Abstract
Dysregulation of inflammation is hypothesized to play a crucial role in the severe complications of COVID-19, with the IL-1/IL-6 pathway being central. Here, we report on the treatment of eight severe COVID-19 pneumonia patients—seven hospitalized in intensive care units (ICUs) in Greece and one non-ICU patient in the Netherlands—with the interleukin-1 receptor antagonist Anakinra. All patients scored positive for the hemophagocytosis score (HScore) and were diagnosed with secondary hemophagocytic lymphohistocytosis (sHLH) characterized by pancytopenia, hyper-coagulation, acute kidney injury, and hepatobiliary dysfunction. At the end of treatment, ICU patients had less need for vasopressors, significantly improved respiratory function, and lower HScore. Although three patients died, the mortality was lower than historical series of patients with sHLH in sepsis. These data suggest that administration of Anakinra may be beneficial for treating severe COVID-19 patients with sHLH as determined by the HScore, and they support the need for larger clinical studies to validate this concept.
Keywords: hemophagocytic lymphohistocytosis, COVID-19, Anakinra, ferritin, respiratory function, pneumonia, SARS-CoV-2, HScore
Graphical Abstract
Complex immune dysregulation in severe COVID-19 suggests the use of immunomodulation therapies. Dimopoulos et al. describe eight cases of COVID-19 patients who all had secondary hemophagocytic lymphohistiocytosis and showed favorable responses in respiratory function upon treatment with the interleukin-1 receptor antagonist Anakinra.
Introduction
The most fearsome complication of pneumonia caused by the novel coronavirus SARS-Cov-2 (COVID-19 illness) is severe respiratory failure leading to mechanical ventilation (MV). The mortality in patients with severe COVID-19 admitted in the intensive care units is reported to be between 50% and 65% (Bhatraju et al., 2020, Chen et al., 2020a, Chen et al., 2020b). The cytokine storm described in these patients (Qin et al., 2020) introduced the concept of attenuation of the hyper-inflammation, with agents targeting pro-inflammatory cytokines, mainly interleukin (IL)-1β and IL-6. Several clinical trials on the efficacy of Anakinra, which targets IL-1β, and of Tocilizumab, Siltuximab, and Sarilumab, which target the IL-6 pathway, are ongoing.
Overproduction of IL-1β by tissue macrophages triggers secondary hemophagocytic lymphohistiocytosis (sHLH), also called macrophage activation syndrome; this is characterized by pancytopenia, hyper-coagulation, acute kidney injury, and hepatobiliary dysfunction. In sepsis, sHLH is a complication leading independently to early death in the first 10 days (Kyriazopoulou et al., 2017); 28-day mortality is reaching 67%. Administration of Anakinra, which is a recombinant soluble receptor antagonist of IL-1β and IL-1α, in patients with signs of sHLH reduced mortality by 30% (Shakoory et al., 2016).
We wanted to assess whether administration of Anakinra would benefit severely ill COVID-19 patients. Since March 2020, seven patients in three intensive care units (ICUs) in Greece and one non-ICU patient in the Radboud University Medical Center (Radboudumc) in the Netherlands with severe COVID-19 were given salvage treatment with Anakinra on the grounds of sHLH diagnosed using the HScore (hemophagocytosis score). Promising indications on treatment efficacy are described here.
Results
All Treated Patients Had Severe Comorbidities and Suffered from sHLH
The seven patients in Greece were treated with 200 mg Anakinra intravenously every eight hours for seven days. The dose regimen was selected since it was administered in patients with sepsis participating in one randomized clinical trial with the acronym PROVIDE (ClinicalTrials.gov NCT03332225). All patients were male, and they suffered from severe comorbidities—mainly coronary heart disease and arterial hypertension (Table 1 ). All patients scored positive by the HScore, which is a classification system using nine different variables; any score greater than or equal to 169 provides 93% sensitivity for the diagnosis of HLH (Fardet et al., 2014). All treated patients had HScores greater than or equal to 169 (Table 2 ). Assays for the detection of cytomegalovirus (CMV) and Ebstein-Barr virus (EBV) in their plasma were negative.
