Abstract
Actin filaments (F-actin) are a key component of eukaryotic cells. Whether serving as a scaffold for myosin or using their polymerization to push onto cellular components, their function is always related to force generation. To control and fine-tune force production, cells have a large array of actin-binding proteins (ABPs) dedicated to control every aspect of actin polymerization, filament localization, and their overall mechanical properties. Although great advances have been made in our biochemical understanding of the remodeling of the actin cytoskeleton, the structural basis of this process is still being deciphered. In this review, we summarize our current understanding of this process. We outline how ABPs control the nucleation and disassembly, and how these processes are affected by the nucleotide state of the filaments. In addition, we highlight recent advances in the understanding of actomyosin force generation, and describe recent advances brought forward by the developments of electron cryomicroscopy.
Abbreviations: ABP, actin-binding protein; cryo-EM, electron cryomicroscopy; NPF, nucleation promoting factor; EM, electron microscopy; G-actin, globular actin; F-actin, filamentous actin
Keywords: Electron cryomicroscopy, Actin, Myosin, Actin-based motility, Actomyosin, Motor protein, Actin-binding protein
1. Introduction
The actin cytoskeleton is a dynamic network of polar filaments serving as a key component of eukaryotic cells. Its many functions include essential processes as diverse as cellular motility, cytokinesis, intra-cellular cargo transport, and endocytosis (for a recent review see [1]). Due to its importance for cell survival, the sequence of actin has changed little during the evolution of the eukaryotic lineage, with a sequence conservation of > 90 % between yeast and human versions of the protein. This makes actin one of the most conserved protein families known to date [2]. Why is this protein so conserved? Recently discovered actin homologs with as little as 20 % sequence identity to eukaryotic actin suggest that this is not due to constraints on the filament structure as these proteins can form filaments that are surprisingly similar to actin filaments (F-actin) [3]. At its core, the function of actin is mechanical with actin filaments used either passively for structural purposes or for force generation. The latter motor function can be achieved in two different ways. By associating with myosin – the partner motor ATPase of actin – the actin cytoskeleton can be used as a scaffold for myosin to generate force. Alternatively, the push associated with the polymerization of F-actin generates force and creates motion [4]. The same protrusive force is used by distant bacterial actin homologs to segregate low copy number plasmids [5]. As such, it is tempting to speculate that this polymerization-coupled force generation was the original function of the actin cytoskeleton. How has evolution created the immense diversity in the function of the eukaryotic actin cytoskeleton? The answer lies in the countless regulatory proteins that control the function of eukaryotic actin within the cell. The combinatorial action of several of these actin-binding proteins (ABPs) is what will ultimately determine whether actin will perform one function or another. Considering this, it is clear why eukaryotic actin is so strictly conserved, while its prokaryotic homologs – with very few ABPs of their own – have diverged so strongly in comparison.
Several decades of biochemical studies have provided us with a rich description of the function of the actin cytoskeleton, and how many actin regulatory proteins diversify its function (reviewed in [[6], [7], [8]]). In contrast, the structural mechanism by which the actin cytoskeleton is remodelled is still poorly understood. Most helical polymers cannot form crystals, and therefore cannot be studied by X-ray crystallography. As such, structural studies of actin filaments have been limited to electron cryomicroscopy (cryo-EM), with the main drawback that until recently this technique was not able to routinely produce reconstructions better than 8 Å resolution. Thus, while the PDB database contains more than 100 structures of actin, most of them correspond to non-polymerizable versions of the protein, or complexes that arrest it in its globular form (G-actin). During the last five years, the cryo-EM field has undergone a revolution [9]. A new generation of detectors capable of directly detecting electrons have pushed the resolutions of reconstructions to levels that rival the results obtained with X-ray crystallography (for a review see [10]).
In this review we summarize the recent advances brought forward by other groups and ours to better understand the structure and function of the actin cytoskeleton. Our main focus will be on the mechanism of actin polymerization and how ABPs fine-tune and remodel the cytoskeleton in cells. We will also evaluate how the field has benefited from recent contributions using cryo-EM.
2. Structure and function of the actin filament
2.1. Nucleotide hydrolysis in F-actin
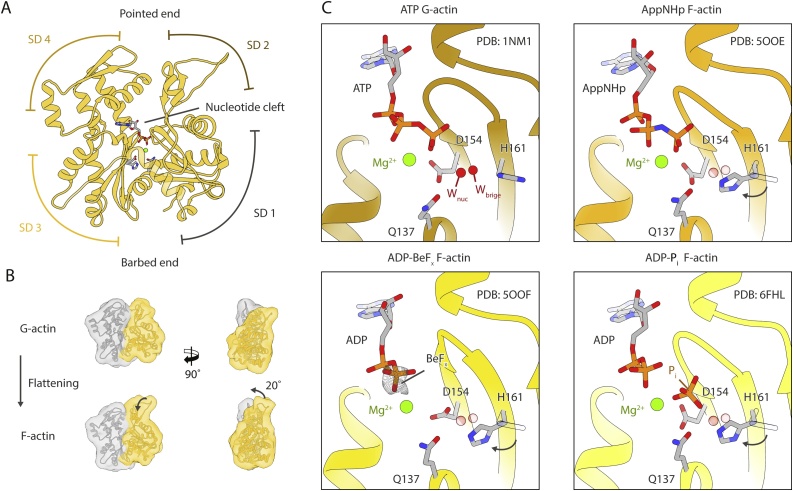
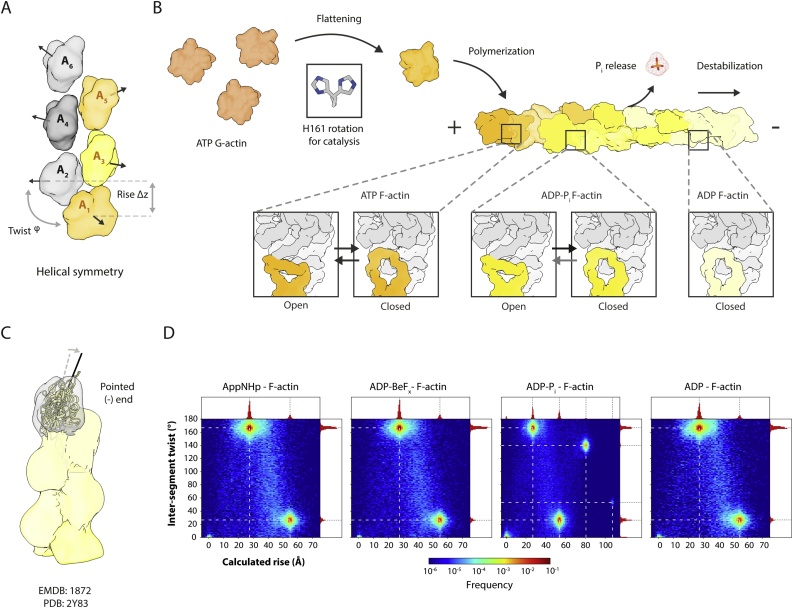
At the beginning of the 90 s, Holmes and colleagues presented the first crystal structure of G-actin [11]. The protein is composed of two main domains, which are often divided into two sub-domains each (SD1 to SD4) (Fig. 1A). In a deep cleft between the domains, actin binds its ligand MgATP (Fig. 1A). The nucleotide is key for stability; in its absence, the actin monomer irreversibly unfolds [12]. During the last decades, several groups have tried to decipher the atomic structure of F-actin. Early on, it was clear that F-actin forms a double-stranded and staggered helix with a right-handed twist (Fig. 2A) [11]. Based on the way in which the filament looks when decorated by myosin, the ends of the filament have been named barbed (SD1 and SD3) and pointed (SD2 and SD4) ends. In 2009, Oda and colleagues could use a model derived from X-ray fibre diffraction studies to show that the actin monomer flattens when incorporated into the filament [13] (Fig. 1B). Confirmation to this model came finally in 2015, when our group solved the first near-atomic resolution structure of the actin filament [14]. Since then, other groups and ours have contributed with high-resolution cryo-EM structures of actin alone and in complex with ABPs ([[15], [16], [17], [18], [19], [20]]). Within the filament, most of the interactions occur between protomers of the same helical strand (intra-strand), where the hydrophobic DNAase-I binding loop (D-loop) is inserted in a hydrophobic cavity between SD1 and SD3 in the next protomer. Additional interactions occur between SD4 and SD3. Inter-strand interactions are sparser, with the most prominent being between the so-called hydrophobic plug and the d-loop of an adjacent protomer ([14,17]).
Fig. 1.
Structural transition upon actin polymerization. (A) Illustration of the actin domain architecture consisting of four subdomains (SD) and a central nucleotide binding cleft. (B) During polymerization actin undergoes a transition from its monomeric (G-actin) to filamentous (F-actin) form. In F-actin SD1 and SD2 (yellow) are rotated ∼20˚ relative to the other domains (gray) closing the central nucleotide-binding cleft. (C) Close-up view into the nucleotide-binding cleft of Mg-ATP-G-actin (PDB: 1NM1) and F-actin bound to either Mg-AppNHp (PDB:5OOE), Mg-ADP-BeFx (PDB: 5OOF) or Mg-ADP-Pi (PDB: 6FHL). The crystal structure of G-actin (top left panel) includes two water molecules (red balls) which are essential for nucleotide hydrolysis. Wnuc is responsible for the nucleophilic attack and Wbridge bridges the catalytic base with Wnuc. Upon the transition to F-actin H161 rotates taking the position of Wbridge and Q137 moves slightly towards the nucleotide. Waters and H161 of ATP G-actin are shown transparently in the other tiles to emphasize the structural transition. As BeFx is not included into the atomic model, its corresponding density (EMDB: 3839) is shown instead (gray mesh). Actin is depicted in shades of yellow depending on the nucleotide state (see Fig. 3 for color legend).
Fig. 2.
Schematic overview of actin polymerization, filament ageing and associated helical symmetry. (A) Actin polymerizes into a helix that can be either described as a right-handed, double-stranded (long pitch) or a left-handed single start (short pitch) filament. The colors were chosen to highlight individual strands of F-actin while the numbering represents consecutive subunits along the short pitch helix. (B) ATP-G-actin adds to the barbed (+) end of the filament undergoing a structural transition to F-actin which includes flattening of its structure and repositioning of side chains essential for catalysis, especially H161 (also see Fig. 1). F-actin has a characteristic nucleotide gradient along the filament, marking the local age of the filament. The age of the filament is transmitted to an essential interaction site at the surface which is made up by the d-loop (shades of yellow) of one subunit and the C-terminal tail of an adjacent subunit (gray). While both the open and the closed d-loop conformation coexist before and after hydrolysis, the occupancy of the closed state increases with age until phosphate release eventually locks the filament in the closed d-loop. (C) Cryo-EM reconstruction of the pointed end of F-actin. The structure shows that the terminal protomer is tilted with respect to the helical axis. (C) The helical symmetry parameters can be directly derived from projection parameters of high-resolution electron microscopy data. The histograms show the per-particle symmetry derived from our original data of F-actin bound to AppNHp (EMDB: 3838), ADP-BeFx (EMDB: 3839), ADP-Pi (EMDB: 4259) and ADP (EMDB: 3835). The rise describes the distance between consecutive subunits of the short-pitch helix while the twist describes the rotation between them (also see Fig. 2A).
Actin is a poor ATPase in its monomeric form. Its catalytic rate is so low (7 10−6 s-1) [21] that no significant ATP hydrolysis occurs in G-actin. Upon polymerization, the ATPase activity of actin increases 42,000 times [22], triggering nucleotide hydrolysis and the subsequent phosphate release. While it is not clear if the nucleotide state of a single protomer in the filament affects its neighbors, a large delay between nucleotide hydrolysis and phosphate release coupled to faster growth from the barbed end results in filaments with a young ATP/ADP-Pi end and an old ADP end [23,24]. Many ABPs can sense the nucleotide state of either F- or G-actin, strongly suggesting that the identity of the nucleotide at the active site elicits conformational changes in actin. These nucleotide-dependent structural differences are key for the function of actin since ABPs that can sense and use them as an age marker to direct cytoskeletal remodeling.
