TPF) chemotherapy.
Methods
Fifty-seven patients with supraglottic squamous cell carcinoma, who were treated by two cycles of TPF induction chemotherapy in our hospital, were enrolled in this study and divided into groups by chemotherapy resistance or chemotherapy sensitivity. The expressions of mRNA and protein of MAPK10, c-Jun and Itga6 in tumor tissues were evaluated by immunohistochemistry. The consistency of mRNA and protein expressions was tested, and the relation with the clinicopathological features was analyzed.
Results
The positive rates of MAPK10 andc-Jun in the tumor tissues of the sensitive group were significantly higher than those of there assistant group, which was 90.48% and 100.00%, respectively. The expression rate of Itga6 was significantly higher in the resistant group, which was 83.33% (P < 0.05). The mRNA levels of MAPK10 and c-Jun were significantly lower in the resistant group than in the sensitive group, whilethemRNA levelof Itga6was significantly higher in the resistant group (P < 0.05). The protein expressions of MAPK10, c-Jun and Itga6 were consistent with their mRNA expressions (P < 0.05). The expressions of MAPK10, c-Jun and Itga6 were not correlatedwithage, gender and tumor diameter (P > 0.05). However, the expressions of MAPK10 and c-Jun were negatively correlated withclinical stage and pathological grading (P < 0.05). Negative correlations between MAPK 10 and Itga6, and between c-Jun and Itga6in tumor tissues were found by Spearman'srank correlation coefficient (P < 0.05). The correlation was also negative in the resistant tumor tissues (P < 0.05).
Conclusion
The MAPK10 and c-Jun expressions were down-regulated, while the Itga6 expression was up-regulated in the chemo-resistant laryngeal carcinoma, and the expression levels of different factors were correlated witheach other. These factorsmight be important biomarkers for predicting outcomes of TPF chemotherapy in laryngeal carcinoma in the future.
Keywords: Larynx carcinoma, Drug resistance, MAPK10, c-Jun, Itga6
Larynx carcinoma is the most common head and neck cancer, accounting for about 25%–30% of all head and neck malignancies.1 Laryngeal carcinoma is the second most common respiratory tract cancer after lung cancer. In China the incidence and mortality of laryngeal carcinoma are 1.1/100000–4.0/100000 and 0.7/100000, respectively, and they keep increasing in some regions.2 Integrated treatment combining surgery, chemotherapy and radiotherapy remains the main option for laryngeal carcinoma. For early-stage laryngeal carcinoma, laser surgery or radiotherapy can preserve the laryngeal anatomy and the swallowing and respiration functions while removing the lesions. But for late-stage laryngeal carcinoma, total laryngectomy may be the only option, which has a great adverse impact on patient's life quality. Non-surgical treatments such as radiotherapy or concurrent radiochemotherapy are associated with the problem of resistance. The development of resistance to radiochemotherapy usually indicates thatthe patients have missed the best timing for surgery. Therefore, larynx-preserving individualized treatment for patients after induction chemotherapy has received growing attention.3 The common induction chemotherapy regimen for laryngeal carcinoma is the docetaxel, cisplatin and 5-fluorouracil (TPF)chemotherapy. But resistance to chemotherapy is the major barrier to improve the efficacy.4 Some resistant patients suffer the pain but gain little benefits from the chemotherapy. It is urgent to develop effective predictors for sensitivity to induction chemotherapy. In our preliminary study, microarray analysis was performed to identify the differentially expressed genes from the laryngeal squamous cell carcinoma tissues in patients sensitive and resistant to induction chemotherapy. Gene enrichment analysis was carried out using Gene Ontology Database and KEGG pathway database. It was found that the expressions of MAPK10, c-Jun and Itga6 were correlated with the sensitivity of laryngeal squamous cell carcinoma to induction chemotherapy.5 In the present study, the protein expressions of MAPK10, c-Jun and Itga6 were detected in the cancer tissues by immunohistochemistry. The correlations between their expressions and clinicopathological parameters of the patients were analyzed. The predictive value of MAPK10, c-Jun and Itga6 for chemotherapy sensitivity of laryngeal carcinoma was determined.
