The research presented in this paper explores the role of the DNA-binding protein Dps as a key defense mechanism of enterohemorrhagic Escherichia coli (EHEC) strains in protecting against killing by the novel antimicrobial peptide WRWYCR. Our results demonstrate that Dps protects against peptide-induced killing of EHEC through direct protection against acid stress and hydroxyl radical formation, both of which are mechanisms targeted by the antimicrobial peptide. This study provides important insights into peptide WRWYCR-mediated killing of EHEC, which could be exploited in the development of more effective antimicrobials through specific targeting of Dps in order to allow a more potent response to the antimicrobial WRWYCR.
KEYWORDS: enterohemorrhagic E. coli, Dps, antimicrobials, acid resistance, DNA damage, dps, EHEC O157:H7, antimicrobial peptide
ABSTRACT
Dps, a DNA-binding protein from starved cells in Escherichia coli, is part of the bacterial defense system that protects DNA against various cellular stresses. Our lab previously demonstrated that a novel antimicrobial peptide, WRWYCR, enhances acid-induced killing of enterohemorrhagic Escherichia coli (EHEC) and ameliorates infection in a Citrobacter rodentium mouse model of EHEC infection. WRWYCR has previously been shown to compromise DNA damage repair and to increase chelatable iron within the cell. These findings, combined with the effects of peptide and acid stress on DNA damage, suggest a key defense role for Dps in peptide-induced killing of EHEC. The goal of this study is to evaluate the role of Dps in peptide-induced killing of EHEC through survival assays and flow cytometric analyses of DNA damage and hydroxyl radical formation. Our results demonstrate that disruption of the dps gene in stationary-phase EHEC O157:H7 cells, but not in exponential-phase cells, enhances acid-, peptide-, and peptide-acid-induced killing relative to that of wild-type (WT) EHEC. Using flow cytometric analysis, we have also demonstrated increased levels of hydroxyl radicals in peptide-treated wild-type EHEC relative to those in the untreated control. Disruption of the dps gene further increases this. These findings indicate that peptide treatment of EHEC enhances the formation of hydroxyl radicals, likely through the Fenton reaction, thereby contributing to the killing action of the peptide, and that dps protects against peptide killing of EHEC. This study provides important insights into peptide WRWYCR-mediated killing of EHEC, which could be exploited in the development of more effective antimicrobials.
IMPORTANCE The research presented in this paper explores the role of the DNA-binding protein Dps as a key defense mechanism of enterohemorrhagic Escherichia coli (EHEC) strains in protecting against killing by the novel antimicrobial peptide WRWYCR. Our results demonstrate that Dps protects against peptide-induced killing of EHEC through direct protection against acid stress and hydroxyl radical formation, both of which are mechanisms targeted by the antimicrobial peptide. This study provides important insights into peptide WRWYCR-mediated killing of EHEC, which could be exploited in the development of more effective antimicrobials through specific targeting of Dps in order to allow a more potent response to the antimicrobial WRWYCR.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) strains represent a class of pathogenic E. coli strains resulting in foodborne illness that is typically associated with clinical symptoms such as abdominal cramps, diarrhea, and hemorrhagic colitis, and which may progress to the fatal systemic sequela known as hemolytic uremic syndrome (HUS) (1–4). There are approximately 95,000 cases of EHEC O157:H7-associated illnesses, 2,000 hospitalizations, and 50 to 60 deaths each year in the United States alone (5–7). The high number of infections can be attributed both to the low infectious dose of EHEC, which is less than 100 cells, and to its high transmissibility (5, 6, 8). These, combined with the limited treatment options currently available, have led to an increased interest in the development of new strategies for EHEC infection, in particular of prevention strategies that target EHEC before it is able to colonize its host.
Our laboratory previously demonstrated that the d-amino acid hexapeptide WRWYCR enhances acid-induced killing of EHEC and ablates infection in a Citrobacter rodentium mouse model of EHEC infection (9, 10). WRWYCR has previously been shown to bind to Holliday junctions during bacterial DNA repair and to prevent their resolution, thereby causing bacterial cell death (11, 12). In an independent pathway, WRWYCR has also been shown to increase chelatable iron within the cell, suggesting that it may promote the formation of hydroxyl radicals through the Fenton reaction, similarly to the action of traditional bactericidal antibiotics (13). While these independent effects of peptide WRWYCR treatment on bacterial cells have been previously characterized, the relationship between the peptide’s different roles in interfering with DNA damage and altering iron availability within the cell is poorly understood.