Table 1.
Demographics, Past History, and Management of Eight Patients with Severe COVID-19
| Pt | Age | Gender | Past history | CCI | Co-administered Drugs | Ventilation at | Norepinephrine (μg/kg/min) | 28-day | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | Glucocorticoids | Hydroxychloroquine | Others | Prone Position | Start | Day 7 | Outcome | ||||
| 1 | 51 | Male | Arterial hypertension | 1 | Hydrocortisone 50mg 6qh x 7 days IV | Yes | Meropenem, teicoplanin, azithromycin | No | 0.13 | 0.08 | Death (day 12) |
| 2 | 74 | Male | DM2, arterial hypertension, BPH | 5 | Hydrocortisone 50mg 6qh x 7 days IV | Yes | Meropenem, teicoplanin, azithromycin | No | 1.11 | 0.59 | Death (day 9) |
| 3 | 67 | Male | CHD, dyslipidemia, arterial hypertension | 3 | Hydrocortisone 250mg single IV infusion | Yes | Meropenem, teicoplanin, azithromycin | No | 0.27 | 0.17 | Alive, weaning from MV day 22 |
| 4 | 84 | Male | CHD, COPD, BPH, dyslipidemia | 10 | None | Yes | Meropenem, teicoplanin, azithromycin | No | 0.78 | 0.44 | Death (day 19) |
| 5 | 56 | Male | Arterial hypertension | 1 | None | Yes | Piperacillin/tazobactam, colistin, azithromycin | Days 1 to 7 (12-20 h/d) | No need | No need | Alive, weaning from MV day 31 |
| 6 | 68 | Male | DM2, CHD, dyslipidemia, arterial hypertension, stroke | 2 | None | Yes | Ceftaroline, azithromycin | No | 1.85 | No need | Alive, on MV |
| 7 | 67 | Male | DM2, CHD, dyslipidemia, arterial hypertension, stroke | 4 | None | Yes | Ceftriaxone, azithromycin | Days 14 to 19 (16 h/d) | 1 | <0.10 | Alive, on MV |
| 8 | 71 | Female | Arterial hypertension, metastatic colon cancer, dyslipidemia | 10 | None | No | Ceftazidime | NA | NA | NA | Alive, discharged day 9 |
Abbreviations; BPH: benign prostate hypertrophy; CCI: Charlson’s comorbidity index; CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; DM2: type 2 diabetes mellitus; IV: intravenous; q6 h: every six hours; NA: non-available.
Table 2.
HScores of the Eight Patients with Severe COVID-19
| Pt | T (°C) | Organomegaly | Number of Cytopenias | Triglycerides (mmol/l) | Fibrinogen (g/l) | Ferritin (ng/mL) | AST (IU/l) | Hemophagocytosis in Bone Marrow | Immuno-suppression | HScore |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39.8 (49) | None (0) | Hb 8.5 g/dl; WBC 2890/mm3 (24) | 2.28 (44) | 0.78 (0) | 5002 (35) | 55 (19) | N/A | No | 171 |
| 2 | 40.0 (49) | Hepatomegaly and splenomegaly (38) | Nil lineage (0) | 4.89 (64) | 0.48 (0) | 1924 (0) | 71 (19) | N/A | No | 170 |
| 3 | 38.5 (33) | Hepatomegaly and splenomegaly (38) | Nil lineage (0) | 1.84 (44) | 0.38 (0) | 3582 (35) | 34 (19) | N/A | No | 169 |
| 4 | 37.5 (0) | Splenomegaly (23) | Hb 8.5 g/dl; WBC 3200/mm3 (24) | 1.53 (44) | 0.80 (0) | 6032 (50) | 36 (19) | N/A | Non-Hodgkin Lymphoma (18) | 178 |
| 5 | 39.5 (49) | Hepatomegaly (23) | WBC 3200/mm3 (0) | 2.32 (44) | 0.64 (0) | 6786 (50) | 241 (19) | N/A | No | 185 |
| 6 | 37.7 (0) | Hepatomegaly and splenomegaly (38) | Nil lineage (0) | 4.22 (64) | 0.57 (0) | 7389 (50) | 265 (19) | N/A | No | 171 |
| 7 | 38.6 (33) | Hepatomegaly and splenomegaly (38) | Hb 8.3 g/dl; WBC 4200/mm3; PLT 48,000/mm3 (34) | 3.29 (44) | 0.77 (0) | 10500 (50) | 332 (19) | N/A | No | 218 |
| 8 | 38.5 (33) | N/A | Hb 6.0 g/dl; WBC 1800/mm3; PLT 31,000/mm3 (34) | 1.80 (44) | 0.76 (0) | >6000 (50) | 615 (19) | N/A | Chemotherapy (18) | 198 |
Numbers in brackets refer to the points allocated per score variable. Abbreviations AST: aspartate aminotransferase; Hb: hemoglobin; PLT: platelet count; N/A: non-available; WBC: total white blood cell count.
