In the era of the pandemic the first goal is reducing COVID-19 risk for staff and patients and at the same time ensuring an optimal anti-cancer treatment avoiding delays.
Radiation therapy is a life-saving treatment and should be guaranteed to all patients with cancer for whom it is indicated.
Definitive chemo-radiotherapy (CRT) is the standard of care for locally advanced oropharyngeal squamous cell carcinoma (OPSCC). To continue curative CRT in patients affected by COVID-19 is subject of debate. Hereby we present a case report of OPSCC patient which was affected by COVID19 after the second Platinum cycle administration. In our multidisciplinary team after infectious disease assessment we decided to continue treatment because of curative intent and the young age of the patient [1].
Xy is a male 62 years old, heavy smokers, nothing in his medical history, who referred in December 2019 with a one-month history of firm, painful lumps (nodule-like structures) on the left side of the neck (level III cervical lymph nodes). Sonography confirmed malignant lymph nodes. The patient underwent an ENT examination with the identification of OPSCC involving the left tonsil and the base of tongue.
A histological examination confirmed the presence of locally advanced oropharynx carcinoma human papillomavirus (HPV) positive.
The patient completed staging assessment with neck computed tomography (CT) and positron emission tomography (PET)- CT scan, and the primary tumor was graded cT4N1 according to the International Union Against Cancer’s 8Th TNM classification system.
Imaging showed that primary tumor from left tonsil involved tongue base (axial diameter of 34 × 29 mm and cranio-caudal extension of 30 mm). It was associated with the presence of some enlarged left cervical lymph node formations close to the vascular structures (the largest of the diameter axial maximum of 19 × 11 mm and cranio-caudal extension of 28 mm).
The multidisciplinary assessment indicated to treat the patient to concurrent CRT according to RTOG schedule.
Treatment started on 24th February. The second Cis-platinum administration was on 16th March 2020.
From 25th March 2020 the patient began to have fever (38.5 °C) and cough.
He performed a negative chest x-ray. For uncontrolled pain and inadequate oral intake it was agreed with the family doctor to increase pain therapy and start hydration at home
On 27 March the fever was 38 °C and a treatment with intravenous Ceftriaxone was started at home by the family doctor.
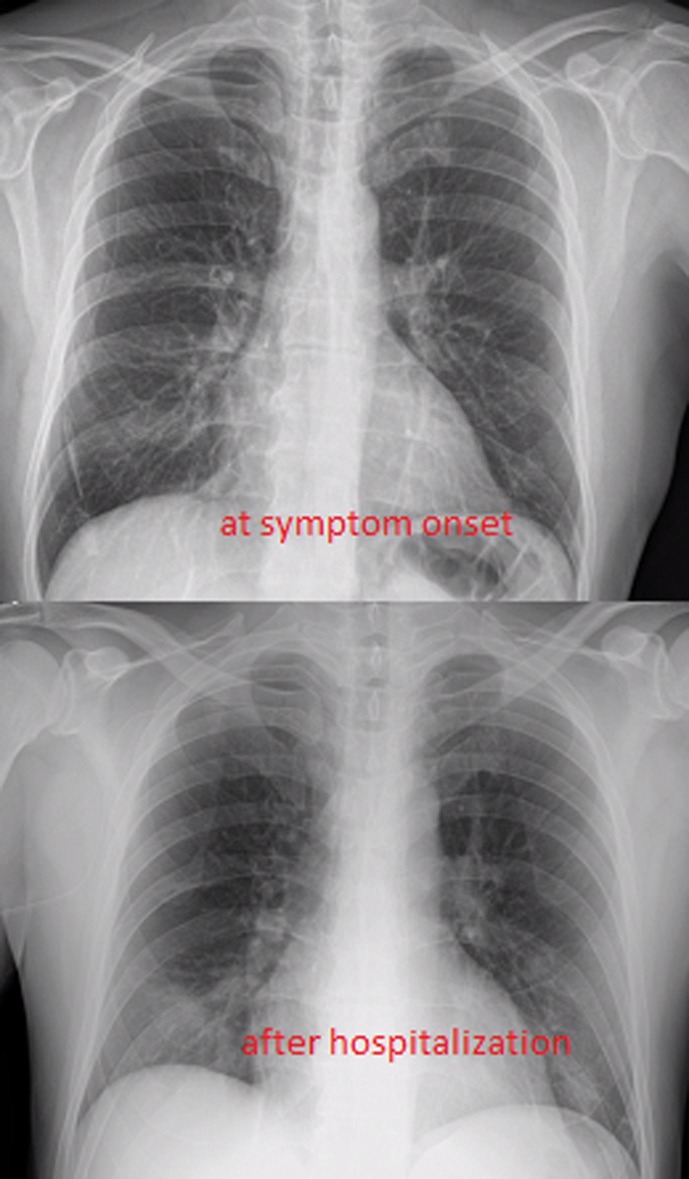
On 29th March, Xy accessed to the emergency room and was hospitalized for dysphagia (cancer and treatment –related), fever and cough (see Fig. 1 ).
Fig. 1.
Chext Xray on symptom onset and after hospitalizaion (mild respiratory symptoms).
Nasal and oropharyngeal swab revealed SARS-CoV-2 infection.
