Abstract
Background
ROS1 gene fusion represents a specific subtype of non‐small cell lung cancer (NSCLC). Crizotinib is recommended for ROS1‐positive NSCLC due to its favorable outcome in published clinical trials. However, due to the low incidence of ROS1‐positive NSCLC, there is limited information on real‐world clinical outcomes in patients treated with either crizotinib or platinum‐based doublet chemotherapy.
Methods
Outcomes were recorded in 102 patients with stage Ⅲb or Ⅳ NSCLC who were treated at four Chinese hospitals between April, 2010 and June, 2019.
Results
Of the 102 patients followed, 71.6% were females, 81.4% were non‐smokers, and 98.0% had adenocarcinoma. First‐line treatment with crizotinib achieved a significantly longer median progression‐free survival (PFS) compared with platinum‐based chemotherapy (14.9 months vs 8.5 months, respectively; P < .001). Next‐generation sequencing (NGS) identified 61 patients who had ROS1 fusion variants, including CD74 (n = 33) and non‐CD74 (n = 28) variants. In patients harboring CD74 fusion variants, the median PFS with first‐line crizotinib treatment was significantly longer than in those harboring non‐CD74 fusion variants (20.1 months vs 12.0 months, respectively; P = .046). However, in patients treated with platinum‐based chemotherapy, there was no significant difference in PFS between the CD74 and non‐CD74 variant groups (8.6 months vs 4.3 months, respectively; P = .115). Overall survival (OS) was not reached.
Conclusions
First‐line therapy with crizotinib is more beneficial than platinum‐based chemotherapy in patients with advanced NSCLC with different ROS1 fusion variants. Patients harboring CD74 fusion variants appear to respond better to crizotinib.
Keywords: crizotinib, efficacy, next‐generation sequencing, non‐small‐cell lung cancer, ROS1
For first‐line treatment in advanced NSCLC with ROS1 rearrangement, crizotinib is more beneficial than platinum‐based chemotherapy. Patients harboring ROS1 CD74 fusion variants who are treated with first‐line crizotinib therapy have better PFS than those harboring non‐CD74 fusion variants. With first‐line platinum‐based chemotherapy, patients in both the CD74 and non‐CD74 fusion variant groups demonstrated a short PFS with no significant difference observed.

1. INTRODUCTION
ROS1 gene fusion has become a new therapeutic target in patients with advanced or metastatic non‐small‐cell lung cancer (NSCLC) in addition to epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene fusions. The proportion of patients with ROS1‐positive NSCLC is lower than those of EGFR‐sensitive mutations and ALK gene fusions, with an overall prevalence of 1%‐2% in the US,1, 2, 3 and approximately 15 000 new cases of ROS1‐positive NSCLC annually.4 In an East Asian population, ROS1 gene fusion has been identified in 2%‐3% of patients with NSCLC.5 Notably, the frequency accounted for 5.7% of patients with triple‐negative (EGFR, ALK, and KRAS wild‐type) NSCLC.6 ROS1 gene fusion is observed predominantly in younger patients, in light smokers (less than 10 pack years), and/or those with a non‐smoking history who have adenocarcinoma.
ROS1 gene fusion was first identified in a NSCLC cell line model, and was observed in a NSCLC patient's specimen by Rikova et al in 2007.7 In a preclinical study, ROS1 gene fusions were shown to be associated with sensitivity to crizotinib therapy, and NSCLC patients harboring ROS1 gene fusions have achieved partial responses to this agent. In an expansion cohort study of the PROFILE 1001 trial, 50 ROS1‐positive NSCLC patients demonstrated a high response rate (72%) and a median progression‐free survival (PFS) of 19.2 months when treated with crizotinib.8 Similarly, in an East Asian study, 127 ROS1‐positive NSCLC patients exhibited a response rate of 71.7% and a median PFS of 15.9 months.9
Currently, crizotinib is approved in many countries as a treatment option for patients with advanced NSCLC who harbor ROS1 gene fusions, based on the results of clinical trials showing its effectiveness in such patients.8, 10, 11, 12 Although some studies have reported that patients with ROS1‐positive NSCLC who are treated with pemetrexed‐based chemotherapy also exhibit good efficacy in PFS,13, 14 these studies were single‐arm, retrospective analyses, and there is a lack of prospective, randomized, phase Ⅲ studies comparing crizotinib with platinum‐based chemotherapy as first‐line treatment for advanced NSCLC due to the rarity of ROS1 rearrangements. In addition, studies reporting the outcomes of crizotinib treatment or platinum‐based doublet chemotherapy in patients with different ROS1 gene fusions have not yet been identified.