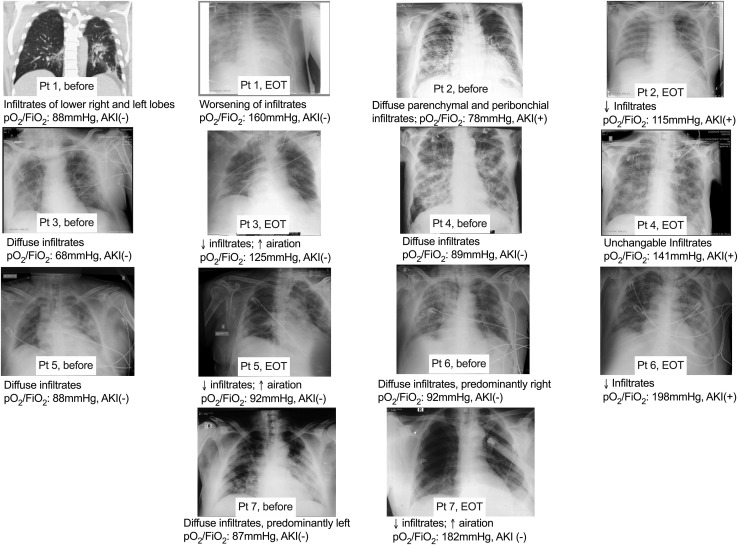
All treated patients had acute respiratory failure, since all had presented with diffuse lung infiltrates in chest X-rays and all had a ratio of partial oxygen pressure to the fraction of inspired oxygen (pO2/FiO2) lower than 100 (Figure 1 ). Only one patient had acute kidney injury.
Figure 1.
Comparative Chest X-rays of the Seven Greek Patients Necessitating Mechanical Ventilation on Day 1 before Start of Anakinra and at the End of Treatment (EOT) with Anakinra
Below each X-ray a brief diagnosis is provided along with the ratio of the partial oxygen pressure to the fraction of inspired oxygen (pO2/FiO2) of that day. The presence (+) or absence (−) of acute kidney injury (AKI) is also noted.
Abbreviations: ↓: decrease; ↑: increase
Anakinra Treatment Was Associated with Improvement of Respiratory Function of Patients under MV
Seven of the described patients started Anakinra treatment after initiation of MV. Three patients died by day 28. All seven patients received treatment with hydroxychloroquine and azithromycin and broad-spectrum antibiotics (Table 1). As shown in Figure 1, lung infiltrates were decreased by the end of treatment (EOT) in five patients and this was accompanied by increase of the pO2/FiO2 ratio. The end of 7-day treatment was accompanied by decrease of the dose of administered vasopressors in six patients (Table 1).
