Abstract
Background
Infectious complications of chimeric antigen receptor (CAR) T-cell immunotherapy in children and young adults have not been well described.
Methods
Medical records of patients ≤26 years old receiving CD19 CAR T-cell infusion (CTI) at a single institution between 2014 and 2017 were reviewed. The number of infections per 100 days-at-risk (infection density) in the 90 days preceding and 0–28 and 29–90 days after CTI was calculated. Poisson regression and Cox analyses were utilized to identify risk factors for infections.
Results
Eighty-three patients received CTI during the study period. Most patients (98%) had refractory or relapsed acute lymphoblastic leukemia (ALL). Infections occurred in 54% of patients in the 90 days before CTI (infection density, 1.23) and in 40% of patients in the first 28 days following CTI (infection density, 2.89). Infection density decreased to 0.55 in the 29–90 days post-CTI. Most infections were bacteremias (39%) or respiratory viral infections (43%). Pre-CTI risk factors associated with infection included prior hematopoietic cell transplantation (HCT), immunoglobulin G (IgG) level <400 mg/dL, and lymphodepletion other than cyclophosphamide plus fludarabine; post-CTI risk factors included higher-severity CRS and IgG <400 mg/dL.
Conclusions
Infection rates in children and young adults receiving CD19 CAR T-cell therapy increase in the first month and then decline. Understanding types and timing of infections and contributing risk factors may help inform prophylactic and monitoring strategies. Specific attention should be given to patients with prior HCT, severe hypogammaglobulinemia, and severe CRS.
Keywords: ALL, CAR T-cell, immunotherapy, pediatric, infection
Indications for immunotherapy using CAR T-cells for malignancies are expanding rapidly. Here we describe the incidence of and risk factors for infections in children, adolescents and young adults receiving CD19 CAR T-cell therapy for ALL. These data may inform prophylactic and monitoring strategies in this high-risk population.
Immunotherapy using chimeric antigen receptor (CAR) T-cells for hematologic malignancies and other diagnoses is increasing rapidly [1]. This approach has resulted in high response rates in children with refractory or relapsed (R/R) B-cell acute lymphoblastic leukemia (B-ALL) [2–4]. The first commercially available CD19 CAR T-cell therapy, tisangenlecleucel (Kymriah), was approved by the Food and Drug Administration in 2017 [5].
Patients under consideration for CAR T-cell therapy are at high risk for infections due to extensive prior antitumor therapies. Before CAR T-cell infusion (CTI), patients receive lymphodepleting chemotherapy that leads to additional cytopenias. Furthermore, CD19 CAR-T cells may deplete normal CD19 B cells in the host, potentially causing prolonged B-cell aplasia and low immunoglobulin G (IgG) levels [6, 7]. Finally, these patients often develop cytokine release syndrome (CRS) following CTI, which may require treatment with anti-interleukin 6 (IL-6) therapies (eg, tocilizumab), steroids, and admission to the intensive care unit (ICU), with associated increased risk for immunosuppression and nosocomial infections [8–10].
The infectious complications of CAR T-cell therapy have not been well studied in children and young adults. Little is known about optimal prophylactic strategies in this setting. Two studies have demonstrated a relatively high rate of infections in adult patients after CD19 CTI, especially in the first 28 days [11, 12]. Patient factors such as underlying ALL, increased number of prior chemotherapy courses, higher CAR T-cell doses, and CRS were associated with higher infection rates. The majority of early infections were bacterial, whereas respiratory virus infections predominated at later time points.
In this study, we examine the epidemiology of and risk factors for infections occurring in the first 90 days after CD19 CTI in children and young adults.
METHODS
Patients
This study was approved by the Institutional Review Board at Seattle Children’s Hospital. We reviewed medical records of patients aged 1–26 years receiving CD19 CAR T cells [2] at our institution between 2014 and 2017. The majority of patients remain under our center’s care for a minimum of 28 days after therapy, as this is the time frame when the majority of complications are expected [11, 12]. Each patient’s lymphodepletion regimen was determined by the treating physician. Bacterial prophylaxis was not routinely prescribed. Patients with a history of prior hematopoietic cell transplantation (HCT) were routinely started on acyclovir prophylaxis if not already receiving it. The type and duration of fungal prophylaxis were prescribed at the discretion of the treating physician. All patients were prescribed prophylaxis for Pneumocystis jirovecii with trimethoprim-sulfamethoxazole or inhaled pentamidine. Intravenous immunoglobulin G (IVIG) supplementation was recommended for patients with IgG levels <400 mg/dL. Empiric antimicrobial therapy was prescribed for fever and neutropenia. Further details of the lymphodepleting regimens and supportive care approaches for patients who developed CRS are in the Supplementary Data.
