Highlights
-
•
Cerebrovascular disease was associated with mortality and severity of COVID-19 (borderline).
-
•
Cardiovascular disease was associated with mortality and severity of COVID-19.
-
•
Gender, age, hypertension, diabetes, and respiratory comorbidities did not influence the associations
-
•
The association between cerebrovascular disease and poor outcome in COVID-19 was not affected by cardiovascular diseases and vice versa .
Keywords: Cardiovascular, Cerebrovascular, COVID-19, Mortality, Severity
Abstract
Background
We conducted a systematic review and meta-analysis to evaluate the latest evidence on the association between cerebrovascular, and cardiovascular diseases and poor outcome in patients with Coronavirus Disease 2019 (COVID-19) pneumonia.
Methods
A comprehensive systematic literature search was performed using PubMed, SCOPUS, EuropePMC, and Cochrane Central Database. The outcome of interest was composite poor outcome that comprised of mortality and severe COVID-19.
Results
A total of 4448 patients were obtained from 16 studies. Cerebrovascular disease was associated with an increased composite poor outcome (RR 2.04 [1.43,2.91], p<0.001; I2: 77%). Subgroup analysis revealed that cerebrovascular disease was associated with mortality (RR 2.38 [1.92,2.96], p<0.001; I2: 0%) and showed borderline significance for severe COVID-19 (RR 1.88 [1.00,3.51], p = 0.05; I2: 87%). Cardiovascular disease was associated with increased composite poor outcome (RR 2.23 [1.71,2.91], p<0.001; I2: 60%), mortality (RR 2.25 [1.53,3.29], p<0.001; I2: 33%) and severe COVID-19 (RR 2.25 [1.51,3.36], p<0.001; I2: 76%). Meta-regression demonstrate that the association was not influenced by gender, age, hypertension, diabetes, and respiratory comorbidities. Furthermore, the association between cerebrovascular disease and poor outcome was not affected by cardiovascular diseases and vice versa.
Conclusion
Cerebrovascular and cardiovascular diseases were associated with an increased risk for poor outcome in patients with COVID-19.
Introduction
At the time of writing this paper, Coronavirus Disease 2019 (COVID-19) was declared a global pandemic, which had infected over 3.3 million people and caused more than 238.000 deaths,1 These numbers are likely to increase by the time of publication. Even though most of the infected individuals have mild or no symptoms, some exhibit more serious complications including severe pneumonia, acute respiratory distress syndrome, and multi-organ failure. Clinical markers can be valuable for the efficient allocation of resources during the pandemic.
Initial reports of COVID-19 cases in China have identified that cerebrovascular and cardiovascular disease were prevalent comorbidities among COVID-19 patients.2 Further study have shown that both cerebrovascular and cardiovascular diseases were associated with a higher incidence of severe COVID-19, which needs to be monitored in the intensive care unit (ICU).3 However, due to the sample size, the report did not reach adequate statistical power for definite conclusions. Nevertheless, these findings lead us to postulate that cerebrovascular and cardiovascular comorbidities might independently be associated with the severity of COVID-19. In this systematic review and meta-analysis, we aimed to evaluate the latest evidence on the association between cerebrovascular andcardiovascular disease and poor outcome in patients with COVID-19.
Methods
Search strategy and study selection
We carried out a comprehensive systematic literature search from PubMed, SCOPUS, EuropePMC, and Cochrane Central Database with the following search terms 1) “COVID-19” OR “SARS-CoV-2” AND “characteristics”, 2) “COVID-19” OR “SARS-CoV-2” AND “cerebrovascular”, and 3) “COVID-19” OR “SARS-CoV-2” AND “cardiovascular”. Two authors independently performed an initial search and screening for relevant articles through title and abstract. Discrepancies were resolved by discussion and discretion of the third author. After removal of duplicates, the potential full-texts were evaluated by applying inclusion and exclusion criteria. The literature search was finalized on April 10th, 2020.