Materials and methods
Subjects
From September 2014 to September 2016, fifty-seven patients with supraglottic squamous cell carcinoma and treated at the department of neck and head surgery of Beijing Tongren Hospital were enrolled. There were 45 males and 12 females, who were aged 42–76 years old with an average of 59.3 ± 6.9 years. Thirty cases were classified as stage Ⅱ, 10 cases as stage Ⅲ, 8 cases as stage ⅣA and 3 cases as stage ⅣB. Paraffin-embedded blocks were collected before the induction chemotherapy. The inclusion criteria were as follows: (1) Histopathologically confirmed as laryngeal squamous cell carcinoma by biopsies; (2) Primary supraglottic carcinoma confirmed by laryngoscopy and CT scan; (3) Having received two cycles of TPF induction chemotherapy; (4) Intact clinicopathological and follow-up data. The following conditions were excluded: (1) Combined with distant metastasis to the lung, bone and brain, or severe infection and cachexia, which caused chemotherapy intolerance; (2) History of malignancies at other systems, or having received surgery, chemotherapy, radiotherapy, immunotherapy or targeted therapy; (3) Neurological or mental disorders, which made the patients incapable of making decision. The research protocol was approved and conducted under the supervision of hospital ethics committee. All patients and their relatives were informed of the objective and significance of the present research, and the informed consent was signed. The patients were divided into chemotherapy resistance group (SD + PD) and chemotherapy sensitivity group (CR + PR). The two groups were comparable in age, gender and stage distribution (P > 0.05, Table 1).
Table 1.
Comparison of baseline information.
| group | Cases (n) | Gender (M/F) | Stage |
|||
|---|---|---|---|---|---|---|
| StageⅡ | StageⅢ | StageⅣa | StageⅣb | |||
| Resistant group | 36 | 28/9 | 17 | 11 | 6 | 2 |
| Sensitive group | 21 | 17/3 | 13 | 5 | 2 | 1 |
| value | 0.679 | 0.426 | −0.728 | |||
| P value | >0.05 | >0.05 | >0.05 | |||
Chemotherapy regimen and efficacyevaluation
All patients received two cycles of TPF chemotherapy consisting of docetaxel 135 mg/m2, day 1; cisplatin 30 mg/m2, day 2-day 4; and 5-Fu 500 mg/m2, day 2-day 6. The above regimen was repeated from day 21 to day 28. Efficacy was evaluated after two cycles of the chemotherapy according to Response Evaluation Criteria in Solid Tumor (RECIST) guideline (version 1.1)6: complete response (CR) was defined as the disappearance of all existing target lesions without the appearance of new lesions; partial response (PR) was defined as the reduction in the sum of the maximum diameter of the lesions by ≥ 50%; stable disease (SD) was defined as the reduction in the sum of the maximum diameter of the lesions below that in PR, or as an increase in the sum of the maximum diameter of the lesions above that in progressive disease (PD); PD was defined as an increase in the sum of the maximum diameter of the lesions by ≥ 20%. Radiotherapy was recommended for patients achieving CR and PR. Surgery with or without postoperative radiotherapy was recommended for chemotherapy-tolerant cases (SD and PD).
Detection methods
Immunohistochemistry
Expressions of MAPK10, c-Jun and Itga6 in the tumor tissues were examined by immunohistochemistry. Anti-MAPK10 polyclonal antibody (concentrated, ab124956, 1:100), anti-Jun monoclonal antibody (concentrated, ab32137, 1:100) and anti-Itga6 polyclonal antibody (concentrated, ab20142, 1:100) were purchased from Abcam (USA). Immunohistochemical detection was performed and the results were interpreted according to the literature.7 Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned and baked. The sections were dewaxed withxylene and dehydrated through an alcohol gradient. Endogenous peroxidase was inactivated by adding 3% H2O2. Antigen retrieval was performed using the hyperbaric citrate method. The sections were added with diluted primary antibody, and for negative control, PBS was added instead of the primary antibody. Reference photos of positive expressions of each protein were provided in the instruction manual. Positive cells were counted using semi-quantitative integration method. Five visual fields were randomly selected under the high-magnification lens ( × 200), and one hundred cells were counted. The results were assessed based on the percentage of positive cells and staining intensity: the scores of 0, 1, 2, 3 and 4 were given to <5%, 5%–24%, 25%–50%, 51%–74% and ≥75% percentages of positive cells, respectively. The staining intensity was classified into no staining, light yellow, brownish yellow and brown, for which the scores of 0, 1, 2, and 3 were given, respectively. The final score was the sum of the above two scores. Final score of 0 was defined as negative (−), 1–2 weakly positive (+), 3–5 moderately positive (++), and 6–7 strongly positive (+++).
