Endoscopic submucosal dissection (ESD) allows for en bloc resection of large superficial lesions throughout the GI tract.1,2 Adequate visualization of the submucosal dissection plane is critically important for both safety and efficacy in ESD. In traditional surgery, an assistant provides effective countertraction. However, this is not feasible with endoscopic resection, wherein countertraction is naturally absent, aside from the effects of gravity acting on the partially resected lesion.
Multiple devices and techniques have been proposed and investigated to enhance visualization of the submucosal space. Internal traction methods include the use of a nylon suture,3 the dental floss-clip traction (or clip-line) method,4,5 the classic clip-rubber band method,6,7 a spring-action S-O clip,8 and the suture pulley method.9,10 External traction devices include large double-balloon devices and robotic platforms.
Technique
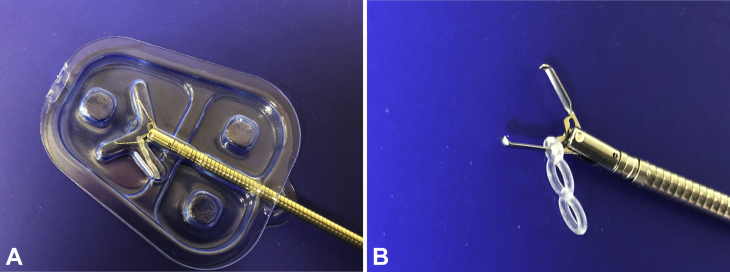
Recently, a novel clip-band traction device was introduced to the United States market (Elastic Traction Device; Micro-Tech Endoscopy USA Inc, Ann Arbor, Mich, USA), specifically indicated for providing retraction in endoscopic resection (Fig. 1). The device consists of 3 interconnected elastic silicone bands (one 1.5-mm ring and two 3.3-mm rings), preloaded onto a standard 11-mm hemostatic clip. The device has a working length of 235 cm, requires a working channel of ≥2.8 mm, and is compatible with standard gastroscopes and colonoscopes.
Figure 1.
The clip-band device. A, The clip-band traction device in its package. B, Open position.
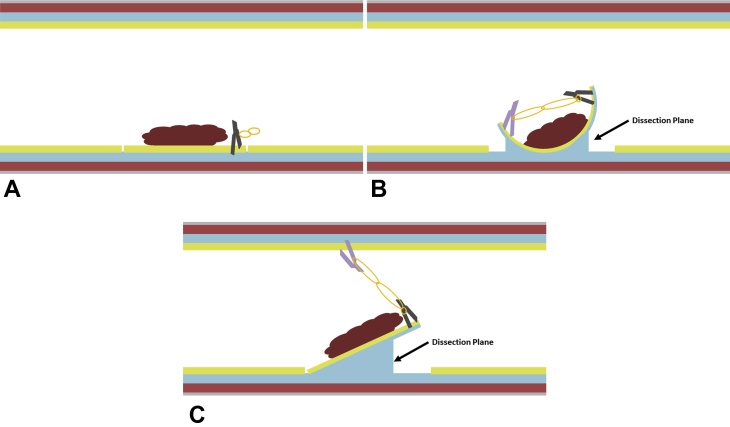
There are 2 potential countertraction strategies (Fig. 2). A full circumferential mucosal incision is first made before device placement. In the manufacturer-specified strategy, the clip-band device is deployed along the mucosal edge. The other end of the elastic band is grasped with a second hemostatic clip, which is then deployed along the mucosal edge on the opposite side of the lesion. This provides countertraction in an edge-toward-center direction. After the resection is complete, both clips are removed along with the specimen.
Figure 2.
The clip-band countertraction technique. A, The clip-band device is deployed along the edge of the lesion. B, In the manufacturer-specified strategy, the second clip is deployed along the opposite edge of the lesion, thus providing countertraction in an edge-toward-center direction. C, In the alternative strategy, the second clip is deployed along the opposite wall of the lumen, thus providing countertraction in an upward/away direction from the area of dissection.
