Pseudomonas aeruginosa is an important opportunistic pathogenic bacterium that causes various acute and chronic infections in human, especially in patients with compromised immunity, cystic fibrosis (CF), and/or severe burn wounds. About 60% of cystic fibrosis patients have a chronic respiratory infection caused by P. aeruginosa. The bacterium is intrinsically highly resistant to antibiotics, which greatly increases difficulties in clinical treatment. Therefore, it is critical to understand the mechanisms and the regulatory pathways that are involved in antibiotic resistance. In this study, we elucidated a novel regulatory pathway that controls the bacterial resistance to fluoroquinolone antibiotics, which enhances our understanding of how P. aeruginosa responds to ciprofloxacin.
KEYWORDS: Fis, pyocin, Pseudomonas aeruginosa, resistance
ABSTRACT
Factor for inversion stimulation (Fis) is a versatile DNA binding protein that plays an important role in coordinating bacterial global gene expression in response to growth phases and environmental stresses. Previously, we demonstrated that Fis regulates the type III secretion system (T3SS) in Pseudomonas aeruginosa. In this study, we explored the role of Fis in the antibiotic resistance of P. aeruginosa and found that mutation of the fis gene increases the bacterial susceptibility to ciprofloxacin. We further demonstrated that genes related to pyocin biosynthesis are upregulated in the fis mutant. The pyocins are produced in response to genotoxic agents, including ciprofloxacin, and the release of pyocins results in lysis of the producer cell. Thus, pyocin biosynthesis genes sensitize P. aeruginosa to ciprofloxacin. We found that PrtN, the positive regulator of the pyocin biosynthesis genes, is upregulated in the fis mutant. Genetic experiments and electrophoretic mobility shift assays revealed that Fis directly binds to the promoter region of prtN and represses its expression. Therefore, our results revealed novel Fis-mediated regulation on pyocin production and bacterial resistance to ciprofloxacin in P. aeruginosa.
IMPORTANCE Pseudomonas aeruginosa is an important opportunistic pathogenic bacterium that causes various acute and chronic infections in human, especially in patients with compromised immunity, cystic fibrosis (CF), and/or severe burn wounds. About 60% of cystic fibrosis patients have a chronic respiratory infection caused by P. aeruginosa. The bacterium is intrinsically highly resistant to antibiotics, which greatly increases difficulties in clinical treatment. Therefore, it is critical to understand the mechanisms and the regulatory pathways that are involved in antibiotic resistance. In this study, we elucidated a novel regulatory pathway that controls the bacterial resistance to fluoroquinolone antibiotics, which enhances our understanding of how P. aeruginosa responds to ciprofloxacin.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that can cause various infections in patients with severe burns, cystic fibrosis (CF), AIDS, or cancer (1–3). In the P. aeruginosa genome, 8% of the genes encode regulators, which might contribute to the bacterium’s ability to quickly adapt to various growth conditions and its resistance to multiple environmental stresses (2, 4).
Pyocins are one type of bacteriocin synthesized by over 90% of P. aeruginosa strains. They are classified into three types, namely, the R, F, and S types (5, 6). Pyocins possess intraspecies and interspecies bactericidal abilities. For example, the spectrum of activity of S-type pyocins is limited to P. aeruginosa, while the R-type pyocins are able to kill other Gram-negative bacteria (7). Smith et al. reported that pyocin S2 displays potent activity against P. aeruginosa biofilms formed by clinical isolates, which provides a potential therapeutic option of utilizing pyocins as a novel antibacterial agent (8). The production of pyocins has a high energy cost, and the release of pyocins is through cell lysis. Therefore, expression of the pyocin genes is under tight control in P. aeruginosa. PrtR is a DNA binding protein that directly represses the expression of prtN, which encodes a positive regulator of the pyocin genes (9, 10). The stability of PrtR is regulated similarly to regulation of the SOS response regulator LexA by RecA. In response to genotoxic agents such as mitomycin C, quinolone antibiotics, UV light, and hydrogen peroxide, the activated RecA triggers the cleavage of PrtR, resulting in upregulation of PrtN and in the subsequent production of pyocins (6, 11, 12). Mutation of pyocin biosynthesis genes increases the bacterial resistance to genotoxic agents, including that to ciprofloxacin (6, 11, 12). The release of pyocins is mainly mediated by PA0614- and PA0629-encoded holin-like and lysozyme-like proteins through cell lysis, accompanied by release of DNA and production of membrane vesicles, which play important roles in biofilm formation (13). In addition, the bactericidal pyocins might enable the producer strain to secure the niche under unfavorable conditions by killing susceptible bacteria (6, 11, 12). Previously, we demonstrated an autorepressive regulatory mechanism of prtR, through which the PrtR protein is maintained at a relatively stable level, thus avoiding the overproduction of pyocins during SOS response (9, 10). Mutations in the pyocin genes increase bacterial resistance to quinolone antibiotics (14).
Factor for inversion stimulation (Fis) is a conserved nucleoid binding protein (NAP) in bacteria (14–18). The Fis protein in Escherichia coli and Salmonella enterica serovar Typhimurium has been shown to facilitate a site-specific DNA inversion reaction and mediate λ phage DNA excision and integration, as well as to influence the transposition frequencies of the transposon Tn5 and insertion sequence IS50 (19–21). In addition, Fis is characterized as a global regulator that affects the topological state of DNA through regulating the gene expression of the DNA gyrase and topoisomerase or directly binding to the promoter region of its target genes (22–27). It also regulates the transcription of rRNA genes, tRNA genes, and other translation-related genes (24–26, 28). In E. coli, the level of Fis largely correlates with the bacterial growth status, e.g., the fis mRNA level peaks at the early logarithmic phase and decreases upon entering the mid-log phase. (27, 28). Factors affecting the bacterial growth status, such as temperature (29, 30), nutrients (31–34), and oxidative stresses (35), have been shown to influence the expression of Fis.
Recently, multiple studies have revealed an important role for Fis in the regulation of virulence factors in various pathogenic bacteria (14, 16, 18, 36–38). Previous studies have depicted the consensus Fis binding sequence as GNNBNwwwwwNVNNC (B, not A; V, not T; w, high proportion of A or T; N, any nucleotide) (39, 40). Our previous research demonstrated that Fis regulates the type III secretion system (T3SS) by directly binding to the upstream region of the exsA gene (encoding the master regulator of the T3SS) in P. aeruginosa (41). In this study, we found that a P. aeruginosa fis::Tn mutant is more susceptible to fluoroquinolone antibiotics and that Fis regulates the expression pyocin biosynthesis genes through prtN. Therefore, our results revealed a novel layer of regulation on pyocin synthesis and correlated with resistance to fluoroquinolone antibiotics.
RESULTS
Fis contributes to bacterial resistance to fluoroquinolones.