Real-time quantitative fluorescence PCR (RT-qPCR)
mRNA expressions of MAPK10, c-Jun and Itga6 in the samples were detected by RT-qPCR. Primers for the target genes and internal reference gene GAPDH were designed and synthesized by Oligobio (Beijing, China) (Table 2). Samples were collected, cryopreserved in liquid nitrogen and homogenated. Total RNA extraction was performed using Trizol reagent (Thermo-Fisher, USA). cDNA was synthesized from total RNA using PrimeScript™ RT Reagent Kit according to the instruction. Amplification was carried out using CFX Connect Real-TimePCRDetection System and KAPA SYBR® FAST qPCR Kit Master Mix (2×) Universal (KAPA BIOSYSTEMS) under the following conditions: 95 °C for 3 min, (95 °C for 15sec, 58 °C for 15sec, 72 °C for 30sec) × 40 cycles, extension at 72 °C for 7min. Relative expression of the target gene was calculated as △NCtgene=(Ctgene-CtGAPDH); amplification fold = 2△Ct; △Ct ≥ 2 was considered positive, and △Ct < 2 negative.
Table 2.
Primers for target genes and β-actin.
| Gene | Primers | Product length |
|---|---|---|
| MAPK10 | Sense:5′-CTG ATG CAG TGC ACG ATC TAC-3′ anti-sense: 5′-AGC GTC GTA CTA GAC GTT GCG AT-3′ |
197 bp |
| c-Jun | Sense:5′-GGGAACAGGTGGCACAGCTA-3′anti-sense: 5′- ACGTTTGCAACTGCTGCGTTA-3′ | 87 bp |
| Itga6 | 5′-CAGTGGAGCCGTGGTTTTG-3′ 5′-CCACCGCCACATCATAGCC-3′ |
113bp |
| GAPDH | Sense 5′- AAACCCATCACCATCTTCCA-3′ Antisense 5′- GTGGTTCACACCCATCACAA-3′ |
198bp |
Statistical analysis
All statistical analyses were performedusing SPSS 20.0 software. Counts were expressed as n (%) and analyzed by χ2 test, Fisher's exact test, or rank sum test. Measurements were expressed as ± s and analyzed by t-test. Correlations between the expressions of the three proteins were analyzed using Spearman's rank correlation coefficient. Consistency between mRNA and protein expressions of the genes were tested by weighted Kappa statistics using SAS 9.0 software. P < 0.05 was considered significant.