In an alternative “traditional ESD countertraction” strategy, the clip-band device is also deployed along the mucosal edge. The other end of the elastic band is grasped with a second hemostatic clip, which is then deployed along the opposite wall of the lumen, slightly distal from the first clip. This provides countertraction in an upward-and-away direction from the area of dissection. After the resection is complete, the lesion is suspended by the second clip, which can be gently removed from the luminal wall with rat-toothed forceps.
Here, we demonstrate both countertraction strategies, and we also introduce a novel defect closure technique using the clip-band traction device (Video 1, available online at www.VideoGIE.org).
Strategy 1: 2 clips on opposite sides of lesion
A 54-year-old woman was referred for resection of a 25-mm flat lesion: a laterally spreading tumor, nongranular (LST-NG) (pseudodepressed, Paris IIa+IIc) at the hepatic flexure. Submucosal lifting was performed with a 6% hetastarch solution. An injectable needle-type ESD knife (DualKnife-J; Olympus America, Center Valley, Pa, USA) was used to complete a full circumferential mucosal incision. The clip-band device was then deployed at the submucosal layer along the anal side of the lesion. Next, the elastic band was clipped to the oral side of the lesion (Fig. 3). The traction provided by the clip-band device revealed a sufficient submucosal dissection plane and allowed en bloc endoscopic resection without perforation. The specimen measured 35 × 35 mm, and the pathologic diagnosis was tubular adenoma with high-grade dysplasia. The margins were negative; thus, complete and curative resection was obtained. The resection was completed in 25 minutes, with a calculated resection speed of 23.1 cm2/hour. The patient was discharged home after the procedure and had an uneventful postoperative course.
Figure 3.
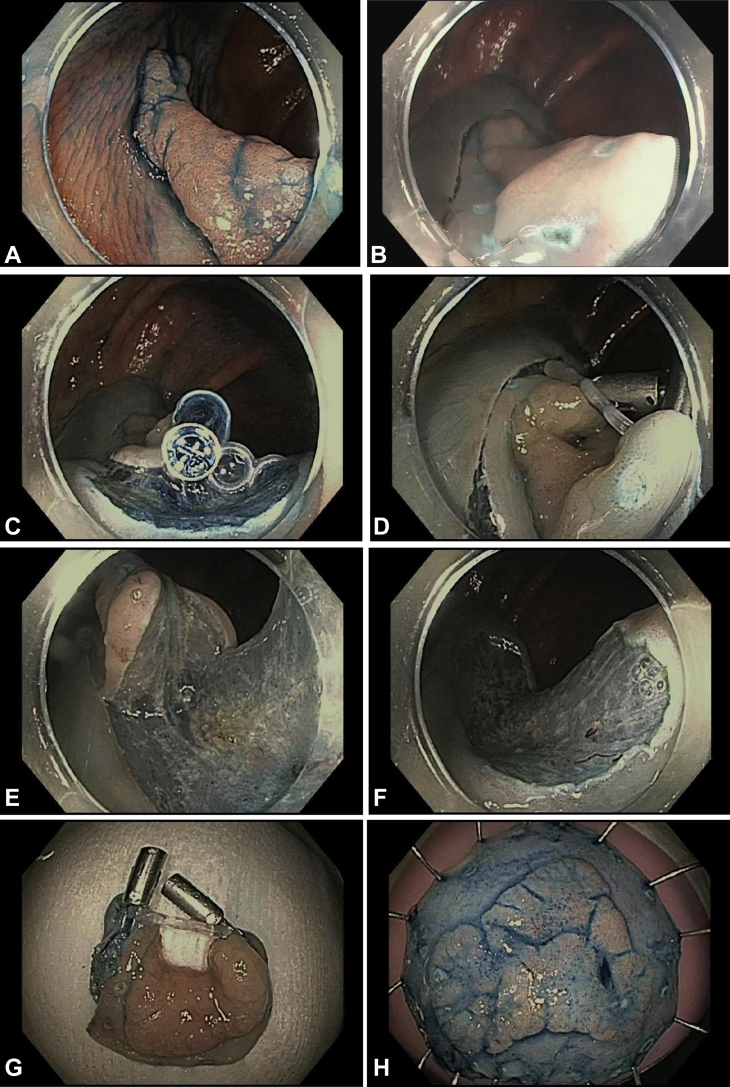
Strategy 1, with 2 clips on opposite sides of the lesion. A, The patient was referred for resection of a 25-mm laterally spreading tumor at the hepatic flexure. B, A full circumferential incision was initially made. C, The clip-band device was deployed at the submucosal layer at the anal side of the lesion and (D) clipped to the opposite mucosal edge, which revealed (E) a suitable dissection plane. F, Endoscopic appearance of resection defect. G, H, Specimen after en bloc resection.