Inclusion and exclusion criteria
In our analysis, we included every study that reported adult COVID-19 patients with information on cerebrovascular or cardiovascular diseases and mortality or clinically validated definition of severe COVID-19.4 We excluded review articles, editorials, correspondence, case reports, case series, pediatric population, and articles in non-English languages.
Data extraction
Two independent authors performed data extraction from the studies. We used standardized forms that included author, year, study design, age, gender, cerebrovascular diseases, cardiovascular diseases, hypertension, diabetes mellitus, mortality, and severe COVID-19.
The definition of cerebrovascular disease used in this meta-analysis was history (comorbidity) of cerebrovascular disease and its synonyms such as stroke and brain infarction. The definition of cardiovascular disease in this meta-analysis was history (comorbidity) of cardiovascular or cardiac disease. Hypertension/coronary heart disease/cardiomyopathy in specific terms was excluded because these diseases often overlap and potentially result in overestimation of cases.
The outcome of interest was composite poor outcome that consisted of mortality and severe COVID-19. Severe COVID-19 patients were defined as patients who had any of the following features during or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of partial pressure of arterial oxygen (PaO2) to fractional concentration of oxygen inspired air (FiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multi organ dysfunction/failure).4
Statistical analysis
The meta-analysis was performed using Review Manager 5.3 (Cochrane Collaboration) and Stata version 16. We used the Mantel-Haenszel formula for calculating dichotomous variables to find risk ratios (RRs), which are reported along with their 95% confidence intervals (CIs). A random-effects model was used for the calculation regardless of the heterogeneity. All P-values in this study were two-tailed, and statistical significance was set at <0.05. A restricted-maximum likelihood random effects meta-regression was performed for age, gender, cardiovascular disease/cerebrovascular disease, hypertension, and diabetes mellitus. Regression-based Harbord's test was implemented to evaluate the small-study effect. An inverted funnel-plot analysis was performed to evaluate the risk of publication bias.
Results
Study selection and characteristics
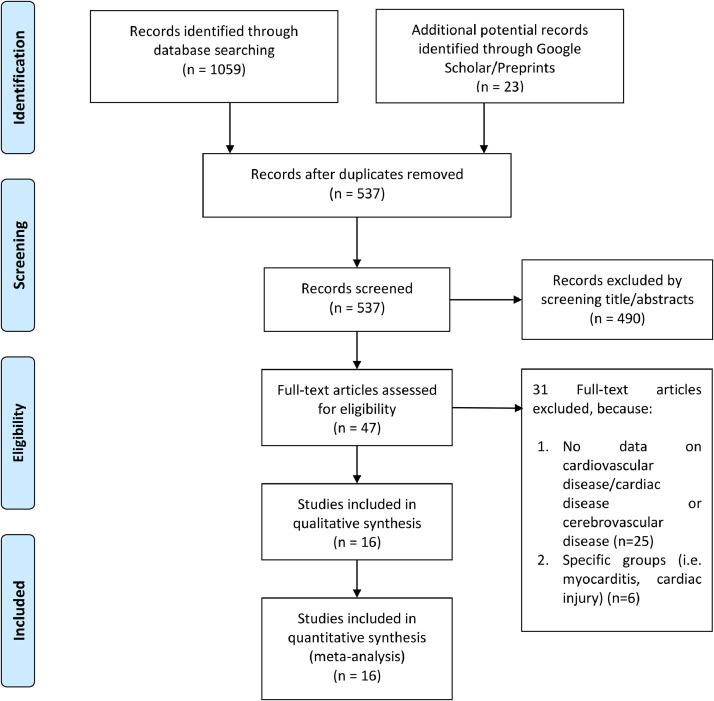
After the omission of duplicates, we were left with 537 patient records, out of 1082 records. After screening the titles and abstracts, 490 records were excluded. After evaluating 47 full-text articles for eligibility, we excluded 31 of them because 1) no outcome for cardiovascular disease/cardiac disease or cerebrovascular disease (n = 25), and 2) specific groups (i.e., myocarditis, cardiac injury) (n = 6). 16 studies were included in the qualitative synthesis and meta-analysis. [Fig. 1 ]. Overall, there were 4448 patients from 16 studies.5 , 6 , 15, 16, 17, 18, 19, 20 , 7, 8, 9, 10, 11, 12, 13, 14 The characteristics of the included studies are displayed in Table 1 . The data for the table are presented in the grouping poor outcome (+) vs poor outcome (-). The forest plot is presented in groupings cerebrovascular disease (+) vs cerebrovascular disease (-) and cardiovascular disease (+) vs cardiovascular disease (-).
