Abstract
In January 2020, China reported a cluster of cases of pneumonia associated with a novel pathogenic coronavirus provisionally named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2). Since then, Coronavirus Disease 2019 (COVID-19) has been reported in more than 180 countries with approximately 6.5 million known infections and more than 380,000 deaths attributed to this disease as of June 3rd , 2020 (Johns Hopkins University COVID map; https://coronavirus.jhu.edu/map.html) The majority of confirmed COVID-19 cases have been reported in adults, especially older individuals with co-morbidities. Children have had a relatively lower rate and a less serious course of infection as reported in the literature to date. One of the most vulnerable pediatric patient populations is cared for in the neonatal intensive care unit. There is limited data on the effect of COVID-19 in fetal life, and among neonates after birth. Therefore there is an urgent need for proactive preparation, and planning to combat COVID-19, as well as to safeguard patients, their families, and healthcare personnel. This review article is based on the Centers for Disease Control and Prevention's (CDC) current recommendations for COVID-19 and its adaptation to our local resources. The aim of this article is to provide basic consolidated guidance and checklists to clinicians in the neonatal intensive care units in key aspects of preparation needed to counter exposure or infection with COVID-19. We anticipate that CDC will continue to update their guidelines regarding COVID-19 as the situation evolves, and we recommend monitoring CDC's updates for the most current information.
Background
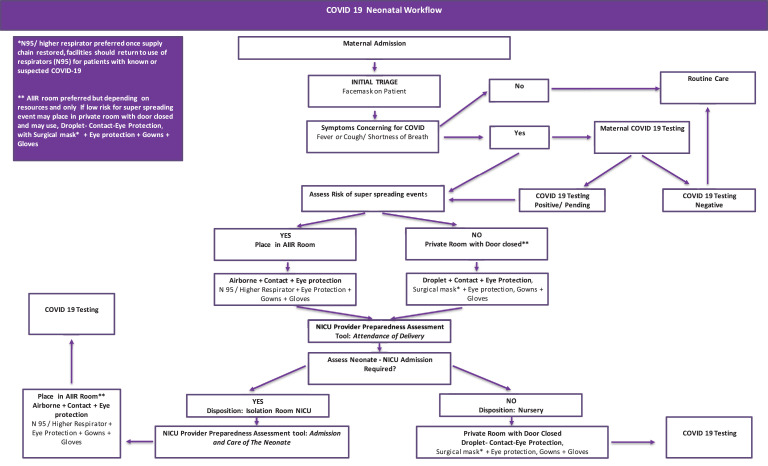
On January 7, 2020, China reported a cluster of cases of pneumonia associated with a novel pathogenic coronavirus named, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2). This novel coronavirus was first identified in Wuhan, Hubei Province, China. On January 30, 2020, the International Health Regulations Emergency Committee of the World Health Organization declared the outbreak a ‘public health emergency of international concern’. Since then, on March 11, 2020, the World Health Organization escalated the declaration to a Pandemic. Currently this viral infection has spread to more than 180 countries, with confirmed positive cases greater than 6.5 million people and associated deaths exceeding380, 000 individuals(Johns Hopkins University COVID map, https://coronavirus.jhu.edu/map.html). The first case of Coronavirus Disease 2019 (COVID-19) was reported in the United States on January 20, 2020.1 Also as of June 3rd, 2020, 1.8 million patients have been confirmed to be infected and almost 106,000 patients have died in the United States (Johns Hopkins University COVID map, https://coronavirus.jhu.edu/map.html), with an ongoing and seriously concerning rapid increase in the number of patients, and deaths associated with COVID-19. Many countries, including the United States, have now reported community spread of COVID-19 (Fig. 1 ).
Fig. 1.
COVID-19 neonatal workflow.
The coronavirus family is a large family of single-stranded RNA viruses that commonly cause infections in humans and other mammals such as bats, cats, cattle, and camels. The SARS-CoV2 virus that causes COVID-19 is a β-Coronavirus with more than half of the sequence similar to previously known Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) that caused epidemics in the last two decades. We have a limited understanding of COVID-19 infections. Based on the published literature and data from epidemics related to other similar viruses, the majority of these infections present with influenza like symptoms with mild upper respiratory and gastrointestinal symptoms, with most cases including cough, breathing difficulty, fever, headache, altered sensation of smell or taste, diarrhea, vomiting, body ache, and rashes. In some cases, COVID-19 can cause serious complications including acute respiratory distress syndrome, sepsis, multi-organ failure, and death.
