Highlights
-
•
The lower BI scores, basic lung diseases, trachea ventilation, tube feeding, and hypoproteinemia are independent risk factors of pneumonia in convalescent patients with stroke.
-
•
The main pathogenic caused pneumonia in convalescent patients with stroke is Gram-negative bacteria.
-
•
There is a high degree of drug resistance among pathogens isolated from pneumonia in convalescent patients with stroke.
Keywords: Pneumonia, Stroke, Logistic regression, Pathogenic microorganism, Rehabilitation, Risk factors
Abstract
Background
Pneumonia is a major complication leading to death after stroke. The risk factors of pneumonia in convalescent patients who have experienced stroke remain poorly defined.
Methods
To identify the risk factors of pneumonia, we applied logistic regression as a statistical method using SPSS23.0 statistical software, based on a sample of 380 patients. And statistical description method was used to analyze pathogens’ characteristics and drug resistance.
Results
Ultimately, the obtained logistic model has statistical significance (χ2(13) = 91.560, P <0.0005). The sensitivity of the model is 41.7%, the specificity is 97.6%, the positive predictive value is 76.9%, and the negative predictive value is 89.8%. The Barthel index (BI) (OR=1.97, 95% CI: 1.01-3.87), basic lung diseases (OR=4.24, 95% CI: 1.02-17.61), trachea ventilation (OR=6.56, 95% CI: 1.18-36.34), feeding tube (OR=6.06, 95% CI: 2.59-14.18), and hypoproteinemia (OR=3.97, 95% CI: 1.56-10.10) were statistically significant (P<0.05). Among patients who have pneumonia, the proportion of gram-positive bacteria, gram-negative bacteria and fungal infection is 10.00%, 54.29%, 5.71% respectively. The study most frequently isolated Pseudomonas aeruginosa (18.57%), followed by Acinetobacter baumannii (10.00%,) and Klebsiella pneumoniae (10.00%). The drug resistance rate of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae to different antibiotics ranged from 0.00-37.77%, 0.00-85.71% and 0.00-57.14%, respectively.
Conclusions
The lower BI scores, basic lung diseases, trachea ventilation, tube feeding, and hypoproteinemia are independent risk factors of pneumonia among convalescent patients with stroke. The main pathogens that caused pneumonia were gram-negative bacteria, and such organisms have different degrees of resistance to drugs.
Introduction
Stroke significantly impacts public health and ranks among the leading causes of death and disabilities, resulting in enormous costs measured in both health care resources and lost productivity. It results primarily from embolus or thrombosis for ischemic stroke and hypertension for hemorrhagic stroke, respectively.1 In 2016, there were 5.5 million deaths and 116.4 million disability-adjusted life-years (DALYs) owed to stroke.2 As reported, the impact of stroke on the Chinese population is more severe compared to average global levels, and the prevalence of stroke continues to surpass that of ischemic heart disease.3 , 4 Intravenous alteplase (rtPA) is an effective treatment for ischemic stroke, and conservative treatment or surgical treatment may be needed for hemorrhagic stroke.5 , 6 Though treatments have been developed to decline stroke mortality rates, the global burden of stroke remains substantial and it is increasing as populations continue to age.
Remarkably, pneumonia is a major complication leading to death after stroke, especially for acute ischemic stroke (AIS) patients.7 Numerous studies report that stroke-associated pneumonia (SAP) has an estimated incidence of 5% to 30% in different countries,[8], [9], [10], [11] and it reached as high as 40% in patients requiring invasive ventilation.12 Immunodepression after stroke, pulmonary aspiration and bacterial infections are major causes of SAP.13 , 14 Many studies have already presented evidence that indicates age, obesity, bilateral lesions, severe neurological deficit, congestive heart failure, dysarthria and dysphagia are risk factors for pulmonary infection among those experiencing acute cerebral infarction.[15], [16], [17] Several scales, including different risk factors, have been developed to evaluate SAP risk in AIS patients.18 , 19
However, most previous research has focused on the risk factors of pneumonia in AIS patients, while few studies have investigated the risk factors of pneumonia in convalescent patients experiencing stroke. The current study used a logistic regression analysis to identify independent risk factors, describe pathogenic microorganism characteristics and investigate the drug resistance of major pathogenic microorganisms of pneumonia in patients experiencing stroke during rehabilitation, with the purpose to provide a reference to prevent pneumonia.
