Abstract
The regulation of cell growth, cell proliferation and cell death is at the basis of the homeostasis of tissues. While they can be regulated by intrinsic and genetic factors, their response to external signals emanating from the local environment is also essential for tissue homeostasis. Tumour initiation and progression is based on the misregulation of growth, proliferation and death mostly through the accumulation of genetic mutations. Yet, there is an increasing body of evidences showing that tumour microenvironment also has a strong impact on cancer initiation and progression. This includes the mechanical constrains and the compressive forces generated by the resistance of the surrounding tissue/matrix to tumour expansion. Recently, mechanical stress has been proposed to promote competitive interactions between cells through a process called mechanical cell competition. Cell population with a high proliferative rate can compact and eliminate the neighbouring cells which are more sensitive to compaction. While this emerging concept has been recently validated in vivo, the relevance of this process during tumour progression has never been discussed extensively. In this review, I will first describe the phenomenology of mechanical cell competition focusing on the main parameters and the pathways regulating cell elimination. I will then discuss the relevance of mechanical cell competition in tumour initiation and expansion while emphasizing its potential opposing contributions to tumourogenesis.
Keywords: Cell competition, Mechanics, Apoptosis, Mechanotransduction, Cancer, Epithelium, Residual stress
1. Introduction
The tight regulation of growth, cell division and cell death is absolutely required to maintain organ shape and functions. The high cell turnover of some organs (e.g., gut cell can be totally renewed within five days [1]) and their regenerative capacity is based on the coordination between cell proliferation, cell growth and cell death. While cell intrinsic pathways that regulate those parameters are very well known, the mechanisms at the basis of their coordination are still poorly understood. This coordination has been mostly studied in the framework of direct cell-cell communication (paracrine signal, contact dependent communication) and long range communication (diffusive molecules, cytonemes). More recently, the contribution of cell shape and local tissue mechanics to this coordination has gained a lot of interest. This is in agreement with the impact of cell mechanics on several central regulators of cell growth and survival [[2], [3], [4]].
The initiation and expansion of tumours is associated with abnormal regulation of cell growth and cell survival. This missregulation is mostly driven by cell intrinsic factors and accumulation of mutations in the genes regulating those processes [5]. Yet, it is nowadays clear that the tumour microenvironment and non-cell autonomous factors also have a strong impact on tumour initiation and progression [6]. This role is particularly relevant in light of the recent reports showing that the probability to observe spontaneous tumorigenic mutations in adult tissues is much higher than the prevalence of cancer [7,8]. Mechanical forces experienced by tumour cells and neighbouring cells is part of those microenvironment effects and were shown to affect every step of tumour progression, from tumour initiation and growth to metastasis formation [9,10].
Cell competition is a process that triggers preferential elimination of one cell population by another through apoptosis [[11], [12], [13]]. This concept was initially discovered more than fourty years ago by Pedro Ripoll and Gines Morata while studying the behavior of mosaic tissues in Drosophila [14]. By inducing Minute mutants (dominant mutations affecting the production of Ribosomes [15]), they showed that Minute/+ heterozygous clones are eliminated from growing tissues when surrounded by wild type (WT) cells. This observation was intriguing since Minute/+ mutant flies showed virtually no defects apart from a slight developmental delay. Later on, other genetic modifications (e.g.: higher levels of the proto-oncogene Myc) were shown to generate “aggressive” clones that could eliminate and fully replace the neighbouring WT cells without visible tissue defects [16,17]. This “supercompetition” was proposed to promote the early expansion of pretumoural cells and tumour initiation in a process akin to field cancerization [18]. The number of tissue and mutations triggering competitive interactions has been constantly increasing in the past few years [11]. It is nowadays also clear that this process is also conserved in mammals [19]. Yet, the mechanisms that trigger preferential elimination of one cell population are still actively debated. Three non-exclusive mechanisms have been proposed to participate to cell elimination: competition for limiting extracellular survival factors, competition driven by contact-dependent death induction and more recently competition triggered by mechanical stress [[20], [21], [22]]. The later came into light recently when it was shown that cell compaction and differential sensitivity to mechanical stress could promote competitive interactions between cells [23,24] and could either eliminate preferentially pretumoural cells [24] or promote the expansion of pretumoural clones [23,25,26]. This so called mechanical cell competition is now emerging as a new category of cell competition which may be relevant in several physiological and pathological contexts [12,[22], [23], [24]].
In this review, I will describe the concept of mechanical cell competition by introducing the key parameters regulating such competitive interactions, and describe recent advances in the identification of the pathways regulating compaction-driven death. I will then describe rapidly the relationship between mechanical cell competition and other mechanisms of cell competition. I will then discuss the relevance of mechanical cell competition in tumour initiation and progression by documenting the effect of the mechanical environment associated with tumour growth, and the impact of residual stress (see Box 1) on tumour progression and intratumoural competition.
Box 1. Definition of the mechanical terms.
Stress: Internal forces exerted by the components of a material on each other. It is a force per unit of area and has the same dimension as pressure (unit: Pascal).
Solid stress: Residual stress that accumulates in the solid components of a tissue following differential growth. This stress is independent of fluid pressure. Residual stress is the stress that remains in a solid material despite the removal of the initial causes of the stress.
Strain: Dimensionless parameter that measures the geometric deformation of a material relative to its initial shape.
Pressure: Force applied per unit of area to the surface of an object orthogonal to its boundaries (unit: Pascal)
Tension: Reaction force applied by a stretched object, opposite of compression (unit: Newton)
Surface tension: Describes the tendency of a fluid surface to minimise its surface area. The amount of energy required to deform this surface is proportional to the surface tension (dimension: energy per unit of area or force per unit of length).
Line tension: Tension at the interface between two fluids or solids. Commonly used to describe tension between two cells (dimension: energy per unit of length). Line tension in an epithelium will be increased by acto-myosin contractility, and reduced by cell-cell adhesion.
Stiffness: Describes how much an object deform in response to a force. Measured by the Young modulus (force per unit of area, in Pascal).
Alt-text: Box 1
2. Cell competition triggered by mechanical stress: from the initial hypothesis to the experimental validation
2.1. Initial hypothesis and theoretical grounds
In a seminal paper, Boris Shraiman proposed more than ten years ago that mechanical stress generated by differential growth could be at the basis of competitive interactions between cells [27]. In many tissues, cell-cell contacts remain tight and the relative displacement of cells is very low. As such, tissues behave like solids which have little capacity to dissipate mechanical stress. In this environment, a relative increase of cell proliferation/growth in a subpopulation should push on the neighbouring cells, which will conversely resist to this pushing. On long time scales, differential growth will build up pressure in the tissue, particularly in the fast growing population and its local environment (Fig. 1A–C). Intuitively, one would expect that such compression will strongly impair the capacity of the fast growing population to expand. There is indeed a growing body of evidences showing that compression/negative strain (see Box 1) can significantly slow down cell proliferation and cell growth [[28], [29], [30], [31], [32], [33], [34], [35]]. How then could this mechanical stress promote the expansion of one population at the expanse of the other? Here is where Boris Shraiman introduced the concept of differential sensitivity to mechanical stress: if the neighbouring slow-growing cells die at lower pressure compared to the fast-growing population, this will be sufficient to trigger preferential elimination of the neighbouring cells. This will lead to space and pressure release and further expansion of the fast growing population (Fig. 1A,D) [27]. This model was particularly relevant for cell competition as most of the mutations associated with competition were affecting growth rate [12]. However, it initially had little impact in the field probably because several important hypotheses of this model remained to be demonstrated experimentally. First, there was at the time no clear evidence that compaction/compression could induce cell death in vivo. Secondly, it would require that different cell types have different sensitivity to mechanical stress, which remained to be demonstrated. Third, it required that stress is not dissipated through cell movements and neighbor exchanges. The later hypothesis was reasonable for many tissues, including the main tissue used to study cell competition: the wing imaginal disc of Drosophila (an epithelial sac of the larvae that will form the adult wing). The higher proliferation rate in the centre of this tissue can generate compaction of the central population and stretching of the cells at the periphery [36,37] (Fig. 1B), suggesting that the mechanical stress is not dissipated by cell movements and neighbour exchanges. Similarly, induction of growth in clones recapitulates the same pattern of deformation: compaction of the fast growing population and stretching of the neighbouring cells [31,36,37]. Yet, this hypothesis may not be valid for all the conditions associated with cell competition. Several competition scenarios (Myc, Minute) were associated with the intermingling of the two cell populations and high cell-cell movements, which should dissipate mechanical stress and prevents its accumulation [[38], [39], [40]].