Strategy 2: first clip on edge of lesion, second clip on opposite wall
A 70-year-old man with a history of surgical hemorrhoidectomy was referred for resection of a massive bulky rectal polyp (LST-granular [mixed, Paris Is+IIa]), spanning three-quarters of the rectal circumference and extending from the dentate line proximally across 2 rectal valves. ESD was performed with the patient under general endotracheal anesthesia because of the length and complexity of the procedure. Submucosal lifting was performed with a 6% hetastarch solution, starting from the anal side of the lesion. An injectable needle-type ESD knife (DualKnife-J; Olympus America) with the assistance of a tapered-tip cap (ST Hood; Fujifilm Medical Systems, Stamford, Conn, USA) was used to create a mucosal incision, and ESD was started by the pocket creation method. Dense submucosal fibrosis was immediately encountered, along with surgical clips from the patient’s prior hemorrhoidectomy. After as much submucosal dissection was completed as possible, additional countertraction was necessary to overcome the sheer size and weight of the lesion. The clip-band device was deployed along the mucosal edge of the lesion and clipped to the opposite wall, thus revealing a suitable dissection plane without requiring the patient to be repositioned (Fig. 4). Submucosal dissection was able to be continued; however, eventually additional countertraction was necessary to move the bulky lesion away from the working area. A second clip-band device was deployed along the anal margin of the lesion and clipped to the opposite wall in the proximal part of the rectum. This revealed a final dissection plane and allowed en bloc endoscopic resection without perforation. The specimen measured 100 × 90 mm, and the pathologic diagnosis was moderately differentiated submucosal adenocarcinoma (T1b) with lymphovascular invasion. The margins were negative; however, given the lymphovascular invasion, the patient was ultimately referred for colorectal surgery. The resection was completed in 4 hours and 59 minutes, with a calculated resection speed of 14.2 cm2/hour. The patient was discharged home after the procedure and had an uneventful postoperative course.
Figure 4.
Strategy 2, with 1 clip on the edge of the lesion and the second clip on the opposite wall. A, The patient was referred for resection of a 100-mm bulky laterally spreading tumor in the rectum, extending from the dentate line across 2 rectal folds. B, The pocket creation method was used for submucosal dissection, which notably encountered dense submucosal fibrosis because of prior surgical hemorrhoidectomy. C, After extensive dissection, additional countertraction was necessary to overcome the sheer size of the lesion. The clip-band device was deployed along the mucosal edge of the lesion and (D) clipped to the opposite wall, which revealed (E) a dissection plane. After additional dissection, additional countertraction was necessary to move the lesion away from the working area. F, A second clip-band device was deployed along the anal margin of the lesion and clipped to the opposite wall in the proximal rectum, which revealed a dissection plane and allowed completion. G, Endoscopic appearance of resection defect. H, Specimen after en bloc resection.