Methods
Study design and patients
This is a retrospective, single-center study of patients who, after stroke, underwent rehabilitation treatment between January to December 2019 in a rehabilitation department. Use the hospital information system (HIS) to collect medical history data, all data are based on medical records. And, a total of 1585 patients who participated in rehabilitation after stroke or other reasons were treated during this period. We screened these patients according to the inclusion and exclusion criteria as follows.
Inclusion and exclusion criteria
The inclusion criteria of stroke patients were as follows: (1) age > = 18 years; (2) hospitalized with a primary diagnosis of stroke including ischemic stroke and hemorrhagic stroke according to guidelines from US and China[20], [21], [22], [23]; (3) stroke confirmed by head computerized tomography (CT) or brain nuclear magnetic resonance imaging (MRI). (4) At the recovery stage of stroke (course of stroke: 1W-6 M), stable condition, and is taking part in rehabilitation treatment. Pneumonia was diagnosed by the clinician team after hospitalization (48 hours later), and based on clinical (lung auscultation and percussion, presence of fever, purulent tracheal secretion), microbiological (tracheal specimens, sputum bacteria detection or culture), and imaging findings. The detailed clinical criteria for judgement included newly emerging lesions or progressively infiltrating lesions in post-stroke chest images combined with more than two of the following clinical symptoms of infection: (1) fever≥38 °C; (2) new cough, productive cough, lung auscultation can hear the rale, or an exacerbation of pre-existing respiratory disease symptoms with or without chest pain; (3) signs of pulmonary consolidation, and/or moist gales; (4) peripheral WBC≥10 × 109/L or ≤4 × 109/L with or without nuclear shift to the left, or elevated C-reactive protein (CRP) and procalcitonin (PCT). The exclusion criteria were as follows: (1) patients with acute stroke (course of stroke: ≤1W), and at the stage of sequelae after stroke (course of stroke: ≥6M) were excluded; (2) only hospital-acquired pneumonia was considered,24 and patients with pneumonia before hospitalization were excluded.
Factors definition and assignments of logistic regression analysis model
The factors of pneumonia in convalescent patients with stroke were divided into 13 related factors including features of patients (gender, age≥60), features of stroke (main diagnosis, course of stroke, Barthel index (BI) scores,25 whether craniotomy surgery was performed due to stroke), basic diseases (diabetes, hypertension, chronic obstructive pulmonary disease (COPD) or other underlying lung diseases), creative operation (indwelling feeding tube, trachea ventilation), use of drugs that may affect immunity (long-term use of immunosuppressive agents or long-term use of glucocorticoids), hypoproteinemia (serum albumin< 35g/L). The course of disease was divided into six grades by month, and the BI scores divided into 4 grades according to nursing dependency. The factors and assignments for the factors of pneumonia in convalescent patients with stroke are shown in Table 1 .
Table 1.
Factors and assignments of pneumonia in convalescent patients with stroke.
| Factors | Subclass | Assignments |
|---|---|---|
| Gender | “1” for “male”; “0” for “female” | |
| Age≥60 | “1” for the presence; “0” for “none” | |
| Main diagnosis | “1” for “ischemic stroke”; “0” for “hemorrhagic stroke” | |
| Course of stroke | 1W-1M | “C1” for the presence |
| 1-2M | “C2” for the presence | |
| 2-3M | “C3” for the presence | |
| 3-4M | “C4” for the presence | |
| 4-5M | “C5” for the presence | |
| 5-6M | “C6” for the presence | |
| Barthel index scores* | ≤40 | “B1” for the presence |
| 41-60 | “B2” for the presence | |
| 61-99 | “B3” for the presence | |
| 100 | “B4” for the presence | |
| Craniotomy surgery | “1” for the presence; “0” for “none” | |
| Diabetes | “1” for the presence; “0” for “none” | |
| Hypertension | “1” for the presence; “0” for “none” | |
| Basic lung diseases# | “1” for the presence; “0” for “none” | |
| Feeding tube | “1” for the presence; “0” for “none” | |
| Trachea ventilation | “1” for the presence; “0” for “none” | |
| Drugs affect immunity | “1” for the presence; “0” for “none” | |
| Hypoproteinemia | “1” for the presence; “0” for “none” |
Barthel index scores: lower scores representing greater nursing dependency.
Basic lung diseases including chronic lung disease includes COPD, asthma, bronchiectasis, and interstitial lung disease.