Results
Expressions of MAPK10, c-Jun and Itga6 in laryngeal squamous cell carcinoma
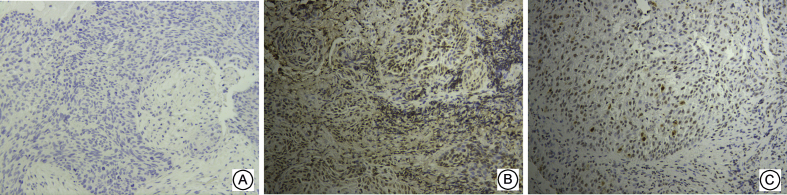
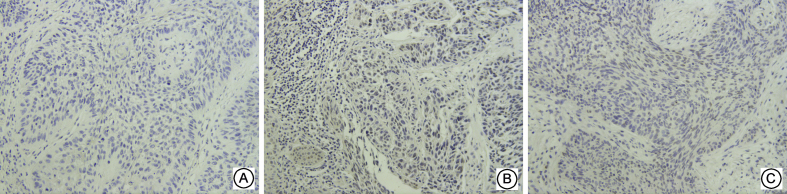
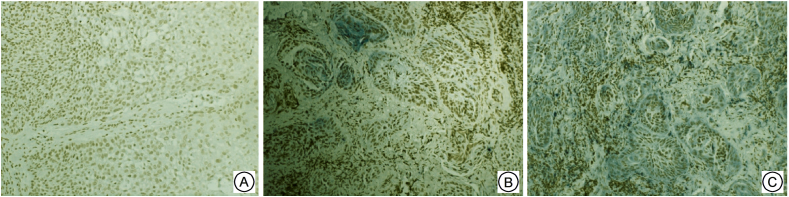
MAPK10 was mainly expressed in the nuclei of the tumor cells and rarely in the cytoplasm. c-Jun was expressed in both the nuclei and cytoplasm, and primarily in the nuclei. Itga6was mainly expressed in the cytoplasm, and the positive cells showed a typical nested distribution (Fig. 1, Fig. 2, Fig. 3). Difference in the expressions of the genes between the resistant and sensitive groups was compared. The positive rates of MAPK10 and c-Jun were higher in the sensitive group, being 90.48% and 100.00%, respectively. Itga6 expression was higher in the resistant group, with a positive rate of 83.33%. The two groups showed significant difference in the expressions of the three proteins (P < 0.05, Table 3).
Fig. 1.
Expressions of MAPK10 in three groups of Supraglottic Laryngeal Squamous Cell Carcinoma ( × 200) A: No expression in negative control group; B: High expression in sensitive group; C: Low expression in resistant group.
Fig. 2.
Expressions of c-Jun in three groups of Supraglottic Laryngeal Squamous Cell Carcinoma ( × 200) A: No expression in negative control group; B: High expression in sensitive group; C: Low expression in resistant group.
Fig. 3.
Expressions of tga6 in three groups of Supraglottic Laryngeal Squamous Cell Carcinoma ( × 200) A: No expression in negative control group; B: Low expression in sensitive group; C: High expression in resistant group.
Table 3.
Expressions of MAPK10, c-Jun and Itga6 in SLSCC.
| Gene | Group | Cases(n) | Expression |
U value | P value | |||
|---|---|---|---|---|---|---|---|---|
| – | + | ++ | +++ | |||||
| MAPK10 | Tolerant group | 36 | 10 | 14 | 9 | 3 | −2.421 | 0.015 |
| Resistant group | 21 | 2 | 6 | 7 | 6 | |||
| c-Jun | Tolerant group | 36 | 4 | 16 | 10 | 6 | −2.136 | 0.033 |
| Resistant group | 21 | 0 | 5 | 11 | 5 | |||
| Itga6 | Tolerant group | 36 | 6 | 8 | 13 | 9 | −2.353 | 0.010 |
| Resistant group | 21 | 8 | 7 | 5 | 1 | |||
Consistency between mRNA and protein expressions of MAPK10, c-Jun and Itga6
The mRNA expressions of MAPK10 and c-Jun in the resistant group were significantly lower than those in the sensitive group (P < 0.05). The mRNA expression of Itga6 in the resistant group was considerably higher than that in the sensitive group (P < 0.05) (Table 4). The positive rates of MAPK10 and c-Jun in the resistant group were lower, which were 75.0% and 86.1%, respectively. The positive rate of Itga6 was higher in the resistant group, which was 80.5%. Consistency test indicated that the mRNA and protein expressions of MAPK10, c-Jun and Itga6 were consistent in the resistant group (Kappa = 0.653,0.722,0.924; P < 0.05).
Table 4.