Of interest is the DNA-binding protein from starved cells in E. coli, Dps, which is an integral part of the bacterial defense system that protects DNA against a variety of assaults (14–16). Specifically, Dps employs a dual mechanism for protection of DNA that involves both physical and chemical modes of protection. Dps can directly bind DNA, although with no apparent sequence specificity, to form a highly stable complex that is resistant to acid and base shock, radiation, and oxidative stress (14, 17–21). The chemical mode of protection exhibited by Dps is attributed to its ferroxidase activity, which indirectly protects DNA through the removal of free Fe2+ from the cytoplasm, thereby reducing the formation of highly reactive oxygen species (hROS) (14, 22, 23).
Together, these modes of protection by Dps, combined with the mechanisms of action of the peptide WRWYCR, suggest a key defense role for Dps in peptide- and peptide-acid-induced killing of EHEC. Consequently, Dps may provide a unique link between the different effects of peptide treatment. This study aims to determine the role of Dps as a protective mechanism during acid, peptide, and peptide-acid treatment of EHEC through its DNA-binding and ferroxidase center abilities.
RESULTS
Peptide WRWYCR behaves as a bactericidal antibiotic.
In order to compare the mode of action of peptide WRWYCR to those of bacteriostatic and bactericidal antibiotics, survival assays of the EHEC EDL933 wild type (WT) were conducted. In the absence of antibiotics, there was a 1.5-log increase in the CFU per milliliter of EHEC during the 3-h growth period. Addition of spectinomycin (a bacteriostatic antibiotic) blocked the growth of bacteria, while addition of either kanamycin (a bactericidal antibiotic) or the antimicrobial peptide WRWYCR caused a significant 5-log decrease in bacterial counts (Fig. 1A).
FIG 1.
Comparison of peptide WRWYCR-induced killing to that of bactericidal and bacteriostatic antibiotics. (A) Survival of stationary-phase EHEC EDL933 in MHB broth containing either no antibiotics, 5 μg/ml kanamycin (bactericidal), 400 μg/ml spectinomycin (bacteriostatic), or 50 μM peptide WRWYCR for 3 h at 37°C, with shaking. An asterisk indicates a significant difference compared to the control sample at each time point; P < 0.05 by 2-way analysis of variance (ANOVA) and Tukey’s multiple-comparison test. Data points represent means ± standard errors of the means (SEMs), n = 2. (B) Flow cytometric analysis of hydroxyl radical formation using hydroxyphenyl fluorescein (HPF) for untreated EHEC (baseline); EHEC exposed to the positive control, 10 mM H2O2; and EHEC exposed to PBS containing either no antibiotics, 5 μg/ml kanamycin (bactericidal), 400 μg/ml spectinomycin (bacteriostatic), or 50 μM peptide WRWYCR and incubated as above. A minimum of 100,000 cells were collected for each sample. Data were analyzed using BD-FACS Diva software version 8.0. Signal peaks refer to hydroxyphenyl fluorescein (HPF)-positive cells. Data from single experiment representative of 3 independent trials. (C) Quantified HPF signal showing the median fluorescence intensity (MFI) ± SEMs from flow cytometric results of 3 independent trials, each with 3 technical replicates. Data for the H2O2 signal and the three antimicrobial treatments were normalized to the untreated control. An asterisk indicates a significant difference compared to the untreated control (UT). P < 0.05 by 2-way ANOVA.
The peptide WRWYCR has been previously shown to increase chelatable iron in E. coli, which has been suggested to occur through the cleavage of iron-sulfur clusters (13). It has also been reported that cleavage of these clusters releases Fe2+ ions into the cell, which, when oxidized, can lead to formation of hydroxyl radicals through Fenton chemistry (24). We therefore examined the formation of hydroxyl radicals in each treatment using flow cytometric analysis and the dye hydroxyphenyl fluorescein (HPF), which is oxidized by hydroxyl radicals with high specificity (25). A solution of 10 mM hydrogen peroxide, known to induce hydroxyl radical formation via Fenton chemistry, was used as a positive control to observe cellular death (Fig. 1B and C). Similarly to the survival assay results described above, the flow cytometric analysis showed a significant increase in hydroxyl radical formation in cells treated with kanamycin and peptide WRWYCR but not in either untreated or spectinomycin-treated cells (Fig. 1B and C). This is consistent with previous findings that show an increase in hydroxyl radical formation in the presence of bactericidal but not bacteriostatic antibiotics (26). Together, these results indicate that peptide WRWYCR behaves similarly to the bactericidal antibiotic kanamycin in its abilities to induce both bacterial cell death and hydroxyl radical formation.