Therefore, in this real‐world study, data on treatment outcomes were analyzed to compare crizotinib with platinum‐based doublet chemotherapy as first‐line treatment in ROS1‐positive NSCLC patients. We further analyzed disease progression patterns and survival outcomes among patients treated with crizotinib treatment and platinum‐based doublet chemotherapy who had different ROS1 gene fusion variants.
2. METHODS
2.1. Patients
In a retrospective, multicenter study, 102 patients with stage IIIb or Ⅳ NSCLC who harbored ROS1 gene fusions were treated at four hospitals in Beijing, China between 24 April 2010 and 6 January 2019. The flow chart of the study is shown in Data S1. ROS1 gene fusion was confirmed by either the fluorescence in situ hybridization (FISH) detection method or by next‐generation sequencing (NGS) technology. Tissue samples originated from the lung (n = 69), lymph nodes (n = 22), pleural effusion (n = 6), and other sites (n = 5). Patients were confirmed as ROS1‐positive by FISH probe methods if there was a red and green split or an isolated signal in a kinase domain in at least 15% of tumor cells (after 50 tumor cells were counted in each sample).
All patients who met the following criteria were eligible: age ≥18 years; stage Ⅲb or Ⅳ NSCLC confirmed only ROS1 gene fusions without other known mutation such as EGFR, ALK, KRAS, MET application, and RET fusion; recurrent or metastatic disease treated with crizotinib or platinum‐based doublet chemotherapy as first‐line therapy; and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 2 or less. Patients with brain metastases at baseline were also included. Patients must not have received any prior treatment for ROS1 gene fusions other than crizotinib, or received synchronous chemoradiotherapy or immune‐directed therapy.
2.2. Treatment
Eligible patients were stratified into two groups on the basis of their treatment regimens. One group received crizotinib at a dosage of 250 mg twice every day. These patients’ disease was assessed approximately every 2 months. The other group received platinum‐based doublet chemotherapy for four or six cycles, followed by maintenance therapy which included bevacizumab combined with pemetrexed, or bevacizumab or pemetrexed alone every 21 days. These disease in those patients was assessed at baseline, after the first dose of treatment, and then after every two treatment cycles. Treatment regimens are summarized in Data S2. Some patients received carboplatin if they could not tolerate cisplatin. Eighteen patients received maintenance treatment with bevacizumab plus pemetrexed, or with pemetrexed or bevacizumab alone.
Treatment with crizotinib or chemotherapy was continued until either radiographic progressive disease (PD) or unacceptable toxicity developed.
2.3. Treatment assessments and definitions
Imaging examinations confirmed the measurable lesions documented by computed tomography (CT) scans of the chest and abdomen, magnetic resonance imaging (MRI) of the brain, or whole‐body bone scans, and the lesions were defined by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Tumor responses were assessed as either a complete response (CR), partial response (PR), stable disease (SD), or PD. The best response of each patient was recorded. The objective response rate (ORR) was defined as patients who showed a CR or PR, and the disease control rate (DCR) was defined as patients who showed a CR, PR, or SD.
The primary endpoint was PFS, which was measured from the first day of treatment initiation to the time of disease progression or death. Overall survival (OS) was calculated from the date of first‐line treatment to death or the last follow‐up. We also recorded the patterns of the first documented treatment failure. Central nervous system (CNS) progression was defined as intracranial failure. Extracranial progression was defined as distant organ metastases other than CNS metastases. Patients with combined extracranial and CNS metastases simultaneously were recorded only in the intracranial group. Patients harboring CD74 fusion variants and other fusion variants were assigned to the CD74‐ROS1 fusion variant group. Smokers were defined as individuals who smoked currently or who reported a smoking history that included at least 100 cigarettes, while non‐smokers were defined as those with a smoking history of less than 100 cigarettes in their lifetime. Smoking history and ECOG performance status data were collected from electronic medical records, along with clinical information and survival outcome data. As this was a retrospective, non‐interventional study, it was exempted from obtaining patients’ informed consent. However, it was approved by an institutional ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (approval 15‐144/1071).
2.4. Statistical analyses
Statistical analyses were performed using SPSS™ version 16.0 (SPSS Inc). Patient's characteristics at baseline were analyzed by applying descriptive statistics. The data were expressed as a percentage for dichotomous variables and analyzed using a Chi‐squared test or Fisher's exact test. The Kaplan‐Meier method was used to analyze the primary endpoint of PFS between groups. Univariate and multivariate analyses were performed using a Cox proportional hazard regression model, and all statistical tests were considered statistically significant if they were two‐tailed and P < .05. Kaplan‐Meier survival curves were created with GraphPad Prism 6.0.