The mRNA Expressions of MAPK10, c-Jun and Itga6 in SLSCC.
| Gene | Group | Cases(n) | Expression level | t value | P value |
|---|---|---|---|---|---|
| MAPK10 | Resistant group | 36 | 0.91 ± 0.50 | 5.802 | <0.05 |
| Sensitive group | 21 | 2.03 ± 0.96 | |||
| c-Jun | Resistant group | 36 | 0.66 ± 0.42 | 7.541 | <0.05 |
| Sensitive group | 21 | 1.97 ± 0.89 | |||
| Itga6 | Resistant group | 36 | 1.86 ± 0.75 | 4.543 | <0.05 |
| Sensitive group | 21 | 0.98 ± 0.62 |
Relationship between the expressions of MAPK10, c-Jun and Itga6 and clinicopathological parameters
The relationship between the expressions of MAPK10, c-Jun and Itga6 and clinicopathological parameters was analyzed. The results showed that the expressions were not correlatedwith age, gender or tumor diameter (P > 0.05); the expressions of MAPK10 and c-Jun were correlated with clinical staging and pathological grading; the higher the severity of the disease, the lower the positive rate was (P < 0.05). The expression of MAPK10 was correlated with lymph node metastasis, and the expression of was higher in the patients with metastasis than that in the patients without metastasis (P < 0.05). The expression of c-Jun was not correlated with lymph node metastasis (P > 0.05). Itga6 was correlated with clinical stage, pathological grade and lymph node metastasis, the more serious the disease, the higher the positive rate (P < 0.05, Table 5).
Table 5.
Relationship between the expressions of MAPK10, c-Jun and Itga6 and clinicopathological parameters[n].
| Clinicopathological parameters | Cases(n) | MAPK10 |
χ2/U value | P value | c-Jun |
χ2/U value | P value | Itga6 |
χ2/U value | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | – | + | – | + | – | ||||||||
| Age | |||||||||||||
| ≥60 | 22 | 17 | 5 | 0.060 | 0.529 | 20 | 2 | 0.236 | 0.503 | 17 | 5 | 0.065 | 0.799 |
| <60 | 35 | 28 | 7 | 33 | 2 | 26 | 9 | ||||||
| Gender | |||||||||||||
| Male | 45 | 33 | 12 | 0.0140 | 0.907 | 37 | 8 | 0.008 | 0.928 | 31 | 14 | 0.173 | 0.681 |
| Female | 12 | 9 | 3 | 10 | 2 | 9 | 3 | ||||||
| Clinical staging | |||||||||||||
| Stage Ⅱ | 30 | 28 | 1 | 9.411 | 0.005 | 36 | 0 | 7.375 | 0.015 | 23 | 13 | 7.035 | 0.010 |
| Stage Ⅲ+stage Ⅳ | 27 | 17 | 11 | 17 | 4 | 20 | 1 | ||||||
| Pathological grading | |||||||||||||
| High | 20 | 19 | 1 | −2.485 | 0.013 | 20 | 0 | −2.650 | 0.006 | 11 | 9 | −2.445 | 0.017 |
| Moderate | 28 | 21 | 7 | 27 | 1 | 24 | 4 | ||||||
| Low | 9 | 5 | 4 | 6 | 3 | 8 | 1 | ||||||
| Tumor diameter | |||||||||||||
| <3 cm | 29 | 22 | 7 | 0.338 | 0.561 | 27 | 2 | 0.001 | 1.000 | 22 | 7 | 0.006 | 0.940 |
| ≥3 cm | 28 | 23 | 5 | 26 | 2 | 21 | 7 | ||||||
| Lymphnode metastasis | |||||||||||||
| Yes | 20 | 11 | 9 | 10.631 | 0.001 | 20 | 0 | 2.325 | 0.286 | 19 | 1 | 6.363 | 0.012 |
| No | 37 | 34 | 3 | 33 | 4 | 24 | 13 | ||||||
Correlations between the expressions of MAPK10, c-Jun and Itga6
Correlations between the expressions of MAPK10, c-Jun and Itga6 were analyzed using Spearman's rank correlation coefficient. A negative correlation was found between the expression of MAPK10 and Itga6 and also between the expression of c-Jun and Itga6, with the correlation coefficients of r = −0.395 (P < 0.05) and r = −0.472 (P < 0.05), respectively. The correlations between the expressions of MAPK 10, c-Jun and Itga6 were analyzed in the tumor tissues for the resistant group. A negative correlation was found between the expression of MAPK10 and Itga6 and also between the expression of c-Jun and Itga6 in the resistant group, with the correlation coefficients of r = −0.443 (P < 0.05) and r = −0.506 (P < 0.05), respectively.