Fig. 1.
PRISMA Flowchart
Table 1.
Characteristics of the included studies
| Authors | Study Design | Samples | Age (Mean/Median) (years) | Male (%) | Cerebrovascular Disease (%) | Cardiovascular Diseases (%) | Hypertension (%) | Diabetes (%) | Respiratory Comorbidities (%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Akbari A 2020 | Observational Retrospective | 13 vs 427 | 48 (overall) | 61.5 vs 56.2 | N/A | 15.3 v 5.4 | 15.3 vs 7.7 | 30.8 vs 6.8 | N/A | Mortality |
| Bai T 2020 | Observational Retrospective | 36 vs 91 | 67 vs 50 | 77.8 vs 57.1 | 5.6 vs 5.5 | 5.6 vs 1.1 | 41.7 vs 23.1 | 13.9 vs 11.0 | N/A | Mortality |
| Cao J 2020 | Observational Retrospective | 17 vs 85 | 72 vs 53 | 76.5 vs 47.1 | 17.6 vs 3.5 | 17.6 vs 2.4 | 64.7 vs 20 | 35.3 vs 5.9 | 23.5 vs 7.1 | Mortality |
| Chen T 2020 | Observational Retrospective | 113 vs 161 | 68.0 vs 51.0 | 73 vs 55 | 4 vs 0 | 14 vs 4 | 48 vs 24 | 21 vs 14 | 10 vs 4 | Mortality |
| Fu L 2020 | Observational Retrospective | 34 vs 166 | <49 (5.9 vs 28.3), 50-59 (23.5 vs 27.1), 60-69 (20.6 vs 31.3), >70 (5 vs 13.2) | 16.2 vs 67.7 | N/A | 8 (cardiac) [overall] | 50.5 [overall] | 68.5 | 4 [overall] | Mortality |
| Luo XM 2020 | Observational Retrospective | 100 vs 303 | 71 vs 49 | 57 vs 44.9 | 22 vs 5.6 | 16 vs 6.6 | 60 vs 17.5 | 25 vs 10.6 | 17 vs 3.6 | Mortality |
| Yuan M 2020 | Observational Retrospective | 10 vs 17 | 68 vs 55 | 47 vs 40 | 10 vs 0 | 30 vs 0 | 50 vs 0 | 60 vs 0 | N/ A | Mortality |
| Guan 2020 | Observational Retrospective | 173 vs 926 | 52.0 vs 45.0 | 57.8 vs 38.2 | 2.3 vs 1.2 | 5.8 vs 1.8 | 23.7 vs 13.4 | 16.2 vs 5.7 | 3.5 vs 0.6 | Severe COVID-19 |
| Hu L 2020 | Observational Retrospective | 172 vs 151 | 65 vs 56 | 52.9 vs 49.7 | 1.7 vs 2.6 | 19.2 vs 5.3 | 38.3 vs 25.8 | 19.2 vs 9.3 | 3.5 vs 0 | Severe COVID-19 |
| Li Q 2020 | Observational Retrospective | 26 vs 299 | 65 vs 49 | 76.9 vs 49.2 | 7.7 vs 0 | 19.2 vs 4.3 | 46.2 vs 22.1 | 19.2 vs 8.4 | 7.7 vs 0.6 | Severe COVID-19 |