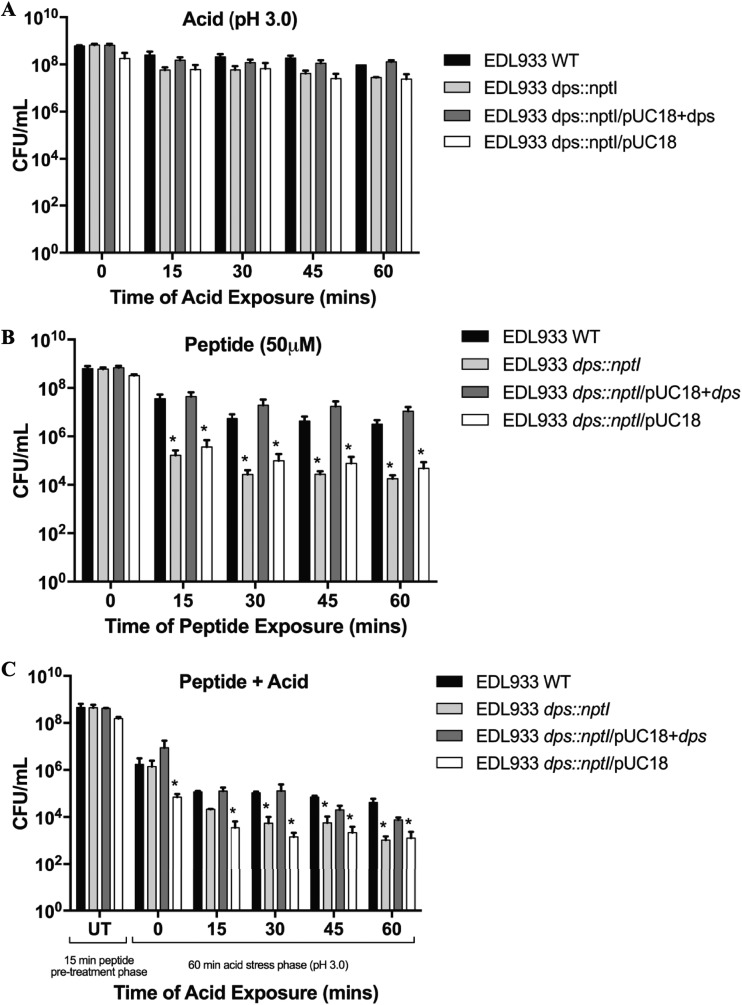
Disruption of dps makes EHEC hypersensitive to peptide and peptide-acid treatment during stationary growth.
To determine the role of Dps in acid, peptide, and peptide-acid treatment of EHEC, survival assays were carried out in the presence of each stress to compare the survival of wild-type EHEC EDL933 to those of the isogenic dps mutant and to the complemented dps mutant. Controls included the EHEC EDL933 WT with pUC18 vector (EDL933/pUC18) and the isogenic dps mutant containing pUC vector (EDL933 dps::nptI/pUC18). When the EHEC EDL933 WT was exposed to acid stress at a pH of 3.0, a time-dependent decrease in viability was seen, with 15% of the bacteria surviving 60 min of exposure (Fig. 2A). Disruption of dps further enhanced this decrease in survival by 1 log, with only 4% of bacteria surviving 60 min of acid exposure. Complementation of the dps gene restored survival to wild-type levels. There was no significant differences in the survival rates across all time points for the dps mutant compared to those for its vector control (EDL933 dps::nptI/pUC18) and, similarly, no significant differences in survival rates for the EDL933 WT compared to its vector control, EDL933 WT/pUC18 (data not shown). Differences in survival rates were more pronounced at a lower pH of 2.5, where a 2-log difference in survival was seen between the wild type and dps mutant (data not shown). However, the use of a pH of 3.0 rather than 2.5 permitted us to more easily see the additive effect of peptide plus acid treatment, which was an important focus for this study.
FIG 2.
Survival of EHEC following acid, peptide, or peptide-acid treatment during stationary growth. Survival assays showing CFU per milliliter after 1 h of acid (pH 3.0) stress (A), peptide WRWYCR (50 μM) treatment (B), or peptide-acid treatment (15-min peptide pretreatment at 50 μM followed by 1 h of acid stress [pH 3.0]) (C). Survival of EDL933 WT/pUC18 showed no significant differences from survival of the EDL933 WT under the same treatments (data not shown). An asterisk indicates a significant difference compared to the EDL933 WT; P < 0.05 by 2-way ANOVA. Data bars represent means ± SEMs, n = 3. Results are from 3 independent trials.