Data Collection
We reviewed medical records for infections occuring in the 90 days before and after CTI. Patients contributed data up to 90 days post-CTI; the date of death; the date of loss to follow-up; or the date of receipt of additional antitumor therapies for relapse, repeat CTI, or as conditioning for HCT, whichever occurred first. Absolute neutrophil (ANC) and lymphocyte (ALC) counts were collected for each patient before lymphodepletion, from all available laboratory data between days 0 and 28 post-CTI, and on day 63 (per study protocols) post-CTI. IgG levels were collected before lymphodepletion and on days 21 and 63 (per study protocols) post-CTI. Treatment for CRS, including use of tociluzimab and/or steroids, was recorded.
Definitions
The primary outcome of interest was infection in the 90 days post-CTI. An infection episode was defined as the presence of clinical findings plus corroborating laboratory, radiographic, histopathologic, and/or microbiologic evidence. Infections were classified as bacterial (bacteremia or site infection), viral (respiratory or other), or fungal (proven or probable) [13]. Infection onset was defined as the day of initial positive diagnostic test or clinical diagnosis. Infections were excluded if they were already present at the time of CTI. Infection severity was categorized as 1 (mild) if no treatment was required, 2 (moderate) if only oral treatment or minimal supportive care was required, 3 (severe) if intravenous therapy or hospitalization was required, 4 if life threatening, or 5 if fatal [14]. Testing for infection was sent based on provider discretion guided by symptoms, further outlined in the Supplementary Data. Further details pertaining to the assessment of the timing, type, and severity of each infection are also described in the Supplementary Data.
The severity of CRS was graded from 1–5 as previously described [15], and definitions of each grade are shown in Supplementary Table 1.
Statistical Analysis
Descriptive statistics (median, range, percentage) are reported for key variables. We calculated the infection density per 100 days-at-risk based on the number of infections per person-days-at-risk and multiplied by 100. This was calculated for the 90 days before CTI and the 0–28- and 29–90-day periods after CTI. The estimates for infection densities are only based on data obtained during the person-days-at risk contributed per patient. We also demonstrate the cumulative incidence of any and each type of infection in the first 28 days after CTI, treating death and loss to follow-up as competing risk events.
Poisson regression was utilized to identify associations between pre-CTI factors and infection density within the first 28 days after CTI. Time-dependent Cox proportional hazards models were used to assess the impact of pre- and post-CTI factors on the time to first infection within 28 days after CTI. For all factors, only data occurring before an infection event were included. For each infection event, the ANC and ALC values from the day of the event were considered as dichotomous data points (ANC < 500 or no, ALC < 300 or no). When a value for that day was not available, the most recently available value was used instead. For both Poisson and Cox regression, variables with a P value of <.25 in univariate models were considered for multivariate adjusted models. Poisson and Cox models were evaluated for overfitting or underfitting and Schoenfeld residuals, respectively. Patients were censored from the study before day 90 post-CTI if they died, were lost to close follow-up, or received further lymphodepleting chemotherapy in the setting of relapse, repeat CTI, or as conditioning for HCT.
RESULTS
Patient Characteristics
Eighty-three patients received CTI during the study period. The median duration of follow-up (range) was 45 (9–90) days. Patient and treatment characteristics are described in Table 1. All patients had underlying B-ALL except for 1 patient with mixed leukemia and 1 with B-cell lymphoma. All patients had relapsed or refractory disease. The median age (range) was 12 (1–25) years. Forty-six patients (55%) had previously undergone allogeneic HCT, and none were on immunosuppresive therapy or had graft-vs-host disease for at least 4 weeks before CTI. Before receipt of lymphodepleting chemotherapy, 18% had an ALC <300 cells/mm3, 35% had an ANC <500 cells/mm3, and 16% had a serum IgG <400 mg/dL. The most common lymphodepletion regimen consisted of fludarabine plus cyclophosphamide (65%). Doses of CAR T cells ranged from 0.5 × 106 to 10 × 106 CAR T cells/kg.
Table 1.