While the X-ray fibre diffraction models contributed to the understanding of the changes of actin upon polymerization, they do not address the question of how the rate of nucleotide hydrolysis is increased in F-actin, nor how the nucleotide bound the active site of F-actin – buried within the protomer – is being read by ABPs. The nature of the catalytic mechanism of actin has been studied by several experimental and theoretical groups [[25], [26], [27], [28], [29], [30], [31]], all providing different candidates for the general base of the reaction. Despite the lack of consensus, three main residues appear to be the most important candidates: Q137, D154, and H161. The Q137A mutation decreases the catalytic activity rate of cardiac actin [27]. The Q137A [32], H161A [26] and the double mutant D154A/D157A [33] are all lethal in yeast, which possesses only one copy of the actin gene, providing evidence for the importance of all of them. Interestingly, the triple mutant Q137A/D154A/H161A can completely abolish the ATPase activity of actin, without modifying its polymerization kinetics [34], arguing for a loose coupling between polymerization and nucleotide hydrolysis. Recently, we have used cryo-EM to obtain near-atomic resolution structures of rabbit α-actin in all its nucleotide states [30]. To obtain the high resolutions at the active site, we developed a hybrid data processing approach that takes advantage of the filamentous nature of the protein without imposing helical symmetry. This allowed us to obtain average resolutions high enough (4.1 – 3.3 Å) to unambiguously determine the nucleotide bound to the protein and the conformation of most residues around it. Regardless of the nucleotide bound to actin, the structure of the filament is strongly conserved. To completely characterize the hydrolysis reaction, we studied the complex with the non-hydrolysable ATP analog AppNHp (also known as AMP-PNP), the transition state analog ADP-BeFx, or the products ADP and Pi, or only ADP. Compared with G-actin, the main difference at the active site is the rotation of H161 towards the γ-phosphate (Fig. 1C). Although the 4.1-Å-resolution map of the ADP state did not completely resolve the active site, we speculated that H161 is in the same conformation also in the ADP state. Based on the high-resolution structures of G-actin of D. discoideum complexed with Li+ and ATP, Vorobiev and colleagues proposed that H161 corresponds to the catalytic base that activates the nucleophilic water for the hydrolysis reaction [26]. A similar conformation of the side chain has been reported by the Kursula lab for the crystal structure of G-actin from Plasmodium berghei [25]. Interestingly, they also presented a structure from Plasmodium falciparum containing a K+ ion at the active site of MgATP-G-actin which they propose is key for the hydrolysis reaction. In our F-actin structures, H161 takes the position corresponding to a water molecule directly bound to the water molecule proposed as catalytic water in the structure of D. discoideum (Fig. 1C). Chou and Pollard later confirmed the reorganization of the active site – including in the ADP state – using chicken α-actin [31].
How is the nucleotide state communicated at the surface of the filaments? While most of the structure of F-actin does not change upon nucleotide hydrolysis, our ADP-BeFx reconstruction shows a new conformation of the intra-strand interface (Fig. 2B). There, the d-loop adopts an open state, creating a hydrophobic pocket that traps the C-terminus of the adjacent monomer in the filament. In contrast, the ADP state shows the previously known closed state of the d-loop. Recently, a similar conformation has been observed in the ADP-actin filaments of Zea mays pollen [35]. In this case, the open d-loop conformation was linked to the stronger mechanical stability of those filaments. This agrees with the increased stability of F-actin upon BeFx binding [36,37]. Notably, our AppNHp and ADP-Pi structures filtered to a lower resolution display a mixture of the two d-loop states [30] suggesting structural flexibility. In line with this, proteolysis, electronic paramagnetic resonance and cross-linking studies have suggested that the d-loop/C-terminus interface has considerable structural flexibility [[38], [39], [40]]. We proposed that this is what ABPs sense as the nucleotide state of the filament. For instance, the ABP coronin is able to sense the nucleotide state of F-actin, strongly preferring ADP-Pi filaments [30,41]. Previous studies showed that coronin binds to the intra-strand interface of the filaments [42], leading the Reisler group to the hypothesis that the protein senses the nucleotide state through the conformation of the C-terminus. In a similar manner, the binding site of cofilin (see section 3.3) includes the intra-strand interface. In this case, the protein strongly prefers ADP to ATP/ADP-Pi actin [43].
The structures of actin with different nucleotides presented by Chou and Pollard could not reproduce our open d-loop state [31]. Unfortunately, they did not include ADP-BeFx in their study. Biochemical data has shown that this nucleotide state has a unique intra-strand interface [37], and in our reconstructions it shows the strongest density for the open d-loop state. Moreover, in their structures, AppNHp seems not completely clean, as evidenced by the much weaker density for the γ-phosphate. AppNHp is known to slowly and spontaneously hydrolyze to AppNH2 in solution [44]. Actin prefers the degradation product over AppNHp [45], effectively enriching AppNH2 over AppNHp. In our hands, we could only saturate the filaments with ∼80 % AppNHp, with the rest corresponding to ∼5 % ADP and 15 % AppNH2 [30]. Thus, their AppNHp structure seems to represent a mixture of nucleotide states that could explain the differences. New reconstructions are needed to confirm these observations.
2.2. Effect of stabilizing toxins
Drug-like toxins that stabilize F-actin have been used for decades in the study of the actin cytoskeleton. For example, due to its cell permeability, a fluorescent version of jasplakinolide (JASP) – conjugated to a silicon-rhodamine derivative – has been recently proposed as an efficient probe for actin imaging [46]. JASP and the widely used toxin phalloidin have similar effects, stabilizing actin filaments, increasing their persistence length and inhibiting phosphate release [47,48]. Our group has recently explored the effect of these molecules on the structure of actin filaments [30,49]. Both toxins bind to the same pocket, with an analogous tryptophan side chain buried between SD4 and SD1 of two consecutive actin protomers. In agreement with their biochemical effect, filaments polymerized from G-ATP-actin and JASP or phalloidin showed additional density at the active site corresponding to Pi. The structures also showed the d-loop in the open conformation. Notably, JASP could stabilize the open d-loop on its own as filaments polymerized from G-ADP-actin and JASP show a clear open d-loop. Thus, JASP should be used with caution as a probe for actin imaging, as it will effectively erase the nucleotide-clock information in the filament. Interestingly, adding phalloidin to aged filaments (F-ADP-actin) does not induce the open d-loop conformation, showing both toxins do not have exactly the same effect [49]. Similarly, a recent structure of a phalloidin-stabilized actomyosin complex showed the d-loop in its closed state [18]. However, myosin directly interacts with the intra-strand interface, raising the interesting possibility that myosin selects the closed d-loop state. Indeed, Zimmerman and colleagues showed that myosin V and VI can differentiate ATP/ADP-Pi filaments from ADP filaments, even in the presence of phalloidin [50]. Myosin VI is particularly interesting, as it walks in the opposite direction than other myosins, and has inverted F-actin-nucleotide preferences [50,51].
2.3. Structural variability and stability of the filaments
In the mid 80 s, Egelman and colleagues proposed a twist disorder of ∼10° that could explain the cross-over distance variability observed in cryo-EM samples of F-actin [52]. His group has further developed the hypothesis of actin acting as a tension sensor, where tension could modulate structural variability [53]. In addition, they proposed that the thin ice necessary for cryo-EM limits the flexibility of the filament allowing high resolution structures but masking the natural variability of F-actin [17]. While this is an interesting hypothesis, it has only been indirectly validated since the ice thickness in actin samples has never been directly measured [17].
Our F-actin cryo-EM data [30] have been collected in different ice thicknesses and the reconstructions have been performed without imposing helical symmetry. Since filaments in helical reconstructions are split into overlapping segments that are then treated as single particles [54], we estimated the local symmetry of F-actin by measuring the twist and distance between each pair of consecutive segments in a filament. None of our samples shows signs of twist variability higher than a few degrees, with an average corresponding to the symmetry measured from the volume (Fig. 2D). Why is the twist in our samples not variable? It is possible that the ice in our samples is still too thin. Alternatively, it is possible that the variability observed in thicker ice is due to low signal to noise ratio (SNR) and not a feature of the filaments. Thicker ice produces lower SNR, decreasing the accuracy of angular assignment, making the estimated parameter distribution broader due to the inherent error. Clearly, the effect of ice thickness on actin requires proper quantitative testing.
Despite the high conservation of eukaryotic actin, small sequence variations can tune the behavior of the filaments. For instance, most apicomplexan actins cannot form long filaments under standard conditions, a feature that is key to their function [55]. These short and dynamic actin filaments associate with myosin to generate force. The force is translated into parasitic motion by a complex array of protein, that together with the actomyosin complex are known as the glideosome (for a review see [56]). In order to understand the reason for apicomplexan actin instability, we used JASP to stabilize long actin filaments of Plasmodium and study them with cryo-EM [16]. The structure is remarkably similar to α-actin, with minor differences in the overall structure that together diminish filament stability. One of the clearest differences comes from the inter-strand interface. There, a methionine residues M270 is replaced by a lysine which we proposed to destabilize the filament. Indeed, a reversal mutation K270 M improves the ability of this actin to form long filaments [25].
2.4. Mechanism of actin polymerization
The process by which pure actin polymerizes is relatively well understood. Filament formation is a highly cooperative process, limited by the unfavorable formation of a tetrameric nucleus [57]. Above a critical concentration, actin polymerizes into μm-long filaments. If the protein is kept in low ionic strength buffers with Ca2+, actin will stay in its G form due to a critical concentration of > 80 μM under these conditions [58]. Conversely, the combination of physiological ionic strengths and Mg2+ drops the critical concentration below 1 μM [59], promoting polymerization. This is due to the strong shielding of the electrostatic potential characteristic of the protein [58] and the specific binding of filament-stabilizing cations [60]. A strong exception to this mechanism is the divergent actin of the apicomplexan parasites. The polymerization of actin of Toxoplasma gondii proceeds without the initial formation of actin nuclei in a non-cooperative process known as isodesmic polymerization [61]. While actins from other apicomplexans – like Plasmodium falciparum – show cooperative polymerization [62] they still fail to form long filaments (see above). Unfortunately, these nuclei are rare and short lived. As such, while differences in F-actin stability can be guessed directly from the structure of the filament, the mechanism of polymerization is more difficult to model on a structural basis. Even after the nucleation step, the polymerization reaction occurs between an end of the filament and an incoming monomer. While the structure of the latter has been well studied using crystallography, little is known about the behavior of G-actin under conditions that favor polymerization. Likewise, several lines of evidence demonstrate that the ends of F-actin are structurally different from the bulk of the filament; they release Pi and exchange nucleotide at a faster rate than the core of the polymer [63]. This suggests that the structures at the ends are different from the filament core, and the mechanism of polymerization cannot be guessed from the structure of the filament alone. In a pioneering study, Narita and colleagues used cryo-EM to study the structure of the pointed end of the filament [64]. Although the final structure was limited to a resolution of ∼20 Å, it was sufficient to show that the terminal protomer indeed adopts a different conformation (Fig. 2C). This conformation should not be compatible with filament elongation, explaining why the kinetics at this end are significantly slower. Unfortunately, no structures of the barbed end have been reported so far. Future studies addressing this and the effects of the nucleotide bound to either end are necessary to fully unravel the mechanism of actin polymerization.