The majority of the confirmed cases with COVID-19 have been reported in adults, especially older individuals with co-morbidities.2 Children have had relatively few infections reported in the literature to date.2, 3, 4, 5, 6
Alt-text: Unlabelled box
The mode of transmission for COVID-19 is known to be via exposure to respiratory droplets and fomites. Person-to-person transmission occurs during close exposure to a person infected with COVID-19 or after contracting it from surfaces with secretions containing viable virus. The role of small respirable particles (aerosols or droplet nuclei), to close proximity transmission is currently not clear. Airborne transmission from person-to-person over long distances is unlikely. Viral shedding has been noticed in the stool samples of infected patients,7 , 8 however, it is uncertain if COVID-19 can be transmitted via the fecal-oral route. Currently, there is no vaccine for COVID-19 and multiple investigators across the globe are working towards creating an effective vaccine to prevent this infection.9
In the face of a growing pandemic with COVID-19, there is an urgent need for proactive preparation and planning to combat COVID-19 in the vulnerable patient population in the neonatal intensive care unit (NICU) and to safeguard patients, their families, and the healthcare personnel (HCP) taking care of them. The aim of this article is to provide a roadmap to the various aspects of preparation needed to safely counter potential COVID-19 infections in the neonatal intensive care unit (NICU) setting. This guidance document is based on the Centers for Disease Control and Prevention's (CDC) current recommendations for COVID-19, and its adaptation to our local resources.
Definitions
The following definitions will be used frequently in this article. They have been adapted from the information at CDC website and they may change in the future due to the evolving situation and understanding of COVID-19.
Person under investigation (PUI)
Any person with suspected exposure or any symptoms consistent with COVID-19 who is currently being investigated is placed under this category.
Super spreading events or aerosols generating procedures
Any procedure that may cause the release of aerosols in the environment puts the person performing them at a higher risk of exposure. Some of the examples include intubation, extubation, open airway suctioning, nebulization, cardiopulmonary resuscitation, continuous positive airway pressure, bag and mask ventilation, and bronchoscopy.
Pregnant women with COVID-19 and vertical transmission to the fetus
We do not have sufficient information from the published literature regarding pregnancy outcomes, birth defects and fetal loss in pregnant women with confirmed COVID-19 infection. There are limited case reports and retrospective reviews from China reporting premature births among mothers with COVID-19 infection during pregnancy. Other viral respiratory infections in mothers during pregnancy may cause increased rates of congenital defects, premature births, and low birth weight among newborns. Ongoing monitoring for outcomes is important in order to learn more about the impact of COVID-19 in both the short-term and the long-term period on the maternal and neonatal population.
Vertical transmission from pregnant women with COVID-19 to fetuses during pregnancy or to newborns during and after delivery is not entirely understood but seems to be less likely based upon the current available data.
Alt-text: Unlabelled box
In the limited data published in scientific reports, outcomes among neonates born to mothers with confirmed COVID-19 remain inconclusive but appear to indicate a lack of in-utero infection or vertical transmission.10 , 11 A case series published by Chen et al. for nine mothers with COVID-19 pneumonia, all of whom had a cesarean section and had healthy newborns10 appears to confirm this. Four of the nine infants were born late preterm at 36 weeks, and one was born early term at 37 weeks gestational age. Six mother-newborn dyads were tested for SARS-CoV-2 in the amniotic fluid, cord blood, neonatal throat swab, and breast milk samples, and all samples tested negative for the virus.
Zeng et al. studied 33 neonates born at Wuhan Children's Hospital, in Wuhan, Hubei Province, China to mothers with confirmed COVID-19. Three neonates among 33 were found to be positive for COVID-19, all delivered by cesarean section.12 Two were born at 40 weeks gestation and developed a fever. The third neonate was born prematurely at 31 weeks gestation due to fetal distress. This study presented limited information regarding a detailed timing of separation of the newborns from the mother and other family members with COVID-19 who may have been in close contact with the newborns after delivery. There was also insufficient information on the method of feeding for the neonates, all of which makes it difficult to determine if the infection among these newborns was vertically or horizontally transmitted. In a case series by Zhu et al. outcomes of 10 neonates, including two pairs of twins, born to mothers with COVID-19 were reported.13 One neonate died, six were born premature (31–34 weeks gestational age), and two were small for their gestational age. None of the newborns that got tested (with nasopharyngeal swabs) for SARS-CoV-2 were confirmed positive. But the authors reported fetal distress, respiratory distress, fever, thrombocytopenia, and abnormal liver function tests in some of these newborns despite testing negative for COVID-19.