Demographic, Laboratory, and Clinical Characteristics
| Baseline Characteristics | No. (% Unless Indicated), n = 83 Unless Specified |
|---|---|
| Median age (range), y | 12 (1–25) |
| Female sex | 33 (40) |
| Race | |
| Asian | 9 (11) |
| White | 48 (57) |
| Hispanic/Latino | 14 (17) |
| Black or Pacific Islander | 2 (2) |
| Other | 4 (5) |
| Unknown | 6 (7) |
| Underlying disease | 81 (98) |
| B-ALL | 1(1) |
| B-cell lymphoma | 1 (1) |
| Mixed phenotype leukemia | |
| Prior allogeneic HCT | 46 (55) |
| Disease status | |
| Refractory | 8 (10) |
| Relapsed | |
| 1 | 29 (35) |
| 2 | 31 (38) |
| 3 | 10 (12) |
| 4 | 5 (6) |
| Hematologic parameters before lymphodepletion, No. (%), median (range) | |
| ALC < 300 cells/mm3 | 15 (18), 826 (0–8476) |
| ANC < 500 cells/mm3 | 28 (34), 1071 (0–11 284) |
| IgG < 400 mg/dL | 13 (16), 617 (143–1480) |
| Pre-CAR T-cell infections (days –90 to –1), No. (%) | 45 (54) |
| Bacterial | 29 (35) |
| Fungal | 15(18) |
| Viral | 20 (24) |
| Antimicrobial prophylaxis, No. (%) | |
| Anti-PJP | 83 (100) |
| Antiviral | 29 (35) |
| Antifungal | 42 (51) |
| Fluconazole | 12 (14) |
| Antimold azole | 21 (25) |
| Echinocandin | 2 (2) |
| Cyclophosphamide/fludarabine lymphodepletiona | 54 (65) |
| Cyclophosphamide only (3 g/m2) | 23 (28) |
| Otherb | 6 (7) |
| Post-CAR T-cell infusion characteristics | |
| Hematologic parameters, No. (%), median (range) | |
| ANC < 500 cells/mm3 | |
| D +7 (n = 82) | 62 (76), 32 (0–3043) |
| D +21 (n = 79) | 38 (48), 624 (0–6636) |
| D +63 (n = 40) | 5 (13), 2061 (0–7730) |
| ALC < 300 cells/mm3 | |
| D +7 (n = 82) | 57 (70), 129 (0–1606) |
| D +21 (n = 79) | 28 (36), 429 (0–3515) |
| D +63 (n = 40) | 8 (20), 567 (0–4183) |
| IgG level < 400 mg/dL | |
| D +21 (n = 78) | 11 (14), 584 (168–1210) |
| D +63 (n = 56) | 16 (29), 455 (190–760) |
| Cytokine release syndrome, any | 74 (89) |
| Grade 1–2 | 66 (79) |
| Grade 3–5 | 8 (10) |
| Corticosteroids and/or tocilizumab | 43 (52) |
| ICU admission by day 28 | 48 (58) |
| Death by day 28 | 3 (4) |
| Infection related | 0 |
| Death by day 90 | 6 (8) |
| Infection related | 1 (1) |
Abbreviations: ALC, absolute lymphocyte count; ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; CAR, chimeric antigen receptor; HCT, hematopoietic cell transplant; IgG, immunoglobulin G; PJP, Pneumocystis jirovecii.
aFludarabine 30 mg/m2 × 4 days plus cyclophosphamide 500 mg/m2 × 2 days (days 1, 2).
bFludarabine only (2%), cyclophosphamide only (1.5 g/m2, 4%), or cyclophosphamide and etoposide (1%).
Details of antimicrobial prophylaxis are outlined in Table 1. At the time of CTI, no patient had a positive blood culture within 48 hours or had fever with clinical signs of infection.
Noninfectious Complications After Lymphodepleting Chemotherapy and CTI
The majority of patients (96%) experienced neutropenia (ANC < 500 cells/mm3) at the time of, or within 7 days of, CTI. Of these, 42% for whom complete data were available did not recover from neutropenia (defined as 2 measurements of ANC >500 cells/mm3) by day 28 post-CTI. Three patients died while still neutropenic in this time period. For the patients who were neutropenic and did recover by day 28 (58%), the median time to recovery was 15 days. Severe hypogammaglobulinemia (IgG <400 mg/dL) was identified in 14% of patients at day 21 (Table 1). By day 63 post-CTI, 13% of 40 patients with available data remained neutropenic and 20% lymphopenic. Of 56 patients with IgG levels available, 29% remained hypogammoglubulinemic with a median IgG level of 455 mg/dL (irrespective of IgG supplementation).
Forty-eight (58%) patients had an ICU admission in the 28 days post-CTI. CRS occurred in 89% of patients at a median (range) of 5 (1–11) days after infusion (Table 1). The majority of these cases were categorized as grade 1 or 2 (79%), with CRS grade 3 or higher occuring in 8 patients (10%). Forty-three (52%) patients received treatment for CRS with either tocilizumab and/or steroids.
Infections Pre-CTI
Forty-five (54%) patients had at least 1 infection in the 90 days before CTI, as detailed in Supplementary Table 2. Forty-eight percent of the infections in this time period were bacterial, and 34% were viral. Nine patients (11%) were being treated for proven or probable fungal infections. The infection density for this time period was 1.23 infections per 100 patient-days-at-risk (Figure 1).