To examine the role of Fis in antibiotic resistance in P. aeruginosa, we determined the MICs of various antibiotics to wild-type PA14 and an isogenic fis::Tn mutant (41, 42). The fis::Tn mutant was slightly more susceptible to tetracycline and tobramycin and more resistant to meropenem and streptomycin (Table 1). However, the fis::Tn mutant displayed a 4-fold decrease in the MICs of ciprofloxacin and ofloxacin. Complementation with a fis gene inserted into the chromosome at the attTn7 site (fis::Tn/att7::fis) restored the bacterial resistance (Table 1). These results suggest that Fis plays an important role in bacterial resistance to fluoroquinolone antibiotics.
TABLE 1.
Bacterial susceptibilities to antibiotics
| Strain | MIC (μg/ml) ofa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| TET | TOB | PB | CAR | MEM | SM | CIP | OFL | |
| PA14 | 25 | 0.25 | 1 | 150 | 1.25 | 25 | 0.3 | 1 |
| fis::Tn strain | 12.5 | 0.125 | 1 | 150 | 2.5 | 50 | 0.075 | 0.25 |
| fis::Tn/att7::fis strain | — | 0.25 | 1 | 150 | 1.25 | 25 | 0.3 | 1 |
TET, tetracycline; TOB, tobramycin; PB, polymyxin B; CAR, carbenicillin; MEM, meropenem; SM, streptomycin; CIP, ciprofloxacin; OFL, ofloxacin; —, not determined due to a TET selection marker in the complemented strain.
Upregulation of pyocin genes leads to increased susceptibility to ciprofloxacin in the fis::Tn mutant.
To understand the role of Fis in the bacterial resistance to ciprofloxacin, we used transcriptome sequencing (RNA-Seq) to compare the gene expression profiles between wild-type PA14 and the fis::Tn mutant under the treatment of ciprofloxacin. The concentration of ciprofloxacin used (0.025 μg/ml) did not affect the growth of either of the strains (data not shown). The multidrug efflux systems and SOS response play important roles in the bacterial resistance to fluoroquinolone antibiotics (43–45). However, the RNA-Seq results revealed no significant differences in the expression of multidrug efflux system- and SOS response-related genes between wild-type PA14 and the fis::Tn mutant (see Table S1 in the supplemental material). We further confirmed the results by real-time PCR assays, which showed similar expression levels of several major multidrug efflux systems and SOS response genes in wild-type PA14 and the fis::Tn mutant, with or without the treatment of 0.025 μg/ml ciprofloxacin, including mexA, mexC, mexE, lexA, recA, and recN (see Fig. S1A and B in the supplemental material). We then treated the bacteria with 0.3 μg/ml ciprofloxacin for 30 min, which resulted in less than 10% killing of the wild-type PA14 and the fis::Tn mutant cells (data not shown). The expression levels of mexA, mexC, mexE, lexA, recA, and recN were similar in the two strains (Fig. S1C and D).
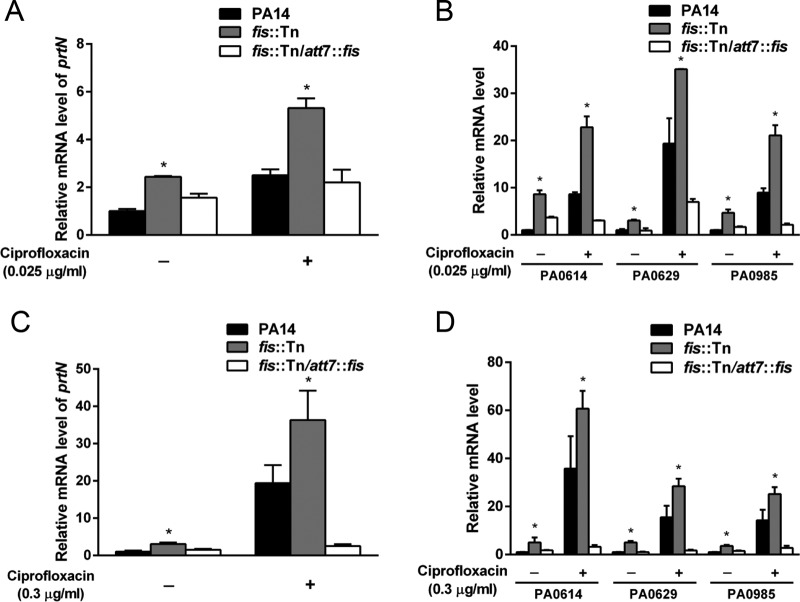
Of note, all of the pyocin biosynthesis genes and the regulatory gene prtN were upregulated in the fis::Tn mutant (Table 2). To confirm the expression of those genes, we used real-time PCR to determine the mRNA levels of prtN, PA0614, PA0629, and PA0985. PA0614 and PA0629 are in the operons of R- and F-type pyocin biosynthesis genes, respectively. PA0985 encodes an S-type pyocin (5, 6). As shown in Fig. 1, the expression of those genes was increased in the fis::Tn mutant in the absence or presence of 0.025 μg/ml (Fig. 1A and B) or 0.3 μg/ml (Fig. 1C and D) ciprofloxacin. Complementation with an fis gene restored the expression levels of the genes in the fis::Tn mutant.
TABLE 2.
mRNA levels of pyocin biosynthesis genes in the fis::Tn mutant compared to those in wild-type strain PA14
| Pyocin type | PA14 gene IDa | PAO1 gene ID | Product | fis::Tn fold change vs wild type | P value |
|---|---|---|---|---|---|
| None | PA14_07950 | PA0610 | Transcription regulatory protein RrtN | 2.66 | 0.03472 |
| R type | PA14_08000 | PA0614 | Pyocin R2, holin | 2.96 | 0.01982 |
| PA14_08010 | PA0615 | Phage baseplate assembly protein V | 2.68 | 0.03293 | |
| PA14_08020 | PA0616 | Bacteriophage protein | 3.06 | 0.01611 | |
| PA14_08030 | PA0617 | Bacteriophage protein | 3.51 | 0.00716 | |
| PA14_08040 | PA0618 | Phage tail protein I | 3.22 | 0.01198 | |
| PA14_08050 | PA0619 | Tail fiber protein | 3.53 | 0.00695 | |
| PA14_08060 | PA0620 | Tail fiber assembly protein | 3.51 | 0.00715 | |
| PA14_08070 | PA0621 | Phage tail sheath protein | 4.46 | 0.00154 | |
| PA14_08090 | PA0622 | Phage tail tube protein | 5.15 | 0.00058 | |
| PA14_08100 | PA0623 | Phage tail assembly protein | 4.63 | 0.00122 | |
| PA14_08120 | PA0624 | Tail length determinator protein | 4.54 | 0.00138 | |
| PA14_08130 | PA0625 | Phage tail protein | 5.16 | 0.00057 | |
| PA14_08140 | PA0626 | Hypothetical protein | 6.23 | 0.00017 | |
| PA14_08150 | PA0627 | Phage late control gene D protein | 4.90 | 0.00082 | |
| PA14_08160 | PA0628 | Lytic enzyme | 4.62 | 0.00127 | |
| PA14_08180 | PA0629 | Hypothetical protein | 5.82 | 0.00034 | |
| PA14_08190 | PA0630 | Hypothetical protein | 5.48 | 0.00082 | |
| F type | PA14_08200 | PA0631 | Hypothetical protein | 3.03 | 0.04734 |
| PA14_08210 | PA0632 | Hypothetical protein | 3.90 | 0.00375 | |
| PA14_08220 | PA0633 | Hypothetical protein | 4.09 | 0.00283 | |
| PA14_08230 | PA0634 | Hypothetical protein | 4.49 | 0.00149 | |
| PA14_08240 | PA0635 | Hypothetical protein | 3.69 | 0.00532 | |
| PA14_08250 | PA0636 | Hypothetical protein | 3.85 | 0.00407 | |
| PA14_08260 | PA0637 | Phage minor tail protein L | 4.28 | 0.00207 | |
| PA14_08270 | PA0638 | Peptidase P60 | 3.70 | 0.00529 | |
| PA14_08280 | PA0639 | Tail assembly protein | 4.21 | 0.00229 | |
| PA14_08300 | PA0640 | Phage-related protein, tail component | 4.32 | 0.00192 | |
| PA14_08310 | PA0641 | Hypothetical protein | 4.07 | 0.00287 | |
| PA14_08320 | PA0642 | Hypothetical protein | 3.81 | 0.00593 | |
| PA14_08330 | PA0643 | Hypothetical protein | 3.97 | 0.0034 | |
| S type | PA14_59220 | Pyocin S5 | 2.92 | 0.02076 |
ID, identifier.