Discussion
The onset of supraglottic carcinoma is usually hidden and most patients are diagnosed at the middleand late stage. Total laryngectomyis the traditional therapy, but it may cause an impairment of the swallowing and speech function. It is necessary to hunt for alternative treatment that can preserve the larynx.8 Multidisciplinary integrated therapy for cancer has made considerable advance in recent years, especially chemotherapy, stereotactic radiotherapy, targeted therapy and immunotherapy. Larynx-preserving therapy has become a possibility for some patients.9 It has been reported that the overall 3-year survival after radiotherapyfor patients responsive to induction chemotherapy was 88.4%, and the progression-free survival was 68.0%. Moreover, patients with normal laryngeal function 3 years later accounted for 77.8%.10 Liao et al11 showed that induction chemotherapy combined with radiotherapy or targeted therapy could preserve the laryngeal function, with little impact on the long-term life quality of the patient. The efficacy was comparable to that of surgery with or without postoperative chemotherapy. The medial survival for the three treatment regimens was 72, 90 and 58 months, respectively. However, not all patients are sensitive to induction chemotherapy. According to Fung et al,12 after 2–3 cycles of DDP plus 5-FU induction chemotherapy for late-stage resectable laryngeal carcinoma, the objective response rate, complete response rate and 3-year survival were 80%, 40%–50% and 68%, respectively. Chemotherapy resistance is the major reason for poor response in laryngeal carcinoma. To preclude chemotherapy for resistant patients, it is important to identify the molecular markers for chemotherapy sensitivity in laryngeal carcinoma, so as to lay the basis for individualized therapy.
In our preliminary study, microarray analysis was applied to the laryngeal squamous cell carcinoma tissues from sensitive and resistant patients, which identified 15 differentially expressedgenes possibly associated with resistance. Among them, MAPK10, c-Jun and Itga6 played the core regulatory role.5 MAPK10 encodes for c-Jun N-terminal kinase 3 (JNK3), a member of the JNK family. Under physiological conditions, MAPK10 is only expressed in the nerve tissues, heart and testicles. JNK3 is believed to be inhibitory on tumor cell proliferation. Deletion of JNK3 is associated with the occurrence and development of brain tumor.13 Here, we detected the mRNA and protein expressions of these three genes by RT-qPCR and immunohistochemistry. The results showed that the MAPK10 expression level decreased in laryngeal carcinoma tissues at a higher level of malignancy. That is, the more advanced the clinical stage, the lower the pathological grade and the more extensive the lymph node metastasis, the greater the downregulation of MAPK10. This further proved the inhibitory effect of MAPK10 on tumor.14 Moreover, the JNK3 expression was significantly higher in the sensitive group than in the resistant group, which implied the role of MAPK10 in the resistance mechanism of laryngeal squamous cell carcinoma.
Itga6 is a transmembrane glycoprotein adhesion receptor, which binds to the extracellular matrix to regulate cell–cell and cell–matrix adhesion. This is the mechanism by which Itga6 is involved in cell division, migration and invasion.15 Study has shown that Itga6 is highly expressed in various types of cancer, which may contribute to chemotherapy resistance in some tumor cells, possibly due to the promoting effect of Itga6 for intracellular adhesion and information transmission.16 In our study, the expression of Itga6 was inversely correlated withclinical stage, pathological grade and lymph node metastasis. The higher the severity of the disease, the higher the expression level of Itga6. Furthermore, its expression was higher in the tumor tissues in the resistant group than in the sensitive group. Thus, Itga6 may resist the killing effect of the chemotherapeutic agent, promote the proliferation and inhibit the apoptosis of tumor cells. It should be pointed out that this result was inconsistent with our findings by microarray analysis, and the reasons remain unclear. We also analyzed the correlations between the expressions of MAPK10, c-Jun and Itga6. The expressions of the three geneswere correlated with each other in the sensitive and resistant groups, but in an opposite way. Given the fact that the mRNA and protein expressions of MAPK10, c-Jun and Itga6 were consistent in the chemotherapy-resistant samples, the reason for the above phenomenon may be attributed to the post-transcriptional modification of mRNA. It is postulated that the MAPK10/c-Jun signaling pathway is involved in the regulation of Itga6 expression on the transcriptional level, but the specific mechanism remains to be further investigated.