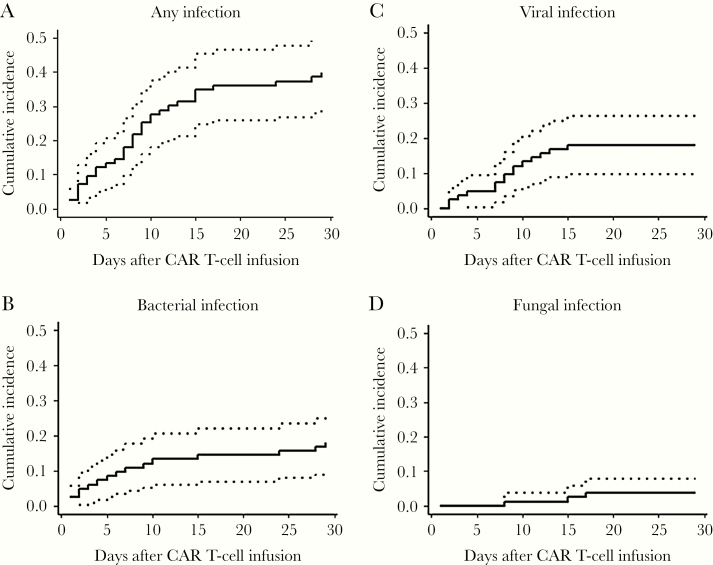
Figure 1.
Cumulative incidence curves of time to first infections in the first 28 days after CAR T-cell therapy. A–D, Cumulative incidences among all patients (n = 83) of any (A), bacterial (B), viral (C), and fungal (D) infections within 28 days after CTI. Dotted lines represent 95% confidence intervals. Death and loss to follow-up were treated as competing risks. Abbreviations: CAR, chimeric antigen receptor; CRS, cytokine release syndrome.
Infections in the First 28 Days Post-CTI
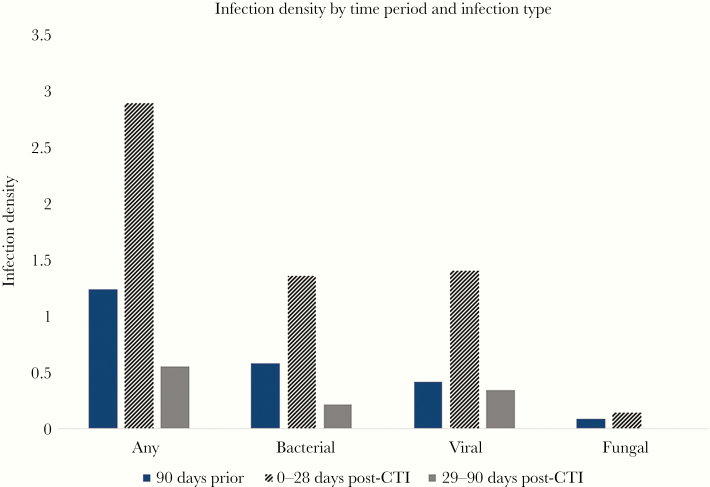
Thirty-three patients (40%) had 37 incident infections in the first 28 days post-CTI (Table 2). Twenty infections (54%) were bacterial, including 16 instances of bacteremia. Of these episodes of bacteremia, 6 were caused by gram-negative organisms, 10 by gram-positive bacteria, and all occurred within the first 15 days post-CTI (Figure 1). Three of the 11 patients who developed bacteremia had infection with an organism that was identified in the pre-CTI period. Sixteen patients had viral infections, the majority of which were due to respiratory viruses (38% of total infections). Two patients developed herpes zoster, with skin manifestations in both; neither patient was taking acyclovir prophylaxis. One patient with pre-CTI probable pulmonary invasive mold infection on mold-active therapy with voriconazole was found to have proven pulmonary infection with Cunninghamella on day 21 post-CTI. It is unclear whether this represented worsening of previous infection or new infection. The cumulative incidences of infections overall and by infection type are in Figure 1. The infection density during the first 28 days was 2.89 infections per 100 days-at-risk (Figure 2).
Table 2.