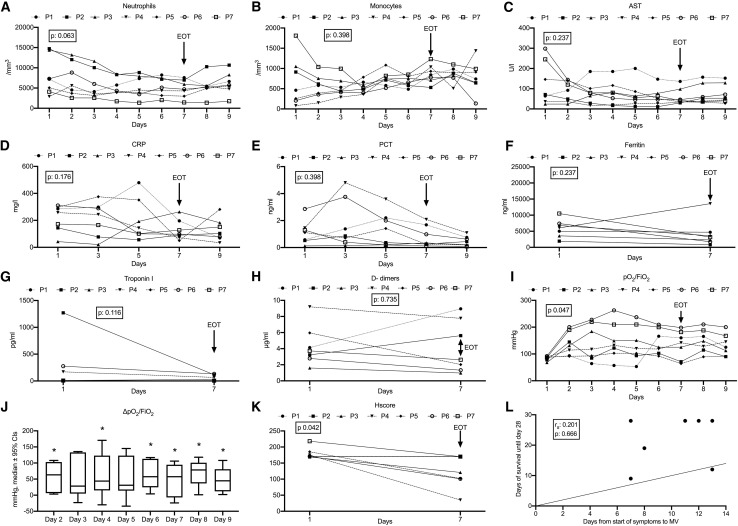
Although treatment with Anakinra seemed to improve the majority of laboratory findings of the patients (Figures 2A–2H) by EOT, which were also maintained two days later, significant decreases from the baseline were mainly found for the pO2/FiO2 ratio, which is an index of respiratory function; this was increased in five patients by EOT and in all seven patients two days later (Figure 2I). This increase ranged between 15% and 117% (Figure 2J). The HScore was also significantly decreased at the EOT (Figure 2K). No association between the time interval from start of symptoms until start of MV and survival time was found (Figure 2L).
Figure 2.
Over-Time Change of Laboratory Values of Each of the Seven Greek Patients Necessitating Mechanical Ventilation
(A) Daily absolute neutrophil count for nine serial days.
(B) Daily absolute monocyte count for nine serial days.
(C) Daily values of aspartate aminotransferase (AST) for nine serial days.
(D) Serum concentrations of C-reactive protein (CRP) on days 1, 3, 5, 7, and 9.
(E) Serum concentrations of procalcitonin (PCT) on days 1, 3, 5, 7 and 9.
(F) Serum concentrations of ferritin on days 1 and 7.
(G) Serum concentrations of troponin I on days 1 and 7.
(H) Plasma concentrations of D-dimers I on days 1 and 7.
(I) Ratio of partial oxygen pressure to fraction of inspired oxygen (pO2/FiO2) for nine serial days.
(J) Boxplots of % changes of the pO2/FiO2 ratio of the seven patients on days 2, 3, 4, 5, 6, 7, 8, and 9 from day 1. P value refer to paired comparisons of the pO2/FiO2 on the indicated days from day 1 by the Wilcoxon test.
(K) HScore (hemophagocytosis score) on days 1 and 7.
(L) Correlation between days from start of symptoms until start of mechanical ventilation (MV) and survival until day 28. The rank of Spearman correlation coefficient (rs) and the respective p value are provided.
Treatment with Anakinra started on day 1. The arrow indicates the end of treatment with Anakinra (EOT: end of treatment). The p value in each panel refers to the paired comparisons between days 1 and 7 by the Wilcoxon test.
The first patient died after 12 days due to refractory shock. Although one strain of Acinetobacter baumannii resistant to carbapenems and colistin was isolated from the tip of the central vein catheter, blood cultures were sterile. The second patient died after nine days due to refractory shock. He had severe hypoxemia accompanied by increase of inflammatory markers and elevated core temperature; blood cultures were sterile. The fourth patient died after 19 days due to refractory shock aggravated by acute kidney injury and anuria; blood cultures were sterile.