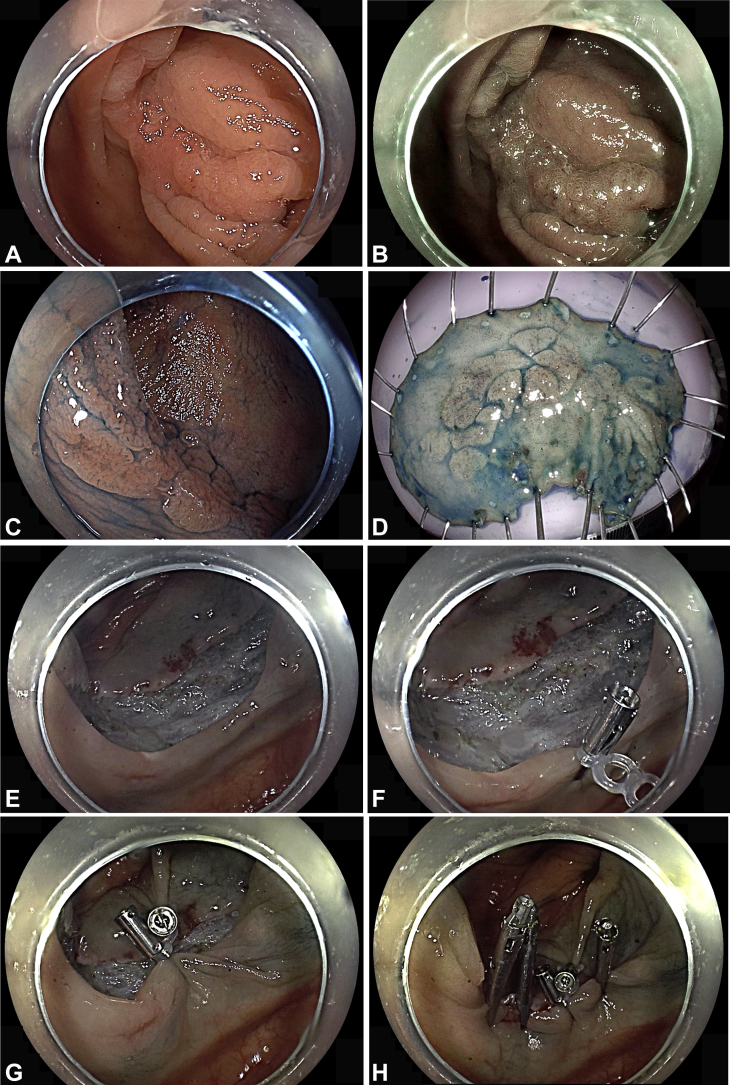
Defect closure by use of the clip-rubber band device
A 78-year-old man who was taking warfarin because of a history of pulmonary embolism was found to have a 60- × 20-mm carpeted lesion (LST-NG) (flat elevated, Paris IIa) in the ascending colon. The patient was heparinized before ESD, and the heparinization was required to be restarted immediately after ESD. After completion of ESD, all visible vessels were cauterized with hemostatic forceps (Coagrasper; Olympus America). Then, defect closure was performed with the clip-band device (Fig. 5). First, the device was deployed at the anal side of the defect. Then, the inner 3.3-mm elastic band was grasped with another clip and was fixed at the oral side of the defect for approximation of the short axis of the defect. This approximation facilitated complete clip closure of the large resection defect. Anticoagulation was restarted immediately after ESD. The patient was discharged home after overnight observation, and he had an uneventful postoperative course.
Figure 5.
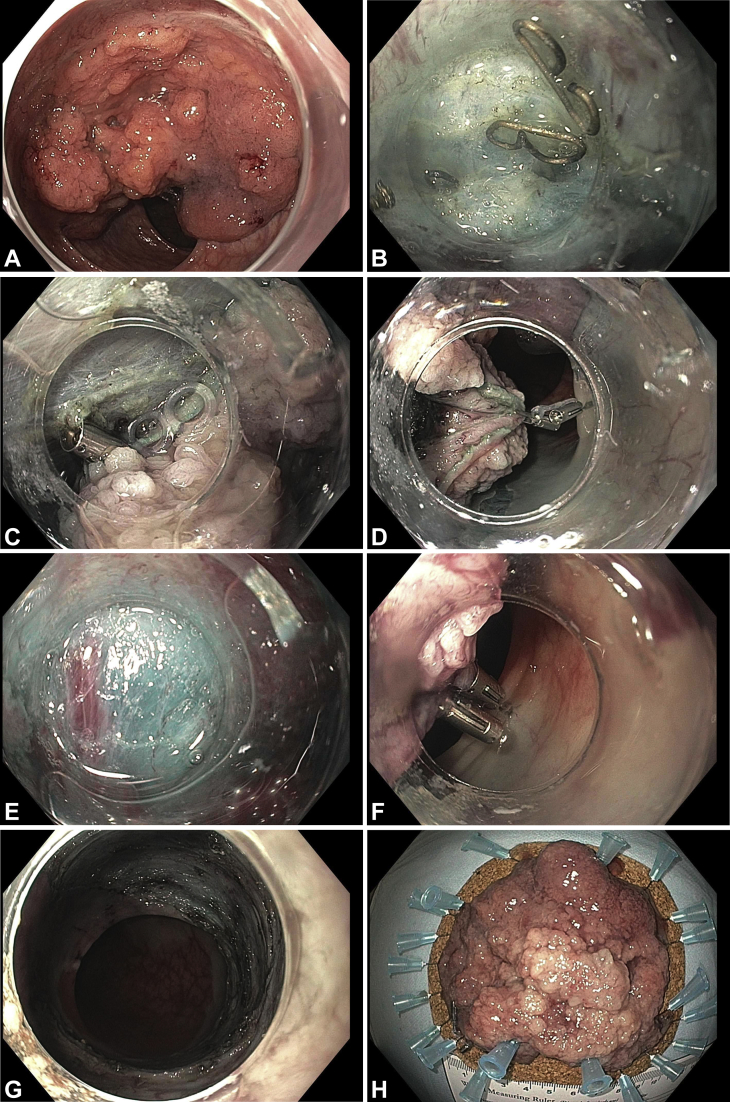
Defect closure. A, The patient was referred for resection of a 60-mm laterally spreading tumor at the hepatic flexure, as seen on white-light imaging (B), blue-light imaging, and (C) chromoendoscopy with indigo carmine. Endoscopic submucosal dissection (ESD) was performed. D, Resected specimen measuring 70 × 30 mm. E, Mucosal defect after ESD. F, The clip-band device was deployed at the anal side of the defect. G, A second clip was deployed at the oral side of the defect for approximation. H, Complete clip closure of the resection defect.
Discussion
The rubber band traction technique was previously described to facilitate ESD by use of a self-made device involving a clip and a standard rubber band.6,7 Recently, a dedicated clip-elastic band device has become available in the United States market, specifically indicated for providing retraction in endoscopic tissue resection. As demonstrated above, effective countertraction was obtained in both strategies, allowing for successful en bloc resection without perforation. A major advantage of the device is that it decreases the reliance on gravity because ESD patients in the United States are often sedated under general endotracheal anesthesia and are thus difficult to reposition.
