Abstract
Background
The current COVID-19 pandemic has changed many medical practices in order to provide additional protection to both our patients and healthcare providers. In many cases this includes seeing patients through electronic means such as telehealth or telephone rather than seeing them in person. Asthma exacerbations cannot always be treated in this way.
Problem
Current emergency unit asthma guidelines recommend bronchodilators be administered by metered dose inhaler (MDI) and spacer for mild-moderate asthma and include it as a choice even in severe asthma, but many emergency units continue to prefer nebulised therapy for patients who urgently require beta-agonists. The utilization of nebulised therapy potentially increases the risk of aerosolization of the coronavirus. Since nosocomial transmission of respiratory pathogens is a major threat in the context of the SARS-CoV-2 pandemic, use of nebulised therapy is of even greater concern due to the potential increased risk of infection spread to nearby patients and healthcare workers.
Practical implications
We propose a risk stratification plan that aims to avoid nebulised therapy, when possible, by providing an algorithm to help better delineate those who require nebulised therapy. Protocols that include strategies to allow flexibility in using MDIs rather than nebulisers in all but the most severe patients should help mitigate this risk of aerosolised infection transmission to patients and health care providers. Furthermore, expedient treatment of patients with high dose MDI therapy augmented with more rapid initiation of systemic therapy may help ensure patients are less likely to deteriorate to the stage where nebulisers are required.
Keywords: Asthma, COVID-19, Exacerbation, Infectious risk, Inhalers, Protocol, Treatment
Abbreviations: MgSO4, Magnesium sulphate; MDI, Metered dose inhaler; SABA, Short acting beta-2-agonist/s; SAMA, Short acting anti-muscarinic agent/s
Background
The SARS-CoV-2 pandemic has created disruptions in the provision of care throughout the world.1 Healthcare providers must incorporate strategies to limit the spread of SARS-CoV-2 within the hospital into their treatment strategies. As we learn more about the transmissibility of SARS CoV-2, and its ability to spread by respiratory droplets, it seems prudent to devise strategies to limit the aerosolization of the virus. Although asthmatic children do not appear to be at a higher risk to develop severe infection, and wheeze does not appear to be a common symptom for paediatric patients with COVID-19,2 the authors are concerned about the potential for nebulisers that are being used in hospitals in treating asthma patients infected with SARS-CoV-2, to transmit contaminated droplet particles to uninfected patients and healthcare workers.
Problem
Nosocomial transmission of respiratory pathogens is always a source of concern especially in the context of highly contagious pathogens like the SARS-CoV-2. During the Severe Acute Respiratory Syndrome (SARS) epidemic in 2003, overcrowding of wards and aerosol-generating procedures were identified as major factors for viral transmission.3 The highest risk procedures appear to be tracheal intubation, non-invasive ventilation, tracheotomy, and manual ventilation before intubation.4 However other procedures may also generate potentially infectious aerosols. Non-invasive ventilation and chest physiotherapy predominantly produce large droplets which fall out on to local surfaces within 1 m (m), whereas nebulisers produce droplets in the small- and medium-size aerosol range which can fall out at greater distances.5
Whereas oxygen therapy via Hudson mask and nasal cannula may disperse exhaled air of patients to 0.4 and 1 m respectively, jet nebulisers can disperse viral particles in exhaled air >0.8 m from the patient3 and remain airborne for more than 30 min, potentially spreading droplets to neighbouring patients and exposing health care workers to additional risk of infection.
It is for these reasons that many expert consensus guidelines on preventing nosocomial transmission during respiratory care for patients with COVID-19 recommend replacing nebulised bronchodilator therapy with bronchodilators given by metered dose inhalers (MDIs) and spacers;6, 7, 8, 9 however, no specific protocols have been published to delineate how to implement this recommendation without adversely affecting patient outcomes. Furthermore, since the availability of rigorous data supporting a specific protocol are lacking, extrapolation from published data is required.
