Graphical abstract
Keywords: Chloroquine, Hydroxychloroquine, Remdesivir
Highlights
-
•
Corona Virus Disease 19 (COVID 19) is caused by novel corona virus (nCoV) & leads to respiratory failure in susceptible individuals.
-
•
Many drugs have been repurposed and are potentially used either prophylactically or therapeutically throughout the world.
-
•
SOLIDARITY trial by WHO promotes clinical trial with respect to four potentially useful repurposed medications.
-
•
The drugs mentioned in SOLIDARITY trial are mainly antivirals except for hydroxychloroquine which is an anti-malarial drug.
-
•
Convalescent plasma therapy is an evolving therapy based on the principle of passive immunity.
-
•
Cytokine storm are believed to lead to deterioration in patients and drugs against the same are also in research.
Abstract
Novel Corona-virus Disease 2019 (nCOVID 19) is caused by a novel virulent corona virus and leads to potentially fatal virulent pneumonia and severe respiratory distress syndrome. It was initially declared as public health emergency if international concern by WHO followed by Pandemic on 12th March 2020. As of 10th April 2020, more than 1.5 million people are affected globally with around 95,000 deaths. Vaccines for this deadly virus are currently under development and many drugs used for other indications have been repurposed and investigated for prophylaxis and treatment of COVID 19. As per SOLIDARITY trial by WHO, some of the most promising candidates include chloroquine phosphate and hydroxychloroquine which are anti-malarial medications, Remdesivir, Lopinavir-Ritonavir combination with or without interferon which are anti-HIV drugs and convalescent plasma therapy. The current evidence of efficacy and ongoing research has been elaborated in the article. Besides, there has been evidence regarding inflammatory pathogenesis of this virus leading to cytokine storm in susceptible individuals. Thus, anti-proinflammatory cytokine drugs like Anakinra and Tocilizumab are undergoing multiple trials and some results are encouraging. Similarly, use of anti-inflammatory cytokines like IL-37 and IL-38 is hypothesised to be useful and is under research. The situation is still evolving and hence there is yet no definitive therapy but to conclude the use of repurposed medications can be a boon till a definitive therapy and vaccines are developed.
1. Background
In December 2019, Wuhan city, the capital of Hubei province in China, became the centre of a virulent disease of pneumonia of unknown cause [1]. By Jan 7, 2020, Chinese scientists had isolated a completely unique coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously referred to as 2019-nCoV), from these patients with virus-infected pneumonia [2]. The outbreak was declared a Public Health Emergency of International Concern on 30 January 2020 by WHO [3]. WHO later designated this SARS-Cov-2 as coronavirus disease 2019 (COVID-19) in February 2020. By March 11, 2020 around 114 countries were affected globally by coronavirus disease then WHO declared COVID-19 as a deadly disease [3]. Although the outbreak is probably going to possess started from a zoonotic transmission event related to an oversized seafood market (Huanan seafood market, Wuhan) that also traded in live wild animals, it soon became clear that efficient person-to-person transmission was also occurring [4]. From phylogenetics analyses undertaken with available full genome sequences, bats and possibly pangolins appear to be the reservoir of COVID-19 virus, but the intermediate host(s) has not yet been identified [5,6].
2. Epidemiology
As of 6 April 2020, over 1,280,000 cases of COVID-19 are reported in over 200 countries and territories, leading to approximately 70,500 deaths. Over 270,000 people have recovered globally [6]. The primary case of the 2019–20 coronavirus pandemic in India was reported on 30 January 2020, originating from China. As of 6 April 2020, the Ministry of Health and Family Welfare have confirmed a complete of 4067 cases, 292 recoveries (including 1 migration) and 109 deaths within the country. Experts suggest the amount of infections may be much higher as India's testing rates are among the bottom within the world. The infection rate of COVID-19 in India is reported to be 1.7, significantly less than within the worst affected countries [7].
3. Clinical features
As show in Fig. 1 , the clinical spectrum of SARS-CoV-2 infection appears to be vague, encompassing asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death [1].
Fig. 1.
Commonly observed signs and symptoms in COVID 19 infection (As shown in Fig. 1, three most common symptoms are fever, dry cough and fatigue followed by other symptoms like anosmia, and headache.