3. RESULTS
3.1. Patients’ baseline characteristics
Of the 102 eligible patients, 73 (71.6%) were females and 29 (28.4%) were males. The median age at diagnosis of advanced NSCLC was 52 years (range 27‐82 years); 92 patients (90.2%) had a good PS score (0 or 1 point), and 83 (81.4%) were non‐smokers. One hundred patients (98.0%) were identified as having lung adenocarcinoma, and 16 presented with CNS metastases at baseline. 88 patients were diagnosed as stage IV, including 74 cases of initial diagnosis and 14 cases of recurrence. Almost half of the patients (n = 56; 54.9%) received oral crizotinib treatment as first‐line therapy, while the remaining 46 patients (45.1%) received a platinum‐based doublet chemotherapy regimen. The characteristics of the two treatment groups well balanced at baseline (Table 1).
TABLE 1.
Baseline characteristics of 102 patients with ROS1‐positive advanced NSCLC
| Characteristics | Total (n = 102) | Crizotinib (n = 56) | Chemotherapy (n = 46) | P‐value |
|---|---|---|---|---|
| Age, y (n, %) | ||||
| ≥60 | 32 (31.4) | 19 (33.9) | 13 (28.3) | .539 |
| <60 | 70 (68.6) | 37 (66.1) | 33 (71.7) | |
| Sex (n, %) | ||||
| Male | 29 (28.4) | 15 (26.8) | 14 (30.4) | .684 |
| Female | 73 (71.6) | 41 (73.2) | 32 (69.6) | |
| Smoking history (n, %) | ||||
| Yes | 19 (18.6) | 8 (14.3) | 11 (23.9) | .214 |
| No | 83 (81.4) | 48 (85.7) | 35 (76.1) | |
| Histological types (n, %) | ||||
| ADC | 100 (98.0) | 55 (98.2) | 45 (97.8) | 1.000 |
| Non‐ADC | 2 (2.0) | 1 (1.8) | 1 (2.2) | |
| Clinical stage (n, %) | ||||
| IIIb | 14 (13.7) | 5 (8.9) | 9 (19.6) | .153 |
| IV | 88 (86.3) | 51 (91.1) | 37 (80.4) | |
| Recurrence | 14 (15.9) | 7 (13.7) | 7 (18.9) | |
| ECOG score (n, %) | ||||
| 0‐1 | 92 (90.2) | 50 (89.3) | 42 (91.3) | 1.000 |
| 2 | 10 (9.8) | 6 (10.7) | 4 (8.7) | |
| Brain metastases at baseline (n, %) | ||||
| Yes | 16 (15.7) | 11 (19.6) | 5 (10.9) | .280 |
| No | 86 (84.3) | 45 (80.4) | 41 (89.1) | |
| ROS1 fusion subtype | ||||
| CD74 | 33 (32.3) | 17 (30.4) | 16 (34.8) | .503 |
| Non‐CD74 | 28 (27.5) | 18 (32.1) | 10 (21.7) | |
| Unknown | 41 (40.2) | 21 (37.5) | 20 (43.5) | |
Abbreviations: ADC, adenocarcinoma; ECOG, Eastern Cooperative Oncology Group; NSCLC, non‐small cell lung cancer.
3.2. First‐line treatment outcomes with crizotinib and platinum‐based chemotherapy
Till the data cutoff date (June 30, 2019), 14 patients (13.7%) had died. The median follow‐up duration from the time of diagnosis to the data cutoff date was 24.9 months (range 6.0‐74.1 months). With first‐line oral crizotinib treatment, 47 patients achieved PR, seven had SD, and two had PD. In patients who received platinum‐based chemotherapy, CR was not observed in any patient, 26 had PR, 20 had SD. The ORR with first‐line crizotinib treatment was significantly better than with platinum‐based chemotherapy (83.9% vs 56.5%, respectively; P = .002), but significant difference was not observed in DCR between the two treatment groups (96.4% vs 100%, respectively; P = .195).
A total of 102 patients were divided into two groups including crizotinib treatment and platinum‐based doublet chemotherapy. The chemotherapy regimens were as follows: pemetrexed/platinum regimens (PP, n = 35), paclitaxel/platinum regimens (n = 5), docetaxel plus cisplatin(n = 2), and gemcitabine plus cisplatin (n = 4). The median PFS was significantly longer in patients who received crizotinib treatment in comparison with those who received platinum‐based doublet chemotherapy regimens (median 14.9 months, 95% CI 10.9‐18.7 months vs 8.5 months, 95% CI 6.8‐10.3 months, respectively; P < .001, Figure 1). We further analyzed the PFS in patients who received PP chemotherapy regimens (median 8.8 months, 95% CI 6.8‐10.8 months). Crizotinib treatment was also superior to PP chemotherapy regimens with significant difference observed between two groups (P < .001). OS was not reached in either treatment group.
FIGURE 1.