Incidence and Severity of Infections After CAR-T-cell Infusion
| Infection Days 0–28 | Infection Days 29–90 | Severity | |||||
|---|---|---|---|---|---|---|---|
| Type of Infection | Events | No. of Patients (%) | Events | No. of Patients (%) | Mild–Moderate | Severe | Life-Threatening/Fatal |
| Any infection | 37 | 33 (40) | 12 | 11 (17) | 21 | 22 | 6 |
| Bacterial infections | 20 | 15 (18) | 5 | 5 (6) | 3 | 16 | 6 |
| Bacteremiaa | 16 | 11 (13) | 3 | 3 (5) | 0 | 13 | 6 |
| Site infectionb | 4 | 4 (5) | 2 | 2 (2) | 3 | 3 | 0 |
| Viral infections | 16 | 16 (19) | 7 | 7 (11) | 19 | 4 | 0 |
| Respiratory virusc | 14 | 14 (17) | 7 | 7(11) | 18 | 3 | 0 |
| Other virus (herpes zoster) | 2 | 2 (2) | 0 | 0 | 1 | 1 | 0 |
| Fungal infectionsd | 1 | 1 (1) | 0 | 0 | 0 | 0 | 1 |
aSix gram-negative infections: Escherichia coli (3), Klebsiella oxytoca (1), Pseudomonas aeruginosa (1), Enterobacter cloacae (1). Thirteen gram-positive infections: Enterococcus faecalis (5), Streptococcus viridans (2), coagulase-negative staphylococci (3), Gordonia species (1), Microbacterium species (1), Clostridium tertium (1).
bSkin and soft tissue infection (3), pneumonia (1), central line site infection (1), proctitis (1).
cRhinovirus/enterovirus (10), parainfluenza (5), respiratory syncticial virus (3), influenza A (1), human metapneumovirus (1), coronavirus (1).
d Cunninghamella pulmonary infection.
Figure 2.
Infection density before and after CAR T-cell therapy. Infection density was calculated as the total number of infection events per patient-days-at-risk multiplied by 100. Only data obtained during person-days-at risk contributed to these estimates. Infection densities are shown for any infection and by pathogen category for the time periods 90 days before CTI and 0–28 and 29–90 days after CTI. Abbreviations: CAR, chimeric antigen receptor; CRS, cytokine release syndrome.
Infections in Days 29–90 Post-CTI
Forty-eight patients contributed data during this time period, with a median duration of follow-up (range) of 51 (29–90) days. There were 7 viral infections in this time period, which comprised the majority of infections (58%). All were due to respiratory viruses (Table 2). Five patients had bacterial infections, and there were no fungal infections. The infection density in the 29–90 days post-CTI decreased to 0.55 per 100 days-at-risk (Figure 2).
Infection Severity
Table 2 demonstrates the severity of infections. Between 0 and 90 days after CTI, mild or moderate infections accounted for 21 of 49 total episodes (43%), most of which were viral infections. There were 22 severe infections (45%) and 6 (13%) life-threatening infections, most of which were bacterial. There were no fatal infections in the first 28 days post-CTI. One death occurred on day 51 post-CTI due to septic shock with Aeromonas hydrophila.
Risk Factors for Infections in the First 28 Days Post-CTI
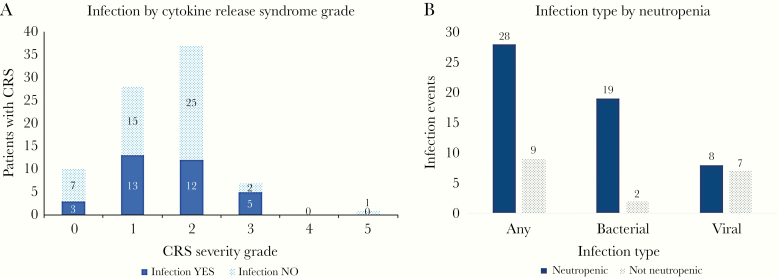
The proportion of patients with any infection in the 28 days post-CTI stratified by CRS grade is shown in Figure 3A. A higher proportion of patients with higher-severity CRS had infections; 30% (95% confidence interval [CI], 7%–65%) had no CRS, 46% had grade 1 (95% CI, 28%–66%), 32% had grade 2 (95% CI, 18%–50%), and 62% had grade ≥3 CRS (95% CI, 24%–91%). Of note, no patients had grade 4 CRS, and only 1 patient had grade 5 CRS and died on day 11 post-CTI. Thus, our understanding of the true impact of higher-grade CRS on infection risk is limited from this cohort. The proportion of patients with infection who were neutropenic at the time of infection is depicted in Figure 3B. Most patients with bacteremia (90%) were neutropenic, whereas viral infections occurred in similar numbers of patients with and without neutropenia.
Figure 3.
Infection events in the first 28 days after CAR T-cell therapy stratified by CRS and the presence of neutropenia. A, Proportion of patients with infection by grade: 0 (no CRS): 30% (95% CI, 7%–65%); 1: 46% (95% CI, 28%–66%); 2: 32% (95% CI, 18%–50%); ≥3: 62% (95% CI, 24%–91%). Of note, the infection episode may have occurred before, during, or after CRS during this time period. B, The number of patients with each infection type stratified by the presence or absence of neutropenia (ANC < 500 cells/µL) at the time of infection. Abbreviations: CAR, chimeric antigen receptor; CI, confidence interval; CRS, cytokine release syndrome.