3. Control of F-actin assembly
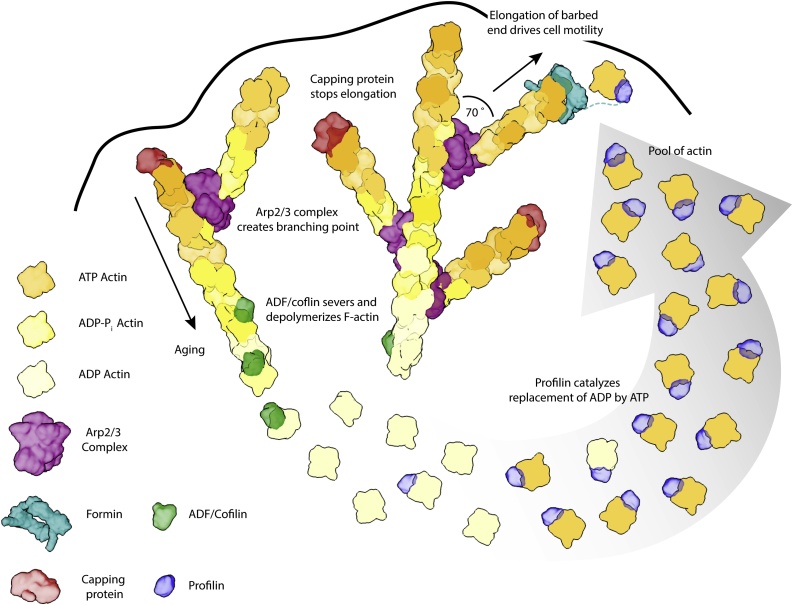
A unique property of actin filaments arises from having different kinetics at both ends. As mentioned before, the faster polymerization at the barbed end combined with nucleotide hydrolysis generates an ADP-bound pointed end. ADP-actin at this end is less stable, triggering depolymerization. G-actin can then exchange its nucleotide and be reincorporated at the barbed end. Once depolymerization and polymerization rates are equalized, the filaments acquire a defined length moving in the direction of polymerization, a process referred to as treadmilling [65,66]. Due to a free G-actin concentration of ∼ 0.1 μM (the critical concentration) the treadmilling speed in vitro is of less than 1 monomer/s [65]. In strong contrast, the speed of actin polymerization can go beyond 1000 monomers/s in vivo [34]. These high polymerization rates therefore cannot only depend on the properties of actin. Instead, the turnover of monomers from the filaments is tightly controlled by the concerted action of several ABPs.
In cells, actin polymerization is used to generate force, with the polymerization of a single actin filament producing pN-magnitude forces [67]. However, only short filaments can withstand the force, as long filaments buckle under tension. Therefore, ABPs are also necessary to produce higher order structures to fine-tune the mechanical properties of the cytoskeleton (Fig. 3). The function of the cytoskeleton is further controlled by the interaction of ABPs with other cellular components, most notably the membrane.
Fig. 3.
Schematic overview of the dendritic nucleation model of actin. The dynamic remodeling of the actin cytoskeleton requires a tight spatial and temporal organization by ABPs. Proteins like capping protein (red) and the Arp2/3 complex (purple) remodel the cytoskeleton by either capping the barbed end or by introducing a branch point from which a new filament can grow. Other proteins like ADF/cofilin (green) and profilin (blue) depolymerize and sever actin or maintain a constant pool of polymerizable actin, respectively. The ATPase activity of actin itself is also essential for the regulation as it results in a nucleotide gradient along the filament which can be sensed by accessory proteins (also see Fig. 2). Actin is depicted in shades of yellow depending on its nucleotide state. The color gradient represents the nucleotide gradient along the filament from the darkest shade (ATP) to brightest shade (ADP). The figure is based on [112].
3.1. Inhibition of nucleation and pointed end elongation
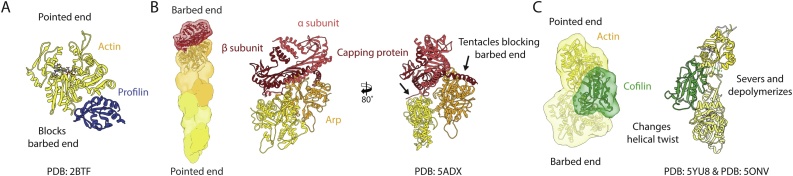
Intracellular actin concentrations reach levels well beyond 50 μM [34,68,69]. To avoid out-of-control polymerization the cell utilizes ABPs to keep a large fraction of actin arrested in its monomeric form [70]. The most prominent of these ABPs is profilin, a protein that binds the barbed end of an actin monomer, inhibiting nucleation (Fig. 3, Fig. 4A). The dissociation constant of the complex is in the sub-μM range. It is even lower for the profilin complex with cytoplasmic actin [34,71]. The protein prefers MgATP-G-actin over ADP-G-actin [72,73], which promotes nucleotide exchange. Structurally, this is due to an opening of the nucleotide-binding cleft of actin induced by the binding of profilin [74,75]. Although profilin binding blocks the barbed end of the monomer, it leaves a free pointed end, ensuring that a pool of MgATP-actin-profilin is available for the elongation of existing free F-actin barbed ends. Upon the G- to F- transition the affinity of profilin to actin drops, resulting in dissociation and allowing barbed end elongation [76]. Besides actin, profilin can additionally bind polyproline stretches, in a pocket far away from the actin-binding site [77,78]. Many ABPs have polyproline regions – most notably the FH1 domain of formins (see section 3.5) – which they use to directly recruit actin from the profilin/actin pool [79].
Fig. 4.
Inhibitors and depolymerizers of F-actin. (A) Profilin (blue, PDB:2BTF) binds and blocks the barbed end of monomeric actin (G-actin). At the same time, profilin promotes the conversion from ADP-G-actin to ATP-G-actin to build a pool of polymerizable actin. (B) Capping protein (red, PDB: 5ADX) binds to the barbed end of the actin filament. It consists of two subunits, α and β, which both have C-terminal tentacles that block the barbed end and stop elongation of actin filaments. (C) Cofilin (green, PDB: 5YU8) preferentially binds to ADP-bound F-actin, where it locates at the interface of two actin subunits within one strand. It changes the helical twist and weakens the interactions within F-actin, eventually depolymerizing and severing actin. As the d-loop of the original model was fragmented, the lower actin subunit in the right panel was replaced by one from PDB: 5ONV to illustrate the proximity of the N-terminus of cofilin (residues missing in shown model are represented schematically as a dashed green line) and the d-loop. Actin is depicted in shades of yellow depending on the nucleotide state (see Fig. 3 for color legend).
Already in the mid 2000s an interesting property of profilin was proposed from single filament studies. At low concentrations the polymerization of profilin-bound actin can be understood as a simple second order reaction. However, the polymerization rate only reaches its maximum at high levels of profilin/actin [80,81], indicating that, under those conditions, the elongation rate is limited by a first order process. Based on energetic considerations, and using several different nucleotides, the Carlier group suggested that nucleotide hydrolysis was necessary to dissociate profilin from the barbed ends of actin, proposing that the energy from ATP breakdown was used in this process, and that the rate of hydrolysis places the upper limit to the polymerization speed [80,81]. However, this was a matter of extensive debate since similar experiments by the Pollard group did not show a speed limit nor a dependence on nucleotide hydrolysis [22]. In a recent study, the Bieling group has shown that, at physiological concentrations of profilin/actin, the polymerization speed indeed reaches a limit. Moreover, a hydrolysis inactive actin mutant which polymerizes with wild type kinetics, shows the same rate limit when polymerized from profilin/actin monomers [34]. Therefore, neither hydrolysis nor Pi release are required to remove profilin from the end of the filament, as has also been suggested by others [82]. Remarkably, using a collection of profilin mutants, the Bieling group demonstrated instead that the rate-limiting step is the dissociation of profilin from the barbed end [34]. What is the role of ATP hydrolysis then? Contrary to the situation in polymerization assays, polymerization in vivo is driven by a steady state concentration of monomeric actin – maintained by ABPs – which depends on nucleotide hydrolysis; ATP breakdown acts in a way as a molecular timer. As such, the function of the actin cytoskeleton is characterized by two coupled reactions: while the energy of filament polymerization itself provides the drive for mechanical work, nucleotide hydrolysis is in charge of maintaining a constant pool of polymerizable monomers for constant growth.
Although profilin is the most common protein maintaining actin in a monomeric state, some cells use thymosin-β4 instead [83]. In contrast to profilin, this protein will cap the monomer at both ends, completely inhibiting polymerization [84]. Due to a partial overlap in their binding sites, binding of profilin or thymosin-β4 should be mutually exclusive [84,85]. This provides the cell with an interesting mechanism to recover actin monomers arrested by thymosin-β4. The affinity of thymosin-β4 for ATP-G-actin is ∼2 μM [86], hence significantly lower than the affinity of profilin. Therefore, under conditions where actin monomers become limiting, profilin can easily recover polymerizable actin by competition [72,84].
3.2. Inhibition of barbed end elongation
Controlling the length of the filaments is a key requirement of many higher-order structures formed by actin. Perhaps the most spectacular example is the sarcomere, where arrays of parallel actin filaments of equal lengths are necessary for proper cellular function. The barbed ends of the filaments sit in a region called the Z-band, where a protein called CapZ or capping protein caps F-actin [87]. Capping protein is not only important for muscle cells; it also controls the length of the cytoplasmic F-actin which will ultimately determine their mechanical properties (Fig. 3, Fig. 4B) (see [88] and references therein).
Capping protein is formed by a heterodimer of two closely related proteins. The bulk of the protein is formed by a long β-sheet surrounded by helices. In addition, each subunit has an additional free helix called tentacle (Fig. 4B). Once bound to the barbed end, it will dissociate very slowly, with a half-life of ∼30 min [89]. In vivo, this might require that capping protein is removed through depolymerization of the filament. However, it has been recently proposed that its interaction with another barbed end binder, formin, can modulate its dissociation kinetics (see section 3.5) [90]. Using cryo-EM, Narita and colleagues obtained a 23-Å-resolution reconstruction of capping protein bound to the filament [91]. Combining their map with the available crystal structure of CapZ [92] they proposed a two-step binding model. The bulk of the protein combined with the α-tentacle are responsible for most of the affinity, binding directly to the barbed end of the penultimate actin monomer (Fig. 4B). The β-tentacle is proposed to bind later, capping the barbed end of the last actin subunit. However, its binding is not necessary for the capping activity of the protein. Besides capping F-actin, capping protein is also part of the dynactin complex. Dynactin is a co-factor for the motor protein dynein, which associates with microtubules [93]. In this complex, capping protein caps a filament formed by centractin (or Arp1), a member of the group of actin related proteins (ARP) (for a review see [94]). A high-resolution structure of the dynactin complex suggests that the binding mode proposed by Narita is correct [95], indeed showing the β-tentacle docked in the barbed end (Fig. 4B). High-resolution structures of capped F-actin barbed ends are now necessary to confirm the model.
3.3. Depolymerizers
In regions of high filament turnover, actin monomers are continuously recycled (Fig. 3). Old regions of the filaments – rich on ADP-actin – are broken down and depolymerized. For actin monomers removed from the filaments, profilin can promote the exchange of their nucleotide and they can then be reincorporated at the growing end. The small globular protein cofilin – a member of the actin depolymerizing factor (ADF)/cofilin family – is and one of the most important proteins for actin recycling. It can bind to G-actin as well as to the side of F-actin. Contrary to profilin, it prefers ADP-actin over ATP/ADP-Pi actin [96,97]. Moreover, the binding of cofilin to ADP-Pi actin increases the Pi release kinetics [98], thus actively modifying the nucleotide state of the filament. Traditionally, the main function of the protein has been understood as to sever filaments to generate new free barbed ends to promote polymerization. However, bulk [96] as well as recent single filament assays have shown that cofilin can also accelerate the depolymerization of filament ends [99,100]. Interestingly, severing is most prominent at low ratios of cofilin:actin [101]. At high concentrations, cofilin stabilizes the filament by forming cofilin patches. Severing only occurs at the border between decorated and undecorated regions, and the cut is preferentially located at the pointed end [99]. In vivo, the function of cofilin is regulated by many mechanisms. Phosphorylation of S3 of cofilin is known to inhibit actin binding and thus to abolish severing [102]. In addition, the protein can only efficiently sever filaments above pH 7 [103]. Changes on the structure of the actin filament have been proposed as the basis for this pH dependence [104]. However, recent single filament studies have suggested that changes in pH modify the rate at which cofilin decorates F-actin, and therefore how many patch borders between decorated and undecorated regions are present in the filament [105].
Structurally, severing is most likely due to a cofilin-induced change in the symmetry of the filament, where the twist decreases from ∼-166.7 to -162° [106] (Fig. 4C). With the help of a ∼9-Å-resolution structure, Galkin and colleagues demonstrated that this is due to a structural change in actin, where the protomers adopt a conformation similar to G-actin [107]. Recently, Tanaka et al. have significantly improved this model with a near-atomic resolution structure of the complex [19]. There, they determined that actin adopts a new conformation, which they named C-actin. The authors further proposed that severing is the result of a mismatch between the symmetries of decorated and undecorated regions. Indeed, the Sindelar lab has used cryo-EM to show that, the transition between cofilin-bound and free regions produces an abrupt change of helical symmetry [108].