Researchers from China tested antibodies IgG and IgM for SARS-CoV-2 in a small case series involving six neonates born to mothers with COVID-19. Using a negative real time reverse transcription- polymerase chain reaction (RT-PCR) test from throat swabs and blood samples they observed virus specific antibodies detected in the serum of all six newborns despite no physical contact with the mother after delivery.14 Two infants had IgG and IgM levels higher than the normal level (<10 AU/mL) and inflammatory cytokine IL-6 was significantly increased in all infants. None of the infants presented with any symptoms as of March 8, 2020. In their editorial comment regarding this case series as well another case report published on the same day,14 , 15 Kimberlin et al. expressed caution in interpreting the results from these reports.16 IgM antibodies have a large polymeric structure and thus do not cross the placenta. When IgM antibodies are found in a newborn, they may reflect fetal production following in-utero infection. IgM enzyme-linked immunosorbent assay testing for congenital infections has a lower sensitivity / specificity than molecular diagnostic tests based on nucleic acid amplification and detection, and may have higher false-positive or false-negative results. One of the case reports described a very rapid decline in the level of IgM antibodies in these newborns,15 which is very unusual for IgM levels in other congenitally acquired infections in which they typically last for a few months. Given this information, it remains unclear if these reports represented a true congenital infection, or just an artifact of resulting antibody levels.
As the majority of currently reported COVID-19 infections in pregnant mothers occurred during their third trimester, it would be prudent to closely follow infants born to the mothers who tested positive during their first and second trimester. Since it is a novel infection just recently identified in humans, there is no information on long-term neurodevelopmental outcomes amongst infants born to mothers with confirmed COVID-19 infection during pregnancy, as well as for infants who get infected themselves.
The following are our recommendations to allow for optimal safety for mothers, their infants, family members and HCP.
Preparation before delivery
Preparation starts with identifying one of two possible scenarios: a mother with confirmed infection with COVID-19, or a mother with a suspected infection or a PUI. The obstetrics team should have guidelines in place for both triage and delivery of the fetus. Infectious Disease (ID) and Infection Prevention and Control (IPC) teams should be consulted for any suspected or confirmed cases. This should be clearly documented in the medical record and communicated to other teams taking care of these mothers in conjunction with the primary obstetrics team, including neonatology. Obstetric teams should have a low threshold for testing of COVID-19 infections in mothers with a new onset of symptoms, including fever, which otherwise would have been worked up for chorioamnionitis, as well as for any other upper respiratory symptom. At some institutions with widespread community transmission, all mothers admitted to the labor and delivery ward are tested for SARS-CoV-2, which may be helpful in identifying asymptomatic patients. This may not be possible at all institutes, as it requires resources for a lot of testing with rapid turnaround times.
Deliveries at-risk for super spreading events or with uncertain progressive clinical courses should be done in a negative pressure airborne infection isolation room (AIIR) with airborne/ contact/ eye-shield precautions (N95 or higher respirator with eye shield or powered air-purifying respirator/ gown/ gloves). Mothers with suspected or confirmed infection, with a stable course and unlikely to require super spreading events, could alternatively stay in a neutral pressure room with droplet/ contact/ eye-shield precautions (surgical mask [N95 or higher respiratory preferred]/ eye shield/ gown/ gloves).
Team members who would attend high-risk deliveries should be identified at the beginning of the shift in order to maintain compliance with Neonatal Resuscitation Program guidelines while ensuring only key personnel are entering the delivery room. Having a fully prepared back-up team outside the delivery room, which enters the room on an as needed basis only, could be beneficial to limit exposure.
A pre-delivery multidisciplinary team huddle, with an optimal dissemination of medical history, estimated infant weight and clear roles assigned to individual NICU team members, especially for full neonatal codes, can be extremely helpful.
Alt-text: Unlabelled box
A clear method of communication between team members inside and outside the delivery room should be established. Ensuring adequate supply of appropriate personal protective equipment (PPE) and identifying a clear location where to procure them (ideally immediately outside and within identified labor and delivery rooms or the operative room) is also valuable. Based on our experience, we have developed a delivery preparation checklist that we have found to be useful and that can be modified for use by other institutions (Table 1 ).
Table 1.