Kaplan‐Meier curves of progression‐free survival (PFS) with crizotinib treatment and platinum‐based chemotherapy in ROS1‐positive advanced non‐small cell lung cancer. The median PFS was significantly longer in patients treated with crizotinib compared with those treated with platinum‐based therapy (median 14.9 mo vs 8.5 mo, respectively; P < .001)
3.3. ROS1 fusion variants and disease progression patterns according to the different variants
NGS was performed on samples from 61 patients, and the ROS1 fusion variants detected were CD74 (n = 30), SDC (n = 11), EZR (n = 7), SLC34A2 (n = 3), ZCCH (n = 2) and other variants (n = 5; one for each variant including SNN, KIAA1217, TFG, MYH9, and CCDC6). Three patients had dual fusion variants with a CD74 fusion variant and another fusion variant (MAGI1 & SLC25A26, CTXN3 & LNCO1184, and STXBP4 & HLF). Two patients had concurrent mutation (one patient has CD74 fusion with TP53 treated with crizotinib, another patient has SDC4 fusion with TP53 treated with chemotherapy). More than half of the ROS1 gene fusion variants were CD74 variants (54.1%). Depending on whether the fusion variant included CD74, patients were divided into two groups: (a) those with CD74 variants and (b) those with non‐CD74 variants. The baseline characteristics of patients in these two groups were comparable (Table 2).
TABLE 2.
Baseline characteristics of patients with the different ROS1 fusion variants
| Characteristics | Total (n = 61) | CD‐74 (n = 33) | Non‐CD74 (n = 28) | P‐value |
|---|---|---|---|---|
| Age, y (n, %) | ||||
| ≥60 | 17 (27.9) | 8 (24.2) | 9 (32.1) | .493 |
| <60 | 44 (72.1) | 25 (75.8) | 19 (67.9) | |
| Sex (n, %) | ||||
| Male | 18 (29.5) | 10 (30.3) | 8 (28.6) | .883 |
| Female | 43 (70.5) | 23 (69.7) | 20 (71.4) | |
| Smoking history (n, %) | ||||
| Yes | 12 (19.7) | 7 (21.2) | 5 (17.9) | 1.000 |
| No | 49 (80.3) | 26 (78.8) | 23 (82.1) | |
| Histological types (n, %) | ||||
| ADC | 59 (96.7) | 31 (93.9) | 28 (100) | .495 |
| Non‐ADC | 2 (3.3) | 2 (6.1) | 0 (0) | |
| Clinical stage (n, %) | ||||
| IIIb | 10 (16.4) | 6 (18.2) | 4 (14.3) | .741 |
| IV | 51 (83.6) | 27 (81.8) | 24 (85.7) | |
| Recurrence | 8 (15.7) | 3 (11.1) | 5 (20.8) | |
| ECOG scores (n, %) | ||||
| 0‐1 | 53 (86.9) | 29 (87.9) | 24 (85.7) | 1.000 |
| 2 | 8 (13.2) | 4 (12.1) | 4 (14.3) | |
| Brain metastases (n, %) | ||||
| Yes | 9 (14.8) | 2 (6.1) | 7 (25.0) | .067 |
| No | 52 (85.2) | 31 (93.9) | 21 (75.0) | |
| First‐line therapy (n, %) | ||||
| Crizotinib | 35 (57.4) | 17 (51.5) | 18 (64.3) | .315 |
| Chemotherapy | 26 (42.6) | 16 (48.5) | 10 (35.7) | |
Abbreviations: ADC, adenocarcinoma; ECOG, Eastern Cooperative Oncology Group.
In ROS1‐positive NSCLC confirmed by NGS technology, 35 patients received crizotinib treatment and 26 received platinum‐based chemotherapy. At the data cutoff date, 18 patients exhibited disease progression in the crizotinib group, but all patients presented disease progression in the chemotherapy group. In the non‐CD74 fusion group, the proportion of patients with intracranial disease progression was higher than those in the CD74 group (33.3% vs 5.9%, respectively; Figure 2A). A total of seven patients treated with crizotinib had intracranial progression. There were six patients in the non‐CD74 group (three patients at baseline and three patients without brain metastases at initial diagnosis) and one patient in the CD74 group during the course of crizotinib treatment. The sites of disease progression in both two groups treated with platinum‐based chemotherapy were mainly extracranial progression (Figure 2B). All three patients who had intracranial progression were in the non‐CD74 group and no patient progressed in the CD74 group.
FIGURE 2.