Anakinra May Prevent Progression of Respiratory Failure
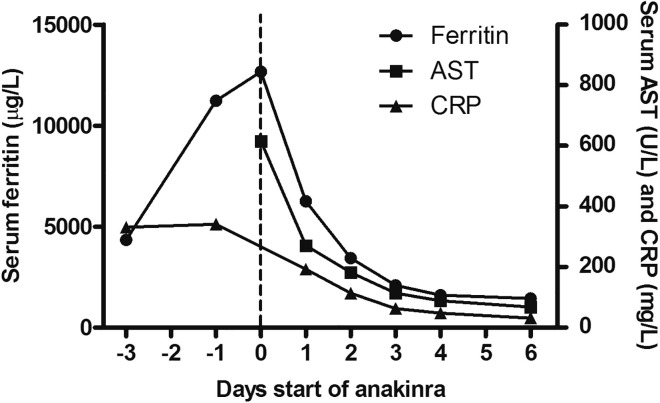
A patient case at the Radboud University Medical Center shows that Anakinra in SARS-Cov-2-infected patients with features of sHLH may prevent progression of respiratory failure and the need for MV. The patient was a 71-year old female with a recurrent ethmoidal adenoid cystic carcinoma and rheumatoid arthritis treated with hydroxychloroquine. She was hospitalized 14 days after the third cycle of palliative chemotherapy (cyclophosphamide, doxorubicin, and carboplatin) with COVID-19. Chest CT showed bilateral ground glass opacities with consolidations (data not shown). Anakinra was administered on day nine of hospitalization because of clinical deterioration with increasing oxygen demand in combination with persistent pancytopenia and rapidly increasing concentrations of serum ferritin (maximum concentration 12,670 ng/mL) and of hepatic aminotransferases. Plasma EBV PCR showed a low load (200 IU/mL), whereas CMV DNA was undetectable. Anakinra (300 mg once daily intravenously for 4 days, followed by 100 mg once daily) resulted in a rapid improvement in clinical condition within 24 h with decrease in oxygen need and decrease in sHLH blood parameters (Figure 3 ). The HScore (presence of organomegaly and hemophagocytosis in bone marrow not scored) decreased from 198 points at baseline to 71 points at EOT, and the patient was discharged home on day nine post start of Anakinra.
Figure 3.
Over-Time Change of Laboratory Values in the Non-ICU Patient
Discussion
Immunomodulation remains a promising strategy for the management of severe COVID-19, since it aims to attenuate the exaggerated inflammatory response of the host. The case series of 7 patients coming from Greece and 1 from the Netherlands reported here supports the concept that Anakinra treatment may improve the respiratory function of patients who have sHLH at the EOT and at follow-up. Patients with severe COVID-19 are reported to experience hyper-coagulation and hyper-inflammation evidenced by increased D-dimers, ferritin, C-reactive protein (CRP), and procalcitonin (PCT). They also experience cardiomyopathy with increased Troponin I (Guan et al., 2020, Huang et al., 2020). All these markers decreased with Anakinra treatment.
All seven patients under MV were male; this could be consistent with reports that the MV complication of COVID-19 is more frequent in male patients (Bhatraju et al., 2020), and may also be a result of chance. The possibility that sHLH is a more common complication of COVID-19 in males cannot be excluded.
A recent position paper suggested measuring HScore in patients with severe COVID-19, encouraging the administration of Anakinra when HScore was diagnostic of sHLH (Mehta et al., 2020). The reported mortality for the three out of seven Greek patients under MV appears high. However, it needs to be taken into consideration that sHLH is accompanied by substantial mortality, as high as 67%, in the case of sHLH developing in patients with sepsis (Kyriazopoulou et al., 2017, Shakoory et al., 2016).
This is not the first time favorable Anakinra responses have been shown in sHLH in adults. Several case series report similar responses of Anakinra treatment in patients with HLH secondary to adult onset Still’s disease (Lenert and Yao, 2016) and systemic infections (Kumar et al., 2017, Wohlfarth et al., 2019). Anakinra was administered subcutaneously and at lower doses than those used herein. It was also co-administered with corticosteroids, antibiotics, and antivirals. These authors highly recommend measuring the HScore when the patient is deteriorating and administering treatment with Anakinra when sHLH is diagnosed.