NICU provider preparedness assessment tool: Attendance at Delivery.
| Patient information |
| Confirm maternal COVID-19 status |
| Obtain pertinent maternal and fetal history |
| Pre-delivery multidisciplinary huddle (NICU and Obstetric team) |
| Isolation status |
| Appropriate isolation room for delivery: |
| Airborne infection isolation rooms for patients who may require aerosol-generating procedures |
| Neutral pressure room with HEPA filter if uncomplicated delivery expected with door closedb |
| Clear signage outside the room regarding infection precautions needed |
| Appropriate staff |
| Identify NICU team members who would enter the delivery room |
| Identify back-up team, who stays outside the room unless needed |
| Clear, planned communication chain for those in and out of delivery room |
| Identify role of team members at delivery |
| Keep a log of every healthcare provider entering and exiting the patient room |
| Personal protective equipment (PPE) |
| Disposable patient examination gloves |
| Disposable isolation gown |
| Respiratory protection (N-95 or higher-level respirator), Face Mask appropriate if no aerosol generating procedures* |
| Appropriate eye protection (e.g. goggles or face shield) |
| Appropriate equipment and medicines for delivery and transport |
| Procedure boxes/supplies checked (code cart, supplies, bag and mask/ neopuff, EKG, umbilical lines) |
| Transport isolette checked and ready to use |
| Medications checked and ready, if not in code cart (epinephrine, normal saline) |
Once supply chain at institution allows, facilities should return to use of respirators (N95) for patients with known or suspected COVID-19.
AIIR preferred, if available. Adapted from Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html.
Preparation in the delivery room
All HCP must enter the delivery room with appropriate PPE and follow recommended infection control practices based on the maternal COVID-19 status and the risk factors for super spreading events. Infant bag-valve mask set-up should include a HEPA filter to minimize aerosols produced during positive pressure ventilation. As mentioned previously, having an identified team available for high and low risk deliveries, with back up team members outside the room, may provide sufficient coverage if help is needed for the mother or infant. Some team members could help in other tasks such as; a runner to the lab/ blood bank, nurses and pharmacists helping with the preparation of medications. After routine care or the need for resuscitation, the family should be updated. There should be a process outlined for cleaning the room and equipment, before and after use, by a suspected or confirmed COVID-19 patient. A detailed debriefing should be done after delivery and the safe disposition of the infant, in order to review how things went and identify areas of improvement. Mock drills and simulation exercises with multidisciplinary teams to evaluate readiness and identify gaps as well as learning opportunities should be considered.
Transport of newborn
Intrafacility transport
There are multiple scenarios that may require the transport of a newborn within a facility; such as transfer from the delivery room to a newborn nursery or to the NICU, for investigations (e.g. magnetic resonance imaging), to the operating room, and to the mother's postpartum room. Infants should be isolated and separated after delivery from mothers who are suspected or confirmed with COVID-19, in order to avoid exposure. Based upon the medical needs, neonates could be transferred to the newborn nursery or to the NICU. There should be clear communication between the delivery team, designated elevator services (if needed), and the recipient unit or area, regarding transfer of an infant. PPE such as gloves and gowns should be changed before transporting the infant, but masks can continue to be worn if not soiled or wet. There should be a dedicated transport isolette that is cleaned appropriately, before and after use, as per hospital disinfection guidelines.
Interfacility transport
Many sick newborns requiring a higher level of care are transferred to regional perinatal centers. It is helpful to engage in preemptive planning, such as using a checklist to prepare for transfer of a neonate born to a PUI or COVID-19 positive mother. Clear communication between the referring and accepting teams should take place, including the condition of the patient, and symptom history of the mother and/or family members who may have close contact. If unsure of the status of the patient with regards to COVID-19, a discussion of the case with the Pediatric ID and IPC teams should take place as soon as the call for a transfer request is received in order to help in ensuring the correct designation of the newborn. All members of the transport team, including the driver, should use appropriate PPE while transporting the infant. Before leaving the patient's room, isolation gowns, and gloves should be changed, but masks may continue to be worn if not soiled or wet as discussed earlier.
Mother and baby contact
We are still learning about various potential modes of transmission, clinical spectrum and duration of viral shedding in a COVID-19 patient. In the absence of sufficient evidence, we suggest taking a cautious approach regarding close contact between a mother with suspected or confirmed COVID-19 and her infant. More data from different geographical regions is required before we know conclusively about epidemiology and vertical transmission of this infection.