Innovation: 1. To predict the chemosensitive biomarkers of supraglottic squamous cell carcinoma and to further study the biological characteristics of supraglottic laryngeal carcinoma. 2. The combination of basic and clinical to explore the sensitivity of laryngeal cancer induction chemotherapy related factors, screening for patients suitable for chemotherapy for induction chemotherapy, to avoid chemotherapy for patients not suitable for chemotherapy, delay the opportunity of other methods of treatment.
Fund program
Sponsored by Beijing Municipal Administration of Hospitals Incubating Program (PX2018009).
Declaration of Competing Interest
There is no conflict of interest.
Edited by Qiong Wu and Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Peller M., Katalinic A., Wollenberg B., Teudt I.U., Meyer J.E. Epidemiology of laryngeal carcinoma in Germany, 1998-2011. Eur Arch Otorhinolaryngol. 2016;273:1481–1487. doi: 10.1007/s00405-016-3922-8. [DOI] [PubMed] [Google Scholar]
- 2.Morshed K., Szymański M., Siwiec H., Gołabek W. Laryngeal cancer in farmers from Lublin region of Poland. Ann Agric Environ Med. 2008;15:13–19. [PubMed] [Google Scholar]
- 3.Gong H.Y., Song Q.F., Hu W.G. A systemic evaluation on the efficacy of preferred chemoradiotherapy or surgical treatment for laryngeal carcinoma. J Evidence-Based Med. 2014;14:229–233. [Google Scholar]
- 4.Janoray G., Pointreau Y., Garaud P. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ± docetaxel for larynx preservation. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv368. pii: djv368. [DOI] [PubMed] [Google Scholar]
- 5.Shi Q., Lian M., Fang J.G. A preliminary analysis on potentially targeted genes of induced chemotherapy in supraglottic laryngeal squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:504–510. doi: 10.3760/cma.j.issn.1673-0860.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H.L. Response evaluation criteria in solid tumors and evolution. J Mod Oncol. 2010;18:839–841. [Google Scholar]
- 7.Liu L., Lu Y., Wang Y. Expression and clinical significance of EGFR, HER2 and CXCR4 in non-small cell lung cancer. Prog Mod Biomed. 2014;14:1069–1073. [Google Scholar]
- 8.Pereira da Silva A., Feliciano T., Vaz Freitas S., Esteves S., Almeida E Sousa C. Quality of life in patients submitted to total laryngectomy. J Voice. 2015;29:382–388. doi: 10.1016/j.jvoice.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Fu X.Y. Chongqing Medical University; 2016. A Meta-Analysis on the Efficacy of Total Laryngectomy and Non-surgical Treatment for Locally Advanced Laryngeal Carcinoma [D] [Google Scholar]
- 10.Matoba T., Ijichi K., Yanagi T. Chemo-selection with docetaxel, cisplatin and 5-fluorouracil (TPF) regimen followed by radiation therapy or surgery for pharyngeal and laryngeal carcinoma. Jpn J Clin Oncol. 2017;47:1031–1037. doi: 10.1093/jjco/hyx115. [DOI] [PubMed] [Google Scholar]
- 11.Liao F., Qin S.K., Gon X.L. Analysis of clinical efficacy in 92 patients with laryngeal carcinoma. Chin Clin Oncol. 2013;18:340–343. [Google Scholar]
- 12.Fung K., Lyden T.H., Lee J. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1395–1399. doi: 10.1016/j.ijrobp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Coffey E.T. Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci. 2014;15:285–299. doi: 10.1038/nrn3729. [DOI] [PubMed] [Google Scholar]
- 14.Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 15.Stewart R.L., O'Connor K.L. Clinical significance of the integrin α6β4 in human malignancies. Lab Invest. 2015;95:976–986. doi: 10.1038/labinvest.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu W., Yang G.L., Xue P., Liu C.M., Guan F. Quantitative analysis of integrin α6 in bladder cancer cell lines. Prog Biochem Biophys. 2015;42:468–475. [Google Scholar]