Motivation
The premise of this report is to develop an algorithm that allows effective treatment of asthma exacerbations whilst protecting other patients and personnel from pathogen exposure. The algorithm is intended to mitigate against the risk of aerosolizing SARS-CoV-2, or any other infectious agent, in all patients, regardless of whether they are suspected of having an infection or not, given the potential for patients to have asymptomatic SARS-CoV2 infection.
Modified algorithm
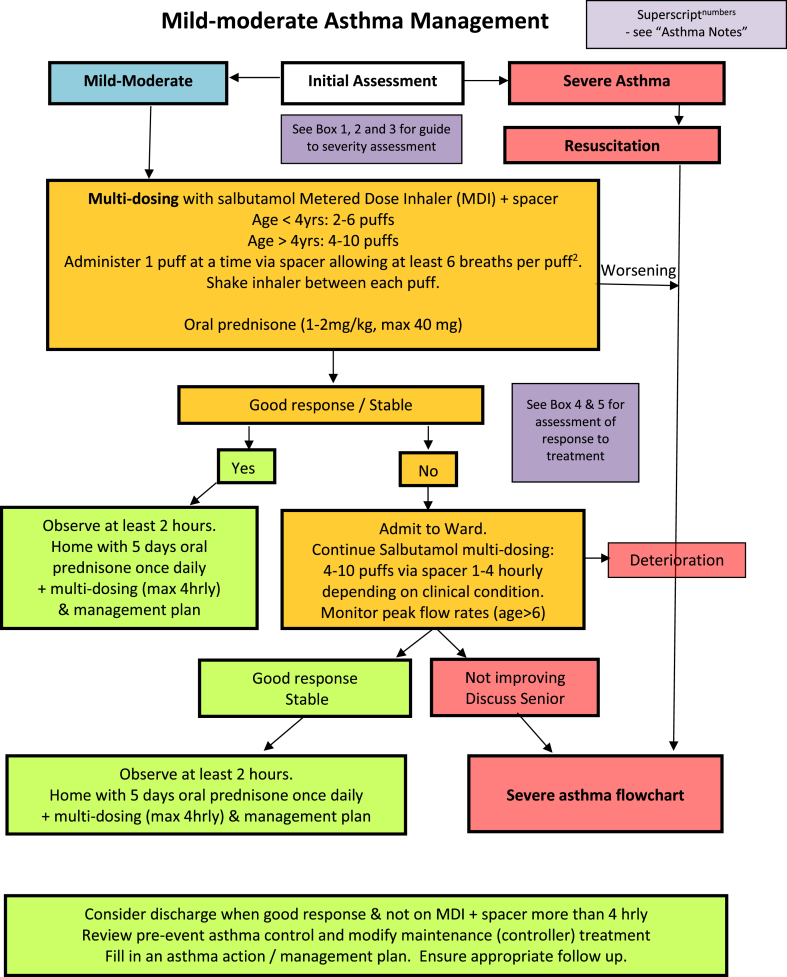
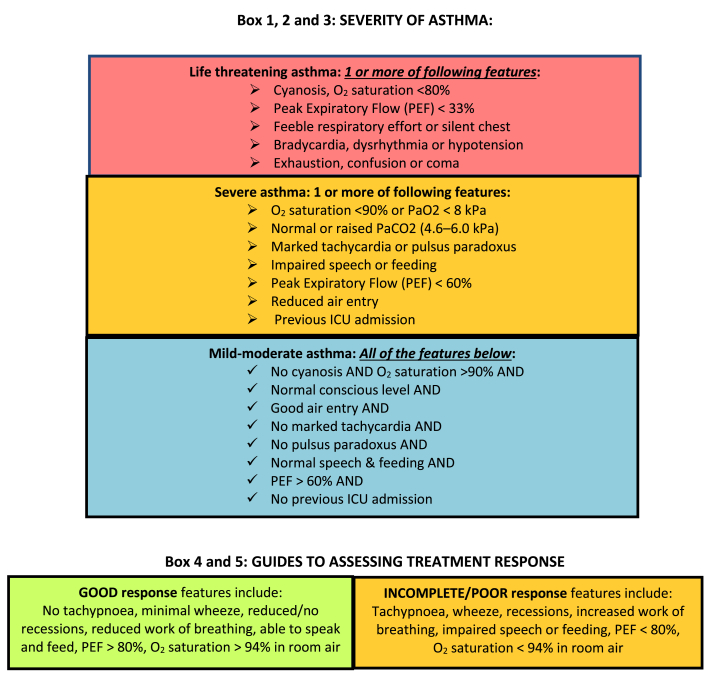
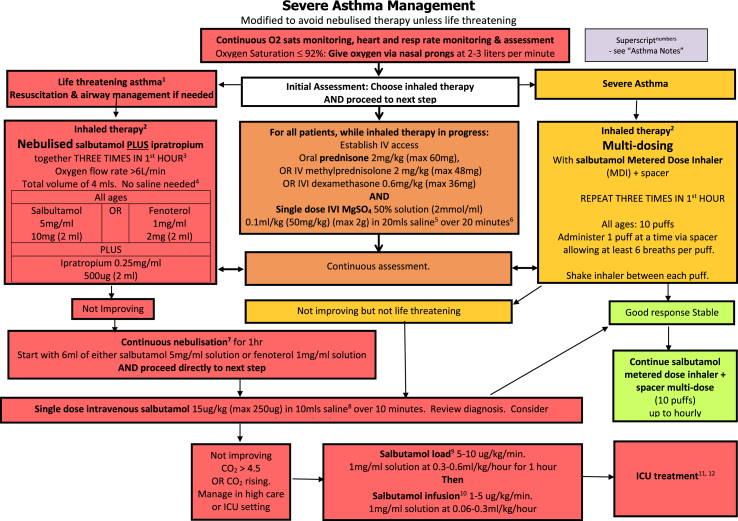
We thus suggest a modified algorithm for the treatment of acute asthma which comprises an algorithm for mild-moderate asthma (Fig. 1), suggested tools for assessing severity and response to treatment (Fig. 2), a severe asthma algorithm (Fig. 3) that includes those with life threatening exacerbations, and a short list of explanatory notes to accompany the severe asthma algorithm. The severe asthma flowchart restricts nebulised therapy to life-threatening asthma, augments treatment by recommending close observation and more rapid initiation of systemic therapy, and allows flexibility to move between nebulised therapy and MDI with spacer therapy in either direction if improvement or deterioration occur. Medication dosages in the flowchart are given for children in mg/kg, with maximal doses that can be given in larger children and adults in brackets.
Fig. 1.
Mild-moderate asthma algorithm
Fig. 2.
Severity and response assessment
Fig. 3.
Severe and life threatening asthma algorithm
Bronchodilator therapy given by MDIs and spacer rather than nebulisers