How does cofilin sense the nucleotide state of the filament? The N-terminus of cofilin is deeply inserted into the intra-strand interface of the filament [19] where it displaces the d-loop, which is disordered in Tanaka’s structure (Fig. 4C). The N-terminus is known to be important for the function of cofilin; removal of the first 5 amino acids [109], or phosphorylation of S3 abolishes severing [102]. Based on our nucleotide state structures of F-actin, we proposed that the changes in the intra-strand interface associated with nucleotide hydrolysis modify the interaction of the N-terminus of cofilin with actin [30]. Instead, Tanaka and colleagues proposed that the presence of the γ-phosphate or Pi at the active site of actin is not compatible with the C-actin conformation, thus inhibiting the binding of cofilin [19]. Determining whether one or both of these explanations are correct requires further investigation.
Besides cofilin, some formins like INF2 (see below) and members of the gelsolin family can also sever filaments. Besides severing, members of the gelsolin family can additionally cap the barbed ends. Contrary to cofilin, these are large proteins, with up to 6 repeats of gelsolin domains (reviewed in [110]). Calcium is required for their function; it activates the proteins and mediates their interaction with F-actin. Only some of the gelsolin domains are involved in the direct capping and severing of the filament, while others are involved in binding to the side of the filament. The structure of gelsolin domains bound to F-actin remains to be solved.
3.4. Actin nucleation through branch formation
To allow for the fast polymerization rates observed in vivo, the nucleation of filaments cannot rely on the rare spontaneous nucleation of actin monomers. Cells bypass this inefficient step by using specific actin nucleators. Each of them has a specific biological function: some will be used to create linear filaments, others to drive the dendritic polymerization needed for cell motility and endocytosis. In some cases they could even be hijacked to drive parasitic motion (reviewed in [111]).
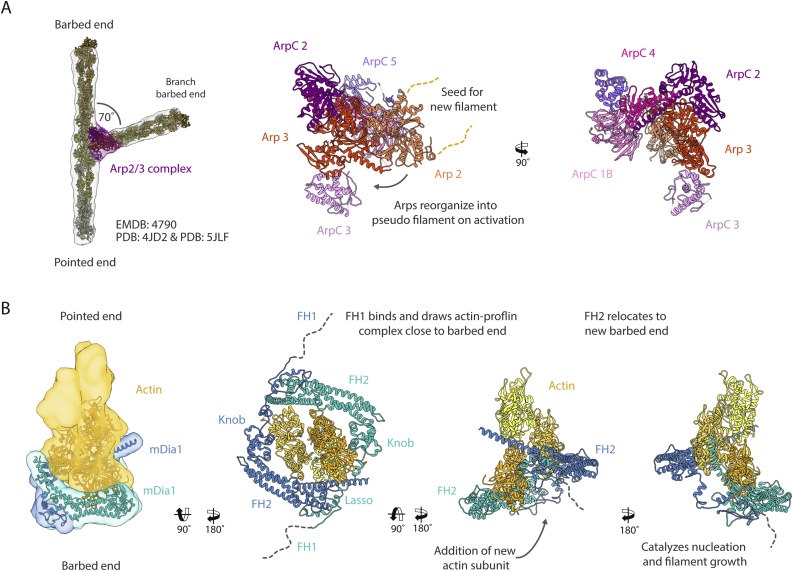
The leading edge of crawling cells is crowded with actin filaments. There, the continuous polymerization of actin against the membrane and its recycling at the other end provides the force for cellular motion (reviewed in [112]). The speed of motion and the mechanical strength generated by the cytoskeleton are both a function of the architecture of the network [88,113]. In order to deliver enough force for motility, the cell creates a densely populated network of branched actin filaments. The branches require an actin nucleator called Arp2/3 complex. The Arp2/3 complex is a 224 kDa multi-subunit oligomer formed by seven proteins (Fig. 5A). At its core, it contains two members of the ARP family – Arp2 and Arp3 – which give the complex its name [114]. The main function of the complex is to nucleate a new filament (daughter) on the side of a pre-existing (mother) one [115] (Fig. 5A). The new filament will grow from there at an angle of ∼70° [116]. Once the branch is formed the polymerization of the nascent filament can be driven by actin polymerases like formins or ENA/VASP.
Fig. 5.
Nucleators of F-actin. (A) The Arp2/3 complex (shades of purple, PDB: 4JD2) binds to the side of actin filaments, creating a branch point which acts as a seed for a branch filament at an angle of ∼70˚. (Left panel) Docking of atomic models of the Arp2/3 complex and F-actin (PDB: 5JLF) into an in situ sub-tomogram average structure (transparent gray, EMDB: 4790). (Middle and right panel) Illustration of domain architecture of the Arp2/3 complex. Arp2 and Arp3 (shades of red) rearrange upon binding to F-actin forming a seed for a new filament. (B) Formins like mDia1 (shades of cyan, model from [34]) bind to the barbed end of actin filaments as a dimer. Their FH2 domains enclose the filament. Their FH1 domains (indicated by dashed lines) are located towards the periphery, where they bind profilin/actin complexes. Actin is depicted in shades of yellow.
On its own, the Arp2/3 complex is rather inactive and will not promote nucleation. In order to go into a productive conformation, the Arp2/3 complex requires binding of additional activators (for a review see [117]). Full activation of the protein requires binding to the mother filament as well as to additional nucleation-promoting factors (NPFs). NPFs are modular proteins which typically contain a V (verprolin or WH2–like), C (connecting) and A (acidic) motif, which are sufficient for their NPF activity (reviewed in [118]).
While many crystal structures of the Arp2/3 complex have been solved [[119], [120], [121]] in the last 15 years, they all represent the inactive conformation. In these structures, Arp2 and Arp3 are far apart, and only part of Arp2 is visible in the electron density, suggesting that the protein is very flexible under these conditions. The lack of crystallographic studies on activated Arp2/3 suggests that the active complex is recalcitrant to crystallization. Thus, in order to understand the structural mechanism of Arp2/3 activation, several groups have approached the problem using single particle electron microscopy. Using reconstructions of the Arp2/3 complex associated with the NPFs cortactin, WAVE, or N-WASp, Xu and colleagues showed that the complex closes upon the binding of the NPFs, bringing Arp2 and Arp3 into an actin dimer-like configuration [122]. Based on these data, they proposed a model where the NPF binds at the pointed end of Arp2 and Arp3. However, recent cross-linking mass spectrometry data disproved this model [123]. Models derived from tomographic reconstructions of reconstituted Arp2/3 branches revealed that all seven subunits of the complex contact the mother filaments. Moreover, the full activation of the complex brings Arp2 and Arp3 into a filament-like conformation that presents a free pseudo-barbed end from which a new filament can polymerize [124]. Being closely related to actin, Arp2 and Arp3 can bind nucleotides at their active site. Moreover, their position as initial seed for polymerization would suggest that their nucleotide state is important for filament stability, just as it is in the actin case. Arp2 and Arp3 seem to be able to hydrolyze ATP, but the hydrolysis of the nucleotide bound to Arp2 appears to be the most important (see [118] and references therein). For instance, hydrolysis in Arp2 triggers debranching [125] and branches created by a hydrolysis deficient Arp2 mutant stay attached longer to the mother filament [126]. Moreover, impairing hydrolysis in either of them negatively affects endocytosis and actin remodeling [126].
Why is high-resolution data on Arp2/3 branches so scarce? The unique characteristics of the Arp2/3 branches make the study of their structure at high resolutions only possible using electron microscopy (EM), although obtaining a high-resolution reconstruction with this sample is still challenging. While it is possible to recreate a dendritic network of filaments on an EM grid, the filaments lay flat on the surface, presenting the same view of the complex. For this reason, most EM studies of Arp2/3 branches deal with the complex on a 2D level [[127], [128], [129]]. In principle, a tomographic reconstruction followed by sub-tomogram averaging of these samples is possible, as it has been done by Rouiller and colleagues [124]. However, since all branches lay flat on the grid, the sub-volumes will share the same missing information and the final reconstruction will therefore contain artefacts. In a recent paper, Jasnin and colleagues used focused-ion-beam milling and electron cryotomography to determine the in-situ structure of the Arp2/3 branches found in the traveling actin waves of D. discoideum [130]. Since daughter filaments were directed either toward or parallel to the basement membrane, they were able to obtain subvolumes of the branch junction in a broad range of orientations, which permitted to recover the missing information due to the missing wedge. Their ∼30-Å-resolution structure agrees with previous reconstructions of the complex, showing the known angle of ∼70°, approximately 8° less than in reconstituted samples [124]. Whether or not this is a systematic difference will require further study.
3.5. Formins, nucleators and polymerases
Another group of important actin nucleators are formins. Contrary to the Arp2/3 complex, they nucleate and polymerize linear actin filaments [131,132]. They are multi-domain proteins that associate tightly with the barbed end of F-actin. The defining features of the family are two conserved domains called formin homology domain 1 and 2 (FH1 and FH2) (Fig. 5B), with the FH2 domain being responsible for the binding to F-actin. They form a tight dimer in a head-to-tail fashion [133], which then surrounds the actin filament [134] (Fig. 5B). Kinetic experiments have shown that in the absence of profilin, binding of the FH1/FH2 domains to the barbed end significantly diminishes the polymerization rate of actin [135]. Interestingly, the protein will stay attached to the growing barbed end, making it a processive or leaky cap. From kinetic data, it has been proposed that the protein populates two states: a closed one that does not allow polymerization and an open one that is polymerization competent [79]. Some formins like mDia1 will spend most of their time in the open state, while others like cdc12 are mostly closed.
The FH1 domain is composed of polyproline track repeats that vary in number and length depending on the specific formin. This is key for the biological function of these proteins. The presence of this polyproline repeats allows the FH1 domain to recruit profilin actin complexes effectively increasing the association rate of profilin/actin to the barbed end [79]. This transforms formins into actin polymerases. Interestingly, recent experiments from the Carlier and Bieling groups have shown that, even in the presence of formin, the rate of actin polymerization reaches a plateau at high profilin/actin concentrations [34,81,82], the limit velocity is just shifted to higher rates (see section 3.1). However, the limiting rates observed by the two groups differ considerably, possibly due to the usage of different actin sources. The Bieling group further demonstrated that just as without formin, the dissociation rate of profilin determines the maximal velocity of polymerization [34] (see section 3.1). Interestingly, the level of the increase in polymerization rate depends on the identity of the formin – particularly its FH2 domain [34] – suggesting that FH1 and FH2 domains control together the polymerization from profilin/actin monomers, as proposed by others as well [81,136].
In an effort to understand how formins control actin polymerization, Otomo and colleagues used X-ray crystallography to determine the structure of the FH2 domain of the yeast formin Bni1p with tetramethylrhodamine-labeled actin (TMR actin) [134]. Although TMR is often used to inhibit actin polymerization, the crystal structure contained actin organized in a helical arrangement. Bni1p decorated the filament, but instead of forming homodimers it formed a helical polymer through domain swapping. The interaction involves two main regions. The knob region of formin targets a crevice formed by SD1 and SD3 of actin which places it at the intra-strand interface. The post region of formin interacts with the inter-strand interface of the filament (Fig. 5B). The helical symmetry of the actin filament observed in the Bni1p structure is different from canonical actin with a rise of 28 Å and a twist of 180°. A recent structure of the FH2 domain of FMNL3 and TMR-actin confirmed most of the actin FH2 domain interactions [137]. However, the structure does not show the helical arrangement, suggesting that this is either a property of Bn1p or a crystallization artefact. Interestingly, using negative stain EM, Gurel and colleagues have recently shown that the FH2 domain of INF2 can decorate the side of an actin filament with canonical symmetry [138]. However, this is an unusual formin that can sever filaments and bind to their sides as part of its function [138]. Moreover, the filament had to be stabilized with phalloidin and BeFx to avoid severing. Thus, it is still not clear whether the FH2 domain of formins can modify the symmetry of the filament under normal conditions.