2.4. Statistical analysis
SPSS23.0 statistical software was used for statistical analysis. Descriptive statistical methods were used for counting data, which were expressed in percentages and constituent ratios. Continuous variables are presented as mean and SD (Mean±SD). The chi-square test was performed on the count data conforming to the normal distribution. Omnibus tests used to assess the model coefficients. The factors with statistically significant differences in the binary logistic regression were applied to multivariate logistic regression analysis if with subclass, and the risk factors of pneumonia in convalescent patients with stroke were obtained by Wald test, and P < 0.05 indicated statistically significant. The odds ratio (OR) and 95% confidence interval (CI) were obtained.
Results
Baseline characteristics of patients
According to the inclusion and exclusion criteria, 380 patients were finally included in the study. There were 271 male (71.31%), and 109 female (28.69%). The average age was 59.67 (59.67±12.61, n=380) years old, range from 25 to 90 years. And, 208 patients were diagnosed with ischemic stroke (54.74%), 172 patients were diagnosed with hemorrhagic stroke (45.26%). There were 61 patients were diagnosed with pneumonia during hospitalization, the incidence of pneumonia in convalescent patients with stroke is 16.1%. Specific data of each factor are presented in Table 2 .
Table 2.
Baseline characteristics of the patients.
| Factors | Subclass | Cases | Data missing/correction | Percentage (%) |
|---|---|---|---|---|
| Age≥60 | 195 | 0, n=380 | 51.32 | |
| Course of stroke | 1W-1M | 164 | 0, n=380 | 43.16 |
| 1-2M | 107 | 28.16 | ||
| 2-3M | 49 | 12.89 | ||
| 3-4M | 29 | 7.63 | ||
| 4-5M | 20 | 5.26 | ||
| 5-6M | 11 | 2.89 | ||
| Barthel index scores | ≤40 | 162 | 67, n=313 | 51.76 |
| 41-60 | 77 | 24.60 | ||
| 61-99 | 50 | 15.97 | ||
| 100 | 24 | 7.67 | ||
| Craniotomy surgery | 95 | 0, n=380 | 25.00 | |
| Diabetes | 101 | 0, n=380 | 26.58 | |
| Hypertension | 345 | 0, n=380 | 90.79 | |
| Basic lung diseases | 17 | 0, n=380 | 4.47 | |
| Feeding tube | 67 | 0, n=380 | 17.63 | |
| Trachea ventilation | 18 | 0, n=380 | 4.74 | |
| Drugs affect immunity | 10 | 5, n=375 | 2.67 | |
| Hypoproteinemia | 81 | 14, n=366 | 22.13 |
3.2. Logistic regression of factors which may induce pneumonia in convalescent patients with stroke
This study evaluated the effects of 13 predictive factors on hospital-acquired pneumonia in patients using binary logistic regression. Ultimately, the obtained logistic model has statistical significance (χ2(13) = 91.560, P <0.0005). The model explained the 45.0% variation (Nagelkerke R2) with or without pneumonia and correctly classified 88.7% of the study patients. The sensitivity of the model is 41.7%, the specificity is 97.6%, the positive predictive value is 76.9%, and the negative predictive value is 89.8%. Among the predictive factors included in the model, the BI scores (OR=1.97, 95% CI: 1.01–3.87), basic lung diseases (OR=4.24, 95% CI: 1.02-17.61), trachea ventilation (OR=6.56, 95% CI: 1.18–36.34), tube feeding (OR=6.06, 95% CI: 2.59-14.18), and hypoproteinemia (OR=3.97, 95% CI: 1.56-10.10) were statistically significant, P<0.05 (Table 3 ). Multivariate logistic regression analysis of the Barthel index showed that patients with severe nursing dependency (BI score<40) had an 8.85-fold (95%CI: 1.16-67.45) increased risk of pneumonia compared with patients who without nursing dependency (Table 4 ).
Table 3.