FIG 1.
Expression levels of pyocin-related genes. The overnight bacterial cultures were diluted 100-fold into fresh LB medium with or without 0.025 μg/ml ciprofloxacin and grown to an optical density at 600 nm (OD600) of 1.0 at 37°C. For the treatment with 0.3 μg/ml ciprofloxacin, the bacteria were grown in LB to an OD600 of 1.0, followed by incubation with the antibiotic for 30 min at 37°C. Total RNA was isolated from the bacteria. The relative mRNA levels of prtN (A, C) and PA0614, PA0629, and PA0985 (B, D) were determined by real-time PCR. rpsL was used as the internal control. The results shown represent data from three independent experiments with similar results. Error bars represent standard deviations. *, P < 0.05 compared by Student's t test to PA14 or the complemented strain.
To test whether the upregulation of the pyocin biosynthesis genes contributes to the higher susceptibility of the fis::Tn mutant, we deleted prtN, PA0629, or the whole F- and R-type pyocin-encoding locus (PA0614 to PA0648). The mutations in wild-type PA14 increased the MIC of ciprofloxacin by 2-fold (Table 3). However, the MIC was increased by 4-fold by the mutations in the fis::Tn mutant (Table 3). These results indicate that the upregulation of pyocin biosynthesis genes in the fis::Tn mutant might contribute to increased susceptibility to ciprofloxacin.
TABLE 3.
Bacterial susceptibility to antibiotics
| Strain | Ciprofloxacin MIC (μg/ml) |
|---|---|
| PA14 | 0.3 |
| fis::Tn strain | 0.075 |
| fis::Tn/att7::fis strain | 0.3 |
| PA14 ΔprtN | 0.6 |
| PA14 ΔPA0629 | 0.6 |
| PA14 ΔPA0614–PA0648 | 0.6 |
| fis::Tn ΔprtN strain | 0.3 |
| fis::Tn ΔPA0629 strain | 0.3 |
| fis::Tn ΔPA0614–PA0648 strain | 0.3 |
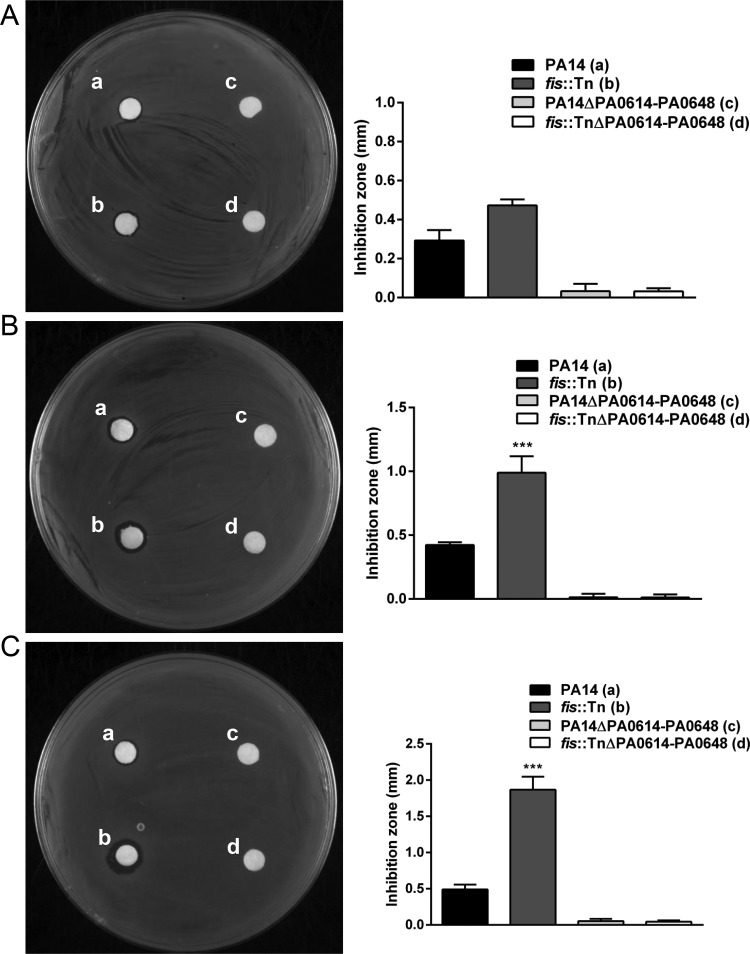
To further confirm the production levels of pyocins, we utilized the wild-type strain PAK as an indicator strain (46). Wild-type PA14 and the fis::Tn mutant were grown without or with 0.025 or 0.3 μg/ml ciprofloxacin. The bacterial supernatants were collected by centrifugation and passed through a 0.2-μm filter. The same amount of the supernatant was spotted on a filter paper, which was placed on a lawn of PAK on an L broth (LB) plate. The inhibition zones from the fis::Tn mutant were bigger than those from wild-type PA14 (Fig. 2A), especially after treatment with higher concentrations of ciprofloxacin (Fig. 2B and C). In addition, deletion of the pyocin biosynthesis locus (PA0614 to PA0648) diminished the inhibition zone (Fig. 2). These results demonstrate that the fis::Tn mutant produces more pyocins than does wild-type PA14.
FIG 2.