When EHEC was exposed to 50 μM peptide WRWYCR treatment, a 2-log decrease in survival was observed for WT bacteria (Fig. 2B). Disruption of dps enhanced this decrease in survival by 2 log, resulting in a total 4-log decrease in survival compared to t = 0, while complementation of dps restored survival to wild-type levels. Survival of the dps mutant containing the empty vector pUC18 matched that of the dps mutant strain, and, similarly, survival of the EDL933 WT matched that of the WT with empty vector. At each time point, the survival of the dps mutant and the dps mutant containing empty vector was significantly lower than the wild-type survival for the same time point.
Finally, when EHEC was pretreated with peptide WRWYCR for 15 min followed by a 60 min exposure to acute acid stress (pH 3.0), there was a greater decrease in survival compared to either acid or peptide treatment alone (Fig. 2C). Reduction in viability was 3.5 log for wild-type EHEC, while disruption of the dps gene further increased this to a 5.5-log decrease in survival at 60 min compared to t = 0. Complementation of dps restored survival to wild-type levels, while the presence of the empty vector resulted in survival rates similar to that of the dps mutant strain. The survival of the dps mutant and the mutant containing empty vector were significantly lower than wild-type survival at time points of 30, 45, and 60 min. Across all time points, survival of EDL933 WT/pUC18 showed no significant differences from survival of the EDL933 WT (data not shown).
Interestingly, when the same experiments were repeated with EHEC cells grown to the mid-exponential phase, there were no significant differences seen between the survival of WT bacteria and that of the dps mutant strain when exposed to either acid, peptide, or peptide-acid treatment (Fig. 3). The difference in response between EHEC cells in the mid-exponential and stationary phases is consistent with reports that Dps is maximally expressed during the stationary growth phase (18).
FIG 3.
Survival of EHEC following acid, peptide, or peptide-acid treatment during exponential growth. Survival assays showing CFU per milliliter after 1 h of acid (pH 3.0) stress (A), peptide WRWYCR (50 μM) treatment (B), or peptide-acid treatment (15-min peptide pretreatment at 50 μM followed by 1 h acid stress [pH 3.0]) (C). Survival of EDL933 WT/pUC18 showed no significant differences from survival of the EDL933 WT under the same treatment conditions (data not shown). An asterisk indicates a significant difference compared to the EDL933 WT; P < 0.05 by 2-way ANOVA. Data bars represent means ± SEMs, n = 3. Results are from 3 independent trials.
Taken together, these findings suggest that disruption of dps during the stationary phase of growth renders EHEC hypersensitive to acid, peptide, and peptide-acid treatment, consistent with previous studies indicating a protective role of Dps against cellular stress (14, 15, 18, 19).
Hydroxyl radical formation is increased following acid, peptide, and peptide-acid treatment of EHEC.
In order to evaluate the role of Dps in hydroxyl radical formation following either acid, peptide, or peptide-acid treatment, flow cytometric analysis with the dye HPF was employed using wild-type EHEC alongside a dps mutant strain. When the EHEC EDL933 WT was treated with peptide WRWYCR, a distinct increase in hydroxyl radical formation was observed compared to that in untreated cells. This increase was evident both in the histograms shown in Fig. 4A and in the results shown in Fig. 4B, where the median HPF signal significantly increased from 1.025 ± 0.127 in the untreated WT control (phosphate-buffered solution [PBS]) to 3.049 ± 0.057 in the peptide-treated WT sample. The increase in hydroxyl radical formation was more pronounced in the absence of dps, with the HPF signal increasing to 4.118 ± 0.089, which was significantly different compared to both the peptide-treated WT and the untreated mutant control. The dps-complemented strain displayed significantly lower levels of hydroxyl radical formation (3.180 ± 0.049) than the dps mutant, consistent with the expectation of levels more similar to those of the wild type. In the dps mutant containing only the pUC vector used to complement the mutant, the HPF signal was similar to that in the dps mutant (3.987 ± 0.090). Taken together, these results suggest that Dps plays an important role in protecting EHEC against peptide-induced hydroxyl radical formation.
FIG 4.