Blood examination were WBC 4.6 K/mcl, Platelets 144 K/mcl Haemoglobin 12.5 g/dL, D-Dimer 10 μg/ml Reactive C protein 123 mg/L pH = 7.5; pCO2 35.1 mmHg; pO2 = 68.6 mmHg.
Table 1 summarizes the results of blood exams.
Table 1.
Blood exam.
| Exam/time | Symptom onset | D2 | D4 | D10 | D20 | |
|---|---|---|---|---|---|---|
| WBC | 4600 | 1880 | 2300 | 6230 | 6700 | K/mcl |
| Lymphocytes | 0.19 | 0.17 | 0.240 | 0.76 | 0.46 | K/mcl |
| CPR | 123.25 | 106.25 | 36.78 | 1.56 | Mg/L | |
| D-dimer | 10.03 | 10.84 | 7.27 | 6.30 | Mcg/mL | |
| SarsCoV2 | Positive | Negative (repeated on d 22) |
Abbreviation: SarsCoV2 = severe acute respiratory syndrome coronavirus 2; D = day.
At the time of hospitalization, Xy had reached a radiotherapy (RT) dose of 50 Gy. The treatment was interrupted for two days to check for eventual evolution to a severe form of the Covid-19.
In fact, it seems that cancer patients are at enhanced risk of serious morbidity, including the need for ventilator support or death (HR 3.56, [95% CI, 1.65–7.69]) [2].
Given the stability of the patient, it was decided to resume radiation therapy on April 1st.
The treatment plan was modified by prescribing to the boost volume 6 fractions of 3 Gy instead of the planned 10 fractions of 2 Gy.
The aim was to achieve a radical dose for the patient but to reduce exposure to the staff of the Radiotherapy department. The patient was treated at the end of the working shift with appropriate protective devices for the staff. The bunker areas was sanitized at the end of the treatment session. Xy received antibiotic treatment with piperacillin tazobactam for 10 days; low molecular weight heparin was administered prophylactically during the hospitalization.
Simultaneous supportive care was administered during the hospitalization; intravenous nutrition of about 1800 k-calories was administered from 29th March to 20th April with polyamine acids + glucose monohydrate + electrolytes + olive oil + medium chain triglycerides + fish oil with a high content of omega-3 acids + soybean oil for parenteral use, a reduced intravenous nutritional therapy of about 550 k-calories was administered to 23rd April 2020.
Pain management consisted of continuous intravenous infusion of hydrochloride morphine 70 mg.
Pain therapy was turned to trans-dermal fentanyl to permit the patient to return home.
However pain control was insufficient therefore the patient was medicated with oral methadone with benefit.
Xy successfully completed radiotherapy on 7th April, while the third Cis-platinum treatment was omitted. Total RT dose to gross tumor volume was 68 Gy, EQD2 69.5 Gy.
At the date of discharge (26th April 2020) ENT examination revealed a clinical complete response.
The patient will perform a neck CT scan in July.
On 19th March 2020 Filippi A et al, reported the experience of a group of Northern Italy radiation therapy departments during this period of crisis; they suggested some practical indications including:
-
–
if a patient had confirmed COVID-19 but is declared healed by the infectious disease team, carefully plan to start or restart treatment according to cancer-related clinical conditions.
-
–
If possible, COVID-19 patients should be treated at the end of the linear accelerator shift to limit the chances of infection for other patients.
-
–
For confirmed COVID-19 patients (or patients waiting for diagnostic confirmation), the waiting and bunker areas should be sanitized at the end of the treatment session [3].
Regarding these indications, we did not wait for the patient's recovery from the infection, while we respected the other recommendations.
The choice to continue RT treatment even during COVID 19 infection reflects the most recent indications of the ASTRO-ESTRO Consensus in which it emerged a strong agreement to continue RT in patients with SARS-CoV-2-related mild symptoms who had completed more than two weeks of treatment and an agreement to continue radiotherapy in patients with SARS-CoV-2-related mild symptoms, irrespective to the received treatment at that point [4].
We decided to omit the third cycle of concomitant chemotherapy because we considered it unsafe with the hypofractionated schedule choosed for RT.
Clinical assessment of patient’s condition and rigid rules for radiotherapy staff and dedicated routes for this patient allowed to proceeding with treatment.
At this time, no clinical HNC-specific data on COVID-19 patients are available – but each choice requires an individualized risk/benefit assessment and a multidisciplinary agreement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Anna Maria Merlotti, Email: Merlotti.a@ospedale.cuneo.it.
Elvio Russi, Email: Russi.e@ospedale.cuneo.it.
References
- 1.Day A.T., Sher D.J., Lee R.C., Truelson J.M., Myers L.L., Sumer B.D. Head and neck oncology during the COVID-19 pandemic: Reconsidering traditional treatment paradigms in light of new surgical and other multilevel risks. Oral Oncol. 2020;6(105) doi: 10.1016/j.oraloncology.2020.104684. 104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippi A.R., Russi E., Magrini S.M., Corvò Renzo. Letter from Italy: First practical indications for radiation therapy departments during COVID-19 outbreak. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson D.J., Palma D., Guckenberger M., Balermpas P., Beitler J.J., Blanchard P. Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.04.016. pii: S0360-3016(20)31034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]