Production levels of pyocins. Indicated strains were grown in LB (A) or treated with 0.025 μg/ml (B) and 0.3 μg/ml (C) ciprofloxacin. The filtered and concentrated bacterial supernatant was spotted on a filter paper that was laid on a lawn of the indicator strain PAK. The size of the inhibition zone was calculated by subtracting the diameter of the filter paper from the diameter of the inhibition circle. The results shown represent data from three independent experiments with similar results. Error bars represent standard deviations. ***, P < 0.001 compared by Student's t test to PA14 or the complemented strain.
PrtR protein level in the fis::Tn mutant.
PrtR and PrtN are the key regulators of pyocin production. Since PrtR directly represses the transcription of prtN (9), the higher mRNA level of prtN in the fis::Tn mutant might be due to a reduced PrtR level. However, real-time PCR assays revealed similar PrtR mRNA levels in PA14 and the fis::Tn mutant in the presence and absence of ciprofloxacin (see Fig. S2A and B in the supplemental material). Next, we examined the protein level of PrtR by utilizing prtR encoding a C-terminal 6×His-tagged product and driven by its native promoter (designated prtR-His) (10). In LB, the PrtR-His levels were similar between wild-type PA14 and the fis::Tn mutant. Treatment with ciprofloxacin resulted in similar levels of degradation of the PrtR-His in both strains (Fig. S2C). These results indicate that Fis might regulate pyocin independently of PrtR.
Fis directly represses the transcription of prtN by binding to its promoter region.
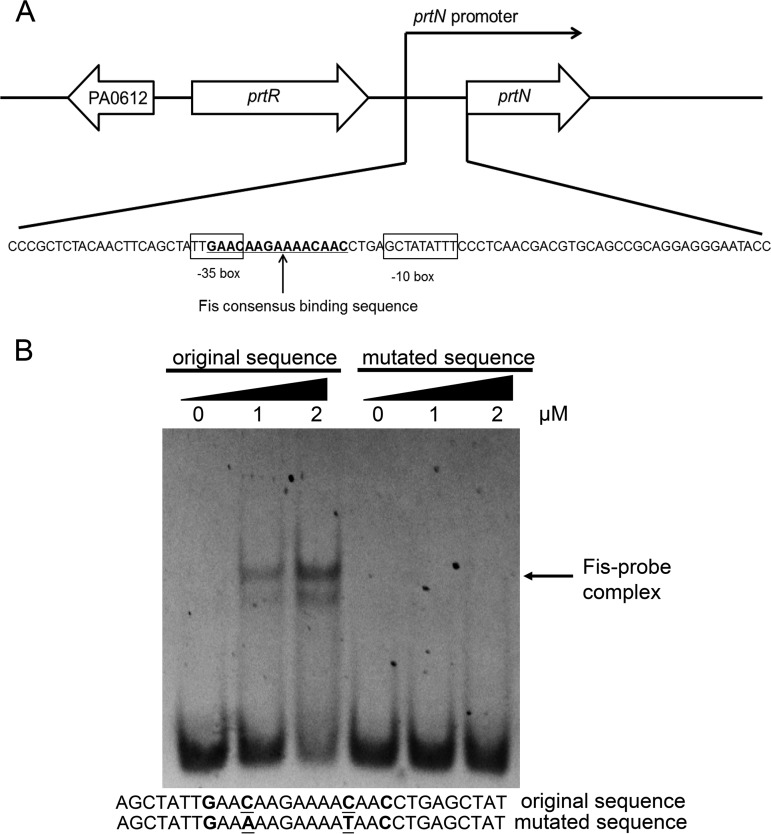
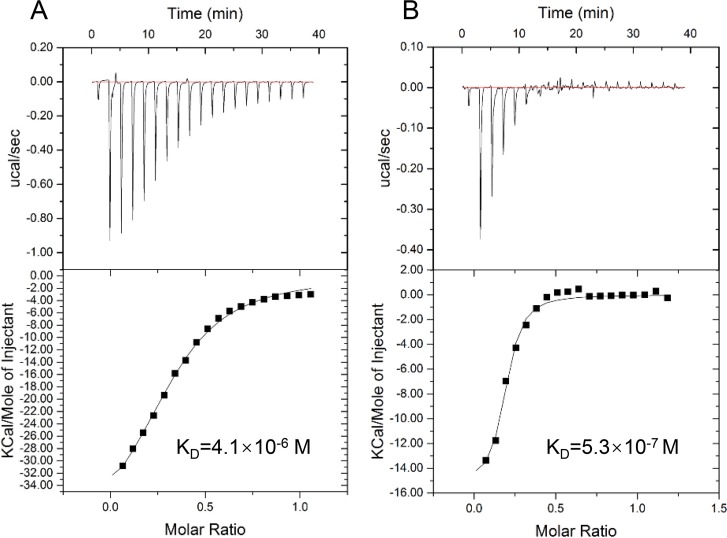
We then examined whether Fis directly regulates the expression of prtN. Sequence analysis revealed a 15-bp potential Fis binding sequence, GAACAAGAAAACAAC, located between the −10 and −35 regions of the prtN promoter (Fig. 3A). An electrophoretic mobility shift assay (EMSA) confirmed the binding between Fis and the predicted sequence (Fig. 3B). Mutation of the consensus motif abolished the binding (Fig. 3B). Isothermal titration calorimetry (ITC) assays confirmed the binding between Fis and the prtN promoter (Fig. 4A); however, the affinity of the binding of Fis to the prtN promoter was approximately 8-fold lower than that of PrtR (Fig. 4B).
FIG 3.
Fis directly binds to the prtN promoter region. (A) Diagram of the genetic organization and putative promoter regions of prtN. The arrow of each open reading frame represents the transcriptional direction. The −10 and −35 regions of the prtN promoter are shown in boxes. The potential Fis binding sequence is underlined. (B) Sequences of the probes used in the electrophoretic mobility shift assay (EMSA) and the consensus Fis binding sequence are shown. Underlined letters represent mutated nucleotides. Purified Fis protein was incubated with the fragment with original or mutated sequence for 30 min at 25°C. The position of the Fis-probe complex is indicated.
FIG 4.
Binding affinities of Fis and PrtR to the prtN promoter region. The binding affinities were measured by isothermal titration microcalorimetry. Shown is isothermal titration microcalorimetry of Fis at 37 μM (A) or of PrtR at 33 μM (B) binding to 0.2 mM DNA probe at 25°C. KD, binding affinity.