The effectiveness of asthma treatment with short acting beta-2-agonists (SABA) given by MDIs with spacer versus nebulisers has been evaluated.10 In a review that includes 39 trials with a total of 1897 children and 729 adults, the authors conclude: “Nebuliser delivery produced outcomes that were not significantly better than metered-dose inhalers delivered by spacer in adults or children.” The authors emphasize that these studies excluded people with life-threatening asthma, and, therefore, their results should not be extrapolated to that patient population. The conclusion of this study was that nebulisers were not superior to MDIs with spacers in preventing hospital admissions. Specifically for children, MDIs with spacers were more effective at reducing the length of stay in the emergency department compared to a nebuliser.10 Notably, home-made spacers have been demonstrated to be equally effective as commercially available spacers for improving oxygen saturation, peak flow rates, and clinical scores while reducing the need for additional treatment and hospital admissions.11
When nebulisers are required, where possible they should be provided in isolation within negative pressure rooms with providers wearing N95 masks, goggles/face shield, and other personal protective equipment required for droplet particle protection. However, to minimize exposure to aerosolised droplets, it is likely safer to use metered-dose inhalers with spacers in lieu of nebulisers in patients suffering asthma exacerbations, particularly in pandemic situations.
The recommended method used in many of the reported studies to deliver SABA via MDI and spacer was to administer four puffs every 10–15 min, while adjusting the therapy to the patient's response. Each puff should be delivered individually via spacer prior to actuating the next puff.