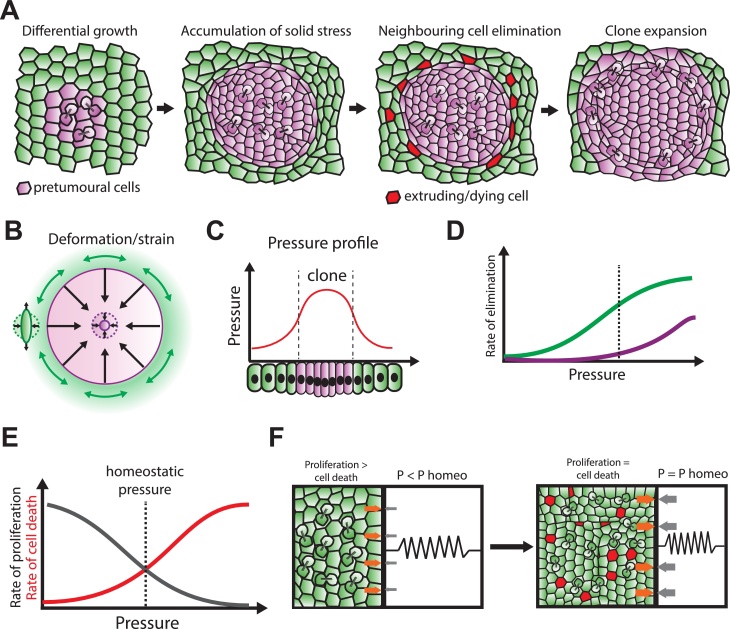
Fig. 1.
Competition for space driven by differential growth and homeostatic pressure.
A: Tissue deformation and cell elimination upon overproliferation of a subpopulation (purple, pretumoural cells) in an epithelium. Red cells are dying/extruding cells in the scenario where green cells are more sensitive to compaction. Cell elimination accelerates purple clone expansion. B: Resulting stress and local deformation (strain) of the cells. The clone (purple) is compressed while the periphery is rather stretched (green). Central cells are homogenously compressed (dotted purple circle: initial shape, plain purple line: final shape), cells at the periphery are stretched tangentially to the clone, and compacted radially (dotted green circle: initial shape, plain green line: final shape). C: Profile of pressure within the tissue (clone margins shown in dashed lines), fast growing cells in purple, slow growing cells in green. Adapted from [27]. D: Hypothetic rate of elimination for a given pressure for the green and the purple cells. The dashed line corresponds to the pressure value at the clone margin. E: Rate of proliferation (grey) and rate of cell death (red) for a given pressure. The dashed line is the cell homeostatic pressure. F: Hypothetical set-up to reveal cell homeostatic pressure (adapted from [41]). A cell population grows in a chamber with a piston. The more cells push on the boundary, the higher the resulting force is (due to the spring compression). The green population expands until pressure reaches the homeostatic pressure (P homeo) where cell proliferation/growth is compensated by cell death (red cells).
Other theoretical frameworks also proposed a role for mechanics in competitive interactions between cells. This includes the concept of homeostatic pressure introduced by M. Basan et al. [41,42], which assumes the existence of a precise pressure at which cell proliferation and growth is perfectly compensated by cell death (Fig. 1E,F). This was based on the assumption that both cell survival and cell proliferation are modulated by pressure. In this framework, cell population in a finite volume will grow until reaching a pressure corresponding to its homeostatic pressure (Fig. 1E,F). However, if one population has a higher homeostatic pressure than another, the former will always eventually eliminate the later, irrespective of the relative growth rate of the two populations in absence of mechanical constrains. While measuring tissue pressure remains a challenging task, the concept of homeostatic pressure could be analogous to the existence of different homeostatic densities between different cell types (see below and [24,43]). In principle, local tissue pressure should correlate positively with cell density and characterization of the density of a cell population at equilibrium should be a good proxy for its homeostatic pressure. The assumptions of those models are similar to the one described in Shraiman’s model, namely that different cell types respond differentially to pressure, and emphasis the role of the balance between proliferation and cell death even in homeostatic tissue.
2.2. Tissue compaction can trigger cell elimination
Those models came again into light when it was shown later that compaction of epithelial cells could trigger their elimination (a key hypothesis of those two models). Using stretchable substrates, Eisenhoffer and colleagues have shown that a 30% increase of density of MDCK cells led to a rapid increase of the rate of cell extrusion (the concerted exit of cells from the epithelial layer [44]) and cell death until the tissue reached back its steady state density [45]. Similarly, Marinari and colleagues showed that a large proportion of cell extrusion occurring in the midline region of the Drosophila pupal notum (a single layer epithelium) could be explained by the local compaction of the tissue [46], which was confirmed later through other experimental approaches [23]. Those initial works validated experimentally for the first time the potential effect of cell and tissue deformation on epithelial cell survival.
Other works also suggested previously that cell compaction could trigger cell elimination. For instance, a reduction of cell surface by changing the spacing of ECM (Extra Cellular Matrix) spots is sufficient to trigger apoptosis in single endothelial cells [35]. Several groups have also exposed single cells to high hydrostatic pressures using pistons. For instance a 10 min exposure to 0.5−0.7 MPa (1 MPa is equal to 10 times the atmospheric pressure) can trigger ganglion neuron cell death [47]. A 30 min exposure to 85–200 MPa is necessary to induce apoptosis 8 h later in lymphoblasts [48]. While those conditions are far away from the physiological pressure levels (several order of magnitudes more that the pressure observed in osteoblasts, 100–300 kPa [49]), they suggest that compression is sufficient to trigger cell death and that different cell types have differential sensitivity to compression, in agreement with the hypothesis described in the previous section. Other experimental set ups have been used to compress cells in more realistic ranges. For instance, high osmolarity has been used to reduce glioma cell volume by 30% for 8 h, which was sufficient to induce apoptosis and maintain effector caspase activation [50]. Alternatively, pressures ranging from 1 kPa to 100 kPa have been applied to 3D cell aggregates (so called spheroid) either by modulating the elastic modulus of the gel in which cells where embedded [[51], [52], [53]] or by modifying the osmolarity of the external media [30]. Interestingly, a pressure of 4 kPa was sufficient to increase apoptosis in spheroid composed of murine mammary carcinoma cells [52] while 7–13 kPa pressure did not affect the rate of apoptosis in spheroid composed of human colon adenocarcinoma cells [30,51]. Altogether, those works confirmed that 3D cell compaction can trigger cell death. Moreover, the results of those studies already suggested that different cell types may have differential sensitivity to compressive forces and compaction, in agreement with the hypothesis required for mechanical competition.
2.3. Experimental validations of mechanical cell competition
Cell competition has been mostly studied in epithelial tissues. As such, the fact that epithelial crowding could induce cell extrusion was particularly relevant [45,46]. However, those two initial studies suggested that extrusion was independent of caspase activation and that cell death was a consequence of anoikis (death triggered by loss of cell-cell and cell-ECM contacts). More specifically, crowding induced extrusion in the Drosophilla pupal notum was suggested to be driven by spontaneous junction remodeling, independently of any dedicated signaling pathways [46,54]. It was therefore difficult to understand how in this framework different cell populations within an epithelium would respond differentially to mechanical compaction. Two later studies confirmed however that mechanical stress could be at the basis of competitive interactions between cells. By studying the interactions between Scribble mutants (a regulator of the apico-basal polarity) and WT MDCK cells, Wagstaff and colleagues observed an active corralling of the mutant cells by the WT cells, which led to Scribble mutant compaction and their elimination [24] (Fig. 2A). Using micropatterns to impose strict boundaries, they observed that homogenous layers of Scribble mutant cells grew to a lower plateau/homeostatic density (see above) compared to WT cells layers, which was concomitant with a higher rate of cell death. Importantly, the high sensitivity of Scribble mutant cells to compaction correlated with higher basal levels of the cell death regulator p53, which further increased upon cell compaction [24]. Those observations suggested that p53 levels could set the homeostatic pressure of cells and that Scribble mutant elimination could be driven by the confrontation of two cell populations with different homeostatic pressures [41]. Accordingly, downregulation of the corralling and cell migration behaviour of WT cells (through E-cad downregulation in Scribble mutants) slowed down but did not abolish Scribble mutant elimination. This suggested that the competitive elimination of Scribble mutants is both driven by their low homeostatic pressure/density compared to WT cells, and their active compaction through WT cell migration.