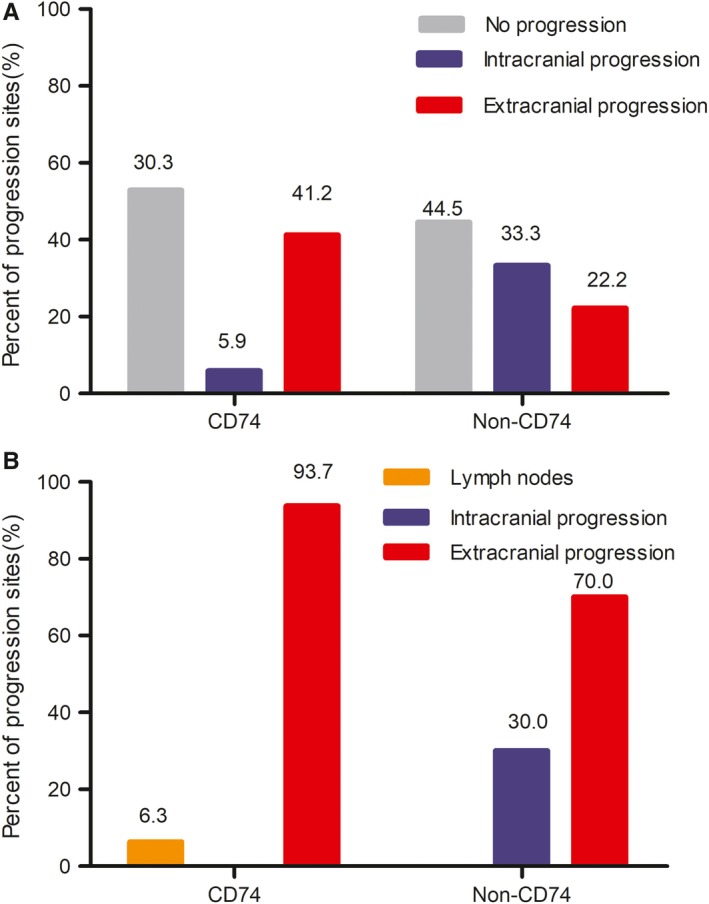
Disease progression patterns treated with crizotinib and platinum‐based therapy according to the different fusion variants. (A) With crizotinib treatment. (B) With platinum‐based chemotherapy
3.4. Survival analyses of crizotinib treatment or platinum‐based chemotherapy with different ROS1 fusion variants
To determine whether different ROS1 gene fusion variants affect the therapeutic response, we analyzed the therapeutic effectiveness of crizotinib and platinum‐based chemotherapy as first‐line treatment with the different variants we identified, including 33 patients with CD74 fusion variants and 28 with non‐CD74 fusion variants. With first‐line crizotinib treatment, the median PFS was significantly longer in patients who harbored CD74 fusion variants than in those with non‐CD74 fusion variants (20.1 months, 95%CI 13.1‐27.0 months vs 12.0 months, 95% CI 9.0‐14.9 months, respectively; P = .046) (Figure 3A). However, no significant difference in PFS between the two groups was observed when platinum‐based chemotherapy was used in first‐line (8.6 months, 95% CI 8.1‐9.2 months vs 4.3 months, 95% CI 2.3‐6.3 months, respectively; P = .115) (Figure 3B). The median OS was not reached in either group.
FIGURE 3.

Kaplan‐Meier curves of progression‐free survival (PFS) with crizotinib treatment and platinum‐based chemotherapy for the different fusion variants. (A) With first‐line crizotinib treatment, the median PFS was significantly longer in patients harboring CD74 fusion variants in comparison with those harboring non‐CD74 fusion variants (20.1 mo; 95% CI 13.1‐27.0 vs 12.2 mo; 95% CI 9.1‐14.9, respectively; P = .046). (B) With first‐line platinum‐based chemotherapy treatment, no statistically significant difference in PFS was observed between patients with the different fusion variants (8.6 mo, 95% CI 8.1‐9.2 vs 4.3 mo, 95% CI 2.3‐6.3, respectively; P = .115)
3.5. Univariate and multivariate analyses for PFS by the Cox regression model
Univariate analysis demonstrated that the PFS in ROS1‐positive NSCLC patients was significantly associated with treatment patterns (crizotinib vs chemotherapy, P < .001) (Table 3). Although there was no statistically significant difference in PFS with brain metastases due to the small sample size, the PFS with crizotinib had shorter PFS in patients with brain metastases than in those without brain metastases (12.0 months vs 15.0 months, P = .249). The status of brain metastases and ROS1 fusion subtype might affect the outcome of PFS. Therefore, the status of brain metastases, ROS1 fusion subtype, and treatment patterns were entered into the multivariate Cox regression model. Multivariate analyses confirmed that ROS1 fusion subtype and treatment pattern were independent predictors of PFS in ROS1‐positive NSCLC patients (P < .05, Table 4).
TABLE 3.