4. Management guidelines
At present clinical management includes infection prevention and control measures and supportive care, including supplementary oxygen and mechanical ventilatory support when indicated. WHO and CDC also recommend safety measures like avoiding direct contact with the patients stricken by acute respiratory infections, frequent washing of hands and people with respiratory infections to hide their nose and mouth while sneezing or coughing. Currently there are many International furthermore as country-specific government management guidelines for COVID-19 getting published and revised subsequently. ‘Clinical management of severe acute infection (SARI) when COVID-19 disease is suspected’ by WHO on March 13, 2020.
‘International pulmonologist’s consensus on COVID-19’ contributed by pulmonologists across the world and ‘Handbook of COVID-19 Prevention and Treatment’ by National health commission of China were published on March 18, 2020. NICE (National Institute for Health and Care Excellence, UK) published its first 3 rapid guidelines on the care of individuals with suspected and confirmed COVID-19, and in patients without COVID-19 on March 21, 2020. ‘Guidelines on Clinical Management of COVID – 19’ by Ministry of Health & Family Welfare, India on March 30, 2020. An array of medication approved for other indications furthermore as several investigational drugs are being studied in several hundred clinical trials that are underway across the world.
5. Potential pharmacotherapies
There aren't any US-FDA, EMA or the other regulatory approved drugs specifically for the treatment of patients with COVID-19. However, on March 22, 2020 the Indian Council of Medical Research (ICMR) recommended the employment of hydroxychloroquine as prophylaxis for asymptomatic health care workers and asymptomatic household contacts of laboratory confirmed cases of COVID-19 [8]. Further, on March 28, 2020 the FDA granted Emergency Use Authorization (EUA) to be used of Chloroquine Phosphate or Hydroxychloroquine Sulphate for Treatment of COVID-19 [9]. WHO has identified a listing of “promising candidates” for COVID-19 treatment which include remdesivir (an investigational agent); lopinavir-ritonavir (approved to be used in HIV) with or without interferon; investigational immunotherapies like monoclonal and polyclonal antibodies; and convalescent sera. In its January 27 statement, WHO failed to support the antimalarial chloroquine or hydroxychloroquine, ribavirin (used for hepatitis), or corticosteroids/steroids for COVID-19 clinical studies [10]. WHO later launched SOLIDARITY trial on March 20, 2020 involving 45 countries and counting which tests four therapy groups: Remdesivir, lopinavir-ritonavir with or without interferon, and hydroxychloroquine. The rationale for selecting these drugs was stated as there was some evidence of effectiveness against the SARS-CoV 2 virus, which caused Covid-19, either in vitro and/or animal studies [11].
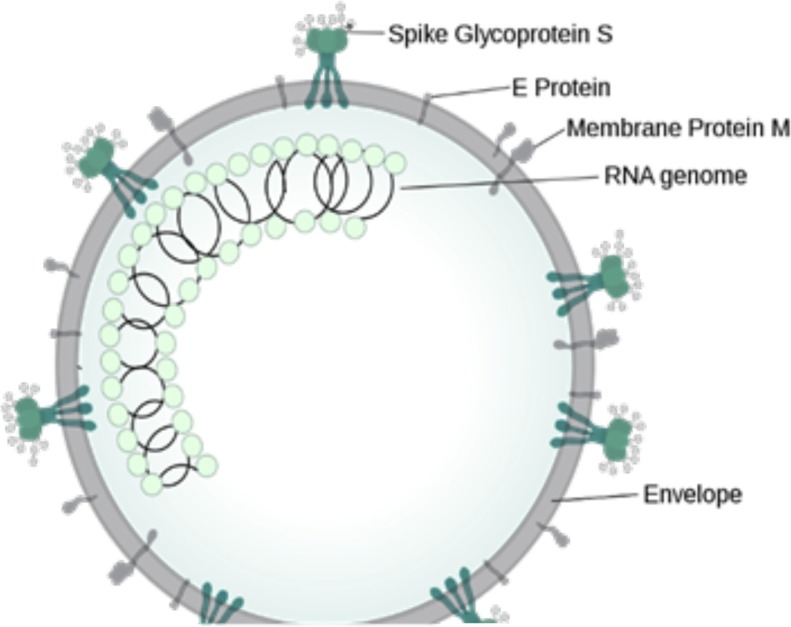
As shown in in the figure, the envelope is like HIV virus consisting of spike glycoprotein S, E protein and membrane protein M which are essential for entry of virus in the cell. This is a RNA virus and thus RNA genome is present in the core.