Infants born to mothers who are a PUI or who have a confirmed COVID-19 diagnosis
These infants should be designated as a PUI. They should be temporarily separated from their mothers and placed in a separate isolation room. In the interest of patients and their families, this is an unfortunate but necessary undertaking. There should be a clear dialog and shared decision making between clinicians and families and the need for such intervention should be explained, preferably before delivery. All efforts should be made to provide support to the mother while she is separated from her newborn. Clinicians should document any refusal to follow recommendations of isolation and document any discussion regarding the risks of infection that could result from failure to isolate the newborn from the mother after birth. Infants not requiring intensive care may be admitted to the Mother/ Baby Unit but remain separated. As mentioned earlier, these rooms should have negative pressure, with clear signage outside the room, and all HCP should take appropriate precautions and use PPE while taking care of these newborn infants. Although AIIR are preferred, neutral pressure rooms could be considered based on the clinical status of the mother and the infant. Newborns should be bathed as soon as possible to remove potential virus contained in secretions that may remain on the skin. There should be a limit to the number of visitors allowed, with a process in place for screening symptoms at every visit. For source containment, mothers should wear a mask whenever the neonate is in the room.
Rooming-in of mother and newborn
If, due to a limitation of a facility's resources, or due to the mother's wishes, a newborn is rooming-in with the mother, there should be a physical barrier, such as a curtain, with a minimum of six feet distance between both patients. The mother or other caregiver should put on a mask and follow strict hand hygiene before and after feeding and when they have any close contact with the newborn. Breastfeeding and handling of breast milk is discussed in the later sections.
Testing for COVID-19 in PUI infants
At our institution, infants are tested for SARS-CoV-2 at 24 h of life, with a second test completed at minimum 24 h apart, or at discharge for a well newborn. For patients in the NICU, a second test is done five days after the first test or earlier on discretion of clinical teams. If both tests are negative and the patient is doing clinically well, the infant can be discharged home. Medical management of a neonate with COVID-19 is beyond the scope of this article and should be done in consultation with the infant's primary medical team and ID teams.
Care in the NICU
Neonates born to mothers with a suspected (PUI) or a confirmed diagnosis of COVID-19 are placed in a separate AIIR. Stable neonates with respiratory support up to 2 l/min by nasal cannula, can be placed in a neutral pressure room with droplet / contact / eye-shield precautions and a HEPA filter. Infants on respiratory support greater than 2 l/min nasal cannula or those that may require an aerosol generating procedure, should be placed in an AIIR with airborne/ contact/ eye-shield precautions. There should be clear signage outside the room regarding infection prevention precautions needed before entering. All HCP who enter the room of a patient with known or suspected COVID-19 should adhere to aforementioned infection prevention precautions and implementing staffing policies to minimize the number of HCP who enter the room is suggested. Only essential personnel should enter the room, for example, physical exams should be limited to the attending physician. Facilities should consider dedicating specific HCP to care for these patients in order to minimize the risk of transmission and exposure to other patients and other HCP. Ideally all suspected patients would be placed in a separate room. If there is more than one patient, COVID-19-suspected or positive patients could be cohorted together with patients of similar status based on availability of local resources and space.
A log of every HCP entering and exiting the patient's room should be kept. In addition, dedicated or disposable non-critical patient-care equipment such as blood pressure cuffs should be used. All PUI or positive infants should have a bag-valve mask with a HEPA filter at the bedside to reduce the risk of aerosol spread if the infant requires positive pressure ventilation. If equipment will be used for more than one patient, cleaning and disinfection between uses should be carried out according to the manufacturer's instructions. Similarly, environmental surfaces and the room should be properly cleaned before and after use. Medical waste should be carefully disposed of in the same way as infectious medical waste.17 Every health facility should assess their own bed surge-capacity and available resources, in order to develop a plan for the placement of a newborn and to adopt a locally suited approach consistent with the recommendations from the CDC. (See Table 2 for our adaptation of CDC recommendations)
Table 2.
NICU provider preparedness assessment tool: Admission and Care of the Neonate.
| Infant Post Delivery Isolation |
| Transport in an isolette with doors closed. |
| Infant placement: Airborne Infection Isolation Room (AIIR) if requires respiratory support OR Neutral pressure room with HEPA filter if no respiratory support/ aerosol generating procedure expectedb |
| Clear signage outside the room regarding infection precautions needed before entering the room |
| Appropriate staff |
| Only essential personnel should enter the room |
| A log of every healthcare provider entering and exiting the patient room should be kept |
| Dedicated primary team as local resources permit |
| Limited sub-specialty team members for exams |
| Personal protective equipment (PPE) |
| Disposable patient examination gloves |
| Disposable isolation gown |
| Respiratory protection (N-95 or higher-level respirator), Face Mask appropriate if no aerosol generating procedures* |
| Appropriate eye protection (e.g. goggles or face shield) |
| Equipment |
| Dedicated or disposable noncritical patient-care equipment (e.g., blood pressure cuffs) |
| Environmental cleaning |
| Ensure proper cleaning and disinfection of environmental surfaces and equipment in the patient room |
| Maternal contact |
| Method to update mother identified and performed (telephone and video conferring preferred, in-person for significant updates / decisions) |
| Family members identified who can receive the updates |
| Feeding method identification |
| Expressed breast milk preferred. Donor breast milk or formula if can not give breast milk for any reason |
Once supply chain at institution allows, facilities should return to use of respirators (N95) for patients with known or suspected COVID-19.
