Highlights
-
•
1,156,863 girls received first dose HPV vaccine between 2011 and 2018 in Rwanda.
-
•
An initial school-grade-targeted catch-up transited to routine 12 yrs vaccination.
-
•
Census-level coverage was 10–40% for girls born in 1993–1995, 50–65% for 1996–2000.
-
•
Census-level coverage was 80–90% for 2001–2006 cohorts vaccinated at 12 years.
-
•
Birth cohorts provide clearer picture of HPV vaccine coverage in Rwanda.
Keywords: Human papillomavirus, Vaccine, Coverage, Cervical cancer, Rwanda
Abbreviations: EPI, Expanded Program on Immunization; HMIS, Health Management Information System; HPV, human papillomavirus; IQR, interquartile range
Abstract
Background
In 2011, Rwanda became the first African nation to implement a national human papillomavirus (HPV) vaccination program, conceived to protect girls aged <15 years (i.e. born ≥1997). After an initial school-grade-targeted catch-up campaign, there was a transition to routine vaccination of 12 year-olds only. We aimed to produce population-level vaccine coverage estimates.
Methods
The Rwandan Expanded Program on Immunization (EPI) collected data on number of eligible girls and HPV vaccines delivered, stratified by calendar year (2011–2018), girl’s age, district and vaccination round. HPV vaccine coverage was estimated by birth cohort (reconstituted using calendar year and age), as a proportion of (1) eligible target, and (2) the 2012 Rwandan census population.
Results
1,156,863 girls received first dose of HPV vaccine between 2011 and 2018, corresponding to 98% of the eligible target. Median vaccination age was 15 years (interquartile range [IQR] 13–16) in 2011–2013 (school grade-targeted catch-up), 13 years (IQR 12–14) in 2014 (transition) and 12 years in 2015–2018 (routine). Population-level coverage versus the census increased from 10 to 40% for girls born in 1993–1995 (median vaccination age = 17 years) to 50–65% for 1996–2000 birth cohorts (14 years), and 80–90% for 2001–2006 birth cohorts (12 years). Coverage trends were similar across provinces and in the capital, Kigali. Second and third round coverage suggested most vaccinated girls completed their recommended dosing regimen (which reduced from 3 to 2 doses in 2015).
Conclusions
Birth cohorts provide a clear picture of population-level HPV vaccine coverage after a pragmatic catch-up campaign, particularly in Rwanda where eligible school grades included wide age ranges. Whilst the catch-up campaign resulted in some coverage gaps in out-of-school teenagers, coverage remains high in cohorts routinely targeted as 12 year-olds.
1. Introduction
Cervical cancer represents by far the most common cancer in Rwandan women, with a high incidence rate typical of many sub-Saharan African settings [1]. In reaction to such a high disease burden, Rwanda became the first African country to initiate a national vaccination program against human papillomavirus (HPV), cervical cancer’s necessary cause.
The goal underpinning the design of the program was to protect as many girls aged <15 years in 2011 (i.e. born from 1997 onwards) as possible, with three doses of quadrivalent HPV vaccine [2]. Thus, following national consultation, it was decided that the program should be initially school-based, targeting girls in primary school grade 6 from 2011, plus two additional catch-up rounds of vaccination of girls in secondary school grade 3 in 2012 and 2013. 2014 was a transition year in which 3 doses were offered to all previously unvaccinated 12–14 year-olds (irrespective of school grade). Then, from 2015 onwards, full transition to an ongoing routine age-based program was made, targeting only 12 year-old girls with a 2-dose schedule, supported by GAVI. Of note, even in the routine program, eligibility continues to be defined primarily through school-based registers, with some community outreach to 12 year-olds not attending school.
Three-dose coverage among eligible girls was reported at 93% in 2011 [3], 97% in 2012 [2] and 97% in 2013 [4]. However, partly as a result of a substantial reconstruction of the Rwandan education system during the preceding decades [5], the age at which children entered and left school, as well as the age ranges represented within school grades, was highly variable. Thus, coverage among eligible girls did not easily translate into coverage by birth cohort. Indeed, in a series of HPV prevalence surveys in 2013/14 designed to target unvaccinated cohorts, vaccination coverage in girls born in 1993–1995 was unexpectedly high: 3–11% in the general population [6] and 6–34% in high-school students [7].