The structural mechanism by which formins control actin polymerization is still not fully understood. While a structure of an FH2 domain bound to an unmodified barbed end is probably necessary to fully understand the structural basis of formin function, two main models have been proposed (for a review see [139]). In the first, named stair-stepping, both FH2 domains of the formin dimer are bound to the last two subunits of the filament. In this conformation the post of one FH2 domain is free to interact with an incoming actin (open state). Once an actin monomer binds, both FH2 domains have their actin-binding site occupied (closed state). The trailing FH2 domain then steps forward and the cycle repeats. In the second model, named stepping second, the FH2 domains always have their actin-binding sites occupied, and formins only step forward once a new actin is bound. The main difference is that in this model the closed state is characterized by a higher twist in the filament, which is incompatible with subunit addition. Using atomic modeling and molecular dynamics simulations, the Voth group has tested this idea [140,141]. While they have observed that the symmetry of the barbed end tends towards twists > 167° they have never observed two distinct conformations, which are required by the stepping second model.
Besides the FH1 and FH2 domains, formins have other regulatory regions which they use to further control their function. For instance, diaphanous formins are characterized by the presence of a diaphanous inhibitory domain (DID) and diaphanous autoregulatory domain (DAD). These two domains interact with each other, inhibiting actin binding. Release of inhibition is mediated by binding to effectors, for instance Rho GTPases [142]. To understand the structural basis of the autoinhibition of the diaphanous formin mDia1, Nezami and colleagues solved the structure of two fragments of the protein that account for most of the molecule [143]. Independently, Otomo and colleagues tackled the problem using a similar strategy [144]. The structures suggested that inhibition stems from the occlusion of the actin-binding sites at the FH2 domain. Unfortunately, their fragments crystallized in a tetramer-like configuration, and the exact connections between the fragments could not be determined. Using negative stain EM, the Goode group determined a low-resolution structure of full length mDia1 [145]. They confirmed one of the possible configurations from the crystal structures, and demonstrated that inhibition is indeed a product of steric hindrance.
4. Actin as a scaffold for force generation
Besides the polymerization-driven push, the motor protein myosin can use the energy from ATP hydrolysis to pull on the actin cytoskeleton. Already in the mid 50 s, actomyosin based force generation – the sliding filament theory – was proposed as the underlying mechanism of sarcomere contractility [146,147]. According to this theory, interleaved myosin and actin filaments slide past each other to contract the cell. Decades later it was finally understood that this is due to the cyclic attachment of myosin to F-actin, pulling and generating force in the process (see [148] for a comment on how the theory developed). Although the phenomenon was first discovered in muscle cells, we now know that myosin homologs are also present in non-muscle cells. Non-muscle myosins are involved in diverse processes including, cargo transport, cellular motility, wound repair, and opisthokont (fungi and animal) cell division (for reviews see [[149], [150], [151]]).
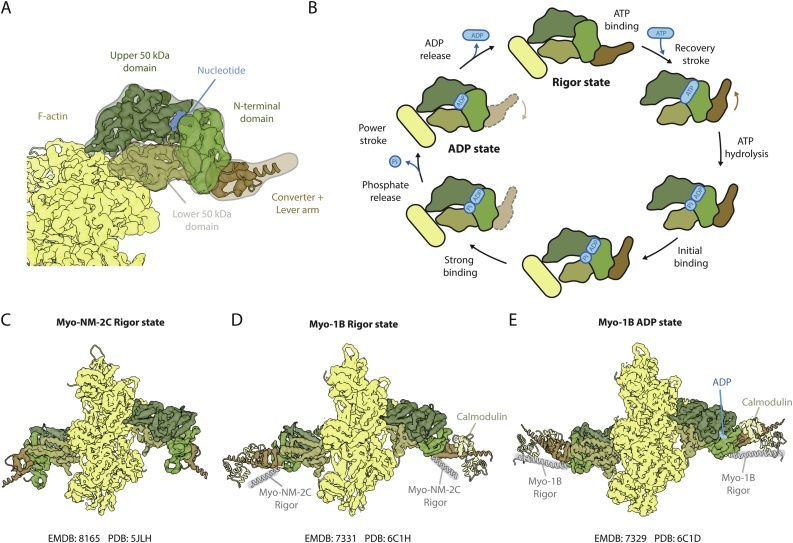
Myosins are composed of heavy and light chains, with the heavy chain making up most of the protein. The heavy chain contains an elongated tail which can be used to dimerize and bind the light chains. In addition, it contains the motor domain which binds to F-actin. The actin-binding site is formed by two regions of the motor domain, known as the upper (U50) and the lower (L50) 50 kDa subdomains, which are separated by a cleft. The motor domain ends with the N-terminal domain and the lever arm (Fig. 6A). The nucleotide binds to a pocket far away from the actin-binding surface, formed in the region between the U50, the N-terminal domain, and a β-sheet known as transducer (Fig. 6A). Although ATP hydrolysis elicits only small structural changes at the active center, they are amplified by the protein, resulting in large changes of the position of the lever arm. It is through this mechanism that myosin couples nucleotide hydrolysis to large motion.
Fig. 6.
Architecture and working cycle of myosin. (A) The motor domain of myosin consists of four major subdomains. The upper (dark green) and lower 50 kDa (khaki) subdomains make up the actin-binding interface and form a central cleft that can open and close. The N-terminal domain (olive green) sits at the hinge region and transmits the nucleotide state in concert with the converter domain (brown) along the lever arm of myosin (brown). (B) Simplified working cycle of myosin illustrating the dependence of the lever arm position and interactions with F-actin on the bound nucleotide (light blue). The lever arm position in the ADP-Pi state relative to the ADP state is not known and thus shown transparently. Cryo-EM structures of (C) myosin NM-2C (EMDB: 8165 PDB: 5JLH) and (D) myosin 1B in the rigor state (EMDB: 7331 PDB: 6C1H). The rigor states of different myosin isoforms do not superimpose (lever arm helix of myosin NM-2C in rigor state shown in transparent gray for comparison). (E) Cryo-EM structure of myosin 1B in complex with ADP (EMDB: 7329 PDB: 6C1D). The lever arm in the ADP state does not superimpose with the one in rigor state (transparent gray) suggesting that the power stroke has not yet completed, but requires ADP release.
In spite of the diverse biological functions of the actomyosin complex, decades of biochemical and structural studies have provided a unified picture of its force generation cycle (Fig. 6B) (reviewed in [152]). In the absence of a nucleotide (rigor state), the motor domain will tightly bind F-actin with its nucleotide-binding pocket closed. Upon ATP binding, the U50 and L50 subdomains open, the affinity for F-actin drops, and myosin detaches. In solution the lever arm of ATP-bound myosin changes to the pre-power stroke position. After nucleotide hydrolysis, ADP-Pi-bound myosin binds F-actin with low affinity. Release of Pi triggers the repositioning of the lever arm – the power stroke – leaving ADP myosin strongly bound to actin. The release of ADP then closes the cycle.
While electron microscopy has been extensively used to study the structure of the actomyosin complex, it was only in 2016 that we could observe, for the first time, the structure of a rigor complex at near atomic resolution [15]. The conformation of myosin agrees well with crystal structures of myosin in the rigor-like state [153,154]. This demonstrates that, in the absence of nucleotide, actin does not significantly affect the structure of myosin. Arguably, one of the most important states in the cycle is ADP-myosin bound to F-actin. The kinetics of ADP release and the affinity of ADP-bound myosin to actin determine whether myosin will be a non-processive motor and stay strongly attached, or a processive motor detaching fast from F-actin (for a review see [155]). The release of ADP can be further regulated by the load on the lever arm (e.g [[156], [157], [158]] and [155] for a review on the subject). This is one of the reasons why processive motors like myosin V can stay attached to F-actin for long distances. In the last years, several groups have used cryo-EM to study the ADP and rigor states of different myosins. Interestingly, the rigor states do not superimpose each other (Fig. 6D). While all ADP states show a lever arm conformation similar to their corresponding rigor, they are all deviated by a few degrees [18,159,160]. This suggests that the power stroke is not complete until nucleotide release. Interestingly, the Sindelar group could identify two distinct conformations in their ADP state [18]. Due to the high resolution of their reconstructions (3.3 and 3.7 Å), it was possible to see that ADP was indeed present in both of them. While one of their ADP states is very similar to rigor, the other shows a different position of the lever arm (Fig. 6E). Moreover, the lever arm position was correlated with the opening of the nucleotide-binding pocket, offering a structural explanation of how the load on the lever arm can affect the ADP release rate. Combined together, those structures are the first direct observation of the different conformations of myosin in its force generation cycle. Solving the structure of the ADP-Pi state is necessary to complete the structural model of the cycle. This will be significantly more challenging, as this corresponds to a low affinity state.
In muscle and non-muscle cells, myosin binding can be modulated by the ABP tropomyosin. In striated muscle, the binding of myosin is further regulated by the troponin complex [161], which controls the position of tropomyosin on the filament in a Ca2+-dependent manner. The structural basis for this process is still missing, and will likely only be resolved using cryo-EM. Until recently, the state-of-the-art structural model for tropomyosin regulation – derived primarily from negative stain microscopy – could be summarized as follows. In the absence of Ca2+, tropomyosin blocks the myosin-binding site (blocked state). Binding of Ca2+ to troponin triggers the azimuthal movement of tropomyosin which exposes the myosin binding sites (closed state), while the binding of myosin moves tropomyosin even further (myosin state). Recently, using our cryo-EM structure of the tropomyosin complex with α-actin we demonstrated that the closed state observed in negative stain is most likely an artefact due to the low pH of uranyl formate or uranyl acetate [14]. This observation has been later corroborated by the Galkin group using purified cardiac F-actin decorated with troponin and tropomyosin (the thin filament) [162]. Interestingly, while the canonical blocked state was absent from their data, they showed that there is a new blocked state (c-blocked state). However, compared to the canonical blocked state this one leaves a large fraction of the myosin-binding site open. Moreover, they showed that tropomyosin occupies three states on cardiac thin filaments, whose relative abundance can be regulated by Ca2+. Notably, their most open state still covers a small fraction of the myosin binding site, suggesting that upon myosin binding a fourth state would be populated. Further studies are necessary to test if this is a universal phenomenon or a special feature of cardiac muscle.
5. Interactions between ABPs fine-tune the actin cytoskeleton
Many ABPs can interact with each other as well as with actin to regulate the cytoskeleton. For instance, cortactin – a nucleation promoting factor – activates the Arp2/3 complex and also binds to the side of the filaments to strengthen the formed branch [163]. In muscle cells, tropomodulin will bind the pointed end and tropomyosin, ensuring the proper alignment of the tropomyosin molecules, which are bound to the side of the filaments [164].
Due to its central role in the process of actin polymerization, the barbed end is a hub for the interaction of many proteins. The binding site for many ABPs is quite similar, with many of them binding to the hydrophobic crevice formed between SD1 and SD3, known as the target-binding or hydrophobic cleft [165]. Due to the overlap of the binding sites of ABPs, their binding has been traditionally understood as competitive. However, new single filament observations have demonstrated that they can interact at the barbed end, modulating the kinetics of each other. For instance, the half-life of capping protein dissociation from actin is ∼30 min [89], which is too slow for the kinetics observed for the whole actin network. The Carlier lab set out to test whether the interaction with formin at the barbed end could be responsible for the dissociation of the protein. Indeed, they demonstrated that a ternary complex including actin, capping protein, and mDia1 exists [90]. A model of the possible complex shows only clashes between the tentacles of capping protein and a helix in the knob region of the FH2 domain. Although in this configuration formin and capping protein would bind with reduced affinity, they are still in the nanomolar range [90]. Most importantly, their binding kinetics to the barbed end are significantly enhanced, which allows fast cytoskeletal remodeling. The same group has also demonstrated that gelsolin and formin can simultaneously bind to the barbed end [81].