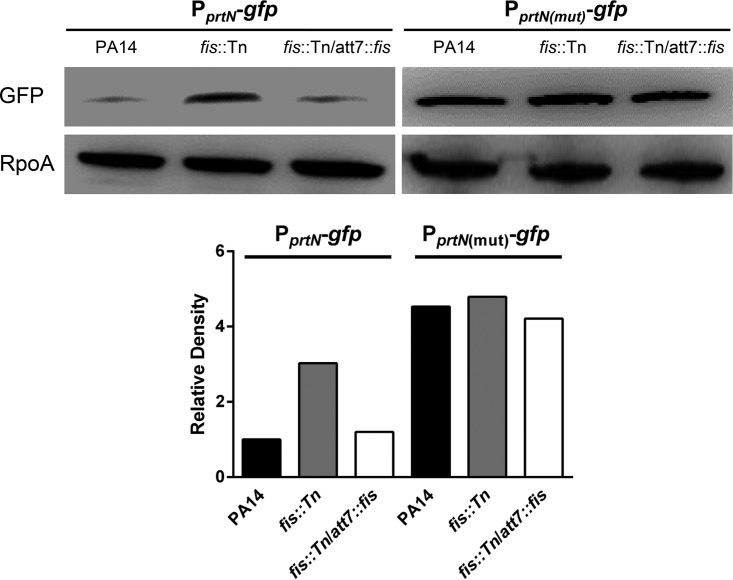
To confirm the regulation of Fis on the transcription of ptrN, we constructed a transcriptional fusion between the promoter of ptrN and a gfp gene, resulting in PprtN-gfp. We then mutated the consensus Fis binding sequence in the PprtN-gfp as shown in Fig. 3B, resulting in PprtN(mut)-gfp. The green fluorescent protein (GFP) level was higher in the fis::Tn mutant. The GFP level was restored in the complemented strain (Fig. 5). The mutation in the Fis binding sequence increased the GFP level in wild-type PA14 and diminished the difference between wild-type PA14 and the fis::Tn mutant (Fig. 5). In combination, these results indicate that Fis directly binds to the promoter region of prtN and represses its expression.
FIG 5.
Expression levels of the gfp transcriptional fusions. Protein levels of the gfp gene driven by the original or mutated prtN promoter. PA14, the fis::Tn mutant, and the complemented strain carrying PprtN-gfp or PprtN(mut)-gfp were grown to an OD600 of 1.0. The levels of GFP were determined by Western blotting. RpoA was used as the loading control. The band intensities were quantified with ImageJ software and normalized to the corresponding RpoA band intensities.
DISCUSSION
In this study, we demonstrated that Fis is involved in bacterial resistance to ciprofloxacin in P. aeruginosa by regulating pyocin biosynthesis genes. The pyocins are produced and released under unfavorable environmental conditions, where the bactericidal pyocins might kill other bacterial strains and secure the niche for the species of the producer cells. In addition, pyocins are involved in the biofilm formation of P. aeruginosa. PA0629 is an endolysin-like protein that degrades peptidoglycan. A previous study revealed that PA0629 contributes to biofilm formation through mediating the release of membrane vesicles, cytosolic contents and extracellular DNA (eDNA), which contribute to the formation and integrity of biofilm (13). Meanwhile, the production and release of pyocins result in lysis of the producer cells (14). Therefore, mutation in the pyocin biosynthesis genes increases the bacterial resistance to fluoroquinolone antibiotics that induce SOS response and degradation of PrtR (14).
Fis is a nucleoid binding protein that has a widespread effect on gene expression through binding with the promoter of target genes (15, 39, 47). Another nucleoid-structuring protein, the heat-stable nucleoid structuring protein (H-NS), also shares the same feature. Numerous studies have demonstrated that H-NS is a universal modulator that plays an important role in mediating the selectively silencing of acquired foreign genes in various bacteria, such as E. coli, S. Typhimurium, Yersinia pseudotuberculosis, etc. (48–52). In P. aeruginosa, the H-NS family proteins MvaT and MvaU have been shown to preferentially bind to the AT-rich regions of the chromosome and control the production of the Pf4 phage and the expression of fimbrial cup gene clusters, as well as expression of genes involved in pyocyanin synthesis and arginine metabolism (53–57).
Here, we found that Fis directly controls the transcription of prtN by directly binding to its promoter region. In the EMSA result, a second band appeared when the Fis concentration was increased to 2 μM. It was previously shown that Fis can bend DNA and promote DNA compaction (58). We suspect that at higher concentration, the Fis protein might bend the DNA to a different conformation, thus resulting in the second band.
Our ITC results indicate that the binding affinity of PrtR to the prtN promoter region is higher than that of Fis. Thus, under normal growth conditions, PrtR might play a major role in repressing the transcription of prtN. When the PrtR protein is cleaved under SOS response, Fis might play a major role in regulating the expression of prtN. Meanwhile, the binding of a regulatory protein to DNA is determined by the amount and the modification of the protein. Previous studies in E. coli revealed acetylation of the Fis protein (59, 60). The autoregulation of PrtR in response to genotoxic reagents (9, 10) further complicates the regulation on prtN. Therefore, further studies are warranted to fully understand the contribution of PrtR and Fis to the regulation of prtN.
In P. aeruginosa, the protein sequence and structures of the F- and R-type pyocins resemble bacterial phage tails. The negative regulator PrtR is a λ cI homologue. In addition, the GC contents of the pyocin genes are lower than the average of the P. aeruginosa genome, suggesting that the pyocin might be derived from a lysogenic phage. Previously, we demonstrated that Fis regulates the T3SS through exsA in P. aeruginosa (41). Chromatin immunoprecipitation sequencing (ChIP-seq) analysis is needed to reveal the global regulatory targets of Fis.
Overall, our results revealed a novel role for Fis in the regulation of pyocin biosynthesis genes, which directly affects bacterial resistance to ciprofloxacin.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains, plasmids, and primers used in this study are listed in Table S2 in the supplemental material. Bacteria were grown in L broth (LB) (5 g of NaCl, 5 g of yeast extract, and 10 g of tryptone per liter; pH ∼7.2 to 7.4) under aerobic conditions at 37°C or on L agar (L broth with 15 g/liter agar). The concentrations of the antibiotics added were as follows: ampicillin at 100 μg/ml, gentamicin at 10 μg/ml, or tetracycline at 10 μg/ml (BBI Life Sciences, China) for E. coli; or carbenicillin at 150 μg/ml, tetracycline at 50 μg/ml, or gentamicin at 50 μg/ml (BBI Life Sciences) for P. aeruginosa. Isopropyl-β-d-1-thiogalactopyranoside (IPTG) (BBI Life Sciences) was added to the culture medium at indicated concentrations when needed.
Plasmid construction.
DNA manipulations were conducted according to standard protocols or to the manufacturer’s instructions for commercial products. Chromosomal gene mutations were generated by homologous recombination as described previously (61). To construct a transcriptional fusion between the prtN promoter and a gfp gene, a 500-bp region upstream of the prtN open reading frame was amplified by PCR from the chromosomal DNA of wild-type PA14. The fragment was cloned into the SacI-BamHI sites of pUCP20 (without the tac promoter) (62), resulting in pUCP20-PprtN. The gfp gene was amplified from the vector pUC18T-mini-Tn7T-gfpmut3 and cloned into the BamHI-PstI sites of pUCP20-PprtN, resulting in pUCP20-PprtN-gfp, where the gfp gene is under the control of the prtN promoter.
RNA sequencing and quantitative real-time PCR.