Defect closure after colorectal ESD is often challenging because ESD defects are often prohibitively large for standard clip closure, and an endoscopic suturing device mounted on a double-channel gastroscope typically cannot reach the right colon segment. Several strategies have been previously reported to facilitate clip closure in ESD, including a loop-clip closure technique that uses a clip and looped nylon string,11 line-assisted clip technique,12 endoloop-assisted clip technique,13 and placement of small incisions around the mucosal defect to provide a better grip for clipping.14 The use of a double-balloon countertraction device has been reported to facilitate endoscopic suturing in the right colon segment by using the device as a conduit.15,16 However, the combination of the double-balloon overtube and suturing device is expensive and not cost effective, considering the current suboptimal reimbursement for ESD. As demonstrated in the video, the clip-band device allows for efficient approximating and subsequently facilitating the clip closure of large resection defects.
Both countertraction strategies with this clip-band device have their respective advantages and disadvantages. With the manufacturer-specified strategy (strategy 1, with 2 clips on opposite sides of the lesion), the advantage is that both clips are still attached to the en bloc specimen; however, countertraction is slowly being lost during submucosal dissection and is virtually absent by the time resection is complete. With the “traditional” countertraction strategy (strategy 2, with 1 clip on the edge of the lesion and the second clip on the opposite wall), the advantage is the ability to provide a stronger and directed countertraction force (with option for using multiple clips); however, the second clip needs to be pulled off the luminal wall after the resection is complete.
In conclusion, the clip-band traction device provides versatile and effective countertraction and a secure defect closure in colorectal ESD. In an environment where numerous countertraction strategies exist, including large expensive devices, the simple clip-band device represents a low-cost yet valuable option to providing effective countertraction and defect closure. Future prospective studies are necessary to compare different modalities of tissue retraction and defect closure in ESD with regard to clinical efficacy and cost effectiveness.