The results of Poisson regression analysis of the impact of baseline patient and clinical variables on infection density within 28 days after CTI are shown in Table 3. Prior HCT and IgG <400 mg/dL were associated with increased infection density but did not reach statistical significance in the adjusted model. Lymphodepletion with combination cyclophosphamide and fludarabine was associated with significantly decreased infection density (rate ratio, 0.46; 95% CI, 0.27–0.77) compared with other regimens in the adjusted model. Age, sex, refractory vs relapsed B-ALL status, CAR T-cell dose, pre-CAR T-cell infections, and prelymphodepletion neutropenia and lymphopenia were not associated with increased infection density.
Table 3.
Pre-CAR T-cell Infusion Factors and Association With Infection Density Within 28 Days of Infusion
| Pre-CAR T Variables | Univariate Ratio of Infection Densities (95% CI) | P Value | Adjusted Ratio of Infection Densities (95% CI)a | P Value |
|---|---|---|---|---|
| Age | 0.99 (0.96–1.03) | .66 | ||
| Sex (male) | 0.93 (0.57–1.54) | .78 | ||
| Prior HCT | 1.69 (1.01–2.92) | .05 | 1.54 (0.92–2.69) | .11 |
| Pre-CAR T infections (day –90 to day 0) | 0.83 (0.51–1.37) | .46 | ||
| Relapsed (vs refractory) ALL | 1.6 (0.66–5.28) | .36 | ||
| Cyclophosphamide/fludarabine (vs other lymphodepletion) | 0.5 (0.30–0.82) | .01 | 0.46 (0.27–0.77) | <.01 |
| Prelymphodepletion parameters | ||||
| IgG < 400 mg/dL | 1.44 (0.77–2.53) | .23 | 1.69 (0.88–3.06) | .09 |
| ALC < 300 cells/mm3 | 1.05 (0.53–1.90) | .88 | ||
| ANC < 500 cells/mm3 | 1.13 (0.68–1.87) | .62 | ||
| CAR T dose (≥5 × 106 cells/kg vs other) | 1.02 (0.49–1.92) | .95 |
Abbreviations: ALC, absolute lymphocyte count; ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; CAR, chimeric antigen receptor; HCT, hematopoietic cell transplant; IgG, immunoglobulin G.
aMultivariate analysis includes variables with P < .25 in univariate analysis.
In a Cox proportional hazards model of the impact of pre- and post-CTI variables on time to first infection, prior HCT (hazard ratio [HR], 2.15; 95% CI, 0.98–4.73) and post-CTI hypogammaglobulinemia (HR, 2.41; 95% CI, 1.02–5.69) were associated with increased infection risk in the first 28 days in the adjusted model (Table 4). This analysis also confirmed the decrease in infection risk with cyclophosphamide and fludarabine lymphodepletion. Severe CRS was associated with an increased risk for infection but did not reach statistical significance in the adjusted model.
Table 4.
Risk Factors for Time to First Infection Within 28 Days of CAR-T-cell Infusion
| Univariate Hazard Ratio (95% CI) | P Value | Multivariate Hazard Ratio (95% CI)b | P Value | |
|---|---|---|---|---|
| Pre-CTI variablesa | ||||
| Prior HCT | 2.23 (1.06–4.70) | .04 | 2.15 (0.98–4.73) | .05 |
| Lymphodepletion with cyclophosphamide plus fludarabine | 0.57 (0.28–1.02) | .05 | 0.45 (0.21–0.96) | .04 |
| Post-CTI variables | ||||
| CRS grade | ||||
| 1–2 (n = 66) | 0.57 (0.22–1.51) | .26 | 0.44 (0.16–1.23) | .11 |
| 3–5 (n = 8) | 2.47 (0.74–8.32) | .14 | 1.81 (0.53–6.15) | .34 |
| Tocilizumab use | 1.15 (0.48–2.77) | .75 | ||
| Steroid use | 0.55 (0.16–1.87) | .34 | ||
| ANC < 500 cells/μLc | 1.14 (0.49–2.65) | .75 | ||
| ALC < 300 cells/μLc | 0.92 (0.37–2.26) | .85 | ||
| IgG < 400 mg/dL | 1.99 (0.99–4.42) | .09 | 2.41 (1.02–5.69) | .04 |
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; CTI, CAR-T-cell infusion; HCT, hematopoietic cell transplant; IgG, immunoglobulin G.
aAll pre-CTI variables included in the Poisson model (Table 3) were included in this analysis, except for pre-CTI IgG, which showed significant collinearity with post-CTI IgG. Only variables meeting the P value criterion for inclusion in the multivariate model (see below) are shown.
bMultivariate model includes variables with P < .25 in the univariate model only.
cANC and ALC data from the day of infection event were utilized when available; otherwise the most recent value was utilized. For each event, each value was considered as a dichotomous variable.
