ABSTRACT
Aim
The aim of this report is to explore the risk factors of XEN stent obstruction, suggesting the need for a stricter control of these factors and highlighting areas for further research.
Background
Despite its proven effectiveness and safety profile, XEN gel stents (Allergan Plc, Dublin, Ireland) can become obstructed. The causes and predicting factors for such obstructions still require further research. While hyphema has long been known to be responsible for secondary glaucoma through trabecular obstruction, it has not been associated, to date, with XEN gel stent obstruction.
Case description
We describe the case of a 55-year-old female patient with primary open-angle glaucoma (POAG) who underwent bilateral XEN gel surgery. Her left eye developed a 2 mm postoperative hyphema, which resolved spontaneously within 8 days. Intraocular pressure (IOP) normalized at 12 mm Hg and increased to 50 mm Hg after 1 month in an otherwise normal-looking eye. Intraoperative examination revealed a nonfunctioning XEN gel stent, which was replaced and sent for laboratory analysis. Macroscopic examination of the tube confirmed obstruction with cellular debris. Tube replacement restored good filtration.
Conclusion
This case report confirms cellular debris as a potential cause of XEN gel stent occlusion, suggesting that aqueous red blood cells (RBCs) could potentially pose a threat to the microstents’ patency even in cases when the bleeding was minimal and self-limited and where the IOP was still controlled at the time of full hyphema resolution. This observation could lead to recommendations for a stricter control of bleeding risk factors prior to microinvasive glaucoma surgery (MIGS), and it raises the question of whether anterior chamber (AC) washout should be advised in postoperative hyphema.
Clinical significance
This case highlights some previously unreported risk factors for XEN stent obstruction and suggests that stricter control of bleeding and monitoring of patients following hyphema could improve surgical outcome.
How to cite this article
Gillmann K, Bravetti GE, Mansouri K. Delayed Obstruction of XEN Gel Stent by Cell Debris in Primary Open-angle Glaucoma: A New Insight into the Pathophysiology of Filtration Device Failure. J Curr Glaucoma Pract 2019;13(3):113–115.
Keywords: Blockage, Glaucoma, Glaucoma surgery, Hyphema, Microinvasive glaucoma surgery, Obstruction, Red blood cells, XEN
BACKGROUND
In recent years, microinvasive glaucoma surgery (MIGS) has emerged as a safer surgical alternative to traditional filtering surgery in mild-to-moderate glaucoma.1 Amongst MIGS options, the XEN gel stent (Allergan Plc, Dublin, Ireland) is 6 mm-long tubes, made of crosslinked gelatin.2 They were designed to be used ab interno to create a drainage canal through the trabecular meshwork between the anterior chamber (AC) and the subconjunctival space. Its 45 μm internal diameter was calculated using Hagen–Poiseuille equation to minimize the risk of hypotony.3 However, despite a good safety profile, the very small lumen of the XEN gel stents has a certain propensity to become obstructed. The exact causes and predicting factors for such obstructions still require further research.4,5
Hyphema has long been known as a potential cause of secondary glaucoma.6 This is assumed to result from clogging of the trabecular meshwork by red blood cells (RBCs) by-products and subsequent fibrotic changes.7 Trabecular obstruction secondary to AC hemorrhages has been observed following several routine ophthalmic procedures and microtrauma;8,9 however, to date, they have not been associated with XEN gel stent obstruction.
CASE DESCRIPTION
In this report, we describe the case of 55-year-old lady of Central African origin with primary open-angle glaucoma (POAG) who underwent bilateral XEN gel surgery.
Initial Presentation
The patient was initially referred by her general ophthalmologist to a tertiary glaucoma center with intraocular pressures (IOP) of 38 mm Hg and 22 mm Hg in the right and the left eyes, respectively. She had been diagnosed with POAG several years before and had been managed with two selective laser trabeculoplasties (SLT) and maximal medical therapy including oral acetazolamide (Vifor Pharma, Bern, Switzerland), despite mediocre self-reported compliance. She had a positive family history for open-angle glaucoma in her mother. At the time of presentation, her best-corrected visual acuity was 10/10 in both eyes on a decimal chart (6/6 Snellen) in slightly myopic eyes (−0.75 D and −1.25 D spherical equivalent in the right and the left eyes, respectively). Slit lamp examination revealed a deep and quiet AC and a clear crystalline lens. Cup/disk ratio was 0.8 in the right eye with an inferior notch and 0.7 in the left eye with a superior notch. Gonioscopy showed bilaterally open angles. Pachymetry was 580 μm and 585 μm in the right and the left eyes, respectively. Automated visual field examination (Octopus, Haag Streit, Koeniz, Switzerland) showed bilateral nasal quadrantanopsia. Optical coherence tomography imaging (Spectralis OCT, Heidelberg Engineering AG, Germany) of the retinal nerve fiber layer (RNFL) showed generally reduced RNFL thicknesses bilaterally, with complete atrophy of the RNFL inferiorly in the left eye. Brain MRI imaging was unremarkable.