Recently, 29 patients with severe COVID-19, the majority of which had pO2/FiO2 less than 100 mmHg and were treated intravenously with Anakinra, were retrospectively analyzed. Their mortality by day 21 was 10%, significantly lower than that of 16 comparators receiving standard-of-care treatment, which was 44% (Cavalli et al., 2020). These patients were less severe than those described herein, since they were not under MV and they had lower ferritin levels. Although HScore was not a criterion for treatment, these published outcomes support the results of our case series showing favorable responses with Anakinra in the respiratory function of patients with COVID-19 who scored positive for the HScore. The one case of the non-ICU patient generates consideration for an early time window, before the need for MV, where immunomodulation may benefit.
Limitations of Study
The main study limitations are the lack of a randomized design and the absence of untreated or placebo-treated comparators, both of which would allow better understanding of the magnitude of the survival benefit coming from Anakinra treatment. Considering these limitations, the presented data in patients with severe COVID-19 and sHLH warrant larger clinical trials to validate these results and to demonstrate the usefulness of anti-IL-1 therapy.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical Commercial Assays | ||
| QIAamp DNA mini kit | QIAgen | 51304 |
| GeneProof EBV PCR kit | QIAgen | 4501063 |
| Genesig PCR CMV kit | Genesig | CMV Easy |
| Human Ferritin ELISA | ORGENTEC Diagnostika GmbH | ORG 5FE |
| Software and Algorithms | ||
| GraphPad Prism | Graphpad Software | https://www.graphpad.com |
| SPSS | IBM | https://www.ibm.com/analytics/spss-statistics-software |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Evangelos J. Giamarellos-Bourboulis (egiamarel@med.uoa.gr)
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Data of this study are available after communication with the Lead Contact
Experimental Model and Subject Details
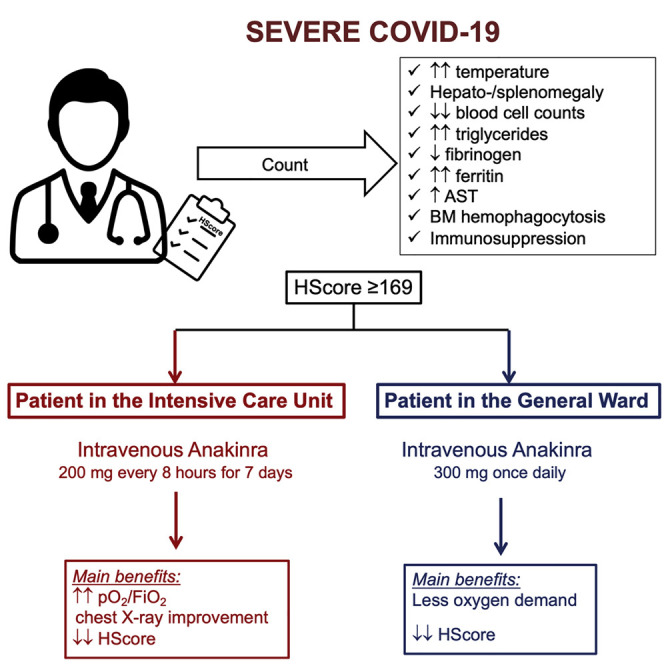
All consecutive patients of both genders between March 21st 2020 and March 28th 2020 in three ICUs in Greece under MV for severe COVID-19 and one non-ICU patient in the Netherlands who were scoring positive by the HScore received salvage intravenous treatment with Anakinra. The dose of Anakinra was 200 mg every eight hours intravenously for seven days for the Greek patients; and 300mg once daily intravenously for 4 days, followed by 100mg once daily subcutaneously until hospital discharge for the Dutch patient. The HScore is developed by experts by the analysis of a cohort of 314 patients and provides points for each of nine variables: degree of core temperature; hepatomegaly and/or splenomegaly; number of cytopenias; concentrations of triglycerides, fibrinogen, ferritin, aspartate aminotransferase; history of immunosuppression; and bone marrow hemophagocytosis. HScore more than or equal to 169 are considered diagnostic for sHLH since they are associated with 93% diagnostic sensitivity (Fardet et al., 2014). Anakinra was administered as off-label salvage treatment in accordance to treatment suggestions for severe COVID-19 and the procedure suggested by the National Public Health Organization (NPHO) of Greece (https://eody.gov.gr/). No randomization to experimental groups was needed according to the study design.