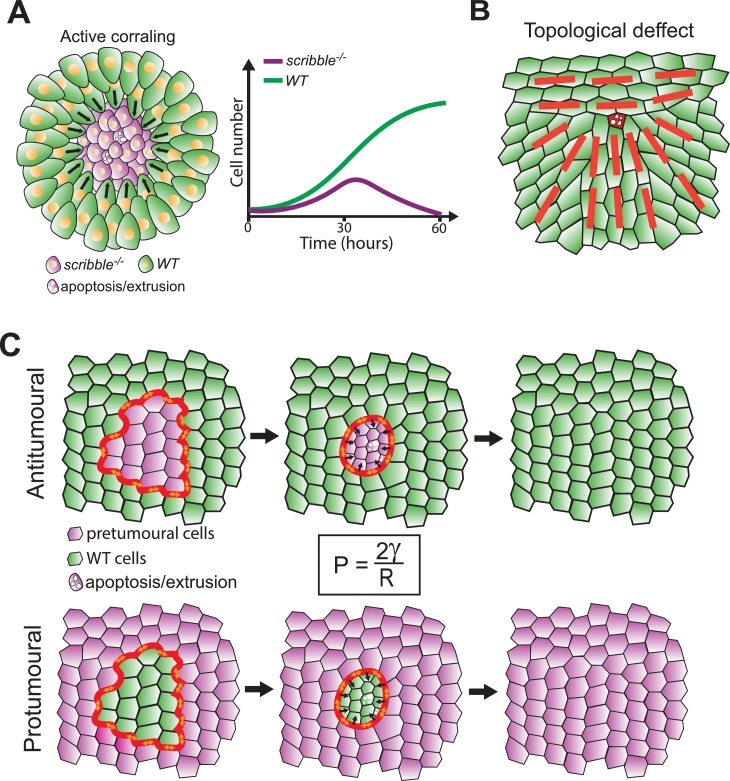
Fig. 2.
Mechanical cell competition scenarios driven by boundary conditions.
A: Left: Cell compaction driven by active corralling: the convergent collective migration of the green population (WT) toward the purple population (here scribble−/− mutant cells) induces cell compaction and cell death. Adapted from [24]. Right: Evolution of cell number of the two populations over time during competition (adapted from [24,43]). B: Spontaneous compaction and cell elimination driven by local topological defects [58]. The red lines show the main local alignment of MDCK cells (behaving like a nematic liquid crystal). The red cell is the dying cell at the tip of the so called “comet like” topological defect. C: Cell compaction and cell elimination driven by clone boundary line tension [62]. Cell misspecification (here induction of an oncogene) leads to the accumulation of acto-myosin at clone boundary (red thick line). The high line tension increases pressure within the clone (see formula) and is higher for small clone radius. The high pressure drives cell elimination until the clone disappear. The same process can either eliminate pretumoural cells if they occupy a small portion of the tissue (top) or induce WT cell elimination (bottom) if the pretumoural cells occupy the majority of the tissue.
Similarly, recent results in the Drosophila pupal notum led to a reassessment of the role of effector caspases in cell extrusion. By inhibiting caspase activation in clones and in the whole tissue, we observed a drastic reduction of the rate of cell extrusion [23]. Moreover, using different live caspase activity sensors, we observed a systematic activation of effector caspases prior to cell extrusion. This suggested that effector caspase activity was required for cell extrusion. As such, cells resistant for apoptosis should be less sensitive to crowding. Accordingly, activation of the oncogene Ras (RasV12) in large clones led clone expansion, neighbouring WT cell compaction and preferential elimination of WT cells [23]. The resistance of clonal cells to compaction is driven by the inhibition of the pro-apoptotic gene hid (Head Involution Defective) by Ras activation [26,55,56]. Importantly, preventing WT cell elimination (through the secretion of EGF by the clones, see below) was sufficient to slow down Ras clone expansion [26].
Those two studies [23,24] confirmed in two independent systems that differential sensitivity to mechanical stress could be at the basis of competitive interactions, and provided the first experimental validation of the initial theoretical studies of mechanical based cell competition [27,41]. Interestingly, those two studies already suggested that mechanical based competition could have both anti and pro-tumoural functions. While it is required for the elimination of pretumoural cells in the first case [24] (scribble mutants can trigger neoplastic growth [57]), it rather promotes pretumoural cell expansion through the elimination of the neighbouring WT cells in the second case [23,26].
3. Mechanical cell competition triggered by cell migration and boundary conditions
We focused so far on mechanical stress generated by differential growth. However other mechanisms can generate cell compaction and cell elimination independently of growth/proliferation. As described above, compaction of Scribble mutant cells is also promoted by the active migration of WT cells toward the mutant cells [24] (Fig. 2A). More recently, spontaneous MDCK cell elimination was observed in zones of convergent movements [58] (Fig. 2B). MDCK cells tend to align locally similar to nematic liquid crystals. However cell migration can generate spontaneous alignment defects (so called +1/2 nematic defects, comet like shape, Fig. 2B) which precede cell extrusion and cell death. Those defects correlated with a local increase of compressive forces (measured by traction force microscopy) and are sufficient to trigger caspase activation and cell extrusion [58]. Those two studies illustrate how collective cell migration can trigger cell compaction and cell elimination.
Alternatively, upregulation of tension at the interface of the two cell populations can also generate compressive forces (Fig. 2C). The shape of cellular interfaces in epithelia can be well approximated by the distribution of surface tension (Box 1) [59]. In this framework, the geometry of interfaces is governed by the same laws that describe the shape of foams and bubbles. This includes the Laplace law which describes the relationship between line tension (Box 1, γ) at the interface between two fluids, the difference of pressure (ΔP) and the radius of the fluid droplet (R) : . Laplace pressure will always exist at the interface, but its magnitude will vary depending on heterotypic adhesion strength (γ) and the curvature of the interface. This law for instance can explain the sorting and the formation of smooth boundaries between different cell lineages upon association [60]. Misspecification of cells in the wing imaginal disc also trigger sorting of the aberrant cells (circular clone shape and smooth clone boundaries) which can either lead to cell elimination [[61], [62], [63], [64]] or the formation of aberrant folds in the tissue (so called “cyst”) [62,63,65]. For instance, this occurs upon local impairment of the Dpp (Decapentaplegic, the orthologue of TGF-β) pathway [[63], [64], [65]] or the Wg (Wingless) pathway [64,66,67], two well-known morphogens that regulate cell fate and cell growth in the wing disc. More recently, Bielmeier and colleagues have shown that a large range of genetic mutations (activation of JAK-STAT or Ras, downregulation of polycomb proteins) in clones could generate cell sorting for large clones, cyst formation for intermediate size or cell elimination for small clones [62]. Those modifications were all associated with an enrichment of actin and the molecular motor Myosin II on the lateral surfaces of the cells at clone boundaries, leading to an increase of clone/WT cells interfacial tension. Increasing interfacial tension at clone boundaries in a 3D vertex model is sufficient to recapitulate the different observed morphologies (sorting for large clones, cyst formation for intermediate size). The role of clone size can be elegantly explained by the Laplace law (see above and Fig. 2C): for a constant line tension, pressure will be inversely proportional to the radius of the clone. Hence small clones should experience much higher pressures than larger ones. While this was not formally tested, this suggests that misspecified cells in small clones will experience high pressure, which may cause their death and their elimination (Fig. 2C). Importantly, in this framework the elimination of cells does not rely so much on their genotype and cell intrinsic properties, but rather on the proportion of tissue occupied by the mutant cells and the WT cells, which set the shape/curvature of the interface [62]. As such, it could either eliminate small patches of pretumoral cells (e.g.: JAK-STAT activation) or eliminate WT cells once the mutant occupies most of the tissue (Fig. 2C). The relative sensitivity to compaction will only set the critical pressure, and therefore the critical clone radius, at which clones start to disappear. Other mechanisms independent of mechanics (such as contact-dependent apoptosis, see below) may also participate to cell elimination and will influence the survival of clones [40,68,69]. This may be particularly relevant for large clones where mechanics should be less efficient. A similar process may be involved in the elimination of isolated pretumoural cells from MDCK cell layers (so called EDAC: Epithelial Defense Against Cancer [70]). While this process was not formally connected to cell compaction and cell death, the strong role of the mechanical properties of the active Ras/WT cell interfaces (illustrated by the role of Ephrins [71], EPLIN [72] or Filamin and Vimentin [73], all impacting the levels of MyoII) and the active compaction of RasV12 cells when surrounded by WT cells [71] could fit with such scenario. Interestingly, interfacial tension was also previously proposed to eliminate early metastatic cells upon seeding in a new tissue [41]. Like in the Drosophila wing disc, the probability of metastatic cell survival would rely on interfacial tension and the size of the cluster of metastatic cells.
In conclusion, I propose that the modulation of interfacial tension associated with cell misspecification can be at the basis of another mechanical cell competition. While the link between cell compaction, pressure and cell elimination was not formally tested in those conditions, it is very likely that the same compaction-driven death phenomenon will occur. Contrary to the first scenarios described above, this alternative mode of compaction does not rely on cell growth and could either eliminate pretumoural cells or promote their expansion depending on the proportion of tissue occupied by them.
4. Molecular pathways sensing compaction and triggering cell elimination
As reported above, the effect of cell compaction on cell survival has been observed in many tissues. As such, there have been intensive efforts deployed to identify the mechanisms involved in compaction sensing and death induction. I will give here a brief description of the pathways involved in compaction-driven death (see Table 1 for summary). An extensive discussion of the mechanisms of compaction sensing can be found elsewhere [74].