6. Pathophysiology and potential therapeutic targets
Fig. 2 is representing the structure of novel corona virus which is like other RNA viruses containing various glycoproteins on the envelope and the RNA genome in the core. As shown in Fig. 3 , the viral cycle is like other viruses consisting of attachment, integration, uncoating, use of cell machinery for replication, assembly and finally release of virions. Steps in coronavirus replication are potential targets for antiviral drugs and vaccines [2]. The spike glycoprotein S of coronavirus is a good candidate for vaccines because neutralizing antibodies are directed against glycoprotein S. Blockade of the specific virus receptor on the surface of the host cell by monoclonal antibodies or other ligands can prevent virus entry. Receptor-induced conformational changes in the S protein can be blocked by peptides that inhibit membrane fusion and virus entry. The polyprotein of the replicase protein is cleaved into functional units by virus-encoded proteinases. Protease inhibitors may block replication. The polymerase functions in a unique membrane-bound complex in the cytoplasm, and the assembly and functions of this complex are potential drug targets. Viral mRNAs made by discontinuous transcription are shown in the cytoplasm with the protein that each encodes indicated at the right. The common 70 base long leader sequence on the 5′ end of each mRNA is shown in red. Budding and exocytosis are processes essential to virus replication that may be targets for development of antiviral drugs.
Fig. 2.
Diagrammatic representation of the structure of novel corona virus.
Fig. 3.
Experimental therapeutic strategies attempt to interfere with different steps in the coronavirus replication cycle. (It shows various targets for potential drugs. Hydroxychloroquine and chloroquine prevent entry into the cell, convalescent plasma is responsible for passive immunity while all the anti-viral drugs and protease inhibitors like Lopinavir are responsible for inhibiting viral replication and processing of viral proteins. Not shown in the figure are various pro-inflammatory cytokines like IL-1 responsible for increased fatality through cytokine storm.
Apart from the pathophysiology of virus itself, it is now believed that the primary cause of mortality in susceptible patients is either due to pro-inflammatory cytokine storm or due to secondary bacterial infection. Secondary bacterial infections can be treated by using various antibiotics. Research is currently focussed for innovation of new therapies to treat this potentially fatal cytokine storm. Besides, most microbes including COVID-19 are known to bind to Toll like Receptor (TLR) which in turn induces a highly proinflammatory cytokine, Interleukin 1 (IL-1). IL-1 is the mediator of fever and fibrosis. This can lead to further deterioration in susceptible COVID positive patients. Thus, drugs supressing IL-1 or IL-1 receptor can be taken into consideration for treatment of COVID -19 [3].
A study has found out that women are much less susceptible to being affected by COVID 19 compared to men. Initially, the reason was hypothesised to be only environmental stating that as smoking is more prevalent in men and hence their respiratory system is compromised leading to greater risk of COVID infection. However, new research suggests that there are some innate differences between both sexes leading to the difference in the susceptibility. The presence of an extra X chromosome in females lead to lower viral load levels, and less inflammation than in men, while CD4 + T cells are higher with better immune response. Besides, women produce higher titre of antibodies which tends to remain in circulation for longer periods. The levels of activation of the immune cells are higher in women than in men, and it is correlated with the trigger of TLR7 and the production of interferon (IFN). TLR7 is higher in women than in men and it’s biallelic expression leads to higher immune responses and increases the resistance to viral infections. TLR7 is expressed in innate immune cells which recognizes single strand RNA virus by promoting the production of antibodies against the virus and the generation of pro-inflammatory cytokines including IL-6 and IL-1 family members. Moreover, in women the production of inflammatory IL-6 after viral infection is lower than in males and is often correlated with a better longevity. In addition, on the X chromosome there are loci that code for the genes involved in the regulation of immune cells such as FOXP3 and transcription factor for T regulatory cells (Treg) involved in virus pathogenesis. The X chromosome influences the immune system by acting on many other proteins, including TLR8, CD40 L and CXCR3 which can be over-expressed in women, and influence the response to viral infections and vaccinations. However, greater production of pro-inflammatory cytokines enhances risk of cytokine storm. Thus, drugs acting against pro-inflammatory cytokines can be theoretically more effective in women compared to men and in future, there may be gender wise differential therapy for treatment of COVID 19 [4].
