Abstract
As a highly infectious respiratory tract disease, coronavirus disease 2019 (COVID-19) can cause respiratory, physical, and psychological dysfunction in patients. Therefore, pulmonary rehabilitation is crucial for both admitted and discharged patients of COVID-19. In this study, based on the newly released pulmonary rehabilitation guidelines for patients with COVID-19, as well as evidence from the pulmonary rehabilitation of patients with severe acute respiratory syndrome, we investigated pulmonary rehabilitation for patients with COVID-19 having complications, such as chronic pulmonary disease, and established an intelligent respiratory rehabilitation model for these patients.
Keywords: Coronavirus disease 2019 (COVID-19), Pulmonary rehabilitation, Intelligence
Coronavirus Disease 2019 (COVID-19) has spread worldwide and has become a global public health emergency. The World Health Organization recently declared the outbreak a pandemic. In accordance with the Law of the People's Republic of China on the Prevention and Control of Infectious Diseases, COVID-19 has been classified as a Category B infectious disease, with prevention and control measures for Category A infectious diseases adopted against the disease. Multiple COVID-19 diagnosis and treatment guidelines have been released by the National Health Commission of the People's Republic of China, all of which have contributed to the gradual control of the epidemic. According to the data released by the National Health Commission, although more than 84,000 patients have been diagnosed with COVID-19, over 78,000 patients have now recovered and have been discharged. Since patients with COVID-19 suffer from various degrees of respiratory, physical, and psychological dysfunction, pulmonary rehabilitation is equally important for both admitted and discharged patients for the treatment of the disease.1 For this reason, several pulmonary rehabilitation guidelines for patients with COVID-19 have been published in China to strengthen the pulmonary rehabilitation of admitted patients and follow-up and health management of discharged patients and thereby help the patients to recover and return to society more promptly and safely.
Pulmonary rehabilitation
Pulmonary rehabilitation refers to the individualized rehabilitation treatment of patients with chronic pulmonary diseases after a detailed assessment. With exercise training as its core, pulmonary rehabilitation comprises comprehensive interventions, including but not limited to psychological and nutritional support, as well as education and behavioral changes.2 The goal of pulmonary rehabilitation is to not only improve the patient's physical and mental conditions but also help the patient return to family and society more promptly.
With the development of evidence-based medicine, progressively more information has been obtained, based on which it is recommended that pulmonary rehabilitation should be the core of standardized management of patients with chronic obstructive pulmonary disease (COPD) and a treatment option for patients with other chronic pulmonary diseases. The “Global Initiative for Obstructive Lung Disease (GOLD)” incorporated pulmonary rehabilitation into the standard treatment for patients with COPD as early as 2001.3 The “Healthy China Initiative (2019–2030)” issued in 2019 has also emphasized the necessity of including pulmonary rehabilitation in a chronic pulmonary disease action plan.4 Furthermore, several high-quality clinical studies have verified the benefits of pulmonary rehabilitation for inpatients, outpatients, and in–home patients. The benefits include improved exercise tolerance in patients with chronic pulmonary diseases, reduced number of hospital admissions and length of hospital stays, enhanced health-related quality of life,5 improved respiratory muscle function and relieved dyspnea,6 alleviated disease-related anxiety and depression,7 and enhanced skeletal muscle function of upper and lower limbs.8,9
Necessity of pulmonary rehabilitation for patients with COVID-19
At present, the main antiviral drugs that are recommended for the treatment of COVID-19 are interferon-α (IFN-α), lopinavir/ritonavir, ribavirin, chloroquine phosphate, hydroxychloroquine sulfate, and arbidol. According to the summary of phased treatment of patients with COVID-19 in China, the clinical efficacy of lopinavir/ritonavir for antiviral treatment is not obvious, and Lopinavir/Ritonavir is associated with several side effects; hence, the drug is not recommended for use at present.10 The indirect evidence for efficacy of Ribavirin and IFN in the treatment of COVID-19 has been mainly derived from the evidence of the efficacy of the drugs in treatment of infections caused by severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS).11,12 However, patients with COVID-19 treated with IFN-α 2b in combination with Ribavirin have no significant improvement in the risk of death compared to those treated with IFN-α 2b alone.13 The latest research shows that chloroquine can effectively inhibit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at the cellular level.14 Furthermore for patients with a history of local epidemiology or other risk factors related to infection (including travel history or exposure to animal influenza virus), oseltamivir can be empirically added to the treatment regimen.15 Some scholars also think that remdesivir, darunavir/cobicistat, and favipiravir could be used in the treatment of COVID-19. At present, a number of randomized controlled trials are in progress to evaluate the efficacy and safety of the abovementioned drugs for the treatment of COVID-19.