Hydroxyl radical formation in peptide-treated EHEC. (A) Flow cytometric analysis of hydroxyl radical formation using hydroxyphenyl fluorescein (HPF) following 50 μM peptide WRWYCR treatment of stationary-phase EHEC for 1 h at room temperature. Histogram peaks refer to HPF-positive cells. Representative of 3 independent trials, each with 3 technical replicates. (B) Quantified HPF signal is reported, showing the median MFI ± SEMs from the flow cytometric data, as in panel A. All data were normalized to t = 0 within each daily experiment. Results are representative of 3 independent trials, each with 3 technical replicates. An asterisk indicates a significant difference compared to the untreated control (UT). # indicates a significant difference compared to peptide-treated EDL933 WT. P < 0.05 by 2-way ANOVA.
DISCUSSION
This is the first study to date to demonstrate a relationship between peptide WRWYCR’s different antimicrobial roles through the protective mechanisms of the DNA-binding protein Dps. The data presented here demonstrate that treatment of EHEC with peptide WRWYCR induces bacterial cell death and increases hydroxyl radical formation to levels similar to those seen with the bactericidal, but not bacteriostatic, class of antibiotics. These results suggest that, similarly to other bactericidal antibiotics, peptide WRWYCR may act by breaking down iron-sulfur clusters within the bacterial cell to release Fe2+, thereby driving the Fenton reaction to form hROS, eventually leading to bacterial cell death (13, 26).
Hydroxyl radicals are generated through Fenton chemistry from the reaction of Fe2+ and hydrogen peroxide and are considered the most harmful oxidative agent for DNA, lipids, and proteins (27). All living cells express a multitude of enzymes that aim to protect against the toxic effects of hydroxyl radical formation, including Dps, which acts by using hydrogen peroxide as an iron oxidant, thereby significantly reducing the production of hydroxyl radicals (27).
When examining the role of Dps in acid, peptide, and peptide-acid treatment of EHEC, survival assays demonstrated that disruption of the dps gene made EHEC hypersensitive to acute acid stress (pH 3.0), peptide treatment (50 μM), or a combination of peptide-acid treatment during the stationary phase of growth but not during the exponential growth phase. Interestingly, the hypersensitivity of the dps mutant to acute acid exposure was profoundly enhanced at a lower pH of 2.5. Since both pH values tested are acidic enough to induce significant levels of DNA damage, the results are consistent with previous reports that Dps directly binds DNA to protect against acid-induced DNA damage (18, 28).
Similarly, the hypersensitivity of the dps mutant to peptide treatment can be explained by the bactericidal activity of the peptide, in which Fe2+ is released and leads to increased levels of hydroxyl radicals within the cell, as shown by flow cytometric analysis. Since dps expression is known to be induced and to sequester Fe2+ in response to hydroxyl radical formation, disruption of dps would result in excess levels of Fe2+ present within the cell, thereby leading to higher levels of hROS, as detected by HPF intensity, and an increase in bacterial cell death (26, 29), both of which we have demonstrated in our results. Complementation of dps in the mutant restored HPF intensity to wild-type levels. Furthermore, the significant changes in HPF intensity across treatment groups are supported by the increase in HPF seen in the positive-control sample treated with H2O2 and exposed to the same treatment conditions and times as all other treatments.
The combination peptide-acid treatment, which we previously demonstrated to be effective at ameliorating infection in a C. rodentium mouse model of EHEC infection (10), combines both of these effects and therefore requires two mechanisms of DNA protection though Dps, namely DNA binding and ferroxidase activity (17). This is supported by the enhanced level of bacterial killing seen in the wild-type strain when exposed to peptide-acid treatment, which is exaggerated in the dps mutant, compared to that seen with either acid or peptide treatment alone.
This study sheds light on one of the protective mechanisms employed by EHEC upon exposure to peptide treatment through Dps. Since the findings of this study demonstrate that Dps acts to protect EHEC against peptide treatment and that disruption of the dps gene results in significantly enhanced levels of bacterial killing, this may suggest a unique strategy to enhance the efficacy of peptide treatment. For example, the combination of peptide treatment with a secondary antibody treatment that neutralizes and/or counteracts the protective effects of Dps may enhance peptide-induced bacterial killing, thereby enhancing its ability as a prevention strategy against EHEC infection. Additionally, while this study demonstrates a unique link between the different antimicrobial effects of peptide WRWYCR through the protective action of Dps, there are likely other protective mechanisms at play. A deeper analysis of these protective mechanisms and antimicrobial resistance mechanisms is vital to fully understanding how EHEC responds to peptide treatment. This insight would allow for the development of combination drug treatments that target the antimicrobial resistance mechanisms at play in order to enhance the efficacy of peptide WRWYCR treatment.