Logistic regression predicting likelihood of pneumonia based on 13 factors.
| Factors | B | S.E. | Wald | df | P | OR | 95%C.I. for OR |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Main diagnosis | 0.35 | 0.51 | 0.46 | 1 | 0.50 | 1.42 | 0.52 | 3.88 |
| Gender | 0.19 | 0.47 | 0.16 | 1 | 0.69 | 1.20 | 0.48 | 3.02 |
| Age≥60 | 0.06 | 0.48 | 0.01 | 1 | 0.90 | 1.06 | 0.42 | 2.69 |
| Course of stroke | 0.06 | 0.16 | 0.12 | 1 | 0.73 | 1.06 | 0.77 | 1.46 |
| Barthel index scores | 0.68 | 0.34 | 3.93 | 1 | 0.05 | 1.97 | 1.01 | 3.87 |
| Craniotomy surgery | 0.54 | 0.49 | 1.18 | 1 | 0.28 | 1.71 | 0.65 | 4.51 |
| Basic lung diseases | 1.45 | 0.73 | 3.96 | 1 | 0.05 | 4.24 | 1.02 | 17.61 |
| Trachea ventilation | 1.88 | 0.87 | 4.63 | 1 | 0.03 | 6.56 | 1.18 | 36.34 |
| Feeding tube | 1.80 | 0.43 | 17.23 | 1 | 0.00 | 6.06 | 2.59 | 14.18 |
| Hypertension | -0.94 | 0.64 | 2.15 | 1 | 0.14 | 0.39 | 0.11 | 1.37 |
| Diabetes | -0.38 | 0.53 | 0.52 | 1 | 0.47 | 0.68 | 0.24 | 1.92 |
| Hypoproteinemia | 1.38 | 0.48 | 8.33 | 1 | 0.00 | 3.97 | 1.56 | 10.10 |
| Drugs affect immunity | 0.62 | 0.91 | 0.47 | 1 | 0.49 | 1.86 | 0.31 | 11.02 |
| Constant | -12.46 | 3.53 | 12.43 | 1 | 0.00 | 0.00 | ||
Table 4.
Multinomial logistic regression to predict pneumonia in convalescent patients with stroke using the Barthel index.
| Pneumonia |or nota | B | S.E. | Wald | df | Sig. | OR | 95% Confidence Interval for OR |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Presence | Intercept | -3.14 | 1.02 | 9.42 | 1.00 | 0.00 | ||||
| Barthel index score | <40 | 2.18 | 1.04 | 4.42 | 1.00 | 0.04 | 8.85 | 1.16 | 67.45 | |
| 41-60 | -0.07 | 1.18 | 0.00 | 1.00 | 0.95 | 0.93 | 0.09 | 9.40 | ||
| 61-99 | -0.04 | 1.25 | 0.00 | 1.00 | 0.97 | 0.96 | 0.08 | 11.12 | ||
| 100 | 0b | 0.00 | ||||||||
The reference category is: none.
This parameter is set to zero because it is redundant.
3.3. Pathogen distribution
Pathogens were identified in 40 (65.6%) of 61 pneumonia patients. 5 patients were identified as gram-positive infection and 5 as gram-negative infection under the microscope, but none identified the pathogen. The pathogen distribution is shown in Table 5 . In patients with pneumonia, the proportion of gram-positive bacteria, gram-negative bacteria and fungal infection is 10.00%, 54.29%, 5.71% respectively. Pseudomonas aeruginosa (18.57%) was isolated most frequently, followed by Acinetobacter baumannii (10.00%) and Klebsiella pneumoniae (10.00%), and Candida albicans (4.29%).
Table 5.
The distribution of pathogenic microbes of pneumonia of convalescent patients with stroke.
| Pathogenic microbes | Cases | Proportion(%) | Classification of Gram staining | Proportion(%) |
|---|---|---|---|---|
| Pseudomonas aeruginosa | 13 | 18.57 | Gram negative bacteria (n=38) | 54.29 |
| Acinetobacter baumannii | 7 | 10.00 | ||
| Klebsiella pneumoniae | 7 | 10.00 | ||
| Escherichia coli | 2 | 2.86 | ||
| Haemophilus influenzae | 2 | 2.86 | ||
| Enterobacter aerogenes | 1 | 1.43 | ||
| Oligotrophic maltophilia | 1 | 1.43 | ||
| Not clear | 5 | 7.14 | ||
| Staphylococcus aureus | 2 | 2.86 | Gram positive bacteria(n=7) | 10.00 |
| Not clear | 5 | 7.14 | ||
| Candida albicans | 3 | 4.29 | Fungus(n=4) | 5.71 |
| Candida glabrata | 1 | 1.43 | ||
| Not clear | 21 | 30.00 | Not clear(n=21) | 30.00 |
| Total* | 70 | 100.00 | n=70 | 100.00 |
A total of 61 people were diagnosed with pneumonia, 9 of whom had 2 microbial infections by microbial cultivation or it was confirmed that gram-positive bacteria and gram-negative bacteria were infected at the same patient under the microscope, so the total number of pathogenic strains was 70.