Univariate survival analyses for PFS
| Variable | B | SE | HR | 95% CI | P |
|---|---|---|---|---|---|
| Age (≥60 vs <60) | −0.008 | 0.255 | 0.992 | 0.602‐1.635 | .976 |
| Gender (male vs female) | 0.006 | 0.257 | 1.006 | 0.608‐1.663 | .982 |
| Smoking history (yes vs no) | 0.103 | 0.290 | 1.109 | 0.629‐1.956 | .722 |
| Histological types (ADC vs Non‐ADC) | −0.948 | 0.724 | 0.388 | 0.094‐1.603 | .291 |
| Clinical stage (IIIb vs IV) | −0.161 | 0.341 | 0.851 | 0.437‐1.661 | .637 |
| ECOG score (0‐1 vs 2 points) | −0.056 | 0.374 | 0.946 | 0.454‐1.970 | .882 |
| Brain metastases at baseline (yes vs no) | 0.123 | 0.306 | 1.131 | 0.622‐2.059 | .686 |
| Fusion subtype (CD74 vs Non‐CD74) | −0.532 | 0.311 | 0.587 | 0.319‐1.082 | .088 |
| Treatment (crizotinib vs chemotherapy) | −1.133 | 0.238 | 0.322 | 0.202‐0.513 | <.001 |
Abbreviations: ADC, adenocarcinoma; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; PFS, progression‐free survival.
TABLE 4.
Predictors of PFS analyzed by multivariate Cox regression
| Variable | B | SE | HR | 95% CI | P |
|---|---|---|---|---|---|
| Brain metastases at baseline (Yes vs No) | 0.088 | 0.325 | 1.092 | 0.578‐2.065 | .786 |
| Fusion subtype (CD74 vs Non‐CD74) | −0.669 | 0.327 | 0.512 | 0.270‐0.972 | .041 |
| Treatment (Crizotinib vs Chemotherapy) | −1.217 | 0.241 | 0.296 | 0.185‐0.475 | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
4. DISCUSSION
Targeted therapies have been recommended as first‐line treatment in patients with advanced, recurrent, or metastatic ROS1‐positive NSCLC, as well as for EGFR mutations or ALK gene fusions. In this study, we analyzed the clinicopathological characteristics and disease progression patterns of NSCLC patients with different ROS1 gene fusion variants who received first‐line treatment with either crizotinib or platinum‐based chemotherapy. Similar to the findings of previous studies,6, 8, 15, 16, 17, 18 we found that ROS1 gene fusions occurred predominately in younger patients, women, and patients with adenocarcinoma without smoking history. At the time of the initial diagnosis, the incidence of brain metastases in the ROS1‐positive NSCLC patients we studied was only 15.7%, which is lower than that of patients with ALK‐rearranged NSCLC.19, 20, 21 For first‐line therapy, in patients treated with crizotinib treatment, disease progression in the brain showed a significant difference between the different ROS1 fusion variants (P < .05); 33.3% of patients in the non‐CD74 variant group had intracranial progression, as compared with only 5.9% of those in the CD74 variant group. In patients treated with platinum‐based chemotherapy, the sites of disease progression were mainly extracranial progression. A reasonable explanation for this finding is that the proportion of patients with brain metastases at baseline treated with crizotinib was higher in the non‐CD74 variant group than those in the CD74 group (27.7% vs 11.8%, respectively). Another reason may be that patients in the non‐CD74 group are prone to intracranial progression. In addition, permeability of crizotinib through the blood–brain barrier may have resulted in higher incidence of brain metastasis.22, 23 In this regard, studies by Kaneda et al24 and Metro et al22 have reported that ratio of crizotinib in cerebrospinal fluid to plasma was very low (only 0.0026 to 0.006).
Our study also provided outcome data for both crizotinib and platinum‐based chemotherapy given as first‐line treatment in patients with different ROS1 fusion variants. In comparison with platinum‐based chemotherapy, crizotinib treatment achieved significantly higher ORR (83.9% vs 56.5%, respectively; P = .002), and PFS (14.9 months vs 8.5 months, respectively; P < .001). The clinical benefit of crizotinib was consistent with those reported by Wu et al9 and Zeng et al25 in ROS1‐positive NSCLC patients in the East Asian population. Univariate and multivariate analyses confirmed that the PFS in ROS1‐positive NSCLC patients was significantly associated with treatment patterns, and crizotinib treatment was more beneficial than chemotherapy. However, several studies have found that ROS1‐positive NSCLC patients appeared to be sensitive to pemetrexed‐based treatment.26, 27 Drilon et al28 reported that ROS1‐positive patients who were treated with pemetrexed‐based chemotherapy had good ORR (78%) and long PFS (23 months), which were higher than our study. A possible reason was that 70% of the patients in the study of Drilon et al28 received maintenance therapy as compared with only 39.1% of patients (18/46) in our study. Another reason may be the differences between eastern and western populations. Although pemetrexed‐based chemotherapy has shown an effective clinical response rate in ROS1‐positive NSCLC patients, it is less efficacious than crizotinib treatment. It is not safe to conclude that platinum‐based chemotherapy was more beneficial than crizotinib treatment for ROS1‐positive advanced NSCLC based on single‐arm, small‐sample size study.