7. Pre-clinical and clinical evidence of drug therapies
A. Chloroquine
-
•
Mechanism of Action: Primarily inhibits the entry, transport and the post-entry stages of SARS-CoV-2. It increases endosomal pH and interferes with the glycosylation of cellular receptor of SARS-CoV and thereby it has the potential to block viral infection. It also inhibits cathepsins, that leads to the formation of the autophagosome which cleaves SARS-CoV-2 spike protein. Furthermore, chloroquine through the inhibition of MAP-kinase interferes with SARS-CoV-2 molecular crosstalk, besides altering the virion assembly, budding and interfering with the proteolytic processing of the M protein.
-
•
In- vitro: Chinese researchers who studied the effect of chloroquine in vitro (using Vero E6 cell line infected by SARS-CoV-2) found chloroquine to be highly effective in reducing viral replication that can be easily achievable with standard dosing due to its favourable penetration in tissues including the lung [5].
-
•
Animal model: Antiviral activity of chloroquine against human coronavirus OC43 infection in new born C57BL/6 mice was highly effective [6].
-
•
Key Clinical Trial: A Chinese study involving more than 100 patients of COVID-19 found chloroquine superior to the control group in reducing symptom duration, exacerbation of pneumonia including radiological improvement and promoting virus-negative seroconversion without any severe side effects [7].
B. Hydroxychloroquine (HCQ)
-
•
Mechanism of Action: Same as that of chloroquine since same structure except for an additional hydroxy moiety in one terminal in HCQ.
-
•
Safety Profile: Addition of hydroxyl molecule makes HCQ less permeable to blood-retinal barrier and allows faster clearance from retinal pigment cell, thereby suggesting a lesser risk of retinal toxicity with HCQ, as compared to chloroquine. Furthermore, the narrow therapeutic and safety index margin with chloroquine makes HCQ a safer option than chloroquine. Long-term clinical safety profile of HCQ is better than that of chloroquine since it allows higher daily dose of HCQ with less drug-drug interactions [5].
-
•
In- vitro: Hydroxychloroquine (EC50 = 0.72 μM) was found to be more potent than chloroquine (EC50 = 5.47 μM) in vitro against SARS-CoV-2 [8].
-
•
Animal model: Whether HCQ works in-vivo is not yet proven for COVID-19 [9].
-
•
Key Clinical Trial: In an open-label study of 36 patients with COVID-19, use of HCQ (200 mg three times per day for 10 days) was associated with a higher rate of undetectable SARS-CoV-2 RNA on nasopharyngeal specimens at day 6 compared with no specific treatment (70 versus 12.5 percent) [10].
C. Remdesivir (RDV)
-
•
Mechanism of Action: It inhibits viral replication through premature termination of RNA transcription.
-
•
In- vitro: It has shown to inhibit SARS-CoV-2 replication in Vero-E6 cells with EC50 at 23.15um.
-
•
Animal model: It has demonstrated to improve pulmonary function and reduce lung viral loads in mice infected with MERS-CoV [11].
-
•
Key Clinical Trial: A single case report by Holshue et al. described clinical improvement after RDV used to treat the first U.S. case of COVID-19. There are several randomized control trials are currently being conducted to evaluate the efficacy and safety of RDV in patients with COVID-19. Two phase III trials initiated in China in February 2020, aimed to evaluate RDV in hospitalized adult patients with mild/moderate (NCT04252664) or severe (NCT04257656) COVID-19 (RDV 200 mg on day 1 and 100 mg once daily for 9 days vs. placebo). Preliminary results of these trials are expected to be announced at the end of April 2020 [12].
D. Lopinavir-ritonavir
-
•
Mechanism of Action: The polyprotein of the replicase protein is cleaved into functional units by virus-encoded proteinases which is inhibited by these protease inhibitor combinations. Thus, inhibiting proteolysis.
-
•
In- vitro: The lopinavir/ritonavir combination was first considered a potentially useful treatment after in-vitro studies showed it had antiviral activity against SARS coronavirus [13].