| Liu Jingyuan 2020 | Prospective Cohort | 17 vs 44 | 56 vs 41 | 58.8 vs 47.7 | N/A | 5.9 vs 0 | 35.3 vs 13.6 | 17.6 vs 4.5 | 17.6 vs 4.5 | Severe COVID-19 |
| Qin 2020 | Observational Retrospective | 286 vs 166 | 61 vs 53 | 54.2 vs 48.2 | 2.8 vs 1.8 | 8.4 vs 1.8 | 36.7 vs 18.1 | 18.5 vs 13.3 | 3.1 vs 1.8 | Severe COVID-19 |
| Wan 2020 | Observational Retrospective | 40 vs 135 | 56 vs 44 | 52.5 vs 54.7 | N/A | 15 vs 1 | 10 vs 9.4 | 22.5 vs 3.1 | 2.5 vs 0 | Severe COVID-19 |
| Wang Dan 2020 | Observational Retrospective | 71 vs 72 | 65 vs 44 | 62 vs 40.3 | 4.2 vs 2.8 | 16.9 vs 5.6 | 43.7 vs 6.9 | 12.7 vs 5.6 | 9.9 vs 4.2 | Severe COVID-19 |
| Wang Y 2020 | Observational Retrospective | 38 vs 72 | ≤40 (7.9 vs 69.4), 41-60 (21.0 vs 18.1), >60 (71.0 vs 12.5) | 63.2 vs 33.3 | 7.9 vs 5.6 | N/A | 39.5 vs 11.1 | 21.0 vs 9.7 | 10.5 vs 2.8 | Severe COVID-19 |
| Zhang Guqin 2020 | Observational Retrospective | 55 vs 166 | 62 vs 51 | 63.6 vs 44.0 | N/A | 23.6 vs 5.4 | 47.3 vs 16.9 | 12.7 vs 9.0 | 7.3 vs 1.2 | Severe COVID-19 |
Table was presented in a grouping of poor outcome (+) vs poor outcome (-)
COVID-19: Coronavirus disease 2019; N/A: Not available
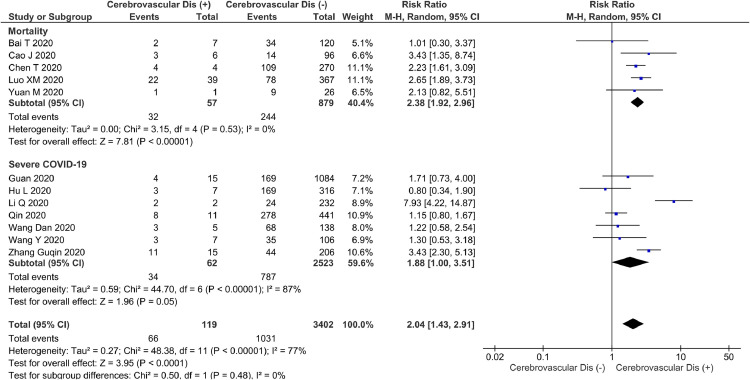
Cerebrovascular disease and poor composite outcome
Cerebrovascular disease was associated with increased poor composite outcome (RR 2.04 [1.43,2.91], p<0.001; I 2: 77%, p<0.001) [Fig. 2 ]. Subgroup analysis revealed that cerebrovascular disease was associated with mortality (RR 2.38 [1.92,2.96], p<0.001; I 2: 0%, p = 0.53). However, cerebrovascular disease showed borderline significance for association with severe COVID-19 (RR 1.88 [1.00,3.51], p = 0.05; I 2: 87%, p<0.001).
Fig. 2.
Cerebrovascular disease and poor patient outcome. Forest-plot showing increased risk of composite poor outcome, including its mortality subgroup and was borderline significant for the severity subgroup.