Structural models have suggested that a decrease in profilin affinity for the barbed end of F-actin might be due to small clashes between the profilin bound to the penultimate actin and the last actin monomer of the filament [76]. Recently, using single filaments assays, the Bieling group has demonstrated that the binding of profilin to the barbed end puts an upper limit to the elongation kinetics of the actin filament where the limiting step is profilin dissociation [34]. Moreover, their data suggests that a ternary complex between actin, profilin and formin exists, placing a new upper limit on the polymerization kinetics [34].
6. Outlook
In the last few years, we have seen tremendous advances in our understanding of cytoskeletal complexes and F-actin-binding proteins. The technical advancement in cryo-EM is largely responsible for this growth. New experiments that investigate proteins that are able to sense the nucleotide state of F-actin are likely to reveal how ABPs read the nucleotide clock and whether this happens through a unified mechanism. Together with structural studies of F-actin, continuous new insight from fluorescence microscopy has also expanded our knowledge of how ABPs control actin polymerization and interact with each other [34,81,90]. The combinatorial control of the function of ABPs opens a new line of investigation that we are only beginning to explore. The experiments we have highlighted in this review have shown that the modulation of the dynamic actin cytoskeleton is incredibly complex. Therefore, its full understanding will require extensive computation modeling, as has been proposed by others before [166]. In addition, we believe that significant emphasis should be put on understanding the structure of filament ends and how they control actin dynamics.
Beyond F-actin, the advancements in cryo-EM will be a gateway to studying the structure of many new ABP structures. Current X-ray structures of many of them – like Arp2/3 and formins – do not show their functionally relevant states. These are only likely to be accessible using cryo-EM. As the hardware limitations that restrict the resolution of these reconstructions are gone, we expect to see a plethora of new ABP structures in the near future.
Acknowledgements
We thank Peter Bieling for his insightful comments on the biochemistry of the actin cytoskeleton. Our work is supported by the Max Planck Society and the European Council under the European Union’s Seventh Framework Programme (FP7/ 2007–2013) (grant 615984 to Stefan Raunser). Sabrina Pospich is supported as a fellow of Studienstiftung des deutschen Volkes.
References
- 1.Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunning P.W., Ghoshdastider U., Whitaker S., Popp D., Robinson R.C. The evolution of compositionally and functionally distinct actin filaments. J. Cell. Sci. 2015;128:2009–2019. doi: 10.1242/jcs.165563. [DOI] [PubMed] [Google Scholar]
- 3.Izoré T., Kureisaite-Ciziene D., McLaughlin S.H., Löwe J. Crenactin forms actin-like double helical filaments regulated by arcadin-2. Elife. 2016;5 doi: 10.7554/eLife.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogilner A., Oster G. Cell motility driven by actin polymerization. Biophys. J. 1996;71:3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdes K., Howard M., Szardenings F. Pushing and pulling in prokaryotic DNA segregation. Cell. 2010;141:927–942. doi: 10.1016/j.cell.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Pollard T.D. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Clainche C., Carlier M.-F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 8.Heintzelman M.B. Gliding motility in apicomplexan parasites. Semin. Cell Dev. Biol. 2015;46:135–142. doi: 10.1016/j.semcdb.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlbrandt W. The resolution revolution. Science. 2014;343:1443–1444. doi: 10.1126/science.1251652. [DOI] [PubMed] [Google Scholar]
- 10.Pospich S., Raunser S. Single particle cryo-EM — an optimal tool to study cytoskeletal proteins. Curr. Opin. Struct. Biol. 2018;52:16–24. doi: 10.1016/j.sbi.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Holmes K.C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 12.Asakura S. The interaction between G-actin and ATP. Arch. Biochem. Biophys. 1961;92:140–149. doi: 10.1016/0003-9861(61)90228-4. [DOI] [PubMed] [Google Scholar]
- 13.Oda T., Iwasa M., Aihara T., Maéda Y., Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457:441–445. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- 14.von der Ecken J., Müller M., Lehman W., Manstein D.J., Penczek P.A., Raunser S. Structure of the F-actin-tropomyosin complex. Nature. 2015;519:114–117. doi: 10.1038/nature14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von der Ecken J., Heissler S.M., Pathan-Chhatbar S., Manstein D.J., Raunser S. cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution. Nature. 2016;534:724–728. doi: 10.1038/nature18295. [DOI] [PubMed] [Google Scholar]
- 16.Pospich S., Kumpula E.-P., von der Ecken J., Vahokoski J., Kursula I., Raunser S. Near-atomic structure of jasplakinolide-stabilized malaria parasite F-actin reveals the structural basis of filament instability. Proc. Natl. Acad. Sci. U.S.A. 2017;114:10636–10641. doi: 10.1073/pnas.1707506114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galkin V.E., Orlova A., Vos M.R., Schröder G.F., Egelman E.H. Near-atomic resolution for one state of F-actin. Structure. 2015;23:173–182. doi: 10.1016/j.str.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentes A., Huehn A., Liu X., Zwolak A., Dominguez R., Shuman H., Ostap E.M., Sindelar C.V. High-resolution cryo-EM structures of actin-bound myosin states reveal the mechanism of myosin force sensing. Proc. Natl. Acad. Sci. U.S.A. 2018;115:1292–1297. doi: 10.1073/pnas.1718316115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka K., Takeda S., Mitsuoka K., Oda T., Kimura-Sakiyama C., Maéda Y., Narita A. Structural basis for cofilin binding and actin filament disassembly. Nat. Commun. 2018;9:1860. doi: 10.1038/s41467-018-04290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwamoto D.V., Huehn A., Simon B., Huet-Calderwood C., Baldassarre M., Sindelar C.V., Calderwood D.A. Structural basis of the filamin A actin-binding domain interaction with F-actin. Nat. Struct. Mol. Biol. 2018;25:918–927. doi: 10.1038/s41594-018-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rould M.A., Wan Q., Joel P.B., Lowey S., Trybus K.M. Crystal structures of expressed non-polymerizable monomeric actin in the ADP and ATP states. J. Biol. Chem. 2006;281:31909–31919. doi: 10.1074/jbc.M601973200. [DOI] [PubMed] [Google Scholar]
- 22.Blanchoin L., Pollard T.D. Hydrolysis of ATP by polymerized actin depends on the bound divalent cation but not profilin. Biochemistry. 2002;41:597–602. doi: 10.1021/bi011214b. [DOI] [PubMed] [Google Scholar]
- 23.Carlier M.F., Pantaloni D. Direct evidence for ADP-inorganic phosphate-F-actin as the major intermediate in ATP-actin polymerization. Rate of dissociation of inorganic phosphate from actin filaments. Biochemistry. 1986;25:7789–7792. doi: 10.1021/bi00372a001. [DOI] [PubMed] [Google Scholar]
- 24.Carlier M.F., Pantaloni D. Binding of phosphate to F-ADP-actin and role of F-ADP-P(i)-actin in ATP-actin polymerization. J. Biol. Chem. 1988;263:817–825. [PubMed] [Google Scholar]
- 25.Kumpula E.-P., Lopez A.J., Tajedin L., Han H., Kursula I. Atomic view into Plasmodium actin polymerization, ATP hydrolysis, and fragmentation. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vorobiev S., Strokopytov B., Drubin D.G., Frieden C., Ono S., Condeelis J., Rubenstein P.A., Almo S.C. The structure of nonvertebrate actin: implications for the ATP hydrolytic mechanism. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5760–5765. doi: 10.1073/pnas.0832273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasa M., Maeda K., Narita A., Maéda Y., Oda T. Dual roles of gln 137 of actin revealed by recombinant human cardiac muscle α-Actin mutants. J. Biol. Chem. 2008;283:21045–21053. doi: 10.1074/jbc.M800570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akola J., Jones R.O. Density functional calculations of ATP systems. 2. ATP hydrolysis at the active site of actin. J. Phys. Chem. B. 2006;110:8121–8129. doi: 10.1021/jp054921d. [DOI] [PubMed] [Google Scholar]
- 29.Freedman H., Laino T., Curioni A. Reaction dynamics of atp hydrolysis in actin determined by ab initio molecular dynamics simulations. J. Chem. Theory Comput. 2012;8:3373–3383. doi: 10.1021/ct3003282. [DOI] [PubMed] [Google Scholar]
- 30.Merino F., Pospich S., Funk J., Wagner T., Küllmer F., Arndt H.-D., Bieling P., Raunser S. Structural transitions of F-actin upon ATP hydrolysis at near-atomic resolution revealed by cryo-EM. Nat. Struct. Mol. Biol. 2018;25:528–537. doi: 10.1038/s41594-018-0074-0. [DOI] [PubMed] [Google Scholar]
- 31.Chou S.Z., Pollard T.D. Mechanism of actin polymerization revealed by cryo-EM structures of actin filaments with three different bound nucleotides. Proc. Natl. Acad. Sci. U.S.A. 2019;116:4265–4274. doi: 10.1073/pnas.1807028115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belmont L.D., Patterson G.M., Drubin D.G. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J. Cell. Sci. 1999;112:1325–1336. doi: 10.1242/jcs.112.9.1325. [DOI] [PubMed] [Google Scholar]
- 33.Wertman K.F., Drubin D.G., Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funk J., Merino F., Venkova L., Heydenreich L., Kierfeld J., Vargas P., Raunser S., Piel M., Bieling P. Profilin and formin constitute a pacemaker system for robust actin filament growth. Elife. 2019;8 doi: 10.7554/eLife.50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren Z., Zhang Y., Zhang Y., He Y., Du P., Wang Z., Sun F., Ren H. cryo-EM structure of actin filaments from Zea mays pollen. Plant Cell. 2019 doi: 10.1105/tpc.18.00973. tpc.00973.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isambert H., Venier P., Maggs A.C., Fattoum A., Kassab R., Pantaloni D., Carlier M.F. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J. Biol. Chem. 1995;270:11437–11444. doi: 10.1074/jbc.270.19.11437. [DOI] [PubMed] [Google Scholar]
- 37.Combeau C., Carlier M.F. Probing the mechanism of ATP hydrolysis on F-actin using vanadate and the structural analogs of phosphate BeF-3 and A1F-4. J. Biol. Chem. 1988;263:17429–17436. [PubMed] [Google Scholar]
- 38.Oztug Durer Z.A., Kudryashov D.S., Sawaya M.R., Altenbach C., Hubbell W., Reisler E. Structural states and dynamics of the D-Loop in actin. Biophys. J. 2012;103:930–939. doi: 10.1016/j.bpj.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oztug Durer Z.A., Diraviyam K., Sept D., Kudryashov D.S., Reisler E. F-Actin Structure Destabilization and DNase I Binding Loop Fluctuations. J. Mol. Biol. 2010;395:544–557. doi: 10.1016/j.jmb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fievez S., Carlier M.F. Conformational changes in subdomain-2 of G-actin upon polymerization into F-actin and upon binding myosin subfragment-1. FEBS Lett. 1993;316:186–190. doi: 10.1016/0014-5793(93)81212-I. [DOI] [PubMed] [Google Scholar]
- 41.Cai L., Marshall T.W., Uetrecht A.C., Schafer D.A., Bear J.E. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128:915–929. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge P., Durer Z.A.O., Kudryashov D., Zhou Z.H., Reisler E. cryo-EM reveals different coronin binding modes for ADP- and ADP-BeFx actin filaments. Nat. Struct. Mol. Biol. 2014;21:1075–1081. doi: 10.1038/nsmb.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhlrad A., Pavlov D., Peyser Y.M., Reisler E. Inorganic phosphate regulates the binding of cofilin to actin filaments. FEBS J. 2006;273:1488–1496. doi: 10.1111/j.1742-4658.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- 44.Yount R.G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an Adenosine triphosphate analog containing a P-N-P linkage. Biochemistry. 1971;10:2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- 45.Cooke R., Murdoch L. Interaction of actin with analogs of adenosine triphosphate. Biochemistry. 1973;12:3927–3932. doi: 10.1021/bi00744a022. [DOI] [PubMed] [Google Scholar]
- 46.Milroy L.-G., Rizzo S., Calderon A., Ellinger B., Erdmann S., Mondry J., Verveer P., Bastiaens P., Waldmann H., Dehmelt L., Arndt H.-D. Selective chemical imaging of static actin in live cells. J. Am. Chem. Soc. 2012;134:8480–8486. doi: 10.1021/ja211708z. [DOI] [PubMed] [Google Scholar]