-
•
Animal model: The lopinavir/ritonavir-treated and interferon-β1b-treated animals had better outcome than the untreated animals, with improved clinical (mean clinical scores ↓50.9 %–95.0 % and ↓weight loss than the untreated animals), radiological (minimal pulmonary infiltrates), and pathological (mild Broncho interstitial pneumonia) findings, and lower mean viral loads in necropsied lung (↓0.59−1.06 log10 copies/glyceraldehyde 3-phosphate dehydrogenase [GAPDH]; P < .050) and extrapulmonary (↓0.11–1.29 log10 copies/GAPDH; P < .050 in kidney) tissues [14].
-
•
Key Clinical Trial: Chan and colleagues compared outcomes in people who received lopinavir/ritonavir as initial treatment, and as rescue therapy, with matched controls; all patients were given ribavirin and steroids according to a standardised protocol. The addition of lopinavir/ritonavir as initial treatment was associated with a statistically significant reduction in the overall death rate and intubation rate compared with matched controls (p < 0·05) [15].
[Note: Camostat Mesylate is another drug of same class (Protease inhibitors) having same mechanism of action, has shown evidence of effectively blocking SARS-CoV-2 in lung cells in-vitro.] [5].
E. Convalescent plasma
-
•
The US-FDA has accepted emergency investigational new drug applications for use of convalescent plasma for patients with severe or life-threatening COVID-19 [16].
-
•
A case series described administration of plasma from donors who had completely recovered from COVID-19 to five patients with severe COVID-19 on mechanical ventilation and persistently high viral titres despite investigational antiviral treatment. The patients had decreased nasopharyngeal viral load, decreased disease severity score, and improved oxygenation by 12 days after transfusion, but these findings do not establish a causal effect. Finding appropriate donors and establishing testing to confirm neutralizing activity of plasma may be logistical challenges [17,18].
F. Drugs Acting Against Pro-Inflammatory Factors
-
a)
Interleukin 1
Interleukin 1 (IL-1) consists of two molecules namely IL-1a and IL-1b, associated with innate immunity. IL-1b is associated with most of the biological pleiotropic properties and hence it is directly referred as IL-1. IL-1 is proinflammatory cytokine and it binds to IL-1 receptor to modulate it’s action. Association with IL-1 receptor leads to recruitment of other pro-inflammatory cytokines. Anakinra is one of the anti-interleukin 1 antagonist which is used to treat rheumatoid arthritis and currently being repurposed for the treatment of COVID-19 [19].
-
•
Mechanism of Action: Inhibits actions of IL-1 leading to inhibition of cytokine storm which is common reason for fatality in COVID-19
-
•
Current Evidence: No current in-vitro and in vivo evidence specifically for COVID 19. Currently, there is no published clinical trial. However, as per the data on clinicaltrials.gov, there are 8 trials using Anakinra against COVID-19 either in recruitment or pre-recruitment stage [20]. One of the major phase 3 trials using this drug is ongoing in Italy and is initiated by the manufacturer (Swedish Orphan Biovitrum) to evaluate efficacy and safety of anakinra or emapalumab with standard of care in reducing hyperinflammation and respiratory distress in patients with COVID-19.
-
b)
Interleukin 6
IL-6 is one of the key pro-inflammatory cytokines. IL-6 activates its downstream Janus kinase (JAK) signal by binding the transmembrane (cis-signalling) or soluble form (trans-signalling) of the IL-6 receptor (IL-6R) and interacting with membrane-bound gp130. Excessive IL-6 signalling leads to a myriad of biological effects that contribute to organ damage, such as maturing naïve T cells into effector T cells, inducing vascular endothelial growth factor (VEGF) expression in epithelial cells, increasing vessel permeability and reduces myocardial contractility [21].
-
•
Mechanism of Action: Tocilizumab is a recombinant humanized monoclonal anti-IL‐6R antibody. It binds both soluble and membrane‐bound IL‐6R to inhibit IL‐6‐mediated cis-signalling and trans-signalling.
-
•
Current Evidence: Case study/series describing use of tocilizumab in patients with COVID-19 have been reported from various areas of the world. In preliminary data from a non-peer reviewed, single-arm Chinese trial involving 21 patients with severe or critical COVID-19 infection, showed rapid fever reduction and a reduced need for supplemental oxygen within few days after receiving tocilizumab (initially given as a single 400-mg dose by IV infusion; this dose was repeated within 12 h in 3 patients because of continued fever). In China: Randomized, multicentre, controlled clinical trial evaluating efficacy & safety in 188 patients with COVID-19 is under way. Results are not yet available. In US/Global scenario, randomized, placebo-controlled trial is in phase 3 (NCT04320615) is in collaboration with the US Health and Human Services Biomedical Advanced Research and Development Authority (BARDA); the study will evaluate safety and efficacy of tocilizumab in combination with standard of care compared with placebo. In this study, there is expectation of enrolment of about 330 patients globally, including in the U.S., beginning in April 2020 [22].
