Abstract
Magnetoencephalographic recordings, external picoTesla Transcranial Magnetic Stimulation (pT-TMS) and a double blind experimental design were used for the evaluation of 10 epilepsy patients (three males, seven females). Their ages ranged from 23 to 48 years. Our results showed an enhancement in the 2-7 Hz frequency range in five out of nine patients who had a statistically significant difference (55%). The pT-TMS could be a significant means for the treatment of epilepsy. Further research should be done prior to have final conclusions.
Keywords:magnetoencephalography, epilepsy, pT-TMS, double blind.
INTRODUCTION
The cerebral cortex produces magnetic fields that can be measured by magnetoencephalography (MEG), a non-invasive technique that records the action of cortical pyramidal cells oriented parallel to the surface of the brain. The magnetic fields created by epileptic foci is about 1 picoTesla (pT) (1 pT=10-12 T).
The pT external transcranial magnetic stimulation (pT-TMS), invented by Anninos and Tsagas (1), has some scientific benefits to patients, as already reported in the literature (2-11). Specifically, using the electronic device (1), patients were able to enhance the irregular (2-7 Hz) frequencies of brain action (2-11). The “Neural Net Model” (11) proposes that pT-TMS causes a temporal modulated neuronal inhibition in regions displaying irregular action in the variety of 2-7 Hz.
By using a double blind experiment is that we have not studied the effects of pT-TMS on epilepsy patients, and our investigation is aiming to notice changes in the subjects’ brain in this status.
MATERIALS AND METHODS
The whole-head 122-channel MEG device has already been described in our previous publications (2-6). There were nine epilepsy participants (three males and six females) aged 23–48, with a mean age of 33.5 years. Patients were referred to our laboratory by practicing neurologists. Participants gave their informed consent for the method and purpose of this research. Troebinger et al (10) used our pT-TMS tool on healthy volunteers with a double blind experimental design (1). We took two-minute MEG to ensure attentiveness for every participant. Our study protocol included two sessions.
In session 1, a two-minute MEG resting state was used for calibration of the pT-TMS tool.
In session 2, a third party set the pT-TMS tool to real or sham stimulation where neither the researcher nor the patient knew anything about its state.
Initially, a two-minute MEG was recorded (run1). Then, a two-minute of real or sham pT-TMS was administered, with participant sitting outside the scanner room. Afterwards, other two-minute MEG resting state data were obtained (run2). Then, pT-TMS stimulation was applied for two minutes. A third party switched the device from sham to real or vice versa. Afterwards, two-minute MEG scan data were recorded (run3).
The software program that detects the amplitude of the primary dominant frequency (MPFD) of MEG power spectra after pT-TMS and application of Fast Fourier Transform (FFT) and the analysis and prediction of sham and real stimulus runs have been described in detail in our previous publications (2-5).
RESULTS
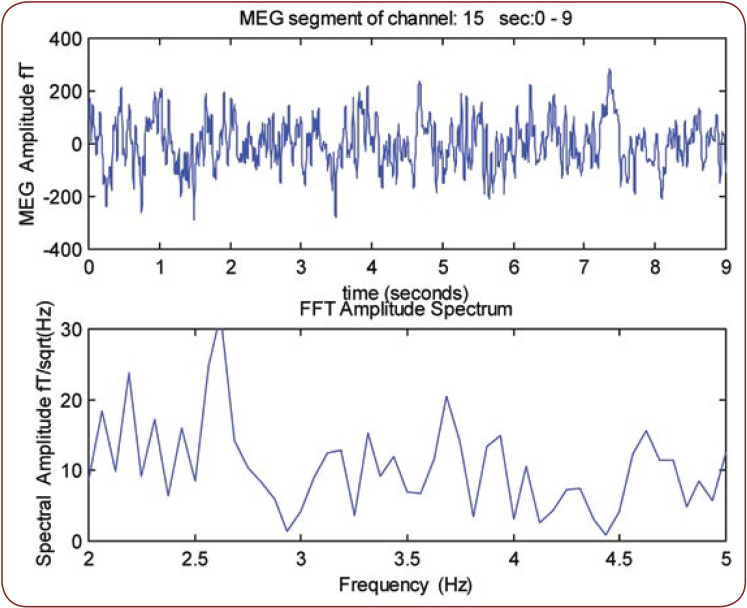
Figure 1 illustrates the MEG records obtained from an epilepsy patient (reference number 1). We observe that the MPFD is 2.7 Hz.