Cardiovascular disease and poor composite outcome
Cardiovascular disease was associated with increased poor composite outcome (RR 2.23 [1.71,2.91], p<0.001; I 2: 60%, p = 0.004) [Fig. 3 ]. Subgroup analysis revealed that cardiovascular disease was associated with both mortality (RR 2.25 [1.53,3.29], p<0.001; I 2: 33%, p = 0.19) and severe COVID-19 (RR 2.25 [1.51,3.36], p<0.001; I 2: 76%, p = 0.001).
Fig 3.
Cardiovascular disease and poor patient outcome. Forest-plot showing increased risk of composite poor outcome, including its mortality and severity subgroup.
Meta-regression
Meta-regression analysis indicated that the association between cerebrovascular disease and composite poor outcome was not influenced by gender (p = 0.714), age (p = 0.872), hypertension (p = 0.575), cardiovascular diseases (p = 0.607), diabetes (p = 0.356) and respiratory comorbidities (p = 0.981).
Meta-regression analysis for cardiovascular disease similarly indicated that the association between cardiovascular disease and composite poor outcome was not influenced by gender (p = 0.722), age (p = 0.910), hypertension (p = 0.218), cerebrovascular diseases (p = 0.502), diabetes (p = 0.062), and respiratory comorbidities (p = 0.703).
Publication bias
The inverted funnel-plot demonstrated a qualitatively asymmetrical shape for both cerebrovascular disease and cardiovascular disease on composite poor outcome. Regression-based Harbord's test demonstrated no indication of small-study effects for cerebrovascular disease (p = 0.579) and cardiovascular disease (p = 0.116) on composite poor outcome.
Discussion
The findings of this study showed that cerebrovascular and cardiovascular diseases were associated with an increased poor outcome in COVID-19. The association was not influenced by gender, age, hypertension, diabetes, and respiratory comorbidities. The association between cerebrovascular disease and poor outcome in COVID-19 patients was not affected by cardiovascular diseases and vice versa.
The presence of underlying cardiac and cerebrovascular pathologies may increase the incidence and severity of infectious diseases, like COVID-19; which has a critical pathomechanism.21 , 22 Acute respiratory infections of viral and bacterial etiologies are well-recognized triggers of cardiovascular events. Hence it is plausible that COVID-19 may either induce new cardiac pathologies and/or aggravate existing cardiovascular diseases.21 , 23 , 24
Angiotensin-converting enzyme 2 (ACE2) is a type 1 transmembrane metalloenzyme and carboxypeptidase that is abundantly present in cardiac epithelial cells, as well as in respiratory, kidney and intestinal tissues. ACE2 plays an important role in the interaction between cardiovascular diseases and COVID-19. It cleaves angiotensin II into angiotensin 1–7, thus exerting more anti-inflammatory and antioxidant actions than inflammatory roles.25 , 26 This enzyme might be embraced by the virus after the activation of viral surface spike (S) protein by the transmembrane protease serine 2 (TMPRSS2).27
One of the mechanisms that potentially mediates cardiovascular disease and higher severity of COVID-19 involves the ACE2 pathway. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are commonly used in individuals with cardiovascular diseases. These drugs upregulate ACE2 expression which consequently facilitates SARS-CoV-2 entry into pneumocytes, and further causes exacerbation/decompensation of the underlying disease.28 The virus downregulates ACE2 expression, limiting its organoprotective effects, thus resulting in renin-angiotensin-aldosterone system (RAAS) dysfunction.25 , 29 Unfortunately, the studies included in the analysis did not provide information regarding the use of ACEI/ARB; thus, the relationship remains inconclusive. Nevertheless, recent evidences demonstrate that cardiac damage might be due to a combination of direct damage and indirect damage through cytokine storm. Additionally, elevation of troponin and N-terminal pro–B-type natriuretic peptide (NT-proBNP) have been associated with increased mortality.30 , 31