3.4. Drug resistance characteristics of main pathogenic microbes
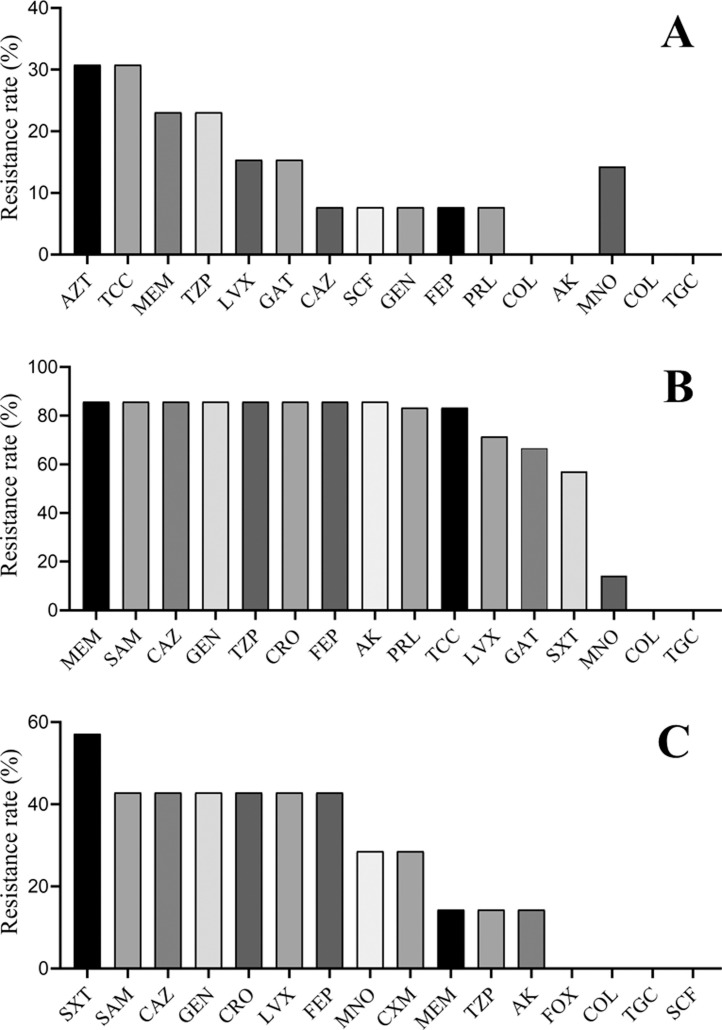
The study listed drug resistance data for the first three pathogens that isolated from pneumonia patients, all gram-negative. The results showed that the resistance rates of Pseudomonas aeruginosa to aztreonam, ticarcillin/clavulanate, meropenem, piperacillin/tazobactam were 30.77%, 30.77%, 23.08%, 23.08%, respectively; while the resistance rates to streptomycin and amikacin were 0.00% (Fig. 1 .A). The resistance rates of Acinetobacter baumannii to meropenem, ampicillin/sulbactam, ceftazidime, amikacin and levofloxacin were 85.71%, 85.71%, 85.71%, 85.71%, 71.43% respectively; and the resistance rates to streptomycin and tegacyclin were 0.00% (Fig. 1.B). The drug resistance rates of Klebsiella pneumoniae to sulfamethoxazole/Trimethoprim, ampicillin/sulbactam, ceftazidime, gentamycin, levofloxacin and meropenem were 57.14%, 42.86%, 42.86%, 42.86%, 42.86%, 14.19% respectively; and to cefoxitin, colistin, tegacyclin and cefoperazone/sulbactam were 0.00% (Fig. 1.C).
Fig 1.
The drug resistance rate of first three pathogens that isolated from pneumonia patients. (A: Drug resistance rate of Pseudomonas aeruginosa, n=13; B: Drug resistance rate of Acinetobacter baumannii, n=7; C: Drug resistance rate of Klebsiella pneumoniae, n=7. SAZT: Aztreonam; TCC: Ticarcillin/clavulanic acid; MEM: Meropenem; TZP: Piperacillin/tazobactam; LVX: Levofloxacin; GAT: Gatifloxacin; CAZ: Ceftazidime; SCF: Cefoperazone/sulbactam; GEN: Gentamicin; FEP: Cefepime; PRL: Piperacillin; COL: Colistin; AK: Amikacin. SAM: Ampicillin/sulbactam; CRO: Cefatriaxone; SXT: Sulfamethoxazole/Trimethoprim; MNO: Minocycline; TGC: Tegafycline; CXM: Cefuroxime; FOX: Cefoxitin.)