In order to clarify this issue and to capture the graduation to a single-cohort age-based immunization approach, we set out to re-assess HPV vaccine coverage at national population level, using a cohort methodology based on 8 years of administrative data from the national Expanded Program on Immunization (EPI). Our principal objectives were to study determinants of vaccine coverage by birth cohort, province and vaccine dose. This information is crucial to understand the successes and challenges of the program implementation, as well as to evaluations of vaccine program effectiveness.
2. Methods
2.1. Data sources
For each year between 2011 and 2018, age-specific data on (1) number of eligible girls and (2) number of delivered HPV vaccines, was collated by the EPI program, Rwandan Ministry of Health. Primary data sources were individual-based registers held at school, compiled by age and/or grade at the health centre level, then at the district level, and finally at the central level. From 2015 onwards, when HPV vaccination entered into the routine EPI program, summary data were additionally recorded in the wider Health Management Information System (HMIS) as for all routine vaccines. Estimates of number of eligible girls and number of delivered HPV vaccines were also stratified by round. However, in 2014, as a result of the transition between the two data collection procedures, data on second and third rounds were not available. Of note, round (1st, 2nd, 3rd) refers to the campaign round in which the vaccines were given, not to the registering of doses at an individual girl level.
Estimates of population size were extracted from the national census undertaken in 2012 (http://statistics.gov.rw), by single year of age, separately for each district of the four Rwandan provinces (North, South, East, West) and the capital Kigali.
Number of eligible girls and number of delivered vaccines were reconstituted into birth cohorts from corresponding age- and calendar year-specific data (see Table 1). Similarly, population-level birth cohorts were reconstituted from age-specific estimates from the 2012 Rwandan census population.
Table 1.
Number of first HPV vaccine doses delivered, population denominators and first dose vaccine coverage, by birth cohort. Rwanda, 2011–2018.
| Birth Year | Number of first dose vaccines delivered (n) |
Cohort denominator (N) |
First dose coverage (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | Eligible | Census | Eligible | Census | |
| 1993 | 5563 | 5563 | 5679 | 94,558 | 98 | 6 | |||||||
| 1994 | 5563 | 10,285 | 15,848 | 16,058 | 122,415 | 99 | 13 | ||||||
| 1995 | 14,841 | 28,397 | 15,846 | 59,084 | 59,058 | 100,833 | 100 | 59 | |||||
| 1996 | 20,405 | 21,900 | 16,384 | 58,689 | 59,304 | 128,765 | 99 | 46 | |||||
| 1997 | 21,186 | 23,928 | 20,450 | 65,564 | 66,619 | 119,641 | 98 | 55 | |||||
| 1998 | 13,542 | 23,314 | 20,907 | 57,763 | 59,059 | 112,035 | 98 | 52 | |||||
| 1999 | 10,663 | 16,804 | 26,154 | 53,621 | 56,595 | 110,257 | 95 | 49 | |||||
| 2000 | 1941 | 11,441 | 21,146 | 79,182 | 113,710 | 115,512 | 158,517 | 98 | 72 | ||||
| 2001 | 163 | 1992 | 12,323 | 64,022 | 78,500 | 80,263 | 113,202 | 98 | 69 | ||||
| 2002 | 22 | 226 | 3241 | 142,192 | 145,681 | 148,215 | 147,619 | 98 | 99 | ||||
| 2003 | 32 | 593 | 114,433 | 115,058 | 119,176 | 143,648 | 97 | 80 | |||||
| 2004 | 109 | 124,820 | 124,929 | 130,695 | 145,925 | 96 | 86 | ||||||
| 2005 | 127,868 | 127,868 | 130,308 | 155,597 | 98 | 82 | |||||||
| 2006 | 134,985 | 134,985 | 137,625 | 164,630 | 98 | 82 | |||||||
| All | 93,889 | 138,319 | 137,153 | 285,396 | 114,433 | 124,820 | 127,868 | 134,985 | 1,156,863 | 1,184,166 | 1,817,642 | 98 | 64 |
| Median age at vaccination, years (IQR) | 15 (13–16) | 13 (12–14) | 12 (12–12) | ||||||||||
Abbreviations: HPV, human papillomavirus; IQR, interquartile range.