The underlying mechanism of increased severity of COVID-19 in patients with cerebrovascular disease remains unclear. It is, therefore, of great interest for further investigations. One plausible explanation for this is that both cerebrovascular and cardiovascular diseases share the same risk factors and often overlapped, hypertension, atrial fibrillation, chronic kidney diseases, and diabetes may increase the risk for various cardio-cerebrovascular diseases. Factors such as diabetes itself have also been shown to increase mortality and severity of COVID-19.22 ACEI/ARB is often used for patients with hypertension and prior stroke, hence, the interplay between these mechanisms may partially explain the association.32 Nevertheless, the presence of hypertension, cardiovascular diseases, and diabetes did not affect the association between cerebrovascular diseases and poor patient outcomes. However, it should be noted that meta-regression itself has limitations in exploring the association between variables, thus facilitating the need for larger cohorts. Besides the indirect association, theoretically, the presence of brain medullary cardiorespiratory or autonomic nervous system dysfunction potentially cause blood pressure and respiratory dysfunction which promotes the risk of contracting opportunistic infections (viral and bacterial).33 The cholinergic pathway suppresses pulmonary innate immunity, increasing the risk of bacterial pneumonia in post-stroke patients.34 Another possible hypothesis for the role of underlying cerebrovascular disease in increasing the risk of poor outcome in COVID-19 patients is the relative immobility in post-stroke patients, which increases the risk for hypercoagulable state that culminates in thrombus formation. Severe SARS-CoV-2 infection itself could induce a dysfunctional hemostatic system leading to a hypercoagulable state, a condition which we commonly encounter in sepsis.35 , 36 Furthermore, a recent evidence of lung pathology in critically ill COVID-19 patients has shown occlusion and microthrombosis in pulmonary blood vessels, which suggests that pulmonary embolism might have a role in increasing the risk of poor patient outcome.37 Unfortunately, none of the included studies specify any functional or motoric status among patients with cerebrovascular disease; thus, any conclusion regarding this hypothesis can neither be proven nor refuted.
Moreover, emerging evidence demonstrates that extra-pulmonary invasion including in the central nervous system, causes substantial neuronal damage.38 Neuroinvasion is one of the possible traits of COVID-19 because of the similarities between SARS-CoV and SARS-CoV-2. Human coronaviruses (OC-43, 229E, MERS, and SARS) and several animal coronaviruses depict this neuroinvasive potential.39 However, the central nervous system normally expresses very low concentrations of ACE2 or dipeptidyl peptidase 4 (DPP4), which is the receptor for SARS-CoV and MERS-CoV entry.40 Possible routes for nervous system involvement after SARS-CoV-2 infection are direct invasion through blood circulation or neuronal pathway, hypoxic injury, immune system damage, ACE2, and the lack of major histocompatibility complex (MHC) antigens in nerve cells. It is currently thought that SARS-CoV-2, together with host immune mechanisms, can turn these infections into persistent diseases with neurological complications, such as viral encephalitis, infectious toxic encephalopathy and acute cerebrovascular disease.41 A single-center observation showed that in 221 COVID-19 patients, 5% developed acute ischemic stroke, 0.5% cerebral venous sinus thrombosis, and 0.5% cerebral hemorrhage. The incidence was higher in older patients with risk factors.42 The authors also stated that an increased inflammatory response and hypercoagulable state was found in patients who developed cerebrovascular events. It has been demonstrated that the risk of poor outcome was higher in patients with prolonged prothrombin time, international normalized ratio, increased thrombin time, and d-dimer which indicates coagulopathy that predisposes patients to thrombotic events.43 Middeldorp et al. reported a 15% (95% CI, 9.3-22) and 34% (95% CI, 23-46) incidence of venous thromboembolism at 7 and 14 days, respectively.44 In patients with intracardiac communication such as patent foramen ovale, this poses a risk for paradoxical thromboembolism. Oxley et al. reported 5 cases of large-vessel stroke in young patients aged <50 years old.45 Two of the reported patients were 33 and 37 years old without risk factors for stroke. Magnetic resonance imaging of the head and neck as well as echocardiographic investigation of the former showed no potential source of thrombus. This suggest that COVID-19 may cause clotting in large vessels of patients not exhibiting risk factors for stroke.