Method Details
The following information was recorded: past-history; co-administered drugs; laboratory data and blood gases for the seven days of treatment and for two more follow-up days; and survival. Complete blood cell counts, serum or plasma concentrations of C-reactive protein, PCT, ferritin, troponin I and D-dimers were measured by automatic analyzers (Abbott Diagnostics). Blood gases were measured by automatic analyzers. Viral load of CMV and EBV was measured in plasma of these patients when sHLH was diagnozed. Viral DNA was isolated using the QIAamp DNA mini kit (QIAGEN) and this was followed by PCR reactions using the Genesig PCR CMV kit (Genesig) and the GeneProof EBV PCR kit (QIAgen). The lower detection limit was 2.77 copies/μl.
Quantification and Statistical Analysis
Results were presented as line graphs separately for every patient. Paired comparisons were done by the Wilcoxon’s rank-signed test. Any value of p below 0.05 was considered significant.
Acknowledgments
The study was funded in part by the Horizon 2020 grant ImmunoSep (#847422) and in part by the Hellenic Institute for the Study of Sepsis. MGN was supported by an ERC Advanced grant (#833247) and a Spinoza grant from the Netherlands Organization for Scientific Research.
Author Contributions
G.D., Q.M., N.M., M.T., A.K., M.M., R.J.V., J.H., A.L. and F.L.V. collected clinical information, reviewed the manuscript, and gave approval of the final version to be submitted M.G.N. conceptualized the study, analyzed the data, drafted the manuscript, and gave approval of the final version to be submitted. M.M. performed PCR experiments, reviewed the manuscript, and gave approval to the final version to be submitted. T.S. performed chest X-ray diagnosis, reviewed the manuscript, and gave approval to the final version to be submitted. E.J.G.B. designed the study, analyzed the data, wrote the manuscript, and gave approval to the final version to be submitted
Declaration of Interests
Mihai G. Netea is supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. Frank L van de Veerdonk is supported by a Vidi grant of the Netherlands Association for Scientific Research. E.J. Giamarellos-Bourboulis has received honoraria from AbbVie USA, Abbott CH, InflaRx GmbH, MSD Greece, XBiotech Inc., and Angelini Italy; independent educational grants from AbbVie, Abbott, Astellas Pharma Europe, AxisShield, bioMérieux Inc, InflaRx GmbH, and XBiotech Inc; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis).
Published: July 8, 2020
References
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., et al. COVID-19 in critically ill patients in the Seattle region—case series. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyper-inflammation: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardet L., Galicier L., Lambotte O., Marzac C., Aumont C., Chahwan D., Coppo P., Hejblum G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Aleem S., Saleh H., Petts J., Ballas Z.K. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J. Clin. Immunol. 2017;37:638–643. doi: 10.1007/s10875-017-0439-x. [DOI] [PubMed] [Google Scholar]
- Kyriazopoulou E., Leventogiannis K., Norrby-Teglund A., Dimopoulos G., Pantazi A., Orfanos S.E., Rovina N., Tsangaris I., Gkavogianni T., Botsa E., et al. Hellenic Sepsis Study Group Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15:172. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenert A., Yao Q. Macrophage activation syndrome complicating adult onset Still’s disease: A single center case series and comparison with literature. Semin. Arthritis Rheum. 2016;45:711–716. doi: 10.1016/j.semarthrit.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., Cron R.Q., Opal S.M. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth P., Agis H., Gualdoni G.A., Weber J., Staudinger T., Schellongowski P., Robak O. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J. Intensive Care Med. 2019;34:723–731. doi: 10.1177/0885066617711386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of this study are available after communication with the Lead Contact