However, some health professionals and patients may not adhere to such recommendations because of limited data using this approach in the most severely affected individuals. This concern is valid and requires us to delineate the circumstances in which nebulisers must be used by considering further stratification of asthma severity.
Stratifying asthma severity
Most emergency department algorithms divide acute asthma into mild-moderate and severe and recommend use of MDI delivered therapy in the mild-moderate category and either MDI delivered therapy or nebulised therapy in severe asthma. Some emergency department algorithms include a third category of life-threatening asthma, which allows further risk-stratification of patients to use MDIs with spacers in mild-moderately affected patients and severely affected asthma patients without immediate life-threatening signs or symptoms. The smaller group of the most severely affected patients is those with life-threatening asthma in whom nebuliser therapy should still be given due to its superior flexibility to deliver oxygen and continuous bronchodilator therapy and to allow concomitant use of short acting anti-muscarinic agents (SAMA).
Augmenting inhaled therapy
For adults with asthma, combining inhaled beta-agonist with SAMA for emergency management of asthma reduces hospitalisation particularly in those with severe attacks.12 Whereas SABA MDIs are uniformly available worldwide, inhaled SAMA such as ipratropium bromide are less commonly available in MDI formulation either as single or combined formulations. Because our suggested algorithm restricts nebulised therapy to only life-threatening episodes, some patients who would formerly have been treated with combination SABA/SAMA therapy will not be able to receive anticholinergics via MDI and spacer.
In addition, we acknowledge that some emergency units may be less familiar with using MDIs and spacers in asthmatics with severe attacks, that shortages of SABA MDIs might occur,1 and that some providers have a perception that MDI therapy is less effective than nebulised therapy,13 factors which might prevent widespread adoption of this algorithm.
The benefit for healthcare workers and other patients using MDI plus spacer must be balanced against the risks to the patient not using nebulised therapy. To mitigate against these concerns, we highlight the need to deliver oxygen concurrently with MDI and spacer therapy; we advocate for strict monitoring of subjects having severe exacerbations; and we recommend acting on those observations by moving between therapy with MDIs and spacers and nebulisers as the patient either improves or deteriorates. In addition, we recommend augmenting inhaled therapy with systemic therapy immediately with corticosteroids and intravenous magnesium sulphate (MgSO4) in all severely affected individuals. Using systemic agents in all severely affected individuals may prevent deterioration in patients to a stage where they require nebulisation and may shorten the use of nebuliser therapy in those patients requiring them for life threatening symptoms.
Systemic agents commonly used to treat acute asthma include oral or intravenous glucocorticosteroids, intravenous magnesium sulphate, a single intravenous dose of salbutamol/albuterol of 15 μg/kg, or a loading dose of 5–10 μg/kg/min salbutamol/albuterol for 1 h followed by infusion at a rate of 1–5 μg/kg/min and intravenous aminophylline.
In the most recent Cochrane review on intravenous MgSO4 for treating adults with acute asthma in the emergency department, MgSO4 reduced hospital admissions and showed some evidence of improvement in lung function.14 The most commonly cited adverse events in the IV MgSO4 groups were flushing, fatigue, nausea and headache, and hypotension, the latter which seems to occur when MgSO4 is given rapidly rather than over 15–30 min. In children, there are fewer studies, but the Cochrane review concluded that treatment with IV MgSO4 reduced the odds of admission to hospital by 68%.15
There is limited evidence to suggest that the use of IV beta2-agonists in children with severe acute asthma improves recovery time and pulmonary index scores and little evidence of benefits for adults with severe acute asthma, with some evidence of increased side effects.16
Hence, we consider IV MgSO4 as the systemic drug of choice to augment bronchodilator therapy in patients with acute asthma attacks receiving SABA via MDI and spacer who would otherwise have received nebulised therapy.
Conclusions
Despite the limitations in the data, we believe that the suggested algorithm is based on scientific evidence, reflects best practice, and should be considered for implementation during pandemic situations where community spread of pathogens is common. They are not strict protocols, and they do not replace the judgment of a senior clinician. These guidelines should not be relied on as a substitute for proper assessment with respect to the particular circumstances of each case and the needs of each patient. Clinicians wishing to implement these guidelines should consider the local skill level available and their local area policies to assess whether modification will be required.