In summary, the findings of this study extend our previous findings of peptide WRWYCR’s potential as an antimicrobial agent by demonstrating its action to be similar to that of the bactericidal class of antibiotics, and they provide a unique link between the two major antimicrobial roles of this peptide through the DNA-binding protein Dps.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Escherichia coli O157:H7 strains used for this study are listed in Table 1. Strains EDL933 ATCC 43895 and EDL933 dps::nptI were kindly provided by Charles Kaspar (University of Wisconsin, Madison, WI). Bacterial glycerol stocks were maintained at −80°C and were streaked onto Luria-Bertani (LB) agar (catalog no. LBL405; BioShop) containing the appropriate antibiotics prior to use in order to obtain single colonies. Overnight cultures were prepared by inoculating single colonies into LB broth containing the appropriate antibiotics and then incubating for 1 to 16 h at 37°C with shaking. Bacterial stresses were carried out in either Mueller-Hinton broth (MHB) or phosphate-buffered solution (PBS) as indicated below.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristicsa | Source or reference |
|---|---|---|
| EDL933, ATCC 43895 | stx1 stx2; serotype O157:H7 isolate from hamburger | 28 |
| EDL933 dps::nptI | Kmr | 28 |
| EDL933 dps::nptI/pUC18+dps | Kmr Apr | This study |
| EDL933 dps::nptI/pUC18 | Kmr Apr | This study |
| EDL933/pUC18 | Apr | This study |
Kmr, kanamycin resistant; Apr, ampicillin resistant.
Antimicrobial peptide WRWYCR.
The peptide WRWYCR was synthesized with a C-terminal amide group, purified to >95% purity at Sigma-Genosys or Bio-Synthesis and dissolved in 50% dimethyl sulfoxide (DMSO), as described previously (12). A WRWYCR stock solution (10 mM) was maintained at −20°C in 50 or 100% DMSO.
Bactericidal versus bacteriostatic antibiotic assay.
An overnight culture of the EDL933 WT was diluted 1:25 in fresh LB broth and grown for 1.5 h to the mid-exponential phase (optical density at 600 nm [OD600], 0.4 to 0.6) at 37°C with shaking. Bacteria were pelleted at 903 × g for 10 min at 4°C to prevent further growth and resuspended in MHB (catalog no. 70192; Sigma-Aldrich) containing either no drug, 5 μg/ml kanamycin, 400 μg/ml spectinomycin, or 50 μM peptide WRWYCR. Samples were incubated at 37°C with shaking (300 rpm) for 3 h. Samples were taken at t = 0, 1, 2, and 3 h, serially diluted in 1× PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO3, and 1.8 mM KH2PO4), plated onto LB agar, and incubated overnight at 37°C for enumeration. Additional samples were taken at t = 3 h and prepared for flow cytometric analysis of hydroxyl radical formation (see below).
Survival assays.
To determine the role of Dps in acid stress of EHEC, overnight bacterial cultures of EHEC were diluted 1:25 (wild-type and EDL933 dps::nptI) or 1:15 (EDL933 dps::nptI/pUC18+dps and EDL933 dps::nptI/pUC18) in fresh LB broth and grown for 1 to 2 h to either the mid-exponential phase (OD600, 0.4 to 0.6) or the early stationary phase (OD600, 0.9 to 1.0) at 37°C with shaking. Bacteria were pelleted as described above, resuspended in 1× PBS (pH 3.0; adjusted with phosphoric acid), and then incubated at room temperature for 1 h, static. Samples were taken at t = 0, 15, 30, 45, and 60 min, serially diluted in 1× PBS, plated onto LB agar, and incubated overnight at 37°C for enumeration.
In order to evaluate the role of Dps in peptide treatment of EHEC, overnight bacterial cultures of EHEC were subcultured as outlined above. Bacteria were pelleted, resuspended in 0.5× PBS containing 50 μM peptide WRWYCR, and then incubated at room temperature for 1 h, static. Samples were taken at t = 0, 15, 30, 45, and 60 min, serially diluted in 1× PBS, plated onto LB agar, and incubated overnight at 37°C for enumeration.