Table 1.
Experimental conditions and pathways involved in compaction sensing and cell death induction.
| Model system | Cause of compaction | Sensing mechanism | Cause of cell death | References |
|---|---|---|---|---|
| MDCK cells | Release of a stretchable substrate | Stretch sensitive channel Piezo | Anoikis | [33,45] |
| Zebrafish embryonic thin | Spontaneous crowding (proliferation) | Stretch-sensitive channel Piezo1 | Anoikis | [33,45] |
| Drosophila pupal notum | Spontaneous crowding (tissue movement) | Spontaneous junction remodeling | Anoikis | [46,54] |
| Drosophila pupal notum | Spontaneous crowding (tissue movement) and near fast growing clones (RasV12) | Downregulation of EGFR/ERK by tissue compaction | Caspase activation, cell extrusion and apoptosis | [23,26] |
| MDCK Scribble mutant and WT MDCK | Growth and collective cell movements | Upregulation of p53 by ROCK and p38 kinases upon density increase | Apoptosis | [24] |
| Spontaneous MDCK extrusion | Topological defects (driven by collective migration) | Nuclear exclusion of YAP driven by compressive forces | Caspase activation, cell extrusion and apoptosis | [58] |
| Murine mammary carcinoma cells spheroid | Growth in a confined environment | Unknown | Mitochondrial apoptotic pathway and necrosis | [52] |
| Glioma cell | Osmotic stress | Volume sensing (pathway unknown) | Caspase activation | [50] |
The first observation of compaction-driven extrusion in epithelia initially suggested that the process was independent of caspases and apoptosis [45,46]. In MDCK cells and in the zebrafish tail, crowding-induced extrusion relies on the activation of the stretch-activated channel Piezo1 [45]. Accordingly, inhibition of Piezo1 by caged morpholinos led to a cell mass accumulation in the Zebrafish embryonic tail, while inhibition of stretch-activated channels with Gadolinium strongly reduced extrusion upon MDCK cell crowding. However, crowding extrusion is likely to be regulated by other unknown factors since Ca2+ influx (which should occur downstream of Piezo activation) is not sufficient to trigger extrusion [33]. Alternatively, crowding extrusion in the Drosophila pupal notum was initially proposed to be driven by spontaneous junction remodelings [46] which could be modulated by junctional tension (Box 1) in the tissue [54].
In other systems, compaction-driven death has been associated with caspase dependent death and apoptosis. Cell volume reduction by osmotic stress triggers the activation of Caspases 3,8 and 9 and apoptosis [50]. Similarly, cell elimination induced at the center of compressed spheroid is both driven by necrosis and Bcl2 (an apoptosis inhibitor) dependent apoptosis [52]. Those results suggested that isotropic compaction can elicit the mitochondrial dependent apoptosis pathway and suggest that some sensing mechanisms must relate compression to caspase activity. The spontaneous extrusion of MDCK cells driven by topological defects can be blocked by the pan caspase inhibitor Z-VAD-FMK and is preceded by Caspase 3 activation (visualized with a live marker) [58]. Caspase activation is driven by the nuclear exclusion of YAP (Yes Associated Kinase) [58], the pro-survival transcriptional co-activator inhibited by the Hippo pathway. Hippo pathway is a central regulator of cell growth and cell survival and is one of the best documented examples of pathways modulated by cell shape and cell mechanics [3]. Hippo pathway is inhibited by cell stretching [28,75,76], while it can be activated by contact inhibition and tissue densification [77]. Similarly, upregulation of cell proliferation/growth in clones in Drosophila wing disc generates compressive forces that lead to an upregulation of Hippo pathway in the clones, which feedbacks negatively on clonal growth [31]. The same feedback may occur during normal development of the wing disc where a progressive upregulation of Hippo correlates with the progressive densification of the tissue [78]. As mentioned previously, elimination of Scribble mutant MDCK cells by WT cells is driven by their compaction and the subsequent upregulation of p53 [24]. This upregulation is driven by the stress kinase p38 and the upregulation of ROCK (an activator of Myosin II) [24]. More recently, we found that the EGFR/ERK pathway is a central regulator of cell survival in the Drosophila pupal notum through the inhibition of the pro-apoptotic gene hid [26]. Using a new live sensor of ERK, we showed that ectopic tissue stretching/compaction could upregulate/downregulate transiently ERK activity. More importantly, activation of Ras in clones led to the compaction and downregulation of ERK in the neighbouring WT cells. This eventually leads to caspase activation, WT cell extrusion and further Ras clone expansion [26]. While we still do not know the molecular basis of the compaction-driven ERK downregulation, those results are in agreement with several reports showing that ERK can be activated by cell/tissue stretching and/or an increase of tension [33,[79], [80], [81], [82]]
Altogether, these results suggest that the compaction sensing mechanisms and the routes used for cell elimination can be quite different depending on the tissue context, the amplitude and timescale of tissue deformations. Yet, they clearly show that several pro-survival and pro-apoptotic pathways can be modulated by changes in cell geometry or changes in tension.
5. Mechanical-based and biochemical-based competition: synergies and antagonisms
We focused so far on the competitive eliminations driven by mechanical stress. However, cell elimination during competition can also be driven by diffusive factors [83,84] or by contact-dependent apoptosis induction [40,68,69,85]. What is the relationship between those biochemical based eliminations and mechanical based eliminations? In principle, mechanical competition driven by differential growth should mostly act in synergy with the other mechanisms of cell elimination. Indeed, most of the genetic backgrounds associated with mechanical based competition (activation of Ras [23,26], activation of Yki [25], Scribble mutants [24,43]) have also been associated with contact dependent elimination or diffusion based elimination. For instance, Ras and Yki activation can trigger the accumulation of the proto-oncogene Myc [86,87], a well-known regulator of contact dependent competition [16,17,40,88]. Similarly, scribble mutant clones are eliminated through the contact-dependent activation of JNK pathway in Drosophila [69]. In these situations, contact and diffusive factors will promote the elimination of the same population as the one driven by mechanical stress. While the relative contribution of those phenomenons was never clearly studied, one can already predict that they will not be favoured in the same conditions. Indeed, contact-dependent cell elimination is enhanced by the high surface of contact between the two cell types [40] and the life-time of those contacts [68]. Overall, a high degree of cell mixing will favour contact-dependent mechanisms [40], while junction remodelings will prevent accumulation of mechanical stress driven by differential growth (see above). Alternatively, the high compactness of clones and the sorting behaviour associated with Ras activation [89] should reduce the contribution of contact-dependent elimination. The situations is even more complex for mechanical competition driven by boundary conditions. Here mechanical stress may act in the opposite direction compared to competitive elimination driven by biochemical cues. For instance, small activated Ras clones can be eliminated from Drosophila wing discs by Laplace pressure [62], while Ras should increase cell aggressiveness through the induction of Myc [87] and induce neighbouring cell elimination. Since the increase of line tension at clone boundaries promotes cell sorting and reduces the surface of contact between the two populations, contact-based elimination should be poorly effective in those conditions. Yet, the contribution of diffusive factors should still be significant and may alter the outcome of mechanical competition driven by boundary contrains.
In summary, the different modes of competition should coexist and may either act in synergy to eliminate one population, or may act antagonistically. The geometry of the interface between the two populations, the size of the clones as well as the mechanical properties of the surrounding tissue (e.g.: proportion of junction remodeling) will modulate the relative contribution of contact-based, diffusive-based and mechanical-based elimination. Assessing the relative contribution of each phenomenon remains very difficult at this stage as it would require the characterization of unique molecular players that can completely shut down one type of competition and not the others.
6. Solid stress in tumor and the impact of mechanical cell competition on tumour progression
Having discussed the theoretical concepts and the experimental validations of mechanical cell competition, it remains unclear whether this process could occur in solid tumours. While the existence of mechanical cell competition has not been formally tested in real tumour, I will try to assess here whether the key ingredients required for such competition are indeed present in tumours and discuss its potential effect on tumour initiation and tumour progression.