G. Anti-inflammatory cytokines as drugs
-
a)
Interleukin 37 (IL-37)
Though it is a member of IL-1 family and is structurally like IL-1, it has anti-inflammatory activity. IL-37 has several mechanisms for immunosuppression but it ultimately leads to suppression of IL-1. It inhibits histocompatibility complex (MHC) molecules and thus inflammation by suppressing IL-1, IL-6, TNF & CCL2 [23]. Currently, there is no evidence regarding safety and efficacy of IL-37.
-
b)
Interleukin 38 (IL-38)
Like IL-37, this is one of the most recently discovered anti-inflammatory cytokine belonging to the family of IL-1. It binds to the receptor of Interleukin 1 receptor type 6 and leads to suppression of inflammation. In-vitro cultures of activated peripheral blood mononuclear cells (PBMCs) are inhibited by IL-38 in the production of several cytokines including IL-1, IL-17 and IL-22. IL-38 gene knock out mice are more susceptible to inflammatory conditions. Currently there are no clinical trials using either IL 38 or its analogues. However, IL-38 can also be a potential new therapy [24].
8. Conclusion
As this is still an evolving topic, only the key medications mentioned in WHO and CDC guidelines as of 9th April 2020 have been incorporated in this review. Besides, we have added potential immunosuppressive therapies in the review as cytokine storm is one of the most common reasons for mortality in susceptible individuals. However, there are many more drugs currently under investigation including drugs like ivermectin, Vitamin C, Baloxavir, colchicine and Tacrolimus. The efficacy and safety of these drugs is still unknown and will be clearer in the coming months.
Funding
None. The research was self funded.
Declaration of Competing Interest
None.
References
- 1.Ahmed S.S. The Corona Virus Disease 2019 (COVID 19): A Review. Journal of Advances in Medicine and Medical Research. 2020;32 [Google Scholar]
- 2.Holmes K.V. SARS coronavirus: a new challenge for prevention and therapy. J. Clin. Invest. 2003;111:1605–1609. doi: 10.1172/JCI18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti P., Gallenga C.E., Tetè G., Caraffa A., Ronconi G., Younes A., Toniato E., Ross R., Kritas S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- 4.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 5.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:241. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 8.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [Published Online, ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiev T. Coronavirus disease 2019 (COVID-19) and anti-rheumatic drugs. Rheumatol. Int. 2020:1. doi: 10.1007/s00296-020-04570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2002. Therapeutic Options for Patients; p. 2002.https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html (Accessed 9 April 2020) [Google Scholar]
- 11.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.-P., Chu H., Zhou J., Chen H., Qin C., Yuen K.-Y. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M.L., Tse M.W., Que T.L., Peiris J.S.M., Sung J., Wong V.C.W., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. [PubMed] [Google Scholar]
- 16.T. Food, Revised Information for Investigational COVID-19 Convalescent Plasma, Food and Drug Administration. 2 (2020) 1–4. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed May 17, 2020).
- 17.Lu C.-C., Chen M.-Y., Chang Y.-L. Potential therapeutic agents against COVID-19: what we know so far. J. Chin. Med. Assoc.: JCMA. 2020 doi: 10.1097/JCMA.0000000000000318. [Published online, ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA – J. Am. Med. Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam Monteagudo L., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020 doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.– List Results Search of: filaggrin | Interventional Studies - List Results - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/results?cond=COVID&term=&type=Intr&rslt=&age_v=&gndr=&intr=Interleukin+1&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&so (accessed May 17, 2020).
- 21.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [Published online, Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASHP . 2020. Assessment for Evidence of COVID Related Treatment.https://www.ahfscdi.com/login (Accessed 1 May 2020) [Google Scholar]
- 23.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 24.van de Veerdonk F.L., de Graaf D.M., Joosten L.A., Dinarello C.A. Biology of IL-38 and its role in disease. Immunol. Rev. 2018;281:191–196. doi: 10.1111/imr.12612. [DOI] [PubMed] [Google Scholar]