The traditional approach to detect ROS1 gene fusion is FISH test, which is the gold standard, but the FISH test does not provide detailed information on ROS1 gene fusion variants. With the development of comprehensive molecular profiling of NSCLC, NGS can detect new fusion variants and more information regarding the fusion variants. Few studies have reported therapeutic outcomes with crizotinib as first‐line treatment for patients with different ROS1 fusion variants. In our study, patients harboring CD74 fusion variants treated with crizotinib treatment tended to have a longer PFS than those harboring non‐CD74 fusion variants (median 20.1 months vs 12.0 months, respectively). Median PFS extended for 8.1 months, and it was significant for the management of advanced or metastatic NSCLC. The finding reported by Michels et al29 indicates that first‐line crizotinib treatment may be more beneficial in patients with CD74 ROS1 fusion variants. Multivariate analyses further demonstrated that ROS1 fusion subtype was a valuable predictor of PFS in ROS1‐positive NSCLC patients. While the finding reported by Li et al30 showed that the median PFS with crizotinib treatment in the CD74 variant group was shorter than that reported in our study. The reasons for these potential discrepancies in results between our data and previous study might be explained by demographic or baseline characteristics. Notably, in our study, 2 patients (11.8%) in the CD74 variant group had a lower incidence of brain metastases at baseline than those in the non‐CD74 variant group (n = 5, 27.7%). All six (16.7%) patients who had brain metastases were in the CD74 group and no patient had brain metastases in non‐CD74 group in Li et al study.30 The PFS of patients treated with crizotinib in the non‐CD74 group was probably shorten by the short PFS in those with brain metastases. Although the status of brain metastases had no statistically significant effect on PFS due to the small sample size, short PFS in our study was founded in patients with brain metastases. A larger‐scale study on brain metastases is warranted. With platinum‐based doublet chemotherapy, patients in both two groups demonstrated short PFS with no significant difference between them.
As our study was a retrospective analysis, there might have some selection bias. In addition, several other limitations must be noted. First, the sample size may have been inadequate due to the low occurrence rate of ROS1 gene fusions. Second, in the CD74 variant group, three patients were found to have ROS1 double fusion variants using NGS, but we did not analyze how these patients responded to crizotinib treatment or to chemotherapy separately for the reason of small number. Third, disease assessments in the two study cohorts were evaluated in different frequency, which might cause PFS bias. However, it was speculated that there was little influence on the results due to significant differences in PFS. Lastly, comparison between patients treated with different lines of crizotinib and chemotherapy was not performed due to the immature nature of the data.
5. CONCLUSIONS
The data from the present study indicate that crizotinib is more beneficial than platinum‐based chemotherapy as first‐line therapy for ROS1‐positive NSCLC. Also, ROS1 gene fusion subtype might be a predictive biomarker for advanced NSCLC. In ROS1‐positive NSCLC patients who harbored CD74 gene fusion variants, crizotinib tended to be more effective than in those who harbored non‐CD74 fusion variants.
CONFLICT OF INTEREST
None of the authors has any no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
All authors can take responsibility for the integrity of the data and the accuracy of the data analysis. Haiyan Xu, Quan Zhang, and Li Liang contributed to concept and design. Junling Li, Zhefeng Liu, Weihua Li, Guangjian Yang, Jianming Ying, and Shucai Zhang contributed to acquisition, analysis, or interpretation. Haiyan Xu, Lu Yang, Fei Xu, and Yan Wang contributed to discussion and revision. All authors read and approved the final manuscript.
Supporting information
Data S1
Data S2
ACKNOWLEDGMENTS
The authors thank the patients, their families, and all of the research members. The authors also thank Dr Min Gao from Pfizer Medical for scientific comments and Content Ed Net, Shanghai Co. Ltd. for editorial assistance with the manuscript.
Xu H, Zhang Q, Liang L, et al. Crizotinib vs platinum‐based chemotherapy as first‐line treatment for advanced non‐small cell lung cancer with different ROS1 fusion variants. Cancer Med. 2020;9:3328–3336. 10.1002/cam4.2984
Haiyan Xu is the first author.
Quan Zhang and Li Liang are co‐first authors.
Haiyan Xu, Quan Zhang, and Li Liang equally contributed to this study.
Jianming Ying, Shucai Zhang, and Yan Wang equally contributed to this study.
DATA AVAILABILITY STATEMENT
The material supporting the conclusion of this study has been included in the article. Some information was provided in the supplementary files, and all other relevant data are available from the corresponding authors of this study.