Finally, to assess the role of Dps in peptide-acid treatment of EHEC, overnight bacterial cultures of EHEC were subcultured as described above. Bacteria were pelleted, resuspended in 0.5× PBS containing 50 μM peptide WRWYCR, and then incubated at room temperature for 15 min, static. Bacteria were pelleted as above, resuspended in 1× PBS (pH 3.0), and incubated at room temperature for 1 h, static. Samples were taken immediately prior to peptide addition (untreated control [UT]) and at t = 0 (immediately prior to acid addition), 15, 30, 45, and 60 min, serially diluted in 1× PBS, plated onto LB agar, and incubated overnight at 37°C for enumeration.
Flow cytometric analysis of hydroxyl radical formation.
Bacterial cultures of EHEC strains were grown to the early stationary phase and exposed to either acid (pH 3.0), peptide WRWYCR (50 μM), peptide-acid (50 μM for 15 min/pH 3.0 for 1 h), or antibiotic stress, as outlined above. Bacteria were pelleted as described above, resuspended in 2% paraformaldehyde (fixative), and incubated at room temperature for 5 min. Following fixation, bacteria were pelleted, washed in 1× PBS, and diluted 1:10 in fresh 1× PBS (pH 7.2) plus 5 μM hydroxyphenyl fluorescein (HPF) (catalog no. H36004; Life Technologies) for 40 min at room temperature, protected from light. The fluorescein isothiocyanate (FITC)-A channel was used to detect the HPF signal, as it has the same excitation and emission spectrum peak wavelengths of approximately 495 nm and 519 nm, respectively. A minimum of 100,000 cells were collected for each sample. Data were analyzed using BD-FACS Diva software version 8.0. HPF signal values are presented in median fluorescence intensity (MFI) units.
Statistical analyses.
All data are represented as means ± standard errors of the means (SEMs) from at least 3 independent experiments. Either one-way or two-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test was used to determine differences among multiple groups. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
The work was supported by an NSERC Discovery Grant (05220) to Debora Barnett Foster and by an Ontario Graduate Scholarship to Tracy Lackraj.
We acknowledge Charles Kaspar, University of Wisconsin (Madison, WI), for generously providing the strains EDL933 ATCC 43895 and EDL933 dps::nptI. We are grateful to Christopher Spring of the Keenan Research Centre for Biomedical Science Research Core Facilities, St. Michael’s Hospital (Toronto, Canada), and Sarah Sabatinos, Department of Chemistry and Biology, Ryerson University (Toronto, Canada), for technical assistance and advice with the flow cytometric analysis.
REFERENCES
- 1.Karmali MA. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev 2:15–38. doi: 10.1128/CMR.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmali MA, Steele BT, Petric M, Lim C. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet (London, England) 321:619–620. doi: 10.1016/S0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen Y, Sperandio V. 2012. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front Cell Infect Microbiol 2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahal EA, Kazzi N, Nassar FJ, Matar GM. 2012. Escherichia coli O157:H7—clinical aspects and novel treatment approaches. Front Cell Infect Microbiol 2:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho NK, Henry AC, Johnson-Henry K, Sherman PM. 2013. Pathogenicity, host responses and implications for management of enterohemorrhagic Escherichia coli O157:H7 infection. Can J Gastroenterol 27:281–285. doi: 10.1155/2013/138673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena T, Kaushik P, Krishna Mohan M. 2015. Prevalence of E. coli O157:H7 in water sources: an overview on associated diseases, outbreaks and detection methods. Diagn Microbiol Infect Dis 82:249–264. doi: 10.1016/j.diagmicrobio.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Serna A 4th, Boedeker EC. 2008. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol 24:38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
- 8.Haas CN, Thayyar-Madabusi A, Rose JB, Gerba CP. 2000. Development of a dose-response relationship for Escherichia coli O157:H7. Int J Food Microbiol 56:153–159. doi: 10.1016/S0168-1605(99)00197-X. [DOI] [PubMed] [Google Scholar]