The three major components of the medical system—prevention, treatment, and rehabilitation—are equally important. The primary clinical manifestations of COVID-19 are fever, cough, dyspnea, and myalgia16; however, severe cases can rapidly progress to acute respiratory distress syndrome (ARDS). In addition, some patients can develop acute myocardial and kidney injuries.17 The latest pathological reports indicate that the predominant pathological changes in early-18 and late-stage patients are diffuse lung injuries, although some patients also suffer from intra-alveolar fibrinous exudate and pulmonary interstitial fibrosis. Moreover, the virus also affects other organs such as the heart, liver, and kidneys to various degrees.19 These changes contribute to hypoxemia and impaired cardiopulmonary and organ functions throughout the body. Currently, evidence on the prognosis of patients with COVID-19 is insufficient, especially for elderly patients in whom the disease is complicated by other basic diseases. It remains unclear whether the impairment of multiple systemic functions is reversible or if the long-term existence of the virus can cause physical dysfunction in these patients. In addition, because COVID-19 has caused a public emergency, patients with COVID-19 may demonstrate different degrees of psychological disorders, such as anger, fear, anxiety, depression, insomnia, and loneliness, as well as a lack of cooperation and abandonment of treatment due to fear of the disease.20 Even when discharged, the patients may experience post-traumatic stress syndrome. Therefore, prompt introduction and continuous availability of pulmonary rehabilitation services is critical for patients with COVID-19.
Evidence for pulmonary rehabilitation of patients with SARS
Follow-up studies have shown that after discharge, patients with severe acute respiratory syndrome (SARS) can still suffer from symptoms, such as restrictive pulmonary dysfunction, palpitations, hand tremors, and exertional dyspnea, all of which affect their daily activities and impair their quality of life.21,22 It has been suggested that these symptoms are associated with prolonged bed rest, adverse effects of steroid medications, and residual pathological changes, such as atelectasis, persistent alveolitis, pulmonary fibrosis, and varying degrees of muscle weakness or dysfunction.23 In addition, a 1-year follow-up of patients with ARDS showed that, survivors of ARDS exhibit persistent functional disability one year after discharge from the intensive care unit. Most patients have extrapulmonary conditions, with muscle wasting and weakness being most prominent.24 Compared with SARS, pathological changes, such as pulmonary fibrosis, have not been dominant in patients with COVID-19; however, we speculate that damage to the lung and other organ systems caused by SARS-CoV-2, especially in severe patients with ARDS, may lead to residual physical dysfunction of varying degrees. Thus, the evidence for pulmonary rehabilitation of patients with SARS provides strong support and reference for the development of pulmonary rehabilitation programs for patients with COVID-19.
Lau et al25 carried out a 6-week pulmonary rehabilitation program for 133 patients with SARS who had been discharged after treatment. Each rehabilitation session was conducted for 1.0–1.5 h, 4–5 times per week. The interventions included 30–40 min of aerobic training at 60%–75% (up to 80%–85% for some subjects) of maximum heart rate predicted using the Chester Step Test to reach a score of 4–6 on the Borg Rating of Perceived Exertion, followed by 3 sets of upper and lower limb resistance exercises at 10–15 RM (repetition maximum, the maximum load that can be repeated with 10–15 movements in each set) and relevant health education. Compared with patients in the control group who only received conventional care, patients in the rehabilitation group showed significant differences in 6-minute walking distance and maximum rate of oxygen consumption during incremental exercise. In addition, the isometric muscle strength of the anterior deltoid muscle and gluteus maximus, the grip strength of both hands (distal muscle strength), and results of the 1-minute curl and push-up test (endurance of abdominal and upper limb muscles) were all substantially elevated in the rehabilitation group. However, although the role-physical, role-emotional, and social function scales of the SF-36 questionnaire had improved within 6 weeks in the rehabilitation group, the pairwise differences were insignificant, possibly due to the extensive physical and psychological impacts of SARS on the patients (especially elderly patients). Furthermore, patients who were not reinstated (predominantly healthcare workers) scored lower on almost all scales (except bodily pain) than patients who were reinstated prior to the completion of the intervention, indicating that prompt reinstatement can help improve the patients’ quality of life. Alternatively, a small sample study in China on 9 discharged patients with SARS who underwent 3 weeks of rehabilitation consisting of respiratory exercises combined with deep breathing and stretching exercises, low-to medium-intensity treadmill aerobic endurance exercise, and direct-current iontophoresis pulmonary physiotherapy, suggested that the perfusion rate for pulmonary function of the patients was significantly different from the baseline value. In addition, the dyspnea level of the rehabilitation group improved considerably compared to that of the control group, while the resting heart rates of both groups recovered to a certain extent.26