AIIR preferred, if available. Adapted from Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html and Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019 (COVID-19) in Inpatient Obstetric Healthcare Settings https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html.
COVID-19 and horizontal transmission to the neonates
Due to widespread community spread in locations such as New York City, many staff members and/ or parents may get infected causing unintended exposure to neonates. The vulnerable patient population in the NICU may be at increased risk for severe infections; thus extreme caution is needed in order to prevent horizontal transmission of COVID-19. At our institution, we mandated that HCP, mothers and caregivers wear a surgical mask in all patient care areas as an extra measure of precaution. Due to high chances of horizontal transmission, any new onset of respiratory distress or fever in an infant in the NICU, with no alternate explanation/ diagnosis, testing for COVID-19 should be strongly considered. In general, if the primary team is thinking of sending a respiratory viral panel, they should consider sending a test for COVID-19. If a PUI infant has a negative COVID-19 test result, prior to ruling out COVID-19 as a diagnosis, an alternative diagnosis should be determined. We recommend consulting IPC and ID teams for further patient specific guidance on repeat testing, possibility of alternative diagnoses and maintenance/ discontinuation of isolation precautions.
Confirmed COVID-19 infection in a neonate
So far, there are only rare case reports of newborns with COVID-19. SARS-CoV-2 attaches to angiotensin converting enzyme-2 (ACE2) receptors to enter cells. There is a concern that due to the presence of ACE2 receptor on neurons, there could be a pertussis-like respiratory center suppression in neonates if they get infected with COVID-19. Therefore all neonates with any degree of illness who are born to mothers with COVID-19 should be observed until their illness is resolved. If there is a new confirmed COVID-19 infection in a neonate, the same guidelines should be adopted for care of a neonate by HCP and family based upon respiratory status and likelihood of infection in a newborn. Although currently inconclusive, the majority of neonatal infections seem to follow horizontal transmission rather than vertical.
Breastfeeding
Breast milk is not contra-indicated for newborns with maternal PUI or confirmed COVID-19 infections.
It is critical to follow adequate infection prevention measures for collecting expressed breast milk. In the limited available literature, COVID-19 has not been detected in breast milk,10 and per current CDC guidelines, expressed breast milk is encouraged. Mothers should be encouraged to express their breast milk to establish and maintain milk supply.
Alt-text: Unlabelled box
A dedicated breast pump should be given to mothers, as well as a mask to wear while pumping.18 Mothers should use strict hand hygiene before, during and after a pumping session. All parts of a breast pump should be thoroughly washed and sanitized as per the manufacturer's instructions in conjunction with CDC guidelines.19 An alternative caregiver or nurse can feed expressed breast milk to the infant. To support breast milk feeding in infants who remain admitted to the NICU after their mothers have been discharged, facilities should devise a plan to allow for breast milk drop-off from an asymptomatic partner or caregiver.
Mothers can start direct breastfeeding when they have been afebrile for a minimum of 72 h without the use of antipyretics and the majority of their respiratory symptoms are resolved. They should be instructed to wear a mask, and to practice strict hand hygiene as well as the thorough cleaning of the breast before and after breastfeeding. If the mother cannot provide breast milk, and there is an option for donor breast milk at the facility, this could be offered. All accepted donor milk is pasteurized and this heat-treatment kills both bacteria and viruses. Feeding options and the clinical plan for an infant should be discussed with the mother ideally before delivery.
Breast milk handling
Although mothers are encouraged to use the strict infection prevention measures, care and consideration to prevent COVID-19 through direct contact with breast milk containers should be considered. With community spread and the potential for many asymptomatic carriers of COVID-19, a cleaning process should be in place when receiving breast milk from all mothers during the COVID-19 pandemic. Every breast milk container received from a PUI or COVID-19 positive mother should be considered as a potential vector. As a result, each container should be cleaned with a disinfectant wipe and placed in a new clean plastic bag.