- 47.Bubb M.R., Spector I., Beyer B.B., Fosen K.M. Effects of jasplakinolide on the kinetics of actin polymerization an explanation for certain in vivo observations. J. Biol. Chem. 2000;275:5163–5710. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 48.Vig A., Ohmacht R., Jámbor É., Bugyi B., Nyitrai M., Hild G. The effect of toxins on inorganic phosphate release during actin polymerization. Eur. Biophys. J. 2011;40:619–626. doi: 10.1007/s00249-010-0659-y. [DOI] [PubMed] [Google Scholar]
- 49.Pospich S., Merino F., Raunser S. Structural effects and functional implications of phalloidin and jasplakinolide binding to actin filaments. BioRxiv. 2019 doi: 10.1101/794495. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann D., Santos A., Kovar D.R., Rock R.S. Actin age orchestrates Myosin-5 and Myosin-6 run lengths. Curr. Biol. 2015;25:2057–2062. doi: 10.1016/j.cub.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells A.L., Lin A.W., Chen L.Q., Safer D., Cain S.M., Hasson T., Carragher B.O., Milligan R.A., Sweeney H.L. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- 52.Egelman E.H., Francis N., DeRosier D.J. F-actin is a helix with a random variable twist. Nature. 1982;298:131–135. doi: 10.1038/298131a0. [DOI] [PubMed] [Google Scholar]
- 53.Galkin V.E., Orlova A., Egelman E.H. Actin filaments as tension sensors. Curr. Biol. 2012;22:R96–R101. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egelman E.H. The iterative helical real space reconstruction method: surmounting the problems posed by real polymers. J. Struct. Biol. 2007;157:83–94. doi: 10.1016/j.jsb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Wetzel D.M., Håkansson S., Hu K., Roos D., Sibley L.D. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol. Biol. Cell. 2003;14:396–406. doi: 10.1091/mbc.e02-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frénal K., Dubremetz J.F., Lebrun M., Soldati-Favre D. Gliding motility powers invasion and egress in Apicomplexa. Nat. Rev. Microbiol. 2017;15:645–660. doi: 10.1038/nrmicro.2017.86. [DOI] [PubMed] [Google Scholar]
- 57.Oda T., Aihara T., Wakabayashi K. Early nucleation events in the polymerization of actin, probed by time-resolved small-angle x-ray scattering. Sci. Rep. 2016;6:34539. doi: 10.1038/srep34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouayrenc J.-F., Travers F. The first step in the polymerisation of actin. Eur. J. Biochem. 1981;116:73–77. doi: 10.1111/j.1432-1033.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- 59.Pollard T.D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 1986;103:2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang H., Bradley M.J., McCullough B.R., Pierre A., Grintsevich E.E., Reisler E., De La Cruz E.M. Identification of cation-binding sites on actin that drive polymerization and modulate bending stiffness. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16923–16927. doi: 10.1073/pnas.1211078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skillman K.M., Ma C.I., Fremont D.H., Diraviyam K., a Cooper J., Sept D., Sibley L.D. The unusual dynamics of parasite actin result from isodesmic polymerization. Nat. Commun. 2013;4:2285. doi: 10.1038/ncomms3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumpula E.P., Pires I., Lasiwa D., Piirainen H., Bergmann U., Vahokoski J., Kursula I. Apicomplexan actin polymerization depends on nucleation. Sci. Rep. 2017;7:12137. doi: 10.1038/s41598-017-11330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujiwara I., Vavylonis D., Pollard T.D. Polymerization kinetics of ADP- and ADP-Pi-actin determined by fluorescence microscopy. Proc Natl Acad Sci U S A. 2007;104:8827–8832. doi: 10.1073/pnas.0702510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narita A., Oda T., Maéda Y. Structural basis for the slow dynamics of the actin filament pointed end. EMBO J. 2011;30:1230–1237. doi: 10.1038/emboj.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wegner A. Head to tail polymerization of actin. J. Mol. Biol. 1976;108:139–150. doi: 10.1016/S0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- 66.Fujiwara I., Takahashi S., Tadakuma H., Funatsu T., Ishiwata S. Microscopic analysis of polymerization dynamics with individual actin filaments. Nat. Cell Biol. 2002;4:666–673. doi: 10.1038/ncb841. [DOI] [PubMed] [Google Scholar]
- 67.Kovar D.R., Pollard T.D. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc. Natl. Acad. Sci. 2004;101:14725–14730. doi: 10.1073/pnas.0405902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koestler S.A., Rottner K., Lai F., Block J., Vinzenz M., Small J.V. F- and G-actin concentrations in lamellipodia of moving cells. PLoS One. 2009;4:e4810. doi: 10.1371/journal.pone.0004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollard T.D., Blanchoin L., Mullins R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2002;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 70.Bray D., Thomas C. Unpolymerized actin in fibroblasts and brain. J. Mol. Biol. 1976;105:527–544. doi: 10.1016/0022-2836(76)90233-3. [DOI] [PubMed] [Google Scholar]
- 71.Kinosian H.J., Selden L.A., Gershman L.C., Estes J.E. Actin filament barbed end elongation with nonmuscle MgATP-actin and MgADP-actin in the presence of profilin. Biochemistry. 2002;41:6734–6743. doi: 10.1021/bi016083t. [DOI] [PubMed] [Google Scholar]
- 72.Pantaloni D., Carlier M.F. How profilin promotes actin filament assembly in the presence of thymosin β4. Cell. 1993;75:1007–1014. doi: 10.1016/0092-8674(93)90544-Z. [DOI] [PubMed] [Google Scholar]
- 73.Vinson V.K., De La Cruz E.M., Higgs H.N., Pollard T.D. Interactions of Acanthamoeba profilin with actin and nucleotides bound to actin. Biochemistry. 1998;37:10871–10880. doi: 10.1021/bi980093l. [DOI] [PubMed] [Google Scholar]
- 74.Porta J.C., Borgstahl G.E.O. Structural basis for profilin-mediated actin nucleotide exchange. J. Mol. Biol. 2012;418:103–116. doi: 10.1016/j.jmb.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chik J.K., Lindberg U., Schutt C.E. The structure of an open state of β-actin at 2.65 Å resolution. J. Mol. Biol. 1996;263:607–623. doi: 10.1006/jmbi.1996.0602. [DOI] [PubMed] [Google Scholar]
- 76.Courtemanche N., Pollard T.D. Interaction of profilin with the barbed end of actin filaments. Biochemistry. 2013;52:6456–6466. doi: 10.1021/bi400682n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferron F., Rebowski G., Lee S.H., Dominguez R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007;26:4597–4606. doi: 10.1038/sj.emboj.7601874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahoney N.M., Janmey P.A., Almo S.C. Structure of the profilin-poly-L-proline complex involved in morphogenesis and cytoskeletal regulation. Nat. Struct. Biol. 1997;4:953–960. doi: 10.1038/nsb1197-953. [DOI] [PubMed] [Google Scholar]
- 79.Vavylonis D., Kovar D.R., O’Shaughnessy B., Pollard T.D. Model of formin-associated actin filament elongation. Mol. Cell. 2006;21:455–466. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romero S., Didry D., Larquet E., Boisset N., Pantaloni D., Carlier M.-F. How ATP hydrolysis controls filament assembly from profilin-actin. J. Biol. Chem. 2007;282:8435–8445. doi: 10.1074/jbc.m609886200. [DOI] [PubMed] [Google Scholar]
- 81.Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D., Carlier M.F. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 82.Jégou A., Niedermayer T., Orbán J., Didry D., Lipowsky R., Carlier M.F., Romet-Lemonne G. Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the properties of profilin. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crockford D., Turjman N., Allan C., Angel J. Ann. N. Y. Acad. Sci. 2010. Thymosin β4: structure, function, and biological properties supporting current and future clinical applications; pp. 179–189. [DOI] [PubMed] [Google Scholar]
- 84.Xue B., Leyrat C., Grimes J.M., Robinson R.C. Structural basis of thymosin- 4/profilin exchange leading to actin filament polymerization. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E4596–E4605. doi: 10.1073/pnas.1412271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irobi E., Aguda A.H., Larsson M., Guerin C., Yin H.L., Burtnick L.D., Blanchoin L., Robinson R.C. Structural basis of actin sequestration by thymosin-β4: implications for WH2 proteins. EMBO J. 2004;23:3599–3608. doi: 10.1038/sj.emboj.7600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlier M.F., Jean C., Rieger K.J., Lenfant M., Pantaloni D. Modulation of the interaction between G-actin and thymosin beta 4 by the ATP/ADP ratio: possible implication in the regulation of actin dynamics. Proc. Natl. Acad. Sci. U.S.A. 2006;90:5034–5038. doi: 10.1073/pnas.90.11.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casella J.F., Craig S.W., Maack D.J., Brown A.E. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J. Cell Biol. 1987;105:371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 89.Wear M.A., Yamashita A., Kim K., Maéda Y., Cooper J.A. How capping protein binds the barbed end of the actin filament. Curr. Biol. 2003;13:1531–1537. doi: 10.1016/S0960-9822(03)00559-1. [DOI] [PubMed] [Google Scholar]
- 90.Shekhar S., Kerleau M., Kühn S., Pernier J., Romet-Lemonne G., Jégou A., Carlier M.-F. Formin and capping protein together embrace the actin filament in a ménage à trois. Nat. Commun. 2015;6:8730. doi: 10.1038/ncomms9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narita A., Takeda S., Yamashita A., Maéda Y. Structural basis of actin filament capping at the barbed-end: a cryo-electron microscopy study. EMBO J. 2006;25:5626–5633. doi: 10.1038/sj.emboj.7601395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamashita A., Maeda K., Maéda Y. Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J. 2003;22:1529–1538. doi: 10.1093/emboj/cdg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schroer T.A. Dynactin. Annu. Rev. Cell Dev. Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 94.Machesky L.M., May R.C. Arps: actin-related proteins. In: dos Remedios C.G., Thomas D.D., editors. Molecular Interactions of Actin. Results and Problems in Cell Differentiation. Springer; Berlin, Heidelberg: 2001. pp. 213–229. [DOI] [PubMed] [Google Scholar]
- 95.Urnavicius L., Zhang K., Diamant A.G., Motz C., Schlager M.A., Yu M., Patel N.A., Robinson C.V., Carter A.P. The structure of the dynactin complex and its interaction with dynein. Science. 2015;347:1441–1446. doi: 10.1126/science.aaa4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carlier M.F., Laurent V., Santolini J., Melki R., Didry D., Xia G.X., Hong Y., Chua N.H., Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maciver S.K., Weeds A.G. Actophorin preferentially binds monomeric ADP-Actin over ATP-bound actin: consequences for cell locomotion. FEBS Lett. 1994;347:251–256. doi: 10.1016/0014-5793(94)00552-4. [DOI] [PubMed] [Google Scholar]
- 98.Suarez C., Roland J., Boujemaa-Paterski R., Kang H., McCullough B.R., Reymann A.-C.C., Guérin C., Martiel J.-L.L., De La Cruz E.M., Blanchoin L. Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr. Biol. 2011;21:862–868. doi: 10.1016/j.cub.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wioland H., Guichard B., Senju Y., Myram S., Lappalainen P., Jégou A., Romet-Lemonne G. ADF/Cofilin Accelerates Actin Dynamics by Severing Filaments and Promoting Their Depolymerization at Both Ends. Curr. Biol. 2017;27:1956–1967. doi: 10.1016/j.cub.2017.05.048. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shekhar S., Carlier M.-F. Enhanced depolymerization of actin filaments by ADF/Cofilin and monomer funneling by capping protein cooperate to accelerate barbed-end growth. Curr. Biol. 2017;27:1990–1998. doi: 10.1016/j.cub.2017.05.036. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pavlov D., Muhlrad A., Cooper J., Wear M., Reisler E. Actin filament severing by cofilin. J. Mol. Biol. 2007;365:1350–1358. doi: 10.1016/j.jmb.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moriyama K., Iida K., Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- 103.Hawkins M., Pope B., Maciver S.K., Weeds A.G. Human actin depolymerizing factor mediates a pH-sensitive destruction of actin filaments. Biochemistry. 1993;32:9985–9993. doi: 10.1021/bi00089a014. [DOI] [PubMed] [Google Scholar]
- 104.Blondin L., Sapountzi V., Maciver S.K., Lagarrigue E., Benyamin Y., Roustan C. A structural basis for the pH-dependence of cofilin F-actin interactions. Eur. J. Biochem. 2002;269:4194–4201. doi: 10.1046/j.1432-1033.2002.03101.x. [DOI] [PubMed] [Google Scholar]