Pulmonary rehabilitation guidelines for patients with COVID-19
Based on front-line expert consensus and references, rehabilitation specialists in China have developed practical and feasible respiratory rehabilitation guidelines for patients with COVID-19. The primary instructions of these guidelines are as follows: (1) The short-term goal of pulmonary rehabilitation is to alleviate dyspnea and relieve anxiety and depression while the long-term goal is to preserve the patient's function to the maximum extent, improve his/her quality of life, and facilitate his/her return to society. (2) It is necessary to perform comprehensive assessments before starting the rehabilitation program. For example, clinical and exercise risk assessments should be performed based on the patient's clinical symptoms, vital signs, auxiliary examinations, imaging, comorbidities, contraindications, etc., whereas quality of life, daily activity endurance, and psychological and nutritional assessments should be conducted in the form of questionnaires. The results of these assessments can then be combined with the patient's aerobic endurance, muscle strength, balance, and flexibility to formulate an individualized and progressive rehabilitation prescription. The prescription content mostly includes: A. Aerobic exercises: walking, fast walking, jogging, swimming, etc., starting from low intensity and gradually improving the intensity and duration, 3–5 times a week, 20–30 min each time. B. Strength training: progressive resistance training is recommended. The training load of each target muscle group is 8–12 RM, 1–3 groups/time. The training interval of each group is 2 minutes, 2–3 times/week, and the training load is increased by 5%–10% every week. C. Balance training: Patients with balance dysfunction should be involved in balance training, including unarmed balance training and balance training instrument. D. Respiratory training: if the patient has symptoms, such as shortness of breath, wheezing, and difficulty in expectoration after discharge, respiratory mode training, such as body position management, adjustment of respiratory rhythm, traction of respiratory muscle group breathing exercise, and expectoration training, should be arranged in combination with the evaluation results. E. Health care training for using traditional Chinese medicine: it is mainly for light and ordinary patients and discharged patients. If there is no contraindication (such as limb dysfunction and abnormal consciousness), it is recommended to carry out Baduan jin, Twenty-four Simplified Tai chi, Six-word Qigong, etc., 30–50 min each time, once a day. (3) All rehabilitation should be carried out under the premise of safety. In case a patient shows peripheral capillary oxygen saturation (SpO2) < 88% or develops symptoms, such as palpitations, sweating, chest tightness, and shortness of breath, which are deemed unsuitable for rehabilitation by the clinician, then the rehabilitation program should be terminated immediately. (4) For mild and moderate cases, rehabilitation interventions should be introduced as early as possible. In contrast, for severe and critical cases, life-saving measures should be prioritized when the patient's condition is unstable or the disease is still progressing. In such cases, pulmonary rehabilitation interventions should be introduced only when the patient's condition has stabilized. In addition, in view of safety and human resources, movement of severely or critically ill patients should be limited to their bed or bedside. Once discharged, patients should continue individualized rehabilitation under the premise of strengthening protection and prevention against other infectious diseases such as cold. (5) Compared with general rehabilitation for patients with chronic diseases, the most distinctive characteristic of rehabilitation for patients with COVID-19 is the infectivity of the disease. Therefore, operations that can increase the risk of infection, such as instructed cough, expiration training, and tracheal compression, should be minimized. A sealed plastic bag should be used to cover the mouth during expectoration to prevent infection. In addition, pulmonary rehabilitation of patients with COVID-19 should be carried out mainly through educational videos, brochures, remote consultations, or online teaching so as to save protective equipment and avoid cross infection. (6) Evaluation and monitoring should be conducted throughout the pulmonary rehabilitation program.27,28
An example of the rehabilitation of a patient with COVID-19 is provided below.