Staff education
As information continues to be fluid during the COVID-19 pandemic and recommendations may frequently change, it can be difficult for the multidisciplinary team to continuously track and follow evolving recommendations. Therefore, clear pathways of communication are needed to update HCP in order to provide care to mothers and infants as well as to allow HCP to educate families on the most up to date information. There should be a pre-defined electronic or physical space where the latest information could be obtained by any HCP if needed. Some of the ways to successfully keep team members up to date may include twice daily multidisciplinary huddles, daily rounding by a multidisciplinary leadership team, and a synthesis document of key practice changes with links to pertinent policies. Education documents for families to take home should also be created and frequently updated with recommendations to allow for standardized teaching to the families. Another important staff education initiative includes information around PPE. All HCP should receive training regarding the correct use, proper donning, doffing, and disposal of any PPE. Additionally, the use of safety monitors in the units which perform live observational audits and coaching, allows for real time corrections and prevention of PPE breaches.
Communication with the mother and other designated family members
While a neonate born to a mother with a confirmed diagnosis or PUI is admitted to the NICU, all medical information should be regularly provided to the mother and other designated family members. For medical and general updates, use of video-based methods to communicate with parents would be a good alternative, if available. For updates requiring in-person conversations, all arrangements should be made to ensure that there is a limited number of staff and family members present in the room, with implementation of complete PPE precautions and adequate safe distancing between individuals to avoid the spread of infection. A possible or confirmed infection in a neonate would cause psychological stress to the family.17 Support should be provided to them by involving social workers, psychologists, and palliative care teams.
Visitation of caregivers
Due to the current COVID-19 pandemic, visitation to our NICU has been restricted to a maximum of two people (only parents, or two people as per individual family situation), with only one person at a time is allowed at the bedside. A similar restricted visitors’ policy was employed at our unit during the recent measles outbreak in the New York City metropolitan area. A medical history of any sickness, fever, and of any upper respiratory symptoms is taken from all caregivers before entering the hospital in order to ensure that only healthy caregivers can visit the patient. Visitors should be asymptomatic and wear a mask while visiting the NICU or well-baby nursery. Family care partners or parents who are allowed to visit, should strictly follow the infection control guidelines and use appropriate PPE while providing care to the newborn. Mothers with COVID-19 should not visit the NICU until they are afebrile without the use of antipyretics for at least 72 h, with improvement of respiratory symptoms and two negative RT-PCR tests for SARS-CoV-2.
Discharge from hospital
If the mother remains afebrile for greater than 24 h and respiratory symptoms have nearly resolved, consider discharge of the medically stable newborn with the mother. If the mother is still febrile or has significant respiratory symptoms a caregiver who does not have symptoms can care for the infant. If the infant will be discharged to a home where there is a PUI or COVID-19 positive mother or partner, it is highly recommended that the infected person should not care for the infant unless there is no alternative. A COVID-19 positive mother or partner should wear a mask, perform strict hand hygiene, and keep a distance of at least 6 feet from the newborn until they are afebrile for 72 h with near resolution of respiratory symptoms and 7 days have passed since the beginning of the symptoms, or two RT-PCR tests for SARS-CoV-2 done a minimum of 24 h apart have been negative. Facilities should develop a process to safely identify and discharge an infant to an alternative caregiver if the mother or partner is unable to care for the infant at the time of discharge. Discharge instructions should be given in the mother's room or via video/ phone conferencing if needed. Infants will be transported to the mother's room or to the family at the hospital lobby for discharge. Parents, caregivers, and other family members should engage in the usual preventive steps to avoid the spread of respiratory infection specifically practicing hand hygiene, covering coughs, and staying up to date on vaccinations, including influenza. The medical team should call the newborn's outpatient pediatric practice to confirm that the office is able to see the newborn. If unable to see the newborn, an alternative practice should be selected. It is important to emphasize that it is unusual for infants to have respiratory symptoms within the first few days of life, and HCP should have a low threshold for considering COVID-19 on the differential, even if the initial test in the newborn may be negative.
Special scenarios
Shortage of PPE such as N95 masks
There is a widespread concern regarding the national shortage of PPE and hence limited supply to hospitals. In particular, many N95 masks are made in, or their raw material for production is obtained primarily from, Hubei province in China, which was the first epicenter for this outbreak. Therefore, there was a backlog of PPE reported by multiple institutions. Critical supplies, including identifying alternative products for PPE, should be continuously assessed. There should be an institution-based plan to conserve and develop re-use protocols for critical supplies including N95 respirators and surgical masks, in order to prepare for extended supply chain disruptions. Transparency to the staff and actions to allow for the HCP team to feel confident and safe while caring for patients is of utmost importance.