Previously, influenza has been shown to increase the risk of stroke.46 These complications can be more severe in the presence of compromised cerebral vasculature. Cerebrovascular diseases often coexist with cardiovascular diseases and their association with poor patient outcomes might be influenced by the latter. Nevertheless, meta-regression demonstrated that the association was not affected by the presence of cardiovascular disease.
Implications for clinical practice
This meta-analysis showed that cerebrovascular and cardiovascular diseases are important risk factors for mortality and severity of COVID-19 patients. Physicians should also be alerted that the risks of opportunistic and bacterial infections are higher in COVID-19 patients with cerebrovascular diseases. We encourage prognostic research to include cerebrovascular and cardiovascular diseases as a component of prognostic models.
Limitations
A limitation of this systematic review and meta-analysis is that a considerable number of included articles were preprints. Most of the studies originated from China and were retrospective in design. Therefore, based on this data, it cannot be established that whether the association is causal. Further prospective cohort studies dedicated for analyzing this matter with adjustment to confounders are needed. The risk of publication bias is high, as shown by the asymmetrical inverted funnel plot. Information regarding the use of chronic medications such as ACEI/ARB is lacking. The included studies only investigated cerebrovascular disease as a comorbidity; recent evidences show that it may be precipitated by COVID-19, and we encourage future studies to explore this matter further. The broad category of cerebrovascular diseases did not differentiate between intracranial hemorrhage, ischemic stroke, and subarachnoid hemorrhage. The location of the cerebral insult was not differentiated, as certain locations of cerebrovascular damage had greater associations with autonomic dysfunction, which are possibly associated with increased mortality and severity of COVID-19 patients.
Conclusions
Our analysis shows that cerebrovascular and cardiovascular diseases are associated with an increased risk of poor outcome in COVID-19 patients. We encourage future studies to investigate this matter with a more detailed cerebrovascular disease classification and adjust the analysis to confounders.
Declaration of Competing Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
Ethics Approval
Not applicable.
Consent to participate
Not applicable
Consent for Publication
All authors consent to the publication of this manuscript.
Availability of Data and Materials
All data available on reasonable request.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributorship Statement
RP conceived and designed the study. IH, MAL, and RP acquired the data, drafted the manuscript, performed data extraction, and interpreted the data. IH, MAL, RP performed extensive research on the topic. JJ and EJW performed data interpretation, extensive research, reviewing, editing, and provide critical revision on the manuscript. All authors contributed to the writing of the manuscript. RP performed the statistical analysis.