The symptoms in epilepsy patients after sham stimulation on the second day in our lab and one month after daily pT-TMS application have been already described in our previous report (6). We noticed that pT-TMS was partially effective in one patient but had no effect in three patients.
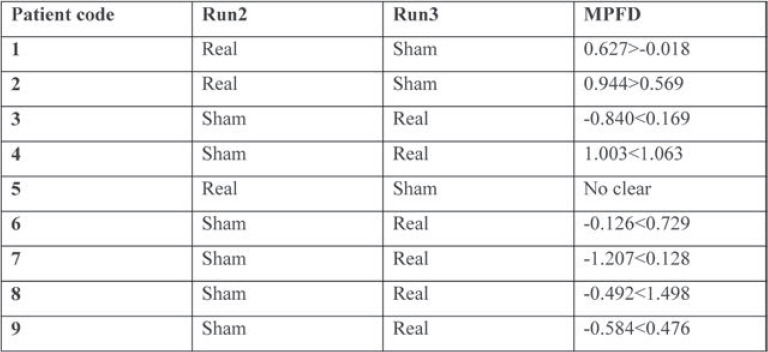
Table 1 shows that the prospect for properly selecting eight or more events with a probability of 0.5 from nine patients is highly statistically significant. In Patient 5, MPFD was not clear and after unblinding, the prediction was 90%.
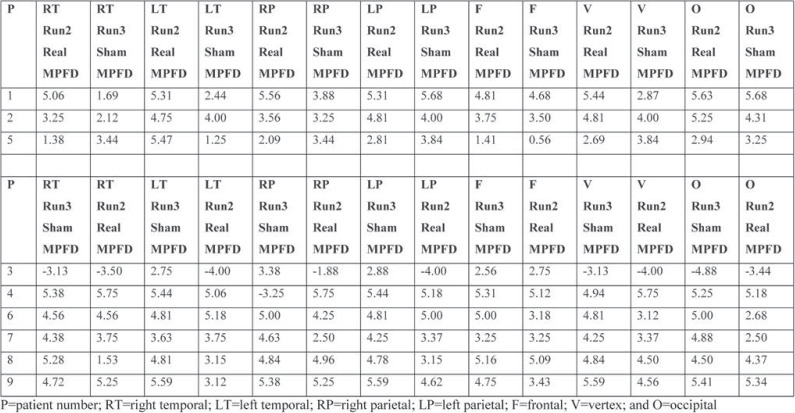
Table 2 shows MPFD from real (run2 in Δf(2)) to sham (run3 in Δf(3)) and real (run3 in Δf(3)) to sham (run2 in Δf(2)) stimulation in the same way, for each brain region according to Table 1.
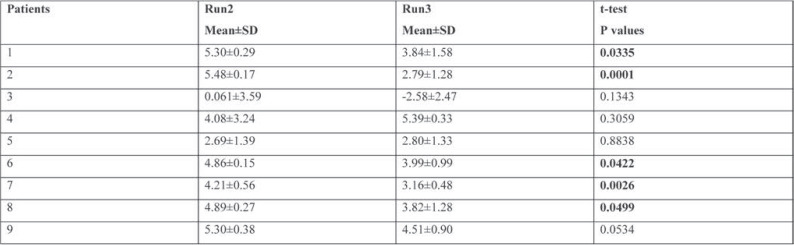
Table 3 summarizes the statistical analysis for the nine participants, with statistical significance for five out of nine (55%).
The corresponding channels to each brain region have been reported in detail in our previous publications (2-6).
DISCUSSION
Given that epileptic foci emit coherent magnetic activity, we attempted to influence the foci with the use of pT-TMS device. The application of pT-TMS to each functional point of seizure activity resulted in a reduction of emitted power from this area and attenuation of epileptic activity.
In our research, we used the schedule described below.
Day 1 – MEG recordings in our lab (run1). We applied sham stimulation, followed by MEG recordings (run3). There were no significant differences between patients’ MEG.
Day 2 – After sham stimulation there was an interview conducted by clinicians. After applying real stimulation, MEG recordings were made (run2). We observed that the patients’ MEG was approximately regular in the best part of them, the majority of irregular frequencies being absent.
Day 3 – After real stimulation, clinicians interviewed patients and confirmed the MEG findings.