6.1. Characterisation of solid stress in tumours
Despite the high rate of proliferation of tumour cells, tumours do not grow exponentially. Many tumour growth curves are rather sigmoid and characterised by an early acceleration of volume expansion, followed by a slow-down and a plateau phase [90,91]. This dynamics can be well fit with a Gompertz growth model, named after a British mathematician who was studying the rate of mortality in the 19th century [92]. While this halt of cell proliferation has been associated with a deprivation of nutrients and oxygen [93], other studies have proposed that mechanical constrains and compression may also halt tumour expansion [51,94]. The first experimental characterization of the compressive forces generated by tumour expansion was performed in vitro using human colon adenocarcinoma cell spheroid embedded in agarose gels with known stifness (Box 1) [51]. In this set up, spheroid growth rate was anti-correlated with the agar stiffness, and the final spheroid size could be well predicted by the final mechanical stress surrounding the spheroid. Interestingly, the pressure on the agar gel was increasing up to 45–120 mmHg (6–16 kPa), which in principle should be sufficient to collapse tumour blood vessels [51] as observed in many solid tumours in vivo [10]. Later studies confirmed that compressive forces could downregulate spheroid growth [30,52,53,94]. While those in vitro studies confirmed that the stress generated by tumour growth could feedback negatively on growth, they do not demonstrate that solid stress can indeed accumulate in tumours in vivo. The existence of solid stress in vivo was confirmed by several independent methods. Partial elimination of human tumour cells using Diphteria toxin could reduce cell density and was sufficient to increase the fraction of open blood and lymphatic vessels in human-tumour xenografts [95]. This is in agreement with a build-up of pressure in tumour generated by cell proliferation. Alternatively, the analysis of human tumour relaxation upon incision has been used to measure stored elastic energy. Residual stress generated by differential growth should lead to the isotropic compression of the internal tissue and an increase of tension tangential to the tumour mass [10] (Fig. 3). Accordingly, a planar cut of excised tumours revealed a contraction of the periphery and bulging out of the central tissue, in agreement with the expected distribution of compression and tension [96,97] (Fig. 3). Assuming a constant elastic modulus in the tumour, the stored energy has been estimated using finite element modeling (e.g.: 0.4–8 kPa for murine tumors [96]), and is in good agreement with the pressure estimated in spheroids [51]. Alternative modes of measurements have confirmed later those ranges of values (tumour slicing, punch of a cylindric void in the tumour) [97,98]. Altogether, those studies confirmed that the accumulation of solid stress is a common phenomenon in solid tumours.
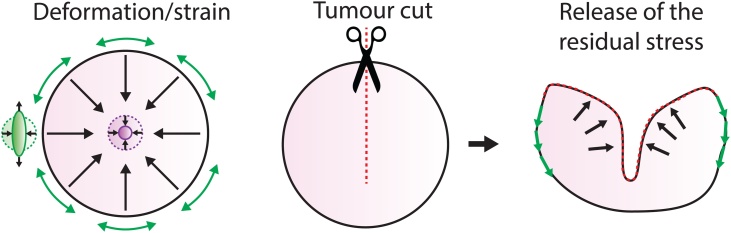
Fig. 3.
Experimental characterisation of the solid stress in tumours.
Left: Expected solid stress accumulated in a solid tumour. Cells are compressed in the center isotropically, while cells at the periphery are stretched tangentially and compacted radially. Middle: Cuting the tumour along the long axis will release the stress. Right: Stress release induces the bulging of the central part (black arrows, compressed zone) and constriction of the periphery (green arrows, stretched area). Adapted from [96]
6.2. Competition for space with the stroma and the neighbouring healthy cells
The accumulation of solid stress in tumours and in the neighbouring tissue could be compatible with a mechanically-driven elimination of the neighbouring cells. However, there is up to know very few studies reporting apoptosis in vicinity of human tumours. A recent report has depicted cleaved-caspase 3 (Cas3) positive cells in the stroma near human colon tumours, breast tumours, lymph node metastasis, lung tumours and brain tumours [99]. The accumulation of Cas3 positive cells correlated with the levels of c-Myc and the nuclear accumulation of YAP in the tumours. This suggested that the proportion of apoptotic cells near tumours may have been underestimated so far. The low sensitivity of most of the assay to detect apoptosis and the absence of data on the very early phases of tumour expansion may prevent a systematic evaluation of the rate of apoptosis near solid tumours. Very recently, neuron death was observed in vicinity of nodular brain tumours in mice [100]. Cell death could be reduced by compression release through brain surgery, or by protecting neurons through lithium treatment. Both treatments led to an improvement of motor coordination [100], suggesting that compression-driven neuronal death can cause neurological dysfunction. Interestingly, the shape of the tumour/stroma interface also correlates with different prognosis. For instance, patients with liver metastasis showing smooth boundaries with a so called “pushing” morphology (deformation of the neighbouring parenchyma) have worst prognosis compared to patients showing a desmoplastic growth (where a rim of collagen surround the tumour) [101,102]. Similarly nodular human brain tumours forming smooth boundaries have worst prognosis compared to tumours with ill-defined margins [100]. Those correlations suggest that the compaction of the neighbouring tissue may be associated with worst prognosis and could be compatible with mechanical cell competition with the neighbouring cells. Moreover, several reports already suggested that tumour cells respond differentially to mechanical stimuli compared to non-transformed cells. For instance, the rate of apoptosis increases for normal NIH3T3 on softer substrates, while H-ras transformed cells are unaffected [103]. Similarly, while normal thyroid cells adjust their elastic modulus to substrate stiffness, cancer thyroid cells do not [104]. Altogether, those studies suggested that all the ingredients of mechanical cell competition are present in human solid tumours. However, the correlation between healthy cell deformation and their elimination was not evaluated, and the contribution of stromal/non-transformed cell death to tumour expansion was never assessed.
6.3. Intratumoural competition for space
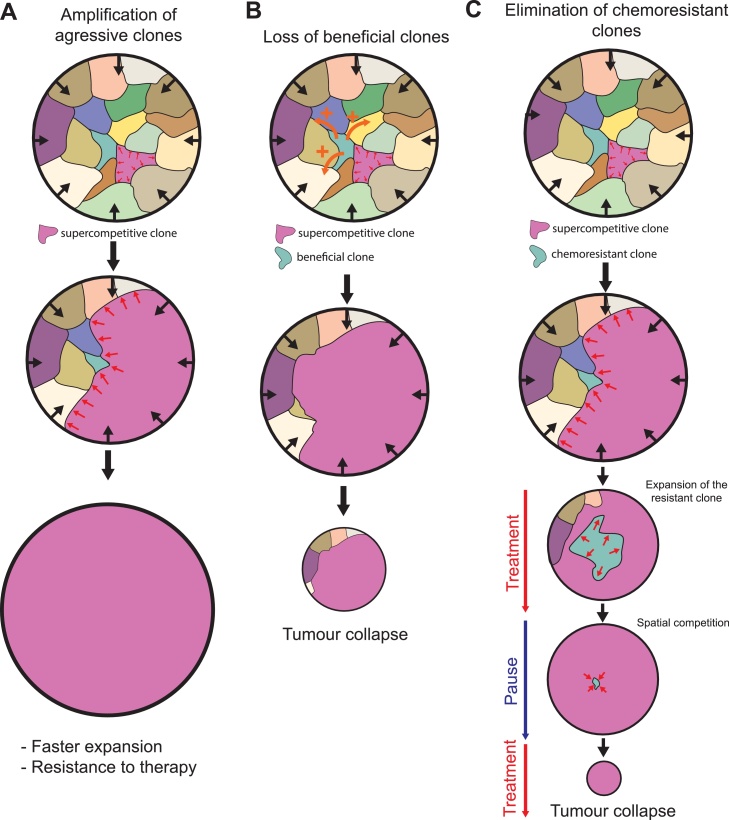
While mechanical cell competition between human tumours and the neighbouring healthy cells remains very hypothetical at this stage, there are currently more evidences for the existence of intratumoural competition (Fig. 4). The rate of apoptosis in tumour spheroid correlates positively with boundary pressure for murine mammary carcinoma cells [52].Compression-driven elimination can be downregulated by Bcl2 [52], suggesting that the sensitivity to the mitochondrial apoptotic pathway could modulate the sensitivity to compressive stress. Recent advances in single cell sequencing have revealed a high diversity of genetic backgrounds and very complex clonal dynamics within a tumour [[105], [106], [107]]. This could be compatible with differential sensitivity to mechanical stress and may be at the basis of clonal competitive interactions within a tumour. Competitive processes between tumour cells have been widely discussed and studied theoretically [[108], [109], [110]], mostly in the framework of clonal interference: namely the reduction of a clonal population growth and survival by another one [111]. Yet, only few studies have clearly characterized competitive interactions between clones [112,113], and none of them have demonstrated the role of spatial constrains and mechanical inputs in such competition. Alternatively, intratumoural competition is also reflected by the process of entosis: the engulfment of a living cell by its neighbor [114,115]. However this process relies on differences in cortical tension between neighbouring cells rather than compressive forces [114,116]. Altogether, those observations strongly suggest that competition for space could be very relevant for clonal competition within a tumour, but a better characterisation of the interactions between clones and the impact of spatial restrictions on those interactions is still missing. What would be then the consequences of such competition on tumour progression? Here again, competitive interactions may have opposing consequences depending on the structure of the tumoural cell population. On one hand, competition for space within the tumour may select for more aggressive clonal population which will overall increase the fitness and the growth of the tumour as a whole [117] (Fig. 4A). Intratumoural competition may also promote the emergence of drug resistant populations which will prevent tumour collapse upon treatment [110] (Fig. 4A). On the other hand, competition for space may eliminate suboptimal cell populations which may not be very proliferative by themselves, but may globally promote tumour growth (Fig. 4B). This was nicely illustrated in the case of breast cancer where a minor cell subpopulation can promote global tumour growth through the secretion of the Interleukin 11 [112]. Accordingly tumour growth can be significantly reduced if this population is outcompeted by faster proliferating competitors. Finally, competition for space could prevent the emergence of drug resistant populations if drug-resistant cells have a lower homeostatic pressure and are outcompeted by non-resistant cells in the absence or at low doses of treatment (Fig. 4C). This was theoretically and experimentally validated for CDK inhibitors treatment on colorectal cancer cells [118]. Here spatial competition reduces the fitness of the drug resistant population and their capacity to replace drug responsive cells during low dose treatment. Note that all those principles could equally apply for contact-dependent and diffusion based competition between chemoresponsive and chemoresistant cells.