XEN Gel Surgery and Follow-up
Bilateral mitomycin C-augmented XEN gel stent implantation was organized with a target IOP ≤18 mm Hg. Surgeries were performed following standard protocols described in the literature. No intraoperative complications were noted, and the patient received a standard postoperative treatment of topical combined tobramycin and dexamethasone (Novartis Pharma, Basel, Switzerland) in decreasing regime. The right eye recovered uneventfully, with IOP normalizing between 12 mm Hg and 16 mm Hg at 3 months, with no additional therapy. The left eye developed a 2-mm hyphema on the first day following surgery, associated with dense RBC in the AC and corneal edema. The initial intraocular pressure was 2 mm Hg, which gradually improved with scopolamine (OmniVision Pharma, Puchheim, Germany) to 9 mm Hg at day 3. After 8 days, the hyphema had completely resolved and IOP was stable at 12 mm Hg. After 1 month, the patient presented to her postoperative follow-up with an IOP of 50 mm Hg in the left eye. On examination, the filtration bleb was shallow but diffuse, the iridocorneal angle was open, the XEN gel stent was well-positioned, and no clear obstruction was visible on its intraocular tip.
XEN Gel Stent Obstruction
Intraocular pressure was reduced using topical and intravenous medications, and an emergency revision procedure was organized in theater. The absence of filtration through the stent was confirmed intraoperatively and the blocked tube was removed. It was sent for macroscopic analysis to confirm the cause of the obstruction and was replaced by a new XEN gel implant. The patient made good recovery without any postoperative complications, and at 1-month, her IOP was stable at 17 mm Hg.
The macroscopic examination confirmed obstruction of the XEN gel stent on its AC extremity, with translucent cell fragments (Fig. 1). No fibrin, blood clot, or other type of tissue could be identified within the obstructed tube.
Fig. 1.
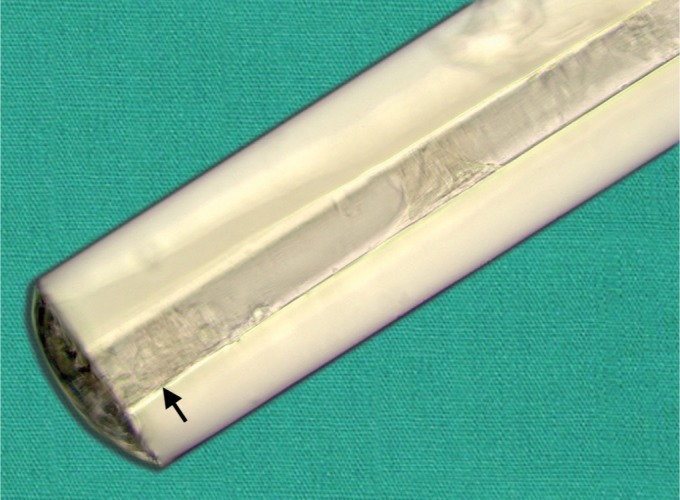
Macroscopic view of the proximal tip of an obstructed XEN gel stent (Allergan Plc, Dublin, Ireland). Multiple cell fragments can be seen within the 45 mm-wide lumen, with a dense aggregate around its anterior chamber opening (arrow) (×126 microscopy, unstained)
DISCUSSION
This case report confirms cell fragments as a potential cause of XEN gel stent occlusion. In this specific case, the exact nature of the cells debris could not be clearly identified microscopically, and they could originate from any type of cells present within the aqueous. In our opinion, however, several factors draw strong suspicions on the theory of the RBC origin: out of the three XEN gel stent implanted in this patient's eyes, two achieved good and durable IOP control, while one became obstructed within just a few weeks of implantation. The only notable difference between the three procedures was the presence of a hyphema and dense AC RBC following the implantation of the stent that did eventually fail. Interestingly, this suggests that AC RBC can present a potential threat to XEN gel stents’ patency, even in cases when the bleeding was minimal and self-limited and where the IOP was still controlled at the time of full hyphema resolution, similarly to what has been observed in sickle cells and ghost cell glaucoma.10–12 Clinically, this observation leads to several recommendations. First, unlike fibrin plugs that are usually visible under gonioscopic examination,11 cell fragments are translucent and can easily mimic a filtering bleb obstruction that would not, however, respond to needling revision. Second, the success rate of XEN gel surgery could potentially be influenced by higher bleeding risks; thus, patients with known bleeding disorders or anticoagulation therapy should be managed accordingly to minimize the risk of postoperative hyphema. Finally, patients presenting with postoperative hyphema should be carefully monitored for XEN gel stent obstruction, as erythrocyte resorption studies have shown that, while 50–70% of aqueous RBC are gradually reabsorbed into the blood stream, over a third of the cells gradually break down within the AC over days.13 Hence, stent blockage by RBC by-products can develop within 1–9 weeks of an intraocular hemorrhage and can cause a delayed rise in IOP.14 More evidence will be needed, however, to determine if early therapeutic actions like an AC washout could reduce the risk of developing subsequent stent obstruction.