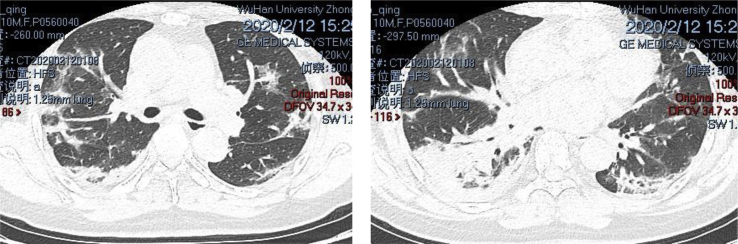
Patient A is a male, 50 years old, and critically ill with COVID-19. Prior to rehabilitation, the patient was treated in the ICU for 1 month before he underwent hormone therapy for over 1 month. The computer tomography (CT) displayed diffuse pulmonary lesions in both lungs, as shown in Fig. 1. Table 1 lists the patient's assessment results prior to rehabilitation, and Table 2 lists the patient's individualized rehabilitation prescription based on the assessment.
Fig. 1.
Diffuse pulmonary lesions in both lungs of the COVID-19 patient.
Table 1.
Assessment results before rehabilitation.
| Assessment Items | Assessment Result | Suggested Issue | |
|---|---|---|---|
| Breath-hold test | Less than 10 seconds (normally 30 seconds) | Impaired lung function | |
| 1-minute step test | Heart rate before and after the test | 102–124 beats/minute | Severely declined cardiopulmonary endurance |
| Blood oxygen change | 97%–94% | ||
| Borg Dyspnea Scale score | 0–2 | ||
| Squat | Cannot complete independently | Lower limb muscle atrophy and reduced muscle function | |
Table 2.
Individualized rehabilitation prescription.
| Prescription Items | Content | Required Equipment |
|---|---|---|
| Improve dyspnea | Diaphragmatic breathing + constricted breathing, 10 minutes, 3 times/day | Bare hands |
| Airway clearance | Expiration training + sputum excretion device, 15 minutes, 1 time/day | Sputum excretion and breathing trainer |
| Respiratory muscle exercise | Non-threshold load training for the inspiratory muscle, started from 3 cm H2O and slowly increased thereafter, 10–15 minutes, 1 time/day | Respiratory muscle trainer |
| Thoracic expansion exercise | Stretching, 5 minutes, 1 time/day | Bare hands |
| Activity | 10–30 minutes, either walking or stepping, target heart rate 124 beats/minute, Borg score 2, blood oxygen no less than 90% | Heart rate monitor and oximeter |
| Resistance exercise | Bare hands, yellow elastic band, big muscle group, 10 minutes/time | Elastic band |
| Nutritional support | Protein powder for muscular dystrophy, 1–12 g/kg | Protein powder |
Thoughts on pulmonary rehabilitation of patients with COVID-19 complicated with chronic pulmonary diseases
For patients with COVID-19 complicated with chronic pulmonary diseases, such as COPD, bronchial asthma, and pulmonary interstitial fibrosis, in addition to performing an assessment and developing a prescription based on the rehabilitation guidelines, the following instructions should be followed: (1) Ensure the continuation of standardized basic medications and a reasonable diet. (2) Promote smoking cessation, flu vaccination, and Streptococcus pneumoniae vaccination. (3) As patients with COVID-19 having chronic pulmonary diseases often have excessive airway secretions, expiration exercises should be performed in addition to general airway clearance exercises to facilitate sputum excretion and reduce the exhaustion due to coughing. In addition, auxiliary techniques, such as the application of oscillatory positive expiratory pressure (OPEP), can be utilized. (4) Appropriate oxygen therapy should be provided during exercises. Patients with chronic pulmonary diseases can develop hypoxemia at rest. Subsequently, when exercising, as the interval for red blood cells to pass through the alveolar capillaries is shortened, the ventilation flow rate disorder increases, and oxygen intake decreases. Meanwhile, an escalated breathing rate causes pulmonary dynamic hyperinflation and gas trapping, which increases the end-expiratory lung volume, dead space ventilation and work of breathing, thereby further reducing blood oxygen saturation. In contrast, introducing oxygen therapy during exercise can meet the elevated metabolic demands, prevent hypoxemia, and reduce pulmonary dynamic hyperinflation, thereby improving the effect of exercise, while allowing an increase in the intensity and duration of the exercise. Hypoxemia during exercise is regarded as the indication for requirement of oxygen therapy (SpO2 at 88%–90% or a relative reduction of 2%–5%, lasting for 0.5–5.0 min). The goal of oxygen therapy is to adjust the oxygen flow rate to maintain the SpO2 within the range of 90%–92%. In order to increase the exercise effect, the oxygen flow rate can be increased according to the exercise intensity to maintain the SpO2 at about 95%.29 (5) Thoracic kyphosis correction: Due to long-term dyspnea, cough, etc., the work of breathing in patients with chronic pulmonary diseases often increases, which leads to the formation of abnormal breathing patterns. The resultant chronic pulmonary hyperinflation usually causes enlargement of the anterior and posterior diameters of the chest, thereby resulting in barrel chest or other chest deformities. A study of 143 young patients with cystic fibrosis showed that in patients over 15 years old, the condition of 77% of females and 36% of males was complicated with thoracic kyphosis deformity of more than 40°.30 Since this deformity can inhibit airway clearance and increase the work of breathing, it is important to incorporate physiotherapy, such as chest and muscle stretching and intensive training, in a comprehensive pulmonary rehabilitation program for thoracic kyphosis correction.