- 9.Lino M, Kus JV, Tran SL, Naqvi Z, Binnington B, Goodman SD, Segall AM, Barnett Foster D. 2011. A novel antimicrobial peptide significantly enhances acid-Induced killing of Shiga toxin-producing Escherichia coli O157 and non-O157 serotypes. Microbiology 157:1768–1775. doi: 10.1099/mic.0.047365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lackraj T, Johnson-Henry K, Sherman PM, Goodman SD, Segall AM, Foster DB. 2016. Novel antimicrobial peptide prevents C. rodentium infection in C57BL/6 mice by enhancing acid-induced pathogen killing. Microbiology 162:1641–1650. doi: 10.1099/mic.0.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kepple KV, Boldt JL, Segall AM. 2005. Holliday junction-binding peptides inhibit distinct junction-processing enzymes. Proc Natl Acad Sci U S A 102:6867–6872. doi: 10.1073/pnas.0409496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunderson CW, Segall AM. 2006. DNA repair, a novel antibacterial target: Holliday junction-trapping peptides induce DNA damage and chromosome segregation defects. Mol Microbiol 59:1129–1148. doi: 10.1111/j.1365-2958.2005.05009.x. [DOI] [PubMed] [Google Scholar]
- 13.Orchard SS, Rostron JE, Segall AM. 2012. Escherichia coli enterobactin synthesis and uptake mutants are hypersensitive to an antimicrobial peptide that limits the availability of iron in addition to blocking Holliday junction resolution. Microbiology 158:547–559. doi: 10.1099/mic.0.054361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almirón M, Link AJ, Furlong D, Kolter R. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev 6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 15.Altuvia S, Almirón M, Huisman G, Kolter R, Storz G. 1994. The dps promoter is activated by OxyR during growth and by IHF and σS in stationary phase. Mol Microbiol 13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A, Kolter R. 1997. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol 179:5188–5194. doi: 10.1128/JB.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiancone E, Ceci P. 2010. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: detoxification of iron and hydrogen peroxide and DNA binding. Biochim Biophys Acta 1800:798–805. doi: 10.1016/j.bbagen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Jeong K, Hung K, Baumler DJ, Byrd JJ, Kaspar CW. 2008. Acid stress damage of DNA is prevented by Dps binding in Escherichia coli O157:H7. BMC Microbiol 8:181. doi: 10.1186/1471-2180-8-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair S, Finkel SE. 2004. Dps protects cells against multiple stresses during stationary phase. J Bacteriol 186:4192–4198. doi: 10.1128/JB.186.13.4192-4198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold AR, Zhou A, Barton JK. 2016. Characterization of the DNA-mediated oxidation of Dps, a bacterial ferritin. J Am Chem Soc 138:11290–11298. doi: 10.1021/jacs.6b06507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antipov SS, Tutukina MN, Preobrazhenskaya EV, Kondrashov FA, Patrushev MV, Toshchakov SV, Dominova I, Shvyreva US, Vrublevskaya VV, Morenkov OS, Sukharicheva NA, Panyukov VV, Ozoline ON. 2017. The nucleoid protein Dps binds genomic DNA of Escherichia coli in a non-random manner. PLoS One 12:e0182800. doi: 10.1371/journal.pone.0182800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceci P, Mangiarotti L, Rivetti C, Chiancone E. 2007. The neutrophil-activating Dps protein of Helicobacter pylori, HP-NAP, adopts a mechanism different from Escherichia coli Dps to bind and condense DNA. Nucleic Acids Res 35:2247–2256. doi: 10.1093/nar/gkm077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huergo LF, Rahman H, Ibrahimovic A, Day CJ, Korolik V. 2013. Campylobacter jejuni Dps protein binds DNA in the presence of iron or hydrogen peroxide. J Bacteriol 195:1970–1978. doi: 10.1128/JB.00059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye J-Z, Su Y-B, Lin X-M, Lai S-S, Li W-X, Ali F, Zheng J, Peng B. 2018. Alanine enhances aminoglycosides-induced ROS production as revealed by proteomic analysis. Front Microbiol 9:29. doi: 10.3389/fmicb.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. 2003. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem 278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 26.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Bellapadrona G, Ardini M, Ceci P, Stefanini S, Chiancone E. 2010. Dps proteins prevent Fenton-mediated oxidative damage by trapping hydroxyl radicals within the protein shell. Free Radic Biol Med 48:292–297. doi: 10.1016/j.freeradbiomed.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 28.Choi SH, Baumler DJ, Kaspar CW. 2000. Contribution of dps to acid stress tolerance and oxidative stress tolerance in Escherichia coli O157:H7. Appl Environ Microbiol 66:3911–3916. doi: 10.1128/AEM.66.9.3911-3916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calhoun LN, Kwon YM. 2011. The ferritin-like protein Dps protects Salmonella enterica serotype Enteritidis from the Fenton-mediated killing mechanism of bactericidal antibiotics. Int J Antimicrob Agents 37:261–265. doi: 10.1016/j.ijantimicag.2010.11.034. [DOI] [PubMed] [Google Scholar]