- 105.Wioland H., Jegou A., Romet-Lemonne G. Quantitative variations with pH of actin depolymerizing Factor/Cofilin’s multiple actions on actin filaments. Biochemistry. 2019;58:40–47. doi: 10.1021/acs.biochem.8b01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGough A., Pope B., Chiu W., Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galkin V.E., Orlova A., Kudryashov D.S., Solodukhin A., Reisler E., Schroeder G.F., Egelman E.H. Remodeling of actin filaments by ADF / cofilin proteins. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20568–20572. doi: 10.1073/pnas.1110109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huehn A., Cao W., Elam W.A., Liu X., De La Cruz E.M., Sindelar C.V. The actin filament twist changes abruptly at boundaries between bare and cofilin-decorated segments. J. Biol. Chem. 2018;293 doi: 10.1074/jbc.AC118.001843. jbc.AC118.001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moriyama K., Yahara I. The actin-severing activity of cofilin is exerted by the interplay of three distinct sites on cofilin and essential for cell viability. Biochem. J. 2002;365:147–155. doi: 10.1042/BJ20020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nag S., Larsson M., Robinson R.C., Burtnick L.D. Gelsolin: The tail of a molecular gymnast. Cytoskeleton. 2013;70:360–384. doi: 10.1002/cm.21117. [DOI] [PubMed] [Google Scholar]
- 111.Lambrechts A., Gevaert K., Cossart P., Vandekerckhove J., Van Troys M. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 2008;18:220–227. doi: 10.1016/j.tcb.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 113.Ofer N., Mogilner A., Keren K. Actin disassembly clock determines shape and speed of lamellipodial fragments. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20394–20399. doi: 10.1073/pnas.1105333108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Machesky L.M., Atkinson S.J., Ampe C., Vandekerckhove J., Pollard T.D. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J. Cell Biol. 1994;127:107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amann K.J., Pollard T.D. The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat. Cell Biol. 2001;3:306–310. doi: 10.1038/35060104. [DOI] [PubMed] [Google Scholar]
- 116.Mullins R.D., Heuser J.A., Pollard T.D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Welch M.D., Mullins R.D. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- 118.Pollard T.D. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 119.Nolen B.J., Pollard T.D. Insights into the influence of nucleotides on actin family proteins from seven structures of Arp2/3 complex. Mol. Cell. 2007;26:449–457. doi: 10.1016/j.molcel.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nolen B.J., Littlefield R.S., Pollard T.D. Crystal structures of actin-related protein 2/3 complex with bound ATP or ADP. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15627–15632. doi: 10.1073/pnas.0407149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robinson R.C., Turbedsky K., Kaiser D.A., Marchand J.B., Higgs H.N., Choe S., Pollard T.D. Crystal structure of Arp2/3 complex. Science. 2001;294:1679–1684. doi: 10.1126/science.1066333. [DOI] [PubMed] [Google Scholar]
- 122.Xu X.P., Rouiller I., Slaughter B.D., Egile C., Kim E., Unruh J.R., Fan X., Pollard T.D., Li R., Hanein D., Volkmann N. Three-dimensional reconstructions of Arp2/3 complex with bound nucleation promoting factors. EMBO J. 2012;31:236–247. doi: 10.1038/emboj.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luan Q., Zelter A., MacCoss M.J., Davis T.N., Nolen B.J. Identification of Wiskott-Aldrich syndrome protein (WASP) binding sites on the branched actin filament nucleator Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E1409–E1418. doi: 10.1073/pnas.1716622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rouiller I., Xu X.P., Amann K.J., Egile C., Nickell S., Nicastro D., Li R., Pollard T.D., Volkmann N., Hanein D. The structural basis of actin filament branching by the Arp2/3 complex. J. Cell Biol. 2008;180:887–895. doi: 10.1083/jcb.200709092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Clainche C., Pantaloni D., Carlier M.F. ATP hydrolysis on actin-related protein 2/3 complex causes debranching of dendritic actin arrays. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6337–6342. doi: 10.1073/pnas.1130513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin A.C., Welch M.D., Drubin D.G. Arp2/3 ATP hydrolysis-catalysed branch dissociation is critical for endocytic force generation. Nat. Cell Biol. 2006;8:826–833. doi: 10.1038/ncb1443. [DOI] [PubMed] [Google Scholar]
- 127.Egile C., Rouiller I., Xu X.P., Volkmann N., Li R., Hanein D. Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions. PLoS Biol. 2005;3 doi: 10.1371/journal.pbio.0030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Espinoza-Sanchez S., Metskas L.A., Chou S.Z., Rhoades E., Pollard T.D. Conformational changes in Arp2/3 complex induced by ATP, WASp-VCA, and actin filaments. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E8642–E8651. doi: 10.1073/pnas.1717594115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Volkmann N., Amann K.J., Stoilova-McPhie S., Egile C., Winter D.C., Hazelwood L., Heuser J.E., Li R., Pollard T.D., Hanein D. Structure of arp2/3 complex in its activated state and in actin filament branch junctions. Science. 2001;293:2456–2459. doi: 10.1126/science.1063025. [DOI] [PubMed] [Google Scholar]
- 130.Jasnin M., Beck F., Ecke M., Fukuda Y., Martinez-Sanchez A., Baumeister W., Gerisch G. The architecture of traveling actin waves revealed by cryo-electron tomography. Structure. 2019;27:1–13. doi: 10.1016/j.str.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 131.Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 132.Sagot I., Rodal A.A., Moseley J., Goode B.L., Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 133.Xu Y., Moseley J.B., Sagot I., Poy F., Pellman D., Goode B.L., Eck M.J. Crystal structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/S0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- 134.Otomo T., Tomchick D.R., Otomo C., Panchal S.C., Machius M., Rosen M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433:488–494. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- 135.Kovar D.R., Harris E.S., Mahaffy R., Higgs H.N., Pollard T.D. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 136.Neidt E.M., Scott B.J., Kovar D.R. Formin differentially utilizes profilin isoforms to rapidly assemble actin filaments. J. Biol. Chem. 2009;284:673–684. doi: 10.1074/jbc.M804201200. [DOI] [PubMed] [Google Scholar]
- 137.Thompson M.E., Heimsath E.G., Gauvin T.J., Higgs H.N., Kull F.J. FMNL3 FH2–actin structure gives insight into formin-mediated actin nucleation and elongation. Nat. Struct. Mol. Biol. 2013;20:111–118. doi: 10.1038/nsmb.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gurel P.S., Ge P., Grintsevich E.E., Shu R., Blanchoin L., Zhou Z.H., Reisler E., Higgs H.N. INF2-mediated severing through actin filament encirclement and disruption. Curr. Biol. 2014;24:156–164. doi: 10.1016/j.cub.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Courtemanche N. Mechanisms of formin-mediated actin assembly and dynamics. Biophys. Rev. 2018;10:1553–1569. doi: 10.1007/s12551-018-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aydin F., Courtemanche N., Pollard T.D., Voth G.A. Gating mechanisms during actin filament elongation by formins. Elife. 2018;7 doi: 10.7554/eLife.37342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baker J.L., Courtemanche N., Parton D.L., McCullagh M., Pollard T.D., Voth G.A. Electrostatic interactions between the Bni1p formin FH2 domain and actin influence actin filament nucleation. Structure. 2015;23:68–79. doi: 10.1016/j.str.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goode B.L., Eck M.J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 143.Nezami A., Poy F., Toms A., Zheng W., Eck M.J. Crystal structure of a complex between amino and carboxy terminal fragments of mDia1: insights into autoinhibition of diaphanous-related formins. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Otomo T., Tomchick D.R., Otomo C., Machius M., Rosen M.K. Crystal structure of the formin mDia1 in autoinhibited conformation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maiti S., Michelot A., Gould C., Blanchoin L., Sokolova O., Goode B.L. Structure and activity of full-length formin mDia1. Cytoskeleton. 2012;69:393–405. doi: 10.1002/cm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huxley H., Hanson J. Changes in the Cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- 147.Huxley A.F., Niedergerke R. Structural changes in muscle during contraction: interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- 148.Andersen O.S. 50-year anniversary of sliding filament. J. Gen. Physiol. 2004;123 doi: 10.1085/jgp.200409079. [DOI] [Google Scholar]
- 149.Vicente-Manzanares M., Ma X., Adelstein R.S., Horwitz A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schwayer C., Sikora M., Slováková J., Kardos R., Heisenberg C.-P. Actin rings of power. Dev. Cell. 2016;37:493–506. doi: 10.1016/j.devcel.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 151.Titus M.A. Myosin-driven intracellular transport. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Houdusse A., Sweeney H.L. How myosin generates force on actin filaments. Trends Biochem Sci. 2016;41:989–997. doi: 10.1016/j.tibs.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Münnich S., Pathan-Chhatbar S., Manstein D.J. Crystal structure of the rigor-like human non-muscle myosin-2 motor domain. FEBS Lett. 2014;588:4754–4760. doi: 10.1016/j.febslet.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 154.Coureux P.-D., Wells A.L., Ménétrey J., Yengo C.M., Morris C.A., Sweeney H.L., Houdusse A. A structural state of the myosin V motor without bound nucleotide. Nature. 2003;425:419–423. doi: 10.1038/nature01927. [DOI] [PubMed] [Google Scholar]
- 155.Walklate J., Ujfalusi Z., Geeves M.A. Myosin isoforms and the mechanochemical cross-bridge cycle. J. Exp. Biol. 2016;219:168–174. doi: 10.1242/jeb.124594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosenfeld S.S., Sweeney H.L. A model of myosin V processivity. J. Biol. Chem. 2004;279:40100–40111. doi: 10.1074/jbc.M402583200. [DOI] [PubMed] [Google Scholar]
- 157.Greenberg M.J., Shuman H., Ostap E.M. Inherent force-dependent properties of β-cardiac myosin contribute to the force-velocity relationship of cardiac muscle. Biophys. J. 2014;107:L41–L44. doi: 10.1016/j.bpj.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Laakso J.M., Lewis J.H., Shuman H., Ostap E.M. Myosin I can act as a molecular force sensor. Science. 2008;321:133–136. doi: 10.1126/science.1159419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gurel P.S., Kim L.Y., Ruijgrok P.V., Omabegho T., Bryant Z., Alushin G.M. cryo-EM structures reveal specialization at the myosin VI-actin interface and a mechanism of force sensitivity. Elife. 2017;6 doi: 10.7554/elife.31125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wulf S.F., Ropars V., Fujita-Becker S., Oster M., Hofhaus G., Trabuco L.G., Pylypenko O., Sweeney H.L., Houdusse A.M., Schröder R.R. Force-producing ADP state of myosin bound to actin. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E1844–E1852. doi: 10.1073/pnas.1516598113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vibert P., Craig R., Lehman W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 162.Risi C., Eisner J., Belknap B., Heeley D.H., White H.D., Schröder G.F., Galkin V.E. Ca2+-induced movement of tropomyosin on native cardiac thin filaments revealed by cryoelectron microscopy. Proc. Natl. Acad. Sci. U.S.A. 2017;114:6782–6787. doi: 10.1073/pnas.1700868114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weaver A.M., Karginov A.V., Kinley A.W., Weed S.A., Li Y., Parsons J.T., Cooper J.A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001;11:370–374. doi: 10.1016/S0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 164.Rao J.N., Madasu Y., Dominguez R. Mechanism of actin filament pointed-end capping by tropomodulin. Science. 2014;345:463–467. doi: 10.1126/science.1256159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dominguez R. Actin-binding proteins – a unifying hypothesis. Trends Biochem. Sci. 2004;29:572–578. doi: 10.1016/j.tibs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 166.Pollard T.D., Cooper J.A. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]