Overnight cultures of bacteria were diluted 100-fold into fresh LB medium with or without 0.025 μg/ml ciprofloxacin and grown to an optical density at 600 nm (OD600) of 1.0 at 37°C. The bacteria were harvested by centrifugation, and total RNA was purified with an RNeasy Protect bacteria minikit (Qiagen, Shanghai, China). The sequencing and data analyses were performed by Genewiz (Suzhou, China). Briefly, the RNA samples were examined with a 2100 Bioanalyzer (Agilent Technologies, USA). Total RNA (1 μg) of each sample was used for library construction. rRNA was depleted with the RiboMinus bacteria module (Invitrogen, USA). Then, the mRNA was fragmented, flowed by construction of a paired-end index library following the manufacturer’s instructions (directional RNA library prep kit for Illumina, NEBNext Ultra). Analysis of the RNA expression was based on the annotations of PA14 (www.pseudomonas.com). The expression levels were calculated with the fragments per kilobase of transcript per million reads (FPKM) method. The P values were calculated with the software edgeR (v3.4.2; Bioconductor).
For quantitative real-time PCR (qRT-PCR), the bacteria were grown with or without 0.025 μg/ml ciprofloxacin to an OD600 of 1.0 at 37°C. For the treatment with 0.3 μg/ml ciprofloxacin, the bacteria at an OD600 of 1.0 were incubated with the antibiotic for 30 min at 37°C. Total RNA was isolated as mentioned previously. cDNA was synthesized from 1 μg of the total RNA using PrimeScript reverse transcriptase (TaKaRa, China) and random primers. The synthesized cDNA was mixed with SYBR Premix Ex Taq II (TaKaRa) and 4 pmol of sense and antisense primers (Table S1) in a 20-μl reaction system. The 30S ribosomal protein coding gene rpsL served as the internal control (41). The CFX Connect real-time system (Bio-Rad, USA) was used to conduct the qRT-PCR assay.
Antibiotic susceptibility assay.
Bacteria were grown overnight in LB at 37°C and diluted into a fresh LB medium to yield an inoculum of approximately 1 × 105 CFU/ml. The method of serial 2-fold dilution in 96-well plates (100 μl/well) was adopted to determine the MICs of various antibiotics for P. aeruginosa (62, 63). The MIC was recorded as the lowest concentration of antibiotic that inhibited visible growth after incubation at 37°C overnight. Assays were repeated at least three times.
Pyocin production assay.
Bacteria were grown in LB with or without 0.025 μg/ml ciprofloxacin to an OD600 of 1.0. An aliquot of the bacteria grown in LB was treated with 0.3 μg/ml ciprofloxacin for 1 h. Supernatant from the same amount of bacteria was collected by centrifugation and filter sterilized. The supernatant was then concentrated 10-fold with an Amicon Ultra 0.5-ml, 10-kDa centrifugal filter unit (Millipore, Germany). Supernatant (20 μl) was spotted on a filter paper, which was then laid on a lawn of PAK cells. The plate was incubated at 37°C for 24 h before measuring the size of the inhibition zone.
Western blotting.
Overnight bacterial cultures of different P. aeruginosa strains were diluted 100-fold in fresh LB with or without 0.025 μg/ml ciprofloxacin and grown at 37°C. When the OD600 reached 0.8 to 1.0, samples from equivalent numbers of bacterial cells were collected. Total bacterial proteins were separated by electrophoresis on an SDS-polyacrylamide gel (12% polyacrylamide), followed by transfer to a polyvinylidene difluoride (PVDF) membrane (Millipore, Germany). The 6×His-tagged PrtR and RpoA were hybridized with a mouse monoclonal anti-His antibody (Millipore, Germany) or an anti-E. coli RNA polymerase α subunit antibody (BioLegend, USA) at room temperature for 1 h. After washing 4 times with phosphate-buffered saline (PBS) containing 0.1% Tween 20, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Promega, USA). To detect the level of GFP, the membrane was hybridized with an HRP-conjugated GFP antibody (GeneTex, USA). Signals were detected with an ECL Plus kit (Millipore, Germany), and the bands were visualized with an X-ray spectrometry (ChemiDoc XRS+) molecular imager (Bio-Rad).
Protein purification.
PA14 cells carrying pMMB67EH-fis-His were grown at 37°C to an OD600 of 0.6 and then induced with 1 mM IPTG for an additional 4 h at 37°C. The bacteria were harvested by centrifugation at 4°C and washed once with 20 mM Tris-HCl (pH 7.9). The cell pellet was stored at −80°C. The frozen pellet was thawed in 10 ml of lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 3 mM β-mercaptoethanol, 0.5% NP-40, and 20 mM imidazole [pH 8.0]) and lysed by ultrasonication. The lysate was centrifuged at 12,000 rpm for 20 min. The 6×His-tagged Fis (Fis-His) protein was purified from the supernatant using a Ni-nitrilotriacetic acid (Ni-NTA) column (Qiagen, Germany), followed by purification with Sephadex G200 molecular sieve chromatography (Bio-Rad).
Electrophoretic mobility shift assay.
The electrophoretic mobility shift assay (EMSA) was performed as previously described with minor modification (10). Briefly, DNA fragments corresponding to the sequences upstream of the prtN genes were synthesized. The DNA fragments (100 ng) were incubated with 0 to 2 mM purified 6×His-tagged Fis protein at 25°C for 30 min in a 20-μl reaction system (10 mM Tris-HCl, 5 mM MgCl2, 5 mM KCl, 0.1% [vol/vol] NP-40, and 1 mM dithiothreitol [pH 7.5]). The samples were loaded onto an 8% native polyacrylamide gel in 0.5 × Tris-borate-EDTA (TBE) buffer (0.044 M Tris base, 0.044 M boric acid, and 0.001 M EDTA [pH 8.0]) that had been prerun for 1 h on ice. Electrophoresis was performed at 100 V for 1.5 h. The gel was then stained with 0.5 μg/ml ethidium bromide in 0.5× TBE. Bands were visualized with a molecular imager (ChemiDoc XRS+; Bio-Rad).
Isothermal titration calorimetry.
ITC was performed with a MicroCal iTC200 instrument (Malvern, UK). The Fis protein at 37 μM or the PrtR protein at 33 μM and the DNA probe at 0.2 mM were dialyzed against the same buffer (100 mM NaCl and 20 mM Tris-HCl [pH 7.5}). The software ORIGIN was used to analyze the raw data.
Statistical analysis.
Each experiment was performed at least three times, and the results are shown as mean ± standard deviation (SD). Student's t test (two-tailed) in Prism (GraphPad Software) was employed to analyze the statistical significance.
Data availability.
The transcriptome sequencing (RNA-Seq) data that support the results of this research have been deposited in the NCBI Sequence Read Archive (SRA) under accession number SRP220299.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Key projects of the National Natural Science Foundation of China (grant 41831287), National Natural Science Foundation of China (grants 31670130, 31970680, 31870130, and 81670766), the National Key Research and Development Project of China (grant 2017YFE0125600), the Tianjin Science and Technology Committee (grant 17JCQNJC09200), and the Fundamental Research Funds for the Central Universities of Nankai University (grants 63191521 and 63191121).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We declare no conflict of interest.