At our institutions, guidelines have been implemented for the use of N95 respirators by HCP when caring for a patient on airborne and airborne-combination precautions. Our institutions developed indications for the safe use and re-use of N95 respirators, if it has a tight fit but not restricting breathing, and is not wet, damaged or contaminated. N95 respirators can be safely stored in a clean paper bag between uses during a shift.
Inadequate resources or shortage of beds
`In a scenario of widespread COVID-19 in a community, there may be an increase in admissions to the NICU, as well as a shortage of staff and hospital beds. There needs to be a local institutional plan in place for such situations, so patients can be cared for safely and transferred out, if needed, with prior preparation for safe transports. Due to significant usage of oxygen while caring for patients with COVID-19 throughout the hospital, a back up system to ensure continuous oxygen supply should be devised. Our institution has established staffing and employee wellness task forces to identify external clinical resources and support our staff who may be asked to care for COVID-19 patients. There are daily multidisciplinary leadership and management calls to set an operational tempo, assess preparedness, and prioritize enterprise-wide action. We developed a plan for our NICU regarding census and staffing, involving clear directions for patient care and when to initiate transferring patients out if needed. There should also be a plan for cohorting of suspected or confirmed cases of COVID-19 in the NICU and well-baby nursery if space becomes an issue. Medical service providers will also be under tremendous psychological pressure due to overwork, shortage of medical resources, patients' poor outcomes, infection among their colleagues, or other detrimental experiences.
Medical staff should be provided with adequate psychological support, as their physical and mental health is crucial in the context of a pandemic.
Alt-text: Unlabelled box
Conclusion
We share this guidance for the preparation of the NICU at a regional perinatal center during the global novel COVID-19 pandemic. Children seem to be less affected with this infection based on the limited published literature, however, one of the most vulnerable pediatric patient populations is cared for in NICUs. Current data on the effects of COVID-19 in fetal life and among neonates after birth is uncertain. As such, there is a need for proactive preparation and planning to manage these viral infections in neonates in ways that safeguard the patients, their families, and the healthcare personnel providing their care. Our understanding of COVID-19 is still evolving; therefore, we strongly suggest continuously monitoring the latest updates from CDC and local health authorities. Every health care facility should evaluate their available resources and make locally suited guidelines to take care of newborns with suspected or confirmed COVID-19, consistent with recommendations from CDC.
Financial disclosure
None of the authors have any financial relationships to disclose.
Declaration of Competing Interest
None of the authors have any conflicts of interest to disclose.
Funding source
No funding was secured.
Contributor Information
Sourabh Verma, Email: sourabh.verma@nyulangone.org.
Rishi Lumba, Email: rishi.lumba@nyulangone.org.
Jennifer L. Lighter, Email: jennifer.lighter@nyulangone.org.
Sean M. Bailey, Email: sean.bailey@nyulangone.org.
Elena V. Wachtel, Email: elena.wachtel@nyulangone.org.
Bgee Kunjumon, Email: bgee.kunjumon@nyulangone.org.
Samantha Alessi, Email: samantha.alessi@nyulangone.org.
Pradeep V. Mally, Email: pradeep.mally@nyulangone.org.
References
- 1.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y., Mo X., Hu Y. Epidemiological characteristics of 2143 pediatric patients with 2019 Coronavirus Disease in China. Pediatrics. 2020;145(6):e20200702. https://nam03.safelinks.protection.outlook.com/?url=https%3A%2F%2Fdoi.org%2F10.1542%2Fpeds.20200702&data=02%7C01%7Cp.alexander%40elsevier.com%7C37772a31617d40c7111508d80ed5a7da%7C9274ee3f94254109a27f9fb15c10675d%7C0%7C0%7C637275658094221653&sdata=uWqsKh5xGMGr8JcDKlihbeMGQ4kuEAZkNLeWvIDlAVU%3D&reserved=0 [Google Scholar]
- 4.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DRAFT landscape of COVID-19 candidate vaccines- 4March 2020. 2020; https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1.
- 10.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng L., Xia S., Yuan W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng H., Xu C., Fan J. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong L., Tian J., He S. Possible VERTICAL TRANSMISSIon of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection be acquired In Utero?: More definitive evidence is needed. JAMA. 2020;323(18):1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Qi H., Bao L. A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units. Lancet Child Adolesc Health. 2020;4(4):258–259. doi: 10.1016/S2352-4642(20)30040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Considerations for inpatient obstetric healthcare settings. 2020; https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html.
- 19.Centers for Disease Control and Prevention. How to keep your breast pump kit clean: the essentials. 2019; https://www.cdc.gov/healthywater/hygiene/healthychildcare/infantfeeding/breastpump.html.