Exploration and implementation of intelligent pulmonary rehabilitation for patients with COVID-19
The strong infectivity of COVID-19 and dispersal of discharged patients make it difficult to realize remote pulmonary rehabilitation. At present, there is insufficient research on the clinical implementation of remote pulmonary rehabilitation. In recent years, rapid development in Internet technology has facilitated the implementation of remote monitoring and mobile intelligence technologies. The use of information and communication technology together with wearable devices has made it possible to practice intelligent, digital remote rehabilitation for patients with chronic pulmonary diseases, the effectiveness and safety of which have been proven non-inferior to those of traditional approaches.31,32 Based on this, we have pioneered the implementation of Internet-assisted pulmonary rehabilitation in Wuhan's mobile cabin hospitals. During the process, rehabilitation content is imported into a dedicated rehabilitation mobile application in text and video formats, including barehanded aerobic training, resistance training with elastic band, and respiratory muscle traction exercise. Once a patient has downloaded the application, he/she can directly obtain an individualized rehabilitation prescription through the application, followed by video training and recordings of rehabilitation exercises. In addition, the mobile app is capable of functions, such as symptom assessment and recording, automatic reminders for medication intake, and health education. All acquired data are transmitted to the management terminal of the medical care platform through information and communication technology, so that the medical staff can remotely monitor and evaluate the information provided by the patient on a regular basis and introduce corresponding measures and interventions. This randomized controlled clinical trial to evaluate the benefits of rehabilitation is still ongoing. It is expected that this remote model will not only reduce direct contact and exposure between doctors and patients, thereby preventing infection and saving protective equipment, but also provide long-term benefits to patients, such as improvement in cardiopulmonary endurance, recovery of physical function, and reduction of anxiety and depression, thereby promoting patients' return to society with an enhanced quality of life.
Conclusion and outlook
The development of rehabilitation medicine has been promoting the transformation of traditional, passive, and fractured medical care models into a human-oriented healthcare system that covers all stages of life. Pulmonary rehabilitation should be provided throughout the diseases management process, regardless of whether the patient is hospitalized or at home. In addition, rehabilitation prescriptions should be individualized based on the patient's specific condition. The effective incorporation of pulmonary rehabilitation into disease management and a patient's daily life, so that it becomes a conscious behavior, can provide long-term benefits to both the patient and his/her family. With the deepening of the understanding of COVID-19, an increasing number of patients have recovered. Pulmonary rehabilitation for these recovered patients has become a major challenge for medical staff, the resolution of which requires multidisciplinary collaboration and joint exploration so that evidence-based, high-quality support can be provided. In addition, the implementation of both traditional and research-based pulmonary rehabilitation provides valuable experience for clinical and rehabilitation medicine practices, which is necessary to implement the “Healthy China” concept.
Funding
This study was supported by a grant from Chinese Academy of Medical Sciences, Research on Prevention and Control System of Chronic Airway Diseases (No. 2019TX320005).