Fig. 4.
Competition for space between tumour clones.
Visualisation of multiple tumoural clones in a constrained solid tumour (black arrow). Competition for space drives the expansion of the purple clone at the expanse of the other clones. The potential consequences on tumour survival/expansion is listed below. A: The selection for aggressive clones globally increases the rate of tumour expansion and may favour chemoresitant populations B: Spatial competition leads to the elimination of suboptimal, yet highly beneficial clones (green clone, orange arrows: secreted pro-survival factor). This can lead to tumour collapse if this population is absolutely required for tumour growth/survival. C: Spatial competition can lead to the elimination of chemoresistant clones (green clone), especially if they are less competitive in absence of treatment. Adaptive therapy (by alternating treatment and pauses) may lead to the full elimination of the chemoresistant population and eventually tumour disappearance once the resistant cells are fully eliminated. Red arrows show either the relative expansion of the resistant population during treatment or its reduction during pauses.
6.4. Therapeutic avenues
While the contribution of spatial competition to tumour initiation and progression remains hypothetical at this stage, one can already discuss whether any relevant therapeutic strategy could emerge from this concept. Two strategies may be applied to overcome/alter spatial competition: modulating the mechanical environment of the tumour, or modulating the pathway involved in compaction sensing and death induction. Once again, these two strategies may have opposing consequences on tumour progression. Pressure release was previously applied on tumour xenograft through targeted tumour cell elimination and a reduction of cell density [95]. While this may promote further tumour expansion through space release, this could also facilitate drug delivery through blood vessel opening [10]. Mechanical insulation of tumour cells through increased line tension at tumour boundary would be another efficient way to increase pressure within a tumour and to slow-down tumour expansion. However, there is to my knowledge no easy way to modulate specifically tumour/healty cells interface tension without affecting key regulators of cell survival/proliferation [89]. Alternatively, targeting the compaction sensing pathway may help to prevent neighbouring cell elimination and slow down tumour expansion. Accordingly, mild activation of the EGFR/ERK pathways in cells neighbouring pretumoural clones (constitutive activation of Ras) can significantly slow down clone expansion [26]. Similarly, global inhibition of apoptosis can prevent neoplastic growth of pretumoural clones in the Drosophila midgut [119] and in the wing imaginal disc [120]. Preventing neuronal death through lithium treatment also reduces neurological dysfunctions associated with brain tumours in mice [100]. As discussed above, this may also have an impact on intratumoural competition whose consequences on tumour growth is much more difficult to predict. Finally, since compaction-driven death may also be required to eliminate early pretumoural cells, targeting compaction sensing pathways may promote new tumour appearance on longer time scales. To conclude, the development of new therapeutic strategies would require a fine assessment of all these effects. The design of a successful treatment would probably rely on the capacity to precisely control in time and space the prevalence of mechanical cell competition.
7. Conclusions
While we start to have several experimental proofs of the concept of mechanical cell competition, there are still many fundamental questions which have not been fully addressed so far. This includes a better molecular and physical characterization of the compaction sensing mechanisms [74], a better assessment of the conditions in which competition for space can occur (genetic background and tissue context), as well as the prevalence of mechanical competition during normal development and tissue homeostasis. As described in the last section of this review, many independent observations suggest that competition for space may occur during tumour initiation and tumour expansion. All the theoretical ingredients required for mechanical cell competition have been described in solid tumours (differential growth and mechanical stress, differential sensitivity to mechanical stress, apoptosis and cell death), and this process is very likely to occur both within the tumour (intratumoural competition) and at the interface between the tumour and the healthy tissue. A better understanding of the impact of spatial constrains on cell competition is therefore highly relevant for the understanding of cancer. In light of the multiple and opposing effects of mechanical cell competition, modeling and experimental approaches clearly incorporating spatial constrains, tumour heterogeneity and the interactions with the neighbouring tissues will be absolutely required to better evaluate those opposing effects. A combination of in silico studies incorporating genetic evolution and mechanical inputs, in vitro studies using mosaic cell culture in 3D spheroid with tunable pressures, and in vivo studies in model organisms with mosaic analysis will help to tackle those burning and exciting questions.
Funding source
Work in my lab is supported by the Institut Pasteur (G5 starting package), the ERC starting grant CoSpaDD (grant number 758457), the cercle FSER (Fondation Schlumberger pour l'Education et la Recherche), and the CNRS. No study sponsors were involved.
Declaration of interests
The author declares no competing interest
Acknowledgements
I would like to thank the members of my lab for critical reading of this manuscript.
References
- 1.Potten C.S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 2.Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 3.Panciera T., Azzolin L., Cordenonsi M., Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irvine K.D., Shraiman B.I. Mechanical control of growth: ideas, facts and challenges. Development. 2017;144:4238–4248. doi: 10.1242/dev.151902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Bissell M.J., Hines W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martincorena I., Roshan A., Gerstung M., Ellis P., Van Loo P., McLaren S., Wedge D.C., Fullam A., Alexandrov L.B., Tubio J.M. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martincorena I., Fowler J.C., Wabik A., Lawson A.R.J., Abascal F., Hall M.W.J., Cagan A., Murai K., Mahbubani K., Stratton M.R. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362:911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher D.T., Alliston T., Weaver V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain R.K., Martin J.D., Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levayer R., Moreno E. Mechanisms of cell competition: themes and variations. J. Cell Biol. 2013;200:689–698. doi: 10.1083/jcb.201301051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent J.P., Fletcher A.G., Baena-Lopez L.A. Mechanisms and mechanics of cell competition in epithelia. Nat. Rev. Mol. Cell Biol. 2013;14:581–591. doi: 10.1038/nrm3639. [DOI] [PubMed] [Google Scholar]
- 13.Nagata R., Igaki T. Cell competition: emerging mechanisms to eliminate neighbors. Dev. Growth Differ. 2018 doi: 10.1111/dgd.12575. [DOI] [PubMed] [Google Scholar]
- 14.Morata G., Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev. Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 15.Kongsuwan K., Yu Q., Vincent A., Frisardi M.C., Rosbash M., Lengyel J.A., Merriam J. A Drosophila Minute gene encodes a ribosomal protein. Nature. 1985;317:555–558. doi: 10.1038/317555a0. [DOI] [PubMed] [Google Scholar]
- 16.Moreno E., Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 17.de la Cova C., Abril M., Bellosta P., Gallant P., Johnston L.A. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 18.Moreno E. Is cell competition relevant to cancer? Nat. Rev. Cancer. 2008;8:141–147. doi: 10.1038/nrc2252. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama T., Fujita Y. Cell competition in mammals - novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol. 2017;48:106–112. doi: 10.1016/j.ceb.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Merino M.M., Levayer R., Moreno E. Survival of the fittest: essential roles of cell competition in development, aging, and Cancer. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Claveria C., Torres M. Cell competition: mechanisms and physiological roles. Annu. Rev. Cell Dev. Biol. 2016;32:411–439. doi: 10.1146/annurev-cellbio-111315-125142. [DOI] [PubMed] [Google Scholar]
- 22.Bras-Pereira C., Moreno E. Mechanical cell competition. Curr. Opin. Cell Biol. 2017;51:15–21. doi: 10.1016/j.ceb.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Levayer R., Dupont C., Moreno E. Tissue crowding induces caspase-dependent competition for space. Curr. Biol. 2016;26:670–677. doi: 10.1016/j.cub.2015.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagstaff L., Goschorska M., Kozyrska K., Duclos G., Kucinski I., Chessel A., Hampton-O’Neil L., Bradshaw C.R., Allen G.E., Rawlins E.L. Mechanical cell competition kills cells via induction of lethal p53 levels. Nat. Commun. 2016;7:11373. doi: 10.1038/ncomms11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi A., Ohsawa S., Umetsu D., Sando Y., Kuranaga E., Igaki T., Fujimoto K. Competition for space is controlled by apoptosis-induced change of local epithelial topology. Curr. Biol. 2018;28:2115–2128. doi: 10.1016/j.cub.2018.05.029. e2115. [DOI] [PubMed] [Google Scholar]