DISCUSSION
This study is the first in-depth description of the frequency, type, severity, and risk factors for infections following CD-19 CAR T-cell therapy in children, adolescents, and young adults. Overall, 33 of 83 patients (40%) experienced 1 or more infections in the first 28 days after CTI. Infection density more than doubled in the first 28 days post-CTI when compared with the 90 days pre-CTI and then decreased in the subsequent 29–90 days post-CTI. Pre-CTI HCT, lymphodepletion with a regimen other then cyclophosphamide plus fludarabine, and post-CTI hypogammaglobulinemia were associated with increased infection.
Although the overall incidence of any infection in the first month is similar to that found in adults with ALL following CTI [12], the infection density of 2.89 infections per 100 days-at-risk was higher in our cohort [11]. As in the adult studies, our patients experienced a predominance of bacterial infections, specifically bacteremias, especially within the first 2 weeks post-CTI. We observed a higher incidence of respiratory viral infections compared with findings in adult CTI recipients, which may be due to the younger age of our patients. Respiratory viral infections are common in pediatric immunocompromised populations [16, 17]. One patient (1%) was diagnosed with a proven invasive mold infection on day 21 post-CTI while receiving voriconazole for previously diagnosed probable invasive mold infection. This individual had concomitant severe neutropenia and mild CRS treated with tocilizumab. It is unclear whether this infection represented new fungal disease or worsening of the previously diagnosed infection.
Our data suggest that prior HCT and hypogammaglobulinemia may be risk factors for infections in the first 28 days post-CTI. Interestingly, preexisting infection and prelymphodepletion lymphopenia and neutropenia did not significantly influence risk of infection. Lymphodepletion with the combination of cyclophosphamide and fludarabine was associated with a decreased incidence of infection in the short term. Although this combination regimen has been associated with improved cancer outcomes after CD19 CAR T-cell therapy in ALL [18], a relationship with decreased infection has not been previously demonstrated. In our cohort, those patients who received CAR T-cell therapy more recently were more likely to have received this combination lymphodepletion. These patients may also have been more likely to have received antibacterial prophylaxis or have received CAR T-cell therapy earlier in their ALL disease process. In addition, cyclophosphamide dosing of 3 g/m2 may have caused more bone marrow suppression than the combination conditioning regimen with a lower cyclophosphamide dose (0.5 g/m2). Unlike previous studies, we did not demonstrate a link between higher CAR T-cell doses and infection. However, our dose range was narrower than those used in previous studies in adult patients.
Prior studies in adult CAR T-cell recipients with lymphoma, chronic lymphocytic leukemia (CLL), and ALL demonstrated that severe CRS (grade 3 or higher) was associated with increased infections [11, 12]. In our patients with ALL, we found a similar association, but this did not reach statistical significance. However, we had fewer patients with severe CRS than in other studies, which may have limited our ability to identify a clear link with infection. The low rates of CRS in this cohort may be related to the different CAR T-cell product and/or an early intervention strategy for CRS [19].
Neutropenia is well known to be associated with increased risk of bacterial infections [20]. In a study of adult CTI recipients, the median time to neutrophil recovery was 6 days, but prolonged cytopenias have been demonstrated previously, even in patients with MRD-negative remission after CAR T-cell therapy [3, 7, 11, 21]. In our study, almost half of the pediatric patients remained neutropenic at day 21, with persistent neutropenia through day 63 in 13% of patients. Given that 90% of bacterial infections in the first 28 days occurred during neutropenia [7], this has important implications for the longer-term management of patients with persistent cytopenias.
For patients in whom records were available for the 29–90-day period post-CTI, infection rates dropped to a rate even lower than in the pre-CTI period. This is despite the fact that sicker patients were less likely to return to their home centers after the first month and therefore were more likely to be included in our analysis. One infection-related death occurred on day 51 due to multiorgan failure attributed to bacterial sepsis. The infection density in the 29–90-day time period in this study was similar to that found beyond 90 days in adult CTI recipients [7].
Persistent B-cell aplasia is a known effect of CD19 CAR T-cell therapy and indeed can serve as a proxy of persistence of CAR T cells themselves [3]. We found that 29% of patients had IgG levels of <400 mg/dL by 63 days post-CTI, and severe hypogammaglobulinemia was associated with increased infection risk. Persistent hypogammoglobulinemia is also a common feature after CTI in adults [6, 11, 22]. Further study of the duration and extent of these deficits could influence infection prevention strategies such as vaccination and more standardized administration of IVIG supplementation.