Additional assumptions were made when distributing doses to birth cohorts. Firstly, 2011–2013 data of eligible girls and number of doses were provided truncated for oldest girls, namely ≥15 for school-grade P6 and ≥18 for S3. We distributed P6 ≥ 15 years among ages 15, 16, 17 and 18 in the ratio 0.44, 0.32, 0.12 and 0.12 based on Ministry of Education estimate of school-grade ages [8]. The category S3 ≥ 18 years was all attributed to 18 year-olds [8]. Secondly, 2014 data for eligible girls and number of doses were provided combined for 13 and 14 year-olds – we thus distributed to 13 and 14 year-olds in a ratio similar to that observed in 2013 (0.55 and 0.45 respectively).
HPV vaccine coverage was estimated according to birth cohort, defined as the number of delivered vaccines as a proportion of (1) eligible girls, and (2) the 2012 Rwandan census population. Given the ±1 year uncertainty in exact birth cohort when converting from calendar year and year of age, data on coverage and median age at vaccination were also expressed as 3-year floating averages.
3. Results
A total of 1,156,863 girls received a first dose of HPV vaccine in Rwanda between 2011 and 2018, representing 98% of eligible girls (n = 1,184,166) (Table 1). Number of first doses delivered per year varied from 93,889 in 2011 up to peak of 285,396 doses in 2014. Median vaccination age was 15 (interquartile range [IQR] 13–16) years in 2011–2013 (during school grade-targeted catch-up), 13 years (IQR 12–14) in 2014 (transition period) and 12 years in 2015–2018 (routine program) (Table 1). A wide number of birth cohorts (~10) received vaccination in 2011–2013, 3 birth cohorts in 2014, and from 2015 onwards, only one. For each birth cohort, population-level coverage was lower than that estimated among eligible girls, particularly in earliest born cohorts (Table 1). Population-level coverage increased from 6% for girls born in 1993 up to a maximum of 99% for those born in 2002.
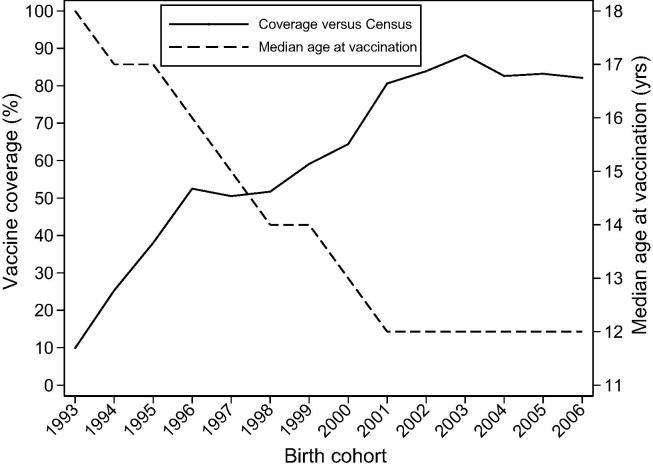
Fig. 1 illustrates trends in population-level vaccine coverage, as well as median age at vaccination, by birth cohort. Population-level coverage increased from 10 to 40% for girls born in 1993–1995, through 50–65% for 1996–2000 birth cohorts, and 80–90% for 2001–2006 birth cohorts. Median age at vaccination decreased steadily from 18 years for girls born in 1993 down to 12 years in girls born in 2001 onwards. Median vaccination age in the three above birth cohort groups was 17 years for 1993–1995, 14 years for 1996–2000, and 12 years for 2001–2006.
Fig. 1.
Trends in first dose HPV vaccine coverage, and in median age at vaccination, by birth cohort. Rwanda 2011–2018. Abbreviation: HPV, human papillomavirus.
Coverage patterns were also estimated at a provincial level (Table 2). The general trend of increasing coverage through the three birth cohort groups (1993–1995, 1996–2000 and 2001–2006) was apparent in all provinces and in the capital, Kigali. There was, however, slightly lower coverage in 1993–1995 birth cohorts in Kigali (10%) compared to the same birth cohort in the rest of the country (23–32%). Kigali also showed lowest coverage in 2001–2006 cohorts (79%), which ranged from 80% to 88% for the rest of the country.
Table 2.