ORCID: https://orcid.org/0000-0003-3998-6551
ORCID: https://orcid.org/0000-0003-1189-8453
ORCID ID: https://orcid.org/0000-0002-1106-0864
References
- 1.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 103. [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J Am Med Assoc.2020:1-9. doi:10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed]
- 4.World Health Organization . 2019. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) [Google Scholar]
- 5.Ali A., Emami A., Javanmardi F., Pirbonyeh N., Fadakar N. Early epidemiological analysis of COVID-19: first report from south of Iran. Res Sq. 2020 doi: 10.21203/rs.3.rs-19915/v1. [DOI] [Google Scholar]
- 6.Bai T., Tu S., Wei Y. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. SSRN Electron J. 2020;6 doi: 10.2139/ssrn.354611. [DOI] [Google Scholar]
- 7.Cao J., Tu W.-J., Cheng W. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. April 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. March 2020:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu L., Fei J., Xiang H.-X. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv. 2020;86(551) doi: 10.1101/2020.03.13.20035329. 2020.03.13.20035329. [DOI] [Google Scholar]
- 10.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. Plos One. 2020;15(3):1–10. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Liu Y., Xiang P. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 2020 doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;53(9):1689–1699. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol.2020:0-1. doi:10.1002/jmv.25783 [DOI] [PMC free article] [PubMed]
- 14.Zhang G., Hu C., Luo L. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.02.20030452. 2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo X., Xia H., Yang W. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 16.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu L., Ph D., Chen S. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Zhou Y., Yang Z., Xia D., Geng S. Clinical characteristics of patients with severe pneumonia caused by the 2019 novel coronavirus in wuhan, china. medRxiv. 2020;(26):1–15. doi: 10.1101/2020.03.02.20029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Li R, Wang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. Res Sq.:1-17. doi:10.21203/rs.3.rs-19680/v1 [DOI] [PMC free article] [PubMed]
- 20.Li Q., Ling Y., Zhang J. Clinical characteristics of SARS-COV-2 infections involving 325 hospitalized patients outside Wuhan. Res Sq. 2020:1–15. doi: 10.21203/rs.3.rs-18699/v1. [DOI] [Google Scholar]
- 21.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 22.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. April 2020 doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madjid M., Miller C.C., Zarubaev V.V. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34 892 subjects. Eur Heart J. 2007;28(10):1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowan L.T., Lutsey P.L., Pankow J.S., Matsushita K., Ishigami J., Lakshminarayan K. Inpatient and outpatient infection as a trigger of cardiovascular disease: the aric study. J Am Heart Assoc. 2018;7(22):1205–1210. doi: 10.1161/JAHA.118.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal R., Bhansali A.COVID-19, diabetes mellitus and ace2: the conundrum. Diabetes Res Clin Pract.2020:108132. doi:10.1016/j.diabres.2020.108132 [DOI] [PMC free article] [PubMed]
- 26.Schiffrin E.L., Flack J., Ito S., Muntner P., Webb C. Hypertension and COVID-19. American Journal of Hypertension. April 2020 doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. March 2020 doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pranata R., Huang I., Lukito A.A., Raharjo S.B.Elevated n-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19 – systematic review and meta-analysis. Postgr Med J. doi:10.1136/postgradmedj-2020-137884 [DOI] [PMC free article] [PubMed]
- 31.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med. April 2020 doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagan S.C., Lee L.A., Hess D.C. Role of angiotensin-converting-enzyme inhibitors in stroke prevention. Am J Heal Pharm. 2003;60(8):788–793. doi: 10.1161/01.str.0000054261.97525.4b. [DOI] [PubMed] [Google Scholar]
- 33.South A.M., Diz D., Chappell M.C. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Circ Physiol. 2020 doi: 10.1152/ajpheart.00217.2020. ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel O., Akyüz L., da Costa Goncalves A.C. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke; A Journal of Cerebral Circulation. 2015;46(11):3232–3240. doi: 10.1161/STROKEAHA.115.008989. [DOI] [PubMed] [Google Scholar]
- 35.Levi M., van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;0(0):1–14. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lue W., Yu H., Gou J., et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). 2020;(March):1-18.
- 38.Desforges M., Le Coupanec A., Dubeau P. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):1–28. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carod Artal F.J. Complicaciones neurológicas por coronavirus y COVID-19. Rev Neurol. 2020;70(09):311. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Bai W., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol. 2020:jmv.25728. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y., Xu X., Chen Z. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/J.BBI.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y., Wang M., Zhou Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Middeldorp S., Coppens M., Haaps T.F.van. Incidence of venous thromboembolism in hospitalized patients with COVID-19. Preprints. 2020 doi: 10.20944/preprints202004.0345.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. April 2020:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren-Gash C., Blackburn R., Whitaker H., McMenamin J., Hayward A.C. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3) doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data available on reasonable request.