- 26.Moreno E., Valon L., Levillayer F., Levayer R. Competition for space induces cell elimination through compaction-driven ERK downregulation. Curr. Biol. 2019;29:23–34. doi: 10.1016/j.cub.2018.11.007. e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shraiman B.I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 29.Helmlinger G., Netti P.A., Lichtenbeld H.C., Melder R.J., Jain R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 30.Delarue M., Montel F., Vignjevic D., Prost J., Joanny J.F., Cappello G. Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys. J. 2014;107:1821–1828. doi: 10.1016/j.bpj.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Y., Heemskerk I., Ibar C., Shraiman B.I., Irvine K.D. Differential growth triggers mechanical feedback that elevates Hippo signaling. Proc. Natl. Acad. Sci. U. S. A. 2016 doi: 10.1073/pnas.1615012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streichan S.J., Hoerner C.R., Schneidt T., Holzer D., Hufnagel L. Spatial constraints control cell proliferation in tissues. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5586–5591. doi: 10.1073/pnas.1323016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gudipaty S.A., Lindblom J., Loftus P.D., Redd M.J., Edes K., Davey C.F., Krishnegowda V., Rosenblatt J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543:118–121. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L., Si G., Huang J., Samuel A.D.T., Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555:103–106. doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C.S., Mrksich M., Huang S., Whitesides G.M., Ingber D.E. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 36.Mao Y., Tournier A.L., Hoppe A., Kester L., Thompson B.J., Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 2013;32:2790–2803. doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legoff L., Rouault H., Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- 38.Simpson P. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev. Biol. 1979;69:182–193. doi: 10.1016/0012-1606(79)90284-7. [DOI] [PubMed] [Google Scholar]
- 39.Li W., Kale A., Baker N.E. Oriented cell division as a response to cell death and cell competition. Curr. Biol. 2009;19:1821–1826. doi: 10.1016/j.cub.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levayer R., Hauert B., Moreno E. Cell mixing induced by myc is required for competitive tissue invasion and destruction. Nature. 2015;524:476–480. doi: 10.1038/nature14684. [DOI] [PubMed] [Google Scholar]
- 41.Basan M., Risler T., Joanny J.F., Sastre-Garau X., Prost J. Homeostatic competition drives tumor growth and metastasis nucleation. HFSP J. 2009;3:265–272. doi: 10.2976/1.3086732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basan M., Prost J., Joanny J.F., Elgeti J. Dissipative particle dynamics simulations for biological tissues: rheology and competition. Phys. Biol. 2011;8 doi: 10.1088/1478-3975/8/2/026014. [DOI] [PubMed] [Google Scholar]
- 43.Bove A., Gradeci D., Fujita Y., Banerjee S., Charras G., Lowe A.R. Local cellular neighborhood controls proliferation in cell competition. Mol. Biol. Cell. 2017;28:3215–3228. doi: 10.1091/mbc.E17-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenblatt J., Raff M.C., Cramer L.P. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 45.Eisenhoffer G.T., Loftus P.D., Yoshigi M., Otsuna H., Chien C.B., Morcos P.A., Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marinari E., Mehonic A., Curran S., Gale J., Duke T., Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–545. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- 47.Quan X., Guo K., Wang Y., Huang L., Chen B., Ye Z., Luo Z. Mechanical compression insults induce nanoscale changes of membrane-skeleton arrangement which could cause apoptosis and necrosis in dorsal root ganglion neurons. Biosci. Biotechnol. Biochem. 2014;78:1631–1639. doi: 10.1080/09168451.2014.932664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takano K.J., Takano T., Yamanouchi Y., Satou T. Pressure-induced apoptosis in human lymphoblasts. Exp. Cell Res. 1997;235:155–160. doi: 10.1006/excr.1997.3666. [DOI] [PubMed] [Google Scholar]
- 49.Thompson W.R., Rubin C.T., Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ernest N.J., Habela C.W., Sontheimer H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J. Cell. Sci. 2008;121:290–297. doi: 10.1242/jcs.017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helmlinger G., Netti P.A., Lichtenbeld H.C., Melder R.J., Jain R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 52.Cheng G., Tse J., Jain R.K., Munn L.L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS One. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alessandri K., Sarangi B.R., Gurchenkov V.V., Sinha B., Kiessling T.R., Fetler L., Rico F., Scheuring S., Lamaze C., Simon A. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14843–14848. doi: 10.1073/pnas.1309482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curran S., Strandkvist C., Bathmann J., de Gennes M., Kabla A., Salbreux G., Baum B. Myosin II controls junction fluctuations to guide epithelial tissue ordering. Dev. Cell. 2017;43:480–492. doi: 10.1016/j.devcel.2017.09.018. e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergmann A., Agapite J., McCall K., Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 56.Kurada P., White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 57.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 58.Saw T.B., Doostmohammadi A., Nier V., Kocgozlu L., Thampi S., Toyama Y., Marcq P., Lim C.T., Yeomans J.M., Ladoux B. Topological defects in epithelia govern cell death and extrusion. Nature. 2017;544:212–216. doi: 10.1038/nature21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lecuit T., Lenne P.F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 60.Amack J.D., Manning M.L. Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science. 2012;338:212–215. doi: 10.1126/science.1223953. [DOI] [PubMed] [Google Scholar]
- 61.Milan M., Perez L., Cohen S.M. Short-range cell interactions and cell survival in the Drosophila wing. Dev. Cell. 2002;2:797–805. doi: 10.1016/s1534-5807(02)00169-7. [DOI] [PubMed] [Google Scholar]
- 62.Bielmeier C., Alt S., Weichselberger V., La Fortezza M., Harz H., Julicher F., Salbreux G., Classen A.K. Interface contractility between differently fated cells drives cell elimination and cyst formation. Curr. Biol. 2016;26:563–574. doi: 10.1016/j.cub.2015.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibson M.C., Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- 64.Adachi-Yamada T., Fujimura-Kamada K., Nishida Y., Matsumoto K. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- 65.Shen J., Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- 66.Widmann T.J., Dahmann C. Wingless signaling and the control of cell shape in Drosophila wing imaginal discs. Dev. Biol. 2009;334:161–173. doi: 10.1016/j.ydbio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Vincent J.P., Kolahgar G., Gagliardi M., Piddini E. Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev. Cell. 2011;21:366–374. doi: 10.1016/j.devcel.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz-Diaz C., Fernandez de Manuel L., Jimenez-Carretero D., Montoya M.C., Claveria C., Torres M. Pluripotency surveillance by myc-driven competitive elimination of differentiating cells. Dev. Cell. 2017;42:585–599. doi: 10.1016/j.devcel.2017.08.011. e584. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto M., Ohsawa S., Kunimasa K., Igaki T. The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature. 2017;542:246–250. doi: 10.1038/nature21033. [DOI] [PubMed] [Google Scholar]
- 70.Kajita M., Fujita Y. EDAC: epithelial defence against cancer-cell competition between normal and transformed epithelial cells in mammals. J. Biochem. 2015;158:15–23. doi: 10.1093/jb/mvv050. [DOI] [PubMed] [Google Scholar]
- 71.Porazinski S., de Navascues J., Yako Y., Hill W., Jones M.R., Maddison R., Fujita Y., Hogan C. EphA2 drives the segregation of ras-transformed epithelial cells from normal neighbors. Curr. Biol. 2016;26:3220–3229. doi: 10.1016/j.cub.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 72.Ohoka A., Kajita M., Ikenouchi J., Yako Y., Kitamoto S., Kon S., Ikegawa M., Shimada T., Ishikawa S., Fujita Y. EPLIN is a crucial regulator for extrusion of RasV12-transformed cells. J. Cell. Sci. 2015;128:781–789. doi: 10.1242/jcs.163113. [DOI] [PubMed] [Google Scholar]
- 73.Kajita M., Sugimura K., Ohoka A., Burden J., Suganuma H., Ikegawa M., Shimada T., Kitamura T., Shindoh M., Ishikawa S. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun. 2014;5:4428. doi: 10.1038/ncomms5428. [DOI] [PubMed] [Google Scholar]
- 74.Valon L., Levayer R. Dying under pressure: cellular characterisation and in vivo functions of cell death induced by compaction. Biol. Cell. 2019;111:51–66. doi: 10.1111/boc.201800075. [DOI] [PubMed] [Google Scholar]