Disclosure
Dr Aihara is a consultant for Boston Scientific, Olympus America, and Fujifilm Medical Systems. Dr Ge disclosed no financial relationships relevant to this publication.
Footnotes
If you would like to chat with an author of this article, you may contact Dr Aihara at haihara@bwh.harvard.edu.
Supplementary data
The use of a clip-band traction device to facilitate colorectal endoscopic submucosal dissection and defect closure.
References
- 1.Ge P.S., Jirapinyo P., Ohya T.R. Predicting outcomes in colorectal endoscopic submucosal dissection: a United States experience. Surg Endosc. 2019;33:4016–4025. doi: 10.1007/s00464-019-06691-4. [DOI] [PubMed] [Google Scholar]
- 2.Akintoye E., Kumar N., Aihara H. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E1030–E1033. doi: 10.1055/s-0042-114774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P.J., Chu H.C., Chang W.K. Endoscopic submucosal dissection with internal traction for early gastric cancer (with video) Gastrointest Endosc. 2008;67:128–132. doi: 10.1016/j.gie.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Abe S., Oda I., Suzuki H. A challenging case of gastric endoscopic submucosal dissection: removal of a sizable cancer through altering patient's position and multiple clip-line traction. VideoGIE. 2019;4:558–560. doi: 10.1016/j.vgie.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida M., Takizawa K., Suzuki S. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video) Gastrointest Endosc. 2018;87:1231–1240. doi: 10.1016/j.gie.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto K., Nagahara A., Ueyama H. Development and clinical usability of a new traction device "medical ring" for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2013;27:3444–3451. doi: 10.1007/s00464-013-2887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parra-Blanco A., Nicolas D., Arnau M.R. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video) Gastrointest Endosc. 2011;74:1137–1141. doi: 10.1016/j.gie.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto R., Hirasawa D., Iwaki T. Usefulness of the S-O clip for gastric endoscopic submucosal dissection (with video) Surg Endosc. 2018;32:908–914. doi: 10.1007/s00464-017-5765-9. [DOI] [PubMed] [Google Scholar]
- 9.Aihara H., Abidi W., Thompson C.C. Countertraction in endoscopic submucosal dissection. VideoGIE. 2016;1:36–37. doi: 10.1016/j.vgie.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge P.S., Thompson C.C., Jirapinyo P. Suture pulley countertraction method reduces procedure time and technical demand of endoscopic submucosal dissection among novice endoscopists learning endoscopic submucosal dissection: a prospective randomized ex vivo study. Gastrointest Endosc. 2019;89:177–184. doi: 10.1016/j.gie.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto N., Beppu K., Matsumoto K. "Loop clip", a new closure device for large mucosal defects after EMR and ESD. Endoscopy. 2008;40:E97–E98. doi: 10.1055/s-2007-995604. [DOI] [PubMed] [Google Scholar]
- 12.Kato M., Takeuchi Y., Yamasaki Y. Technical feasibility of line-assisted complete closure technique for large mucosal defects after colorectal endoscopic submucosal dissection. Endosc Int Open. 2017;5:E11–E16. doi: 10.1055/s-0042-121002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Q., Chen T., Zhong Y.S. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329–334. doi: 10.1055/s-0032-1326214. [DOI] [PubMed] [Google Scholar]
- 14.Otake Y., Saito Y., Sakamoto T. New closure technique for large mucosal defects after endoscopic submucosal dissection of colorectal tumors (with video) Gastrointest Endosc. 2012;75:663–667. doi: 10.1016/j.gie.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Kantsevoy S.V., Wagner A., Mitrakov A.A. Rectal reconstruction after endoscopic submucosal dissection for removal of a giant rectal lesion. VideoGIE. 2019;4:179–181. doi: 10.1016/j.vgie.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S., Momose K., Hara H. Facilitating endoscopic submucosal dissection: double balloon endolumenal platform significantly improves dissection time compared with conventional technique (with video) Surg Endosc. 2019;33:315–321. doi: 10.1007/s00464-018-6336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The use of a clip-band traction device to facilitate colorectal endoscopic submucosal dissection and defect closure.