First dose HPV vaccine coverage, by birth cohort and by province.
| Province | Census population, N (first dose vaccine coverage, %) |
||
|---|---|---|---|
| 1993–1995 | 1996–2000 | 2001–2006 | |
| East | 73,854 (23) | 151,374 (52) | 221,443 (80) |
| Kigali | 38,694 (10) | 56,123 (54) | 69,037 (78) |
| North | 54,145 (25) | 113,386 (62) | 150,925 (88) |
| South | 74,025 (32) | 154,163 (53) | 214,531 (83) |
| West | 77,088 (29) | 154,169 (57) | 214,685 (86) |
| All | 317,806 (25) | 629,215 (56) | 870,621 (84) |
Abbreviation: HPV, human papillomavirus.
With the exception of the transition year of 2014, data allowed a comparison of completion rates by round (Table 3). Overall, estimated coverage was similar in all three rounds, suggesting that vaccinated girls tended to complete their recommended dosing regimen. For birth cohorts 1993–1995 and 1996–2000, however, there were actually slightly more doses offered at round 2 and round 3 than at round 1. Of note, 2003–2006 cohorts were primarily targeted after the shift to a two-dose regimen, so round three coverage was close to nil (Table 3).
Table 3.
Completion rates, by birth cohort and vaccination round.
| Birth Cohorts | Census population N | Number of vaccines delivered, n (vaccine coverage, %) |
||
|---|---|---|---|---|
| Round 1 | Round 2 | Round 3 | ||
| 1993–1995 | 317,806 | 80,495 (25) | 89,136 (28) | 92,633 (29) |
| 1996–1999# | 470,698 | 235,637 (50) | 255,413 (54) | 249,680 (53) |
| 2003–2006# | 609,800 | 502,840 (82) | 475,704 (78) | 583 (<1) |
Abbreviation: HPV, human papillomavirus.
Because of missing data on rounds 2 and 3 in 2014, data on birth cohorts 2000, 2001 and 2002 (that were targeted in 2014) are excluded from this table.
4. Discussion
This work provides a robust picture of population-level HPV vaccine coverage in Rwanda, eight years after program initiation. Given the step-wise roll out, including an initial catch-up campaign targeting school grades of wide age range, followed by a transition to an age-based approach, clarity is brought by estimating coverage at a birth cohort level. This birth cohort approach shows that whilst the school-based catch-up campaign resulted in some coverage gaps, likely explained by out-of-school teenagers, coverage remains high (80–90%) in all cohorts routinely targeted as 12 year-olds.
Previously, the coverage of the Rwandan HPV vaccine program has been reported as a proportion of eligible girls, of whom > 95% were successfully vaccinated [2], [3], [4]. This was supported by 94% first dose HPV coverage reported by a 2017 immunization coverage survey among 1066 girls in secondary schools across Rwanda [9]. Coverage is lower, however, when estimated versus the 2012 census population. This difference does not derive from estimates of vaccine doses delivered (the same numerator is used in both reporting approaches), but rather the definition of the eligible population (see methods). In the 2011–2013 catch-up campaign, lists of eligible girls in targeted P6 and/or S3 school-grades were provided by schools to the health authorities. Of note, these lists did not include out-of-school teenagers.
Furthermore, school lists revealed a wide range of ages in P6 and S3. This was a result of an important reconstruction of the Rwandan education system during the preceding decades [5]. This reform offered all Rwandans the opportunity to receive the full statutory number of years of state education, irrespective of age, following a post-genocide period of disruption. Thus, the number of doses delivered to grades P6 and S3 in 2011–2013 was spread over a higher, and wider, range of birth cohorts (median age of vaccination 15 years), than the initial aim of the program.
Discrepancies between eligible and census-based coverage are much smaller for cohorts targeted as 12 year-olds by the routine HPV vaccine program, i.e those girls born from 2002 onwards. This is due to high school attendance for 12 year-olds, and the simplification associated with an age-, rather than grade-, based definition of eligibility. Of note, even in the routine program, lists of eligible 12 year-old girls continue to be generated primarily by schools. Whilst some outreach was also performed for out-of-school 12 year-old girls by community health workers, coverage achieved by this approach in 2011 was reported to be much lower than in school-enrolled girls [3], and the definition of the eligible population less clear.