Standardization of antimicrobial prophylaxis in patients receiving CTI may help prevent infections. Specifically, we identified 2 patients who developed herpes zoster in the first month after CTI, both of whom had not received antiviral prophylaxis. We have now instituted a policy of antiviral prophylaxis with acyclovir for all patients starting at lymphodepletion and continuing for at least 100 days. We are also instituting standard appoaches to antibiotic prophylaxis during neutropenia in patients who are expected to have a prolonged duration (>7 days) of ANC ≤200/mm3. Ensuring that prophylaxis covers previously identified infections is an important consideration. Indeed, 3 of the 11 patients with bacteremia in the 0–28-day post-CTI period had preceding bacteremia with the same organism. Although there was only 1 episode of invasive fungal disease, our standard approach to antifungal prophylaxis has also evolved. Fluconazole is given to all patients during neutropenia; for patients with a history of mold infection, we consider starting or continuing a mold-active azole. The optimal type and duration of prophylactic regimens need further study [21].
This study has limitations given the retrospective design and use of data from a single center. Analyses of infection events beyond 28 days after CTI included fewer patients due to loss to follow-up after patients returned to their primary oncologists. This may have resulted in biasing analyses during this time period toward sicker patients. Our patients received variable antimicrobial prophylaxis regimens, and our analysis was not powered to perform risk factor analysis for each type of infection individually; risks may vary for viral, bacterial, and fungal infections. It is possible that patients with lymphopenia and neutropenia were more likely to receive antimicrobial prophylaxis, which may have altered our ability to detect an association between these factors and infection outcomes.
Assigning causality for the infections in the early period after CTI is difficult. Many of the infections may not be directly attributable to the product itself given the underlying risk factors, the conditioning lymphodepleting chemotherapy, and the development and treatment of CRS. In fact, the infection characteristics in adult patients were found to be similar to ALL patients receiving salvage chemotherapy [11]. In addition, these data may not be generalizable to CAR T-cell treatments for other hematologic or solid malignancies. Finally, the use of different CAR T-cell constructs or concurrent antitumor therapies could likely affect infection risk as well.
Increasing use of CAR T-cell therapy for the treatment of malignancies is leading to improvements in survival for high-risk children with ALL, while also posing risks for infections, particularly during the early period after CTI. Our results suggest that viral, bacterial, and fungal prophylaxis and lymphodepletion regimens need to be carefully considered in the setting of CAR T-cell therapy. Patients with previous HCT, hypogammaglobulinemia, and severe CRS may be at highest risk. Further study of patients with standardized prophylactic regimens and long-term follow-up will be useful for optimizing treatment guidelines for these high-risk children.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the Center for Clinical and Translational Research, Seattle Children’s Hospital, Seattle, Washington (to S.V.), and the National Institute of Allergy and Infectious Diseases (K23 AI119133 to J.A.H. and K23 AI114844 to A.W.).
Potential conflicts of interest. J.A.H. has served as a consultant for Nohla Therapeutics, Inc., Amplyx, and Gilead and has received research support from Nohla Therapeutics, Karius, and Takeda (formerly Shire), all unrelated to this research. A.W. has served as a consultant for Kyorin Pharmaceuticals, unrelated to this research. R.G. has served as a consultant for Novartis, unrelated to this research. J.A.E. has served as a consultant to Sanofi Pasteur and Meissa Vaccines and has received research support from AstraZeneca, Chimerix, GlaxoSmithKline, Merck, and Novavax, all unrelated to this research. S.B.V. and P.Q. have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018; 379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017; 129:3322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. FDA approval brings first gene therapy to the United States. 2017. Available at: https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states. Accessed February 1, 2020. [Google Scholar]
- 6. Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T - and a side order of IgG, to go? - immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev 2019; 38:100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant 2020; 26:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev 2019; 34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 2019; 16:45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fishman JA, Hogan JI, Maus MV. Inflammatory and infectious syndromes associated with cancer immunotherapies. Clin Infect Dis 2019; 69:909–20. [DOI] [PubMed] [Google Scholar]
- 11. Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018; 131:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JH, Romero FA, Taur Y, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis 2018; 67:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019:ciz1008. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young JH, Logan BR, Wu J, et al. ; Blood and Marrow Transplant Clinical Trials Network Trial 0201 Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant 2016; 22:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danziger-Isakov L, Englund J, Green M, et al. Cytomegalovirus in pediatric hematopoietic stem cell transplantation: a case-based panel discussion of current challenges. J Pediatric Infect Dis Soc 2018; 7:72–4. [DOI] [PubMed] [Google Scholar]
- 17. Fisher BT, Danziger-Isakov L, Sweet LR, et al. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatric Infect Dis Soc 2018; 7:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126: 2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 2019; 134:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 2005; 142:979–95. [DOI] [PubMed] [Google Scholar]
- 21. Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of chimeric antigen receptor T cells in B cell acute lymphoblastic leukemia: an expert opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2019; 25:e76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant 2020; 26:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.