CONCLUSION
In conclusion, this report confirms that XEN gel stents can become obstructed from cell fragments. It appears that postoperative AC bleed could be a potential risk factor for such type of stent blockage, and every effort should be made to minimize the risk of perioperative bleed. More research is needed to determine if and when AC washout should be performed, in the event of an hyphema, to reduce the risk of XEN gel stent obstruction.
CLINICAL SIGNIFICANCE
This case highlights some previously unreported risk factors for XEN stent obstruction and suggests that stricter control of bleeding and monitoring of patients following hyphema could improve surgical outcome.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Yook E, Vinod K, Panarelli J. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147–154. doi: 10.1097/ICU.0000000000000457. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary A, Salinas L, Guidotti J, et al. XEN gel implant: a new surgical approach in glaucoma. Expert Rev Med Devices. 2017;15(1):47–59. doi: 10.1080/17434440.2018.1419060. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Mansouri K, Guidotti J, Rao H, et al. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN gel implant surgery: 1-year results. J Glaucoma. 2018;27(2):140–147. doi: 10.1097/IJG.0000000000000858. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Dervenis N, Mikropoulou A, Dervenis P, et al. Dislocation of a previously successful XEN glaucoma implant into the anterior chamber: a case report. BMC Ophthalmol. 2017;17(1):148. doi: 10.1186/s12886-017-0540-1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillmann K, Mansouri K, Bravetti GE, et al. Chronic intraocular inflammation as a risk factor for XEN gel stent occlusion: a case of microscopic examination of a fibrin-obstructed XEN stent. J Glaucoma. 2018;27(8):739–741. doi: 10.1097/IJG.0000000000001002. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Sihota R, Kumar S, Gupta V, et al. Early predictors of traumatic glaucoma after closed globe injury. Arch Ophthalmol. 2008;126(7):921–926. doi: 10.1001/archopht.126.7.921. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Gragg J, Baker MB. Hyphema. StatPearls. Treasure Island (FL): StatPearls Publishing; 2018. [Google Scholar]
- 8.Thompson JM, Chang JS, Bermudez-Magner JA, et al. Ghost cell glaucoma following sutureless scleral-fixated posterior chamber intraocular lens placement. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):111–113. doi: 10.3928/23258160-20150101-22. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Elgin U, Sen E, Teke MY, et al. Microtrauma-induced recurrent hyphema and secondary glaucoma associated with chronic acetylsalicylic acid use. Int Ophthalmol. 2012;32(1):89–92. doi: 10.1007/s10792-012-9517-5. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Goldberg MF. The diagnosis and treatment of secondary glaucoma after hyphema in sickle cell patients. Am J Ophthalmol. 1979;87(1):43–49. doi: 10.1016/0002-9394(79)90190-9. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Saldanha C. Human erythrocyte acetylcholinesterase in health and disease. Molecules. 2017;22(9):E1499. doi: 10.3390/molecules22091499. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman EA, Mousa A, Al-Mansouri SM, et al. Glaucoma after open-globe injury at a tertiary care university hospital: cumulative causes and management. J Glaucoma. 2016;25(3):e170–e174. doi: 10.1097/IJG.0000000000000162. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Hørven I. Erythrocyte resorption from the anterior chamber of the human eye. Acta Ophthalmol (Copenh) 1963;41:402–412. doi: 10.1111/j.1755-3768.1963.tb03549.x. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Campbell DG, Simmons RJ, Grant WM. Ghost cells as a cause of glaucoma. Am J Ophthalmol. 1976;81(4):441–450. doi: 10.1016/0002-9394(76)90299-3. DOI: [DOI] [PubMed] [Google Scholar]