Conflict of interest
None.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Diagnosis and Treatment Guideline for Novel Coronavirus Pneumonia . 7th ed. 2020. The People's Republic of China: National Health Commission of the People's Republic of China, General Office of the National Health Commission.http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml at. [Google Scholar]
- 2.Spruit M.A., Singh S.J., Garvey C. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels R.A., Buist A.S., Calverley P.M. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 4.Healthy China Initiative (2019–2030) 2019. The People's Republic of China: National Health Commission of the People's Republic of China.http://www.nhc.gov.cn/guihuaxxs/s3585u/201907/e9275fb95d5b4295be8308415d4cd1b2.shtml at. [Google Scholar]
- 5.COPD Working Group Pulmonary rehabilitation for patients with chronic pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12:1–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Charususin N., Gosselink R., Decramer M. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax. 2018;73:942–950. doi: 10.1136/thoraxjnl-2017-211417. [DOI] [PubMed] [Google Scholar]
- 7.Gordon C.S., Waller J.W., Cook R.M., Cavalera S.L., Lim W.T., Osadnik C.R. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest. 2019;156:80–91. doi: 10.1016/j.chest.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Calik-Kutukcu E., Arikan H., Saglam M. Arm strength training improves activities of daily living and occupational performance in patients with COPD. Clin Res J. 2017;11:820–832. doi: 10.1111/crj.12422. [DOI] [PubMed] [Google Scholar]
- 9.Tarigan A.P., Pandia P., Mutiara E., Pradana A., Rhinsilva E., Efriyandi E. Impact of lower-limb endurance training on dyspnea and lung functions in patients with COPD. Open Access Maced J Med Sci. 2018;6:2354–2358. doi: 10.3889/oamjms.2018.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Su N., Shen C., Jiang A.D. Literature analysis on the efficacy and safety of lopinavir/ritonavir in viral infectious diseases (in Chinese) Herald Med. 2020;3:1–15. [Google Scholar]
- 11.Khalid M., Al Rabiah F., Khan B., Al Mobeireek A., Butt T.S., Al Mutairy E. Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. 2015;20:87–91. doi: 10.3851/IMP2792. [DOI] [PubMed] [Google Scholar]
- 12.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong D.S., Wang R.R., Ye Z.Q., Rao Y.F., Xu K.J., Lu X.Y. The effect of interferon combined with ribavirin on the risk of death in patients with Severe Coronavirus Infection: a Systematic Review based on previous MERS-CoV studies (in Chinese) Chin J Hosp Pharm. 2020:1–7. [Google Scholar]
- 14.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T., Chen G., Guo W. A quick guidelinefor novel coronavirus pneumonia (Trial 3rd Edition) (in Chinese) Herald Med. 2020;39:305–307. [Google Scholar]
- 16.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notice on Issuing Psychological Crisis Intervention Guidelines for the Novel Coronavirus Pneumonia Epidemic . 2020. The People's Republic of China:National Health Commission of the People's Republic of China.http://www.gov.cn/zhengce/zhengceku/2020-01/27/content_5472433.html at. [Google Scholar]
- 21.Hui D.S., Joynt G.M., Wong K.T. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng C.K., Chan J.W., Kwan T.L. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59:889–891. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 24.Herridge M.S., Cheung A.M., Tansey C.M. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 25.Lau H.M., Ng G.Y., Jones A.Y., Lee E.W., Siu E.H., Hui D.S. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust J Physiother. 2005;51:213–219. doi: 10.1016/S0004-9514(05)70002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z., Wang N.H., Lan Y., Li M., Chen R. Rehabilitation for the patients with severe acute respiratory syndrome in remission (in Chinese) Chin J Rehabil Med. 2004;10:15–17. [Google Scholar]
- 27.Chinese Association of Rehabilitation Medicine Respiratory rehabilitation committee of Chinese association of rehabilitation medicine, cardiopulmonary rehabilitation group of Chinese society of physical medicine and rehabilitation. Recommendations for respiratory rehabilitation of COVID-19 in adults (in Chinese) Chin J Tubere Respir Dis. 2020;43:308–314. [Google Scholar]
- 28.Xie Y.X. Rehabilitation treatment of patients with COVID-19 (in Chinese) Rehabil Med. 2020;30:1. [Google Scholar]
- 29.Nonoyama M.L., Brooks D., Lacasse Y., Guyatt G.H., Goldstein R.S. Oxygen therapy during exercise training in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007:CD005372. doi: 10.1002/14651858.CD005372.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson R.C., Specter B.B. Kyphosis and fractures in children and young adults with cystic fibrosis. J Pediatr. 1994;125:208–212. doi: 10.1016/s0022-3476(94)70194-6. [DOI] [PubMed] [Google Scholar]
- 31.Rassouli F., Boutellier D., Duss J., Huber S., Brutsche M.H. Digitalizing multidisciplinary pulmonary rehabilitation in COPD with a smartphone application: an international observational pilot study. Int J Chronic Obstr Pulm Dis. 2018;13:3831–3836. doi: 10.2147/COPD.S182880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourne S., DeVos R., North M. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014580. [DOI] [PMC free article] [PubMed] [Google Scholar]