Day 10 – Patients underwent MEG and were then evaluated by clinicians. As most of the patients reported a worsening of their pretreatment status, we advised their relatives to use pT-TMS at home every night (23:00 pm). After one month of pT-TMS application, every epilepsy patient was re-evaluated by MEG and then interviewed by clinicians, who found a benefit from this treatment.
Magnetic fields are effective on seizure activity due to their transport characteristics and alterations they produce in the biological membrane properties and stability (9). Given the complex cellular systemic and neuroendocrine effects of magnetic fields on organic systems, we can accept alternative explanations responsible for such effects.
CONCLUSION
Based on the 55% effect of pT-TMS, we conclude that this method may be a significant tool for the treatment of epilepsy patients. Further studies are needed before reaching a final conclusion.
Conflicts of interest: none declared.
Financial support: This study was funded by a partnership between GGET (General Secretariat of Research and Technology, GR) and ERGO AEBE, INC, GR (Grant Number: 80623).
FIGURE 1.
The MEG record of nine seconds obtained from an epilepsy patient (No 1). The primary dominant frequency is 2.7 Hz.
TABLE 1.
The highly statistically significant possibility to correctly select eight or more events, each with a probability of 0.5, from nine patients
TABLE 2.
Effect of the maximum primary dominant frequency in real and sham stimulations for each patient, according to the order of stimulation (run2 sham or run3 sham) of Table 1
TABLE 3.
Statistical analysis for the nine patients with the values summarised in Table 2. The results are statistically significant at the level of 0.05 (marked bold)
Contributor Information
Photios ANNINOS, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Nicolia ANNINOU, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Adam ADAMOPOULOS, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Athanasia KOTINI, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Triandafillos GEMOUSAKAKIS, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Nicolaos TSAGAS, bDepartment of Electrical Engineering, Engineering Faculty, Democritus University of Thrace, Greece.
References
- 1.Anninos PA, Tsagas N. Electronic apparatus for treating epileptic individuals. US patent 5453072. 1995.
- 2.Anninos P, Kotini A, Adamopoulos A, Tsagas N. Frequency analysis for the effect of pico Tesla-Transcranial Magnetic Stimulation in epilepsy patients using Magnetoencephalography. J Rare Dis Res Treat. 2018;3:22–28. [Google Scholar]
- 3.Anninos P, Adamopoulos A, Tsagas N, Kotini A. Pico-Tesla Transcranial Magnetic Stimulation on Migraine patients. A Double Blind Experimental Design. Enliven: Neurol Neurotechnol. 2018;5:1–8. [Google Scholar]
- 4.Anninos P, Adamopoulos A, Kotini A, Tsagas N. Pico-Tesla external TMS on dystonia patients with a double blind experimental design. A MEG study. EC Neurology. 2017;6:245–253. [Google Scholar]
- 5.Anninos P, Adamopoulos A, Kotini A, Tsagas N. Pico-Tesla Transcranial Magnetic Stimulation on Depression patients with double blind experimental design. Neurol Neurother. 2017;2:000112. [Google Scholar]
- 6.Anninos P, Adamopoulos A, Kotini A, Tsagas N. The effects of pico-Tesla external TMS on epilepsy patients. A MEG study. Curr Neurobiol. 2017;8:78–88. [Google Scholar]
- 7.Anninos PA, Tsagas N, Sandyk R, Derpapas K. Magnetic stimulation in the treatment of partial seizures. Int J Neurosci. 1991;3-4:141–171. doi: 10.3109/00207459109167029. [DOI] [PubMed] [Google Scholar]
- 8.Anninos P, Kotini A, Adamopoulos A, Tsagas N. Magnelic stimulation can modulate seizures in epileptic patients. Brain Topog. 2003;1:57–64. doi: 10.1023/a:1025610516767. [DOI] [PubMed] [Google Scholar]
- 9.Anninos PA, Tsagas N, Jacobson JI, Kotini A. The biological effects of magnetic stimulation in epileptic patients. Panminerva Med. 1999;3:207–215. [PubMed] [Google Scholar]
- 10.Troebinger L, Anninos P, Barnes G. Neuromagnetic effects of pico Tesla stimulation. Physiol Meas. 2015;9:1901–1912. doi: 10.1088/0967-3334/36/9/1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anninos PA, Tsagas N, Adamopoulos A. A brain model theory for epilepsy and the mechanism for treatment with experimental verification using SQUID measurements. In: Cotterill RM (ed). Models of brain function. New York: Cambridge University Press. 1986. pp. 405–421.