- 75.Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 76.Elosegui-Artola A., Andreu I., Beedle A.E.M., Lezamiz A., Uroz M., Kosmalska A.J., Oria R., Kechagia J.Z., Rico-Lastres P., Le Roux A.L. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–1410. doi: 10.1016/j.cell.2017.10.008. e1314. [DOI] [PubMed] [Google Scholar]
- 77.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan Y., Alegot H., Rauskolb C., Irvine K.D. The dynamics of hippo signaling during Drosophila wing development. Development. 2018 doi: 10.1242/dev.165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirata H., Gupta M., Vedula S.R., Lim C.T., Ladoux B., Sokabe M. Actomyosin bundles serve as a tension sensor and a platform for ERK activation. EMBO Rep. 2015;16:250–257. doi: 10.15252/embr.201439140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sano T., Kobayashi T., Negoro H., Sengiku A., Hiratsuka T., Kamioka Y., Liou L.S., Ogawa O., Matsuda M. Intravital imaging of mouse urothelium reveals activation of extracellular signal-regulated kinase by stretch-induced intravesical release of ATP. Physiol. Rep. 2016;4 doi: 10.14814/phy2.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aoki K., Kondo Y., Naoki H., Hiratsuka T., Itoh R.E., Matsuda M. Propagating wave of ERK activation orients collective cell migration. Dev. Cell. 2017;43:305–317. doi: 10.1016/j.devcel.2017.10.016. e305. [DOI] [PubMed] [Google Scholar]
- 82.Aoki K., Kumagai Y., Sakurai A., Komatsu N., Fujita Y., Shionyu C., Matsuda M. Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Mol. Cell. 2013;52:529–540. doi: 10.1016/j.molcel.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Senoo-Matsuda N., Johnston L.A. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila myc. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18543–18548. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alpar L., Bergantinos C., Johnston L.A. Spatially restricted regulation of Spatzle/Toll signaling during cell competition. Dev. Cell. 2018;46:706–719. doi: 10.1016/j.devcel.2018.08.001. e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhiner C., Lopez-Gay J.M., Soldini D., Casas-Tinto S., Martin F.A., Lombardia L., Moreno E. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell. 2010;18:985–998. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Ziosi M., Baena-Lopez L.A., Grifoni D., Froldi F., Pession A., Garoia F., Trotta V., Bellosta P., Cavicchi S., Pession A. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prober D.A., Edgar B.A. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 2002;16:2286–2299. doi: 10.1101/gad.991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Claveria C., Giovinazzo G., Sierra R., Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- 89.Bosveld F., Guirao B., Wang Z., Riviere M., Bonnet I., Graner F., Bellaiche Y. Modulation of junction tension by tumor suppressors and proto-oncogenes regulates cell-cell contacts. Development. 2016;143:623–634. doi: 10.1242/dev.127993. [DOI] [PubMed] [Google Scholar]
- 90.Benzekry S., Lamont C., Beheshti A., Tracz A., Ebos J.M., Hlatky L., Hahnfeldt P. Classical mathematical models for description and prediction of experimental tumor growth. PLoS Comput. Biol. 2014;10 doi: 10.1371/journal.pcbi.1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freyer J.P. Role of necrosis in regulating the growth saturation of multicellular spheroids. Cancer Res. 1988;48:2432–2439. [PubMed] [Google Scholar]
- 92.Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. London. 1825;115 doi: 10.1098/rstb.2014.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grimes D.R., Kannan P., McIntyre A., Kavanagh A., Siddiky A., Wigfield S., Harris A., Partridge M. The role of oxygen in avascular tumor growth. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montel F., Delarue M., Elgeti J., Malaquin L., Basan M., Risler T., Cabane B., Vignjevic D., Prost J., Cappello G. Stress clamp experiments on multicellular tumor spheroids. Phys. Rev. Lett. 2011;107 doi: 10.1103/PhysRevLett.107.188102. [DOI] [PubMed] [Google Scholar]
- 95.Padera T.P., Stoll B.R., Tooredman J.B., Capen D., di Tomaso E., Jain R.K. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 96.Stylianopoulos T., Martin J.D., Chauhan V.P., Jain S.R., Diop-Frimpong B., Bardeesy N., Smith B.L., Ferrone C.R., Hornicek F.J., Boucher Y. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nia H.T., Liu H., Seano G., Datta M., Jones D., Rahbari N., Incio J., Chauhan V.P., Jung K., Martin J.D. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 2016;1 doi: 10.1038/s41551-016-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nia H.T., Datta M., Seano G., Huang P., Munn L.L., Jain R.K. Quantifying solid stress and elastic energy from excised or in situ tumors. Nat. Protoc. 2018;13:1091–1105. doi: 10.1038/nprot.2018.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Giacomo S., Sollazzo M., de Biase D., Ragazzi M., Bellosta P., Pession A., Grifoni D. Human Cancer cells signal their competitive fitness through MYC activity. Sci. Rep. 2017;7:12568. doi: 10.1038/s41598-017-13002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seano G., Nia H.T., Emblem K.E., Datta M., Ren J., Krishnan S., Kloepper J., Pinho M.C., Ho W.W., Ghosh M. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 2019 doi: 10.1038/s41551-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eefsen R.L., Vermeulen P.B., Christensen I.J., Laerum O.D., Mogensen M.B., Rolff H.C., Van den Eynden G.G., Hoyer-Hansen G., Osterlind K., Vainer B. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin. Exp. Metastasis. 2015;32:369–381. doi: 10.1007/s10585-015-9715-4. [DOI] [PubMed] [Google Scholar]
- 102.Van den Eynden G.G., Bird N.C., Majeed A.W., Van Laere S., Dirix L.Y., Vermeulen P.B. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis. 2012;29:541–549. doi: 10.1007/s10585-012-9469-1. [DOI] [PubMed] [Google Scholar]
- 103.Wang H.B., Dembo M., Wang Y.L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 2000;279:C1345–1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 104.Rianna C., Radmacher M. Comparison of viscoelastic properties of cancer and normal thyroid cells on different stiffness substrates. Eur. Biophys. J. 2017;46:309–324. doi: 10.1007/s00249-016-1168-4. [DOI] [PubMed] [Google Scholar]
- 105.Tabassum D.P., Polyak K. Tumorigenesis: it takes a village. Nat. Rev. Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 106.Lawson D.A., Kessenbrock K., Davis R.T., Pervolarakis N., Werb Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018;20:1349–1360. doi: 10.1038/s41556-018-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tissot T., Thomas F., Roche B. Non-cell-autonomous effects yield lower clonal diversity in expanding tumors. Sci. Rep. 2017;7:11157. doi: 10.1038/s41598-017-11562-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nowell P.C. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 109.Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waclaw B., Bozic I., Pittman M.E., Hruban R.H., Vogelstein B., Nowak M.A. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature. 2015;525:261–264. doi: 10.1038/nature14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Visser J.A., Rozen D.E. Clonal interference and the periodic selection of new beneficial mutations in Escherichia coli. Genetics. 2006;172:2093–2100. doi: 10.1534/genetics.105.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marusyk A., Tabassum D.P., Altrock P.M., Almendro V., Michor F., Polyak K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514:54–58. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yates L.R., Gerstung M., Knappskog S., Desmedt C., Gundem G., Van Loo P., Aas T., Alexandrov L.B., Larsimont D., Davies H. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat. Med. 2015;21:751–759. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Overholtzer M., Mailleux A.A., Mouneimne G., Normand G., Schnitt S.J., King R.W., Cibas E.S., Brugge J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 115.Fais S., Overholtzer M. Cell-in-cell phenomena in cancer. Nat. Rev. Cancer. 2018;18:758–766. doi: 10.1038/s41568-018-0073-9. [DOI] [PubMed] [Google Scholar]
- 116.Sun Q., Luo T., Ren Y., Florey O., Shirasawa S., Sasazuki T., Robinson D.N., Overholtzer M. Competition between human cells by entosis. Cell Res. 2014;24:1299–1310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee S.W., Morishita Y. Possible roles of mechanical cell elimination intrinsic to growing tissues from the perspective of tissue growth efficiency and homeostasis. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bacevic K., Noble R., Soffar A., Wael Ammar O., Boszonyik B., Prieto S., Vincent C., Hochberg M.E., Krasinska L., Fisher D. Spatial competition constrains resistance to targeted cancer therapy. Nat. Commun. 2017;8:1995. doi: 10.1038/s41467-017-01516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suijkerbuijk S.J., Kolahgar G., Kucinski I., Piddini E. Cell competition drives the growth of intestinal adenomas in Drosophila. Curr. Biol. 2016;26:428–438. doi: 10.1016/j.cub.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eichenlaub T., Cohen S.M., Herranz H. Cell competition drives the formation of metastatic tumors in a Drosophila model of epithelial tumor formation. Curr. Biol. 2016;26:419–427. doi: 10.1016/j.cub.2015.12.042. [DOI] [PubMed] [Google Scholar]