In 2013/2014, a series of HPV prevalence surveys were undertaken in Kigali, the Rwandan capital, as a collaboration between the Rwandan Ministry of Health and the International Agency for Research on Cancer. The aim of these surveys was to provide a picture of HPV epidemiology in unvaccinated Rwandan women, and to serve as a baseline for future evaluations of HPV vaccine effectiveness. In a first survey, of cervical specimens taken from sexually active women aged 18–69 years living in Kigali [6], 3–11% of girls born in 1993–95 reported to have received HPV vaccine. This was unexpected at the time, but matches with the 10% population-level coverage for the 1993–1995 birth cohort estimated for Kigali City by the current work. In a second, school-based survey, of HPV in urine samples [7], coverage was 6–34% in the 1993–1995 birth cohort. This is consistent with a higher coverage in teenagers attending school at the time of the catch-up program. Of note, the current work revealed that coverage in 1993–1995 birth cohorts is even higher outside Kigali (23–32%), driven by a higher average age in P6 and S3 in the rural provinces compared to the capital.
Of note, WHO-UNICEF also compile HPV vaccine coverage according to an approach based on the cohort turning 15 years in each reporting year (2011 to 2018 currently available for Rwanda [10]). For birth cohorts targeted routinely as 12 year-olds, (e.g. 2002 and 2003 cohorts, turning 15 years-old in 2017 and 2018, respectively) our (99% and 83%) and WHO-UNICEF (>100% and 80%) coverage estimates are consistent. However, the number of vaccines delivered (and vaccine coverage estimates) for birth cohorts targeted in the catch-up and transition phases are considerably lower in the WHO-UNICEF reporting system than those estimated here. This highlights the requirement for data stratified both by year and by age for accurate birth cohort attribution following a roll out that targets multiple cohorts.
This analysis is associated with some limitations. Firstly, data was provided at the level of campaign round, rather than by a vaccination registry linking doses at an individual level. This is a regular feature of immunization coverage reporting. However, given that the number of doses given at the second and third rounds were slightly higher than those given at the first (at least for the catch-up campaign), it highlights that at least some girls must have received their first dose at later rounds, and that first dose vaccine coverage could be a few percentage points higher. The under-estimate cannot be very large, however, given the already very high overall estimate of first dose coverage (>95%) among the eligible population. Indeed, population-level coverage gaps are driven mainly by non-eligible (i.e. out of school) girls.
Secondly, we provide population-level coverage and median age at vaccination according to three-year floating averages. This approach provides smoother trends that are likely to better reflect the uncertainties associated with constituting birth cohorts from calendar year and girl's year of age (i.e. we did not have data on girl's exact date of birth, nor exact date of vaccination). Of note, this approach does not affect total number of vaccines delivered, nor general coverage trends, only how vaccines were assigned across adjacent cohorts.
Experiences from demonstration projects and national HPV vaccination programs (of which Rwanda was the first) in low income countries, have reported that school-based vaccination programs can achieve vaccine coverage of above 90% [11], particularly in settings with high primary school girls' attendance. However, a number of challenges in identifying and enumerating the target populations have been reported, including high rates of absenteeism, difficulty in determining age, and complications of a grade-based approach [11].
This experience from Rwanda illustrates how these implementation challenges are compounded at higher, and wider, target ages at vaccination. Whereas the catch-up campaign clearly resulted in some coverage gaps in out-of-school teenagers, coverage in cohorts routinely targeted as 12 year-olds at primary school was consistently very high (>80%). Although the overarching aim of the Rwandan program was to protect all girls born ≥ 1997 (i.e. all those younger than 15 years in 2011), some pragmatic choices, namely that of a primarily school-based approach, had to be made in the face of these important implementation challenges. This meant that approximately half of girls born in 1997–1999 were in the targeted school grades at the relevant moment and received vaccination during the catch-up campaign. Nevertheless, this 50% coverage may translate into a larger impact on HPV prevalence (and cervical cancer risk) at the population level, given the strong additional effects of herd immunity in HPV transmission [12], [13].