REFERENCES
- 1. Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378‐381. [DOI] [PubMed] [Google Scholar]
- 2. Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non‐small cell lung cancer. Clin Cancer Res. 2012;18:4570‐4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu Y, Zhang X, Lin X, et al. Clinicopathological features and clinical efficacy of crizotinib in Chinese patients with ROS1‐positive non‐small cell lung cancer. Oncol Lett. 2019;17:3466‐3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gainor JF, Shaw AT. Novel targets in non‐small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK‐RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim HR, Lim SM, Kim HJ, et al. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol. 2013;24:2364‐2370. [DOI] [PubMed] [Google Scholar]
- 7. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190‐1203. [DOI] [PubMed] [Google Scholar]
- 8. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371:1963‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y‐L, Yang J‐H, Kim D‐W, et al. Phase II study of crizotinib in East Asian patients with ROS1‐positive advanced non–small‐cell lung cancer. J Clin Oncol. 2018;36:1405‐1411. [DOI] [PubMed] [Google Scholar]
- 10. Juan O, Popat S. Crizotinib for ROS1 patients: one small step in biomarker testing, one giant leap for advanced NSCLC patients. Lung Cancer. 2017;2014:131‐133. [DOI] [PubMed] [Google Scholar]
- 11. Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992‐999. [DOI] [PubMed] [Google Scholar]
- 12. Kazandjian D, Blumenthal GM, Luo L, et al. Benefit‐risk summary of crizotinib for the treatment of patients with ROS1 alteration‐positive, metastatic non‐small cell lung cancer. Oncologist. 2016;21:974‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song Z, Su H, Zhang Y. Patients with ROS1 rearrangement‐positive non‐small‐cell lung cancer benefit from pemetrexed‐based chemotherapy. Cancer Med. 2016;5:2688‐2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y‐F, Hsieh M‐S, Wu S‐G, et al. Efficacy of pemetrexed‐based chemotherapy in patients with ROS1 fusion‐positive lung adenocarcinoma compared with in patients harboring other driver mutations in East Asian populations. J Thorac Oncol. 2016;11:1140‐1152. [DOI] [PubMed] [Google Scholar]
- 15. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu J, Lin Y, He X, et al. Comparison of detection methods and follow‐up study on the tyrosine kinase inhibitors therapy in non‐small cell lung cancer patients with ROS1 fusion rearrangement. BMC Cancer. 2016;16:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheffler M, Schultheis A, Teixido C, et al. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6:10577‐10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y‐F, Hsieh M‐S, Wu S‐G, et al. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol. 2014;9:1171‐1179. [DOI] [PubMed] [Google Scholar]
- 19. Ou S‐h i, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK‐positive NSCLC. Ann Oncol. 2014;25:415‐422. [DOI] [PubMed] [Google Scholar]
- 20. Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene‐addicted non‐small‐cell lung cancer. J Thorac Oncol. 2012;7:1807‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gainor JF, Tseng D, Yoda S, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1‐positive non‐small‐cell lung cancer. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Metro G, Lunardi G, Floridi P, et al. CSF concentration of crizotinib in two ALK‐positive non‐small‐cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10:e26‐e27. [DOI] [PubMed] [Google Scholar]
- 23. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443‐e445. [DOI] [PubMed] [Google Scholar]
- 24. Kaneda H, Okamoto I, Nakagawa K. Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement‐positive non‐small‐cell lung cancer. J Thorac Oncol. 2013;8:e32‐e33. [DOI] [PubMed] [Google Scholar]
- 25. Zeng L, Li Y, Xiao L, et al. Crizotinib presented with promising efficacy but for conconmitant mutation in next‐generation sequencing‐identified ROS1‐rearranged non‐small‐cell lung cancer. Onco Targets Ther. 2018;11:6937‐6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang Y, Wakelee HA, Neal JW, et al. Relationship of driver oncogenes to long‐term pemetrexed response in non‐small‐cell lung cancer. Clin Lung Cancer. 2015;16:366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riess JW, Padda SK, Bangs CD, et al. A case series of lengthy progression‐free survival with pemetrexed‐containing therapy in metastatic non‐small‐cell lung cancer patients harboring ROS1 gene rearrangements. Clin Lung Cancer. 2013;14:592‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drilon A, Bergagnini I, Delasos L, et al. Clinical outcomes with pemetrexed‐based systemic therapies in RET rearranged lung cancers. Ann Oncol. 2016;27:1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michels S, Massutí B, Schildhaus H‐U, et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1‐rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14:1266‐1276. [DOI] [PubMed] [Google Scholar]
- 30. Li Z, Shen L, Ding D, et al. Efficacy of crizotinib among different types of ROS1 fusion partners in patients with ROS1‐rearranged non‐small cell lung cancer. J Thorac Oncol. 2018;13:987‐995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data S2
Data Availability Statement
The material supporting the conclusion of this study has been included in the article. Some information was provided in the supplementary files, and all other relevant data are available from the corresponding authors of this study.