Conceptualization: Yuqing Long, Weihui Wu; formal analysis: Yuqing Long, Yongxin Jin; funding acquisitions: Yongxin Jin, Fang Bai, Zhihui Cheng, Weihui Wu; investigation: Yuqing Long, Weixin Fu, Su Wang; methodology: Yuqing Long, Xuan Deng, Weihui Wu; supervision: Zhihui Cheng, Weihui Wu; writing—original draft: Yuqing Long; writing—review & editing: Zhihui Cheng, Weihui Wu.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Govan JR, Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. doi: 10.1128/MMBR.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 3.Pukatzki S, Kessin RH, Mekalanos JJ. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc Natl Acad Sci U S A 99:3159–3164. doi: 10.1073/pnas.052704399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moradali MF, Ghods S, Rehm BH. 2017. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel-Briand Y, Baysse C. 2002. The pyocins of Pseudomonas aeruginosa. Biochimie 84:499–510. doi: 10.1016/s0300-9084(02)01422-0. [DOI] [PubMed] [Google Scholar]
- 6.Ghequire MG, De Mot R. 2014. Ribosomally encoded antibacterial proteins and peptides from Pseudomonas. FEMS Microbiol Rev 38:523–568. doi: 10.1111/1574-6976.12079. [DOI] [PubMed] [Google Scholar]
- 7.Filiatrault MJ, Munson RS Jr, Campagnari AA. 2001. Genetic analysis of a pyocin-resistant lipooligosaccharide (LOS) mutant of Haemophilus ducreyi: restoration of full-length LOS restores pyocin sensitivity. J Bacteriol 183:5756–5761. doi: 10.1128/JB.183.19.5756-5761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith K, Martin L, Rinaldi A, Rajendran R, Ramage G, Walker D. 2012. Activity of pyocin S2 against Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 56:1599–1601. doi: 10.1128/AAC.05714-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui H, Sano Y, Ishihara H, Shinomiya T. 1993. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J Bacteriol 175:1257–1263. doi: 10.1128/jb.175.5.1257-1263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Z, Shi J, Liu C, Jin Y, Li K, Chen R, Jin S, Wu W. 2014. PrtR homeostasis contributes to Pseudomonas aeruginosa pathogenesis and resistance against ciprofloxacin. Infect Immun 82:1638–1647. doi: 10.1128/IAI.01388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penterman J, Singh PK, Walker GC. 2014. Biological cost of pyocin production during the SOS response in Pseudomonas aeruginosa. J Bacteriol 196:3351–3359. doi: 10.1128/JB.01889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang W, Small DA, Toghrol F, Bentley WE. 2005. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics 6:115. doi: 10.1186/1471-2164-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Carcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB. 2016. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun 7:11220. doi: 10.1038/ncomms11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falconi M, Prosseda G, Giangrossi M, Beghetto E, Colonna B. 2001. Involvement of FIS in the H-NS-mediated regulation of virF gene of Shigella and enteroinvasive Escherichia coli. Mol Microbiol 42:439–452. doi: 10.1046/j.1365-2958.2001.02646.x. [DOI] [PubMed] [Google Scholar]
- 15.Rossiter AE, Browning DF, Leyton DL, Johnson MD, Godfrey RE, Wardius CA, Desvaux M, Cunningham AF, Ruiz-Perez F, Nataro JP, Busby SJ, Henderson IR. 2011. Transcription of the plasmid-encoded toxin gene from enteroaggregative Escherichia coli is regulated by a novel co-activation mechanism involving CRP and Fis. Mol Microbiol 81:179–191. doi: 10.1111/j.1365-2958.2011.07685.x. [DOI] [PubMed] [Google Scholar]
- 16.Kelly A, Goldberg MD, Carroll RK, Danino V, Hinton JC, Dorman CJ. 2004. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology 150:2037–2053. doi: 10.1099/mic.0.27209-0. [DOI] [PubMed] [Google Scholar]
- 17.Steinmann R, Dersch P. 2013. Thermosensing to adjust bacterial virulence in a fluctuating environment. Future Microbiol 8:85–105. doi: 10.2217/fmb.12.129. [DOI] [PubMed] [Google Scholar]
- 18.Lenz DH, Bassler BL. 2007. The small nucleoid protein Fis is involved in Vibrio cholerae quorum sensing. Mol Microbiol 63:859–871. doi: 10.1111/j.1365-2958.2006.05545.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RC, Bruist MF, Simon MI. 1986. Host protein requirements for in vitro site-specific DNA inversion. Cell 46:531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- 20.Ball CA, Johnson RC. 1991. Multiple effects of Fis on integration and the control of lysogeny in phage lambda. J Bacteriol 173:4032–4038. doi: 10.1128/jb.173.13.4032-4038.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinreich MD, Reznikoff WS. 1992. Fis plays a role in Tn5 and IS50 transposition. J Bacteriol 174:4530–4537. doi: 10.1128/jb.174.14.4530-4537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider R, Travers A, Kutateladze T, Muskhelishvili G. 1999. A DNA architectural protein couples cellular physiology and DNA topology in Escherichia coli. Mol Microbiol 34:953–964. doi: 10.1046/j.1365-2958.1999.01656.x. [DOI] [PubMed] [Google Scholar]
- 23.Travers A, Muskhelishvili G. 2005. DNA supercoiling—a global transcriptional regulator for enterobacterial growth? Nat Rev Microbiol 3:157–169. doi: 10.1038/nrmicro1088. [DOI] [PubMed] [Google Scholar]
- 24.Auner H, Buckle M, Deufel A, Kutateladze T, Lazarus L, Mavathur R, Muskhelishvili G, Pemberton I, Schneider R, Travers A. 2003. Mechanism of transcriptional activation by FIS: role of core promoter structure and DNA topology. J Mol Biol 331:331–344. doi: 10.1016/s0022-2836(03)00727-7. [DOI] [PubMed] [Google Scholar]
- 25.Choi HS, Kim KS, Park JW, Jung YH, Lee Y. 2005. Effects of FIS protein on rnpB transcription in Escherichia coli. Mol Cells 19:239–245. [PubMed] [Google Scholar]
- 26.Bokal AJ, Ross W, Gaal T, Johnson RC, Gourse RL. 1997. Molecular anatomy of a transcription activation patch: FIS-RNA polymerase interactions at the Escherichia coli rrnB P1 promoter. EMBO J 16:154–162. doi: 10.1093/emboj/16.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallik P, Paul BJ, Rutherford ST, Gourse RL, Osuna R. 2006. DksA is required for growth phase-dependent regulation, growth rate-dependent control, and stringent control of fis expression in Escherichia coli. J Bacteriol 188:5775–5782. doi: 10.1128/JB.00276-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin DJ, Cagliero C, Zhou YN. 2012. Growth rate regulation in Escherichia coli. FEMS Microbiol Rev 36:269–287. doi: 10.1111/j.1574-6976.2011.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filutowicz M, Ross W, Wild J, Gourse RL. 1992. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol 174:398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gille H, Egan JB, Roth A, Messer W. 1991. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res 19:4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato SM, Orman MA, Brynildsen MP. 2013. Metabolic control of persister formation in Escherichia coli. Mol Cell 50:475–487. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Browning DF, Cole JA, Busby SJ. 2004. Transcription activation by remodelling of a nucleoprotein assembly: the role of NarL at the FNR-dependent Escherichia coli nir promoter. Mol Microbiol 53:203–215. doi: 10.1111/j.1365-2958.2004.04104.x. [DOI] [PubMed] [Google Scholar]