Vaccine effectiveness in these same 1997–1999 catch-up cohorts has recently been reported in Kigali [14]. In high-school students 18–20 years old in 2017, of whom 89% reported HPV vaccination (consistent with high coverage among eligible school attending population), an 86% reduction in vaccine-targeted HPV types (6/11/16/18) was observed in comparison to unvaccinated girls of the same age in 2013/14 [14]. Furthermore, significant cross-protection against non-vaccine-targeted oncogenic HPV types was also reported [14]. Future HPV prevalence surveys in the general Rwandan female population are expected to provide additional population-level evidence on the effect of herd immunity on out-of-school girls, as well as on impact in highly protected cohorts vaccinated as 12 year-olds. The timing and design of these impact studies will be strongly informed by current estimates on birth cohort coverage.
5. Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Funding
This work was supported by the Bill & Melinda Gates Foundation, Seattle, WA (http://www.gatesfoundation.org/) [grant number OPP1053353].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.04.021.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Arbyn M., Weiderpass E., Bruni L., de Sanjose S., Saraiya M., Ferlay J. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2019 doi: 10.1016/s2214-109x(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binagwaho A., Ngabo F., Wagner C.M., Mugeni C., Gatera M., Nutt C.T. Integration of comprehensive women's health programmes into health systems: cervical cancer prevention, care and control in Rwanda. Bull World Health Organ. 2013;91(9):697–703. doi: 10.2471/Blt.12.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binagwaho A., Wagner C.M., Gatera M., Karema C., Nutt C.T., Ngabo F. Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90(8):623–628. doi: 10.2471/BLT.11.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatera M., Bhatt S., Ngabo F., Utamuliza M., Sibomana H., Karema C. Successive introduction of four new vaccines in Rwanda: High coverage and rapid scale up of Rwanda's expanded immunization program from 2009 to 2013. Vaccine. 2016;34(29):3420–3426. doi: 10.1016/j.vaccine.2015.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Appui Rwanda. Le système scolaire au Rwanda. [03 Jan 2020]; 2009. Available from: http://www.appuirwanda.org/040-le-systeme-scolaire-au-rwanda-2009.html.
- 6.Ngabo F., Franceschi S., Baussano I., Umulisa M.C., Snijders P.J., Uyterlinde A.M. Human papillomavirus infection in Rwanda at the moment of implementation of a national HPV vaccination programme. BMC Infect Dis. 2016;16(1):225. doi: 10.1186/s12879-016-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi S., Chantal Umulisa M., Tshomo U., Gheit T., Baussano I., Tenet V. Urine testing to monitor the impact of HPV vaccination in Bhutan and Rwanda. Int J Cancer. 2016;139(3):518–526. doi: 10.1002/ijc.30092. [DOI] [PubMed] [Google Scholar]
- 8.MINEDUC, UNICEF. Understanding Dropout and Repetition in Rwanda. Kigali, Rwanda: Laterite Ltd; 2017 [03 Jan 2020]. Available from: http://www.rencp.org/wp-content/uploads/2018/09/DROPOUT-STUDY-FULL-REPORT.pdf.
- 9.Expanded Program on Immunization (EPI) - Rwandan Ministry of Health. Rwanda Routine Immunization Coverage Survey; 2017.
- 10.World Health Organization, UNICEF. Immunization, Vaccines and Biologicals [31 Mar 2020]. Available from: https://www.who.int/immunization/monitoring_surveillance/data/en/.
- 11.Gallagher K.E., Howard N., Kabakama S., Mounier-Jack S., Burchett H.E.D., LaMontagne D.S. Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007–2016. Papillomavirus Res (Amsterdam, Netherlands) 2017;4:72–78. doi: 10.1016/j.pvr.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baussano I., Lazzarato F., Ronco G., Franceschi S. Impacts of human papillomavirus vaccination for different populations: A modeling study. Int J Cancer. 2018;143(5):1086–1092. doi: 10.1002/ijc.31409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisson M., Bénard É., Drolet M., Bogaards J.A., Baussano I., Vänskä S. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–e17. doi: 10.1016/S2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baussano I., Sayinzoga F., Tshomo U., Tenet V., Vorsters A., Heideman D.A.M. Impact of Human Papillomavirus vaccination in Rwanda and Bhutan: Evidence from Urine-based Impact Monitoring Surveys. Emerg Infect Dis. 2020 doi: 10.3201/eid2701.191364. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.