In addition to considering these emergency guidelines, we also recommend that asthma be kept well under control to reduce the possibility of exacerbations that may lead to patients attending emergency departments and potentially putting themselves at risk.1,8,9 Current Global Initiative for Asthma (GINA) guidelines recommend that combination inhaled corticosteroids with formoterol be used as a preferred single maintenance and rescue therapy, as studies have shown that using this as patient initiated rescue therapy provides comparable benefit to a SABA for relief of smooth muscle dysfunction in addition to providing increased topical corticosteroid protection against worsening airway inflammation.17
Funding
Not applicable.
Consent for publication
N/a.
Availability of data and materials
N/a.
Ethics approval
N/a.
Author contributions
ML conceived of the project and designed the algorithms. All authors contributed to development of the recommendations, writing of the text and review of the article
Declaration of Competing Interest
No authors have competing interests relevant to this publication.
Acknowledgements
To the Board of Directors of the World Allergy Organization for support in development of this article.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Shaker M., Oppenheimer J., Grayson M. COVID-19: pandemic contingency planning for the Allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020 May;8(5):1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012. Epub 2020 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu X., Zhang L., Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020 doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D.S. Severe acute respiratory syndrome (SARS): lessons learnt in Hong Kong. J Thorac Dis. 2013;5(2):S122–S126. doi: 10.3978/j.issn.2072-1439.2013.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonds A.K., Hanak A., Chatwin M. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14(46):131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 6.Respiratory care committee of Chinese Thoracic Society. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia] Zhonghua Jiehe He Huxi Zazhi. 2020;17:E020. doi: 10.3760/cma.j.issn.1001-0939.2020.0020. [DOI] [PubMed] [Google Scholar]
- 7.Global initiative for asthma . 2020. Covid-19: GINA Answers to Frequently Asked Questions on Asthma Management.https://ginasthma.org/covid-19-gina-answers-to-frequently-asked-questions-on-asthma-management/ [Internet] Available from: [Google Scholar]
- 8.Abrams E., T'Jong G.Y.C. 2020. Canadian Pediatric Society Practice Point: Paediatric Asthma and COVID-19.https://www.cps.ca/en/documents/position/paediatric-asthma-and-covid-19 [Internet] Available from: [Google Scholar]
- 9.Abrams E.M., Szefler S.J. Managing asthma during COVID-19: an example for other chronic conditions in children and adolescents. J Pediatr. 2020 Apr 21 doi: 10.1016/j.jpeds.2020.04.049. pii: S0022-3476(20)30528-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cates C.J., Welsh E.J., Rowe B.H. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052. doi: 10.1002/14651858.CD000052.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Martinez C., Sossa M., Lozano J.M. Commercial versus home-made spacers in delivering bronchodilator therapy for acute therapy in children. Cochrane Database Syst Rev. 2008;2:CD005536. doi: 10.1002/14651858.CD005536.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkland S.W., Vandenberghe C., Voaklander B., Nikel T., Campbell S., Rowe B.H. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst Rev. 2017;1:CD001284. doi: 10.1002/14651858.CD001284.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoo S.M., Tan L.K., Said N., Lim T.K. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855–860. doi: 10.4187/002013209793800411. [DOI] [PubMed] [Google Scholar]
- 14.Kew K.M., Kirtchuk L., Michell C.I. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014;5:CD010909. doi: 10.1002/14651858.CD010909.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths B., Kew K.M. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4:CD011050. doi: 10.1002/14651858.CD011050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travers A.H., Milan S.J., Jones A.P., Camargo C.A., Jr., Rowe B.H. Addition of intravenous beta 2 -agonists to inhaled beta 2 -agonists for acute asthma. Cochrane Database Syst Rev. 2012;12:CD010179. doi: 10.1002/14651858.CD010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kew K.M., Karner C., Mindus S.M., Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013;12:CD009019. doi: 10.1002/14651858.CD009019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/a.