Discussions
This study revealed that about 16.1% stroke patients in convalescent period will develop pneumonia during hospitalization. Lower BI scores, with basic lung diseases, trachea ventilation, tube feeding, and hypoproteinemia are concerned to be independent risk factors for pneumonia. No statistical significance was observed for age, gender, type of stroke, diabetes, and hypertension. Among the patients with pneumonia, gram-negative bacilli were the most common, followed by Gram-positive bacilli and fungi. Gram-negative bacteria are common to Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae; Gram-positive bacteria are more common to Staphylococcus aureus; fungi are mainly Candida albicans. The results suggested that the resistance rate of Pseudomonas aeruginosa to monocyclic aztreonam was the highest, about 30.77%, and it was sensitive to ceftazidime and cefoperazone/sulbactam, but no resistance to colistin and amikacin. Acinetobacter baumannii has a high resistance rate to carbapenems, penicillins, cephalosporins, and aminoglycoside antibacterials, up to 85.71%, and was sensitive to colistin and tigecycline. The resistance rate of Klebsiella pneumoniae to sulfamethoxazole/trimethoprim is 57.14%, and the resistance rates to third-generation cephalosporins and quinolones are more than 40%; to cefoxitin sodium, colistin, tigecycline, Cefoperazone and sulbactam are not resistant.
The BI score has been used as a nursing dependency assessment in many fields, such as rehabilitation after stroke, after cardiovascular disease, after joint surgery, and Parkinson's disease.[26], [27], [28], [29] Usually, a lower BI score means higher nursing dependence. As it reported, a BI score ≤80 is associated with a higher mortality in patients with community acquired pneumonia (CAP) and can be a useful tool to predict CAP mortality in general population.30 Besides, negative correlation was found between BI scores and the existence of pneumonia complications in acute stroke patients.31 The result of this study is consistent with previous reports, but no statistical significance was observed between moderate or mild nursing dependency and non-nursing dependency. Researches presented that chronic respiratory diseases, such as COPD and bronchiectasis increase pneumonia risk.24 Imbalance of airway microbial flora in patients with underlying lung diseases and possible long-term use of inhaled corticosteriod may the causes of pneumonia.[32], [33], [34] In this study, the use of hormones and immunosuppressants were also observed as possible risk factors, but they did not show statistical significance, probably because of the limited number of cases (n=10) in which the drugs were used in the study subjects. It may also be related to the type of medication and the course of treatment. Some stroke patients may require continuous tracheal ventilation due to the damage of respiratory center. Studies have reported that about 18% of tracheal ventilation patients develop pneumonia, and pneumonia was associated with prolonged ventilation.35 Although tracheotomy can lead to pneumonia, some studies have reported that if patients have to tracheal ventilation, it can reduce the incidence of ventilator-associated pneumonia both in children and old patients.[36], [37] Dysphagia is a common symptom seen in stroke patients. In order to provide nutritional support, dysphagic patients after stroke usually need an appropriate feeding method, indwelling nasogastric tubes are routinely used. But it was reported that prolonged use of nasogastric tube feeding may have negative effects, which is also confirmed in the results of this study, since the risk of aspiration or bacterial calibration may increase with prolonged nasogastric tube feeding.38 Intensive evaluation of dysphagia and removal of the nasogastric tube in the early stages of stroke might reduce pneumonia incidence and mortality.39 Hypoalbuminemia is common in hospitalized patients and is associated with adverse clinical outcomes. Researches have suggested that hypoproteinemia leads to height of inflammatory factors and can be used as a risk factor for predicting mortality for acute diseases.40 The results of this study presented that hypoalbuminemia increases the risk of pneumonia by 3.97 times, this may be related to dysphagia and the decrease of living ability, or infection in the convalescent period of stroke, which may lead to the lack of nutrition supply or excessive albumin consumption, while much remains to be studied.