- 33.Browning DF, Grainger DC, Beatty CM, Wolfe AJ, Cole JA, Busby SJ. 2005. Integration of three signals at the Escherichia coli nrf promoter: a role for Fis protein in catabolite repression. Mol Microbiol 57:496–510. doi: 10.1111/j.1365-2958.2005.04701.x. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Gil G, Bringmann P, Kahmann R. 1996. FIS is a regulator of metabolism in Escherichia coli. Mol Microbiol 22:21–29. doi: 10.1111/j.1365-2958.1996.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein-Fischer D, Elgrably-Weiss M, Altuvia S. 2000. Escherichia coli response to hydrogen peroxide: a role for DNA supercoiling, topoisomerase I and Fis. Mol Microbiol 35:1413–1420. doi: 10.1046/j.1365-2958.2000.01805.x. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg MD, Johnson M, Hinton JC, Williams PH. 2001. Role of the nucleoid-associated protein Fis in the regulation of virulence properties of enteropathogenic Escherichia coli. Mol Microbiol 41:549–559. doi: 10.1046/j.1365-2958.2001.02526.x. [DOI] [PubMed] [Google Scholar]
- 37.Schechter LM, Jain S, Akbar S, Lee CA. 2003. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect Immun 71:5432–5435. doi: 10.1128/iai.71.9.5432-5435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lautier T, Nasser W. 2007. The DNA nucleoid-associated protein Fis co-ordinates the expression of the main virulence genes in the phytopathogenic bacterium Erwinia chrysanthemi. Mol Microbiol 66:1474–1490. doi: 10.1111/j.1365-2958.2007.06012.x. [DOI] [PubMed] [Google Scholar]
- 39.Cho BK, Knight EM, Barrett CL, Palsson BO. 2008. Genome-wide analysis of Fis binding in Escherichia coli indicates a causative role for A-/AT-tracts. Genome Res 18:900–910. doi: 10.1101/gr.070276.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hancock SP, Stella S, Cascio D, Johnson RC. 2016. DNA sequence determinants controlling affinity, stability and shape of DNA complexes bound by the nucleoid protein Fis. PLoS One 11:e0150189. doi: 10.1371/journal.pone.0150189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng X, Li M, Pan X, Zheng R, Liu C, Chen F, Liu X, Cheng Z, Jin S, Wu W. 2017. Fis regulates type III secretion system by influencing the transcription of exsA in Pseudomonas aeruginosa strain PA14. Front Microbiol 8:669. doi: 10.3389/fmicb.2017.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorr T, Lewis K, Vulic M. 2009. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet 5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaber JW, Hochhut B, Waldor MK. 2004. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 427:72–74. doi: 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- 45.Webber MA, Piddock LJ. 2003. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 46.Kohler T, Donner V, van Delden C. 2010. Lipopolysaccharide as shield and receptor for R-pyocin-mediated killing in Pseudomonas aeruginosa. J Bacteriol 192:1921–1928. doi: 10.1128/JB.01459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hancock SP, Ghane T, Cascio D, Rohs R, Di Felice R, Johnson RC. 2013. Control of DNA minor groove width and Fis protein binding by the purine 2-amino group. Nucleic Acids Res 41:6750–6760. doi: 10.1093/nar/gkt357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorman CJ. 2004. H-NS: a universal regulator for a dynamic genome. Nat Rev Microbiol 2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 49.Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, Hinton JC. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog 2:e81. doi: 10.1371/journal.ppat.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 51.Atlung T, Ingmer H. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol 24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 52.Heroven AK, Nagel G, Tran HJ, Parr S, Dersch P. 2004. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol Microbiol 53:871–888. doi: 10.1111/j.1365-2958.2004.04162.x. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Liu X, Tang K, Wang P, Zeng Z, Guo Y, Wang X. 2019. Excisionase in Pf filamentous prophage controls lysis-lysogeny decision-making in Pseudomonas aeruginosa. Mol Microbiol 111:495–513. doi: 10.1111/mmi.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castang S, Dove SL. 2012. Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. J Bacteriol 194:5101–5109. doi: 10.1128/JB.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMackin EAW, Marsden AE, Yahr TL. 2019. H-NS family members MvaT and MvaU regulate the Pseudomonas aeruginosa type III secretion system. J Bacteriol 201:e00054-19. doi: 10.1128/JB.00054-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallet I, Diggle SP, Stacey RE, Camara M, Ventre I, Lory S, Lazdunski A, Williams P, Filloux A. 2004. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J Bacteriol 186:2880–2890. doi: 10.1128/jb.186.9.2880-2890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castang S, McManus HR, Turner KH, Dove SL. 2008. H-NS family members function coordinately in an opportunistic pathogen. Proc Natl Acad Sci U S A 105:18947–18952. doi: 10.1073/pnas.0808215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skoko D, Yoo D, Bai H, Schnurr B, Yan J, McLeod SM, Marko JF, Johnson RC. 2006. Mechanism of chromosome compaction and looping by the Escherichia coli nucleoid protein Fis. J Mol Biol 364:777–798. doi: 10.1016/j.jmb.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinert BT, Iesmantavicius V, Wagner SA, Scholz C, Gummesson B, Beli P, Nystrom T, Choudhary C. 2013. Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol Cell 51:265–272. doi: 10.1016/j.molcel.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. 2014. Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS One 9:e94816. doi: 10.1371/journal.pone.0094816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Long Y, Liu Y, Liu Y, Chen R, Shi J, Zhang L, Jin Y, Yang L, Bai F, Jin S, Cheng Z, Wu W. 2016. HigB of Pseudomonas aeruginosa enhances killing of phagocytes by up-regulating the type III secretion system in ciprofloxacin induced persister cells. Front Cell Infect Microbiol 6:125. doi: 10.3389/fcimb.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen F, Chen G, Liu Y, Jin Y, Cheng Z, Liu Y, Yang L, Jin S, Wu W. 2017. Pseudomonas aeruginosa oligoribonuclease contributes to tolerance to ciprofloxacin by regulating pyocin biosynthesis. Antimicrob Agents Chemother 61:e02256-16. doi: 10.1128/AAC.02256-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptome sequencing (RNA-Seq) data that support the results of this research have been deposited in the NCBI Sequence Read Archive (SRA) under accession number SRP220299.