The World Health Organization (WHO) has published a list of priority pathogens posing the greatest threat to human health, including Acinetobacter baumannii, Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae, such as Klebsiella pneumoniae. In addition, since the outbreak of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) at the end of 2019, which has caused infections and deaths of millions of people worldwide, the new coronavirus infection appears to be another major threat to human health.41 Unlike viral pneumonia, refractory bacterial pneumonia is mostly caused by drug-resistant bacteria, and the infectivity is not as strong as viral pneumonia. The treatment of bacterial pneumonia still depends on powerful antibacterial drugs, while the treatment of new coronavirus pneumonia has not yet found special drugs, and the focus of prevention and treatment is to reduce contact transmission and rely on your own strong immunity before the vaccine was successfully developed.[42], [43] The drug resistance of microbes is a challenging problem in the world, and it is often faced with the situation of no drug available. Incidence of infection caused by drug resistance organism is increasing in hospital, and this will enhance medical financial burden.44 And it was considered to be caused by overuse of antimicrobial. Besides, the drug resistance rate varies in different regions, a higher rates of drug resistance was observed in developing countries.45 , 46 In this study, Acinetobacter baumannii showed a high resistance rate to most antibacterial drugs, up to 85.7%; and the drug resistance rate of Klebsiella pneumoniae to SXT reaches 57.1%. Fortunately, neither of the two is resistant to tegacyclin or myxomycin at present. But, the high bacterial resistance rate still poses a serious threat to clinical antibacterial treatment in this hospital. Some scholars have suggested that strict antibacterial drug management may be beneficial for inhibiting bacterial resistance,[47], [48] while the development of new antibacterial drugs is also imminent at the same time. In recent years, although much researches have been done on the mechanism of bacterial resistance,[49], [50], [51] and some new pathways to combat bacterial resistance have also been discovered,[52], [53] the confrontation between humans and bacteria will never stop.
Conclusions
Though limited by unicentric study, limited by sample size, and there may be some other factors not included in the observation, the current data contributes to our current understanding of the lower BI scores, basic lung diseases, trachea ventilation, tube feeding, and hypoproteinemia are independent risk factors of pneumonia in convalescent patients with stroke. Severe nursing dependency also heightens the risk of pneumonia. The main pathogens that cause pneumonia were gram-negative bacteria, and they exhibit different degrees of drug resistance.
Declaration of Competing Interest
We declare there to be no conflicts of interest.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies among the public, commercial, or not-for-profit sectors.
Ethics statement
This study was approved by the Medical Ethics Committee of the hospital (NO:2019054) and patients records were anonymized before analysis.
References
- 1.Knight-Greenfield A., Nario JJQ, Gupta A. Causes of acute stroke: a patterned approach. Radiol Clin North Am. 2019;57:1093–1108. doi: 10.1016/j.rcl.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minh Nguyen Gregory A., Johnson Catherine Owens. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study. The Lancet Neurology. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., Jiang B., Sun H. The burden of stroke in China: Results from a nationwide population-based epidemiological survey. PLoS One. 2018;13:1–11. doi: 10.1371/journal.pone.0208398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Simiao, Wu Bo, Liu Ming. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 5.Del Zoppo GJ, Saver J.L., Jauch E.C. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hankey G.J. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S., Selim M.H., Caplan L.R. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 8.Westendorp W.F., Nederkoorn P.J., Vermeij J.D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlas R.S., Clark A.B., Bettencourt-Silva J.H. Pneumonia and risk of serious adverse outcomes in hospitalized strokes in Thailand. J Stroke Cerebrovasc Dis. 2019;28:1448–1454. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Bruening T., Al-Khaled M. Stroke-Associated Pneumonia in Thrombolyzed patients: incidence and outcome. J Stroke Cerebrovasc Dis. 2015;24:1724–1729. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Karlinski M.A., Bembenek J.P., Baranowska A. Infections diagnosed after admission to a stroke unit and their impact on hospital mortality in Poland from 1995 to 2015. J Stroke Cerebrovasc Dis. 2018;27:1775–1782. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 12.de Montmollin E., Ruckly S., Schwebel C. Pneumonia in acute ischemic stroke patients requiring invasive ventilation: Impact on short and long-term outcomes. J Infect. 2019;79:220–227. doi: 10.1016/j.jinf.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Liu Dan-Dan, Chu Shi-Feng, Chen Chen. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP) Neurochem Int. 2018;114:42–54. doi: 10.1016/j.neuint.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Chen L.F., Chang C.Y., Hsu L.C. Bacterial pneumonia following acute ischemic stroke. J Chin Med Assoc. 2013;76:78–82. doi: 10.1016/j.jcma.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Maeshima S., Osawa A., Hayashi T. Elderly age, bilateral lesions, and severe neurological deficit are correlated with stroke-associated pneumonia. J Stroke Cerebrovasc Dis. 2014;23:484–489. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Sjahrir H., Siregar I.K.D.H. Accuracy comparison of age, atrial fibrillation, dysphagia, stroke severity, sex (A2DS2) and acute ischemic stroke-associated pneumonia score (AIS-APS) to predict pneumonia in acute ischemic stroke. J Neurol Sci. 2017;381:998. [Google Scholar]
- 17.Tziomalos K., Kostaki S., Papagianni M. Obesity is an independent risk factor for pneumonia in patients admitted with acute ischemic stroke. Atherosclerosis. 2018;275:e75. [Google Scholar]
- 18.Helmy T.A., Abd-Elhady M.A., Abdou M. Prediction of Ischemic Stroke-Associated Pneumonia: A Comparison between 3 Scores. J Stroke Cerebrovasc Dis. 2016;25:2756–2761. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S., Marchina S., Massaro J. ACDD(4) score: A simple tool for assessing risk of pneumonia after stroke. J Neurol Sci. 2017;372:399–402. doi: 10.1016/j.jns.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Powers W.J., Rabinstein A.A., Ackerson T. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 21.Chinese Stroke Society Chinese Society Of Neurology Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chinese J Neuromed. 2018;51:666–682. [Google Scholar]
- 22.Rd Hemphill JC, Greenberg S.M., Anderson C.S. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the american heart association/American stroke association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 23.Chinese Stroke Society Chinese Society Of Neurology Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage 2014. Chin J Neurol. 2015;48:435–444. [Google Scholar]
- 24.Lanks C.W., Musani A.I., Hsia D.W. Community-acquired pneumonia and hospital-acquired pneumonia. Med Clin North Am. 2019;103:487–501. doi: 10.1016/j.mcna.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Quinn T.J., Langhorne P., Stott D.J. Barthel index for stroke trials: development, properties, and application. Stroke. 2011;42:1146–1151. doi: 10.1161/STROKEAHA.110.598540. [DOI] [PubMed] [Google Scholar]
- 26.Bouwstra H., Smit E.B., Wattel E.M. Measurement properties of the Barthel index in geriatric rehabilitation. J Am Med Dir Assoc. 2019;20:420–425. doi: 10.1016/j.jamda.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Galeoto G., Formica M.C., Mercuri N.B. Evaluation of the psychometric properties of the Barthel Index in an Italian ischemic stroke population in the acute phase: a cross-sectional study. Funct Neurol. 2019;34:29–34. [PubMed] [Google Scholar]
- 28.Motoki H., Nishimura M., Kanai M. Impact of inpatient cardiac rehabilitation on Barthel Index score and prognosis in patients with acute decompensated heart failure. Int J Cardiol. 2019;293:125–130. doi: 10.1016/j.ijcard.2019.06.071. [DOI] [PubMed] [Google Scholar]
- 29.Taghizadeh G., Martinez-Martin P., Meimandi M. Barthel Index and modified Rankin Scale: Psychometric properties during medication phases in idiopathic Parkinson disease. Ann Phys Rehabil Med. 2019 doi: 10.1016/j.rehab.2019.08.006. inpress. [DOI] [PubMed] [Google Scholar]
- 30.Murcia J., Llorens P., Sanchez-Paya J. Functional status determined by Barthel Index predicts community acquired pneumonia mortality in general population. J Infect. 2010;61:458–464. doi: 10.1016/j.jinf.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Fujita R., Kato R., Nishio M. The influence of complications found in an acute period of stroke patient on Barthel Index score at a recovery-care-unit. Physiotherapy. 2015;101:e1098–e1099. [Google Scholar]
- 32.Dima E., Kyriakoudi A., Kaponi M. The lung microbiome dynamics between stability and exacerbation in chronic obstructive pulmonary disease (COPD): Current perspectives. Respir Med. 2019;157:1–6. doi: 10.1016/j.rmed.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Yang M., Du Y, Chen H. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019 doi: 10.1016/j.intimp.2019.105950. Inpress. [DOI] [PubMed] [Google Scholar]
- 34.Finney L., Berry M., Singanayagam A. Inhaled corticosteroids and pneumonia in chronic obstructive pulmonary disease. Lancet Respir Med. 2014;2:919–932. doi: 10.1016/S2213-2600(14)70169-9. [DOI] [PubMed] [Google Scholar]
- 35.Rello J., Lorente C., Diaz E. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest. 2003;124:2239–2243. doi: 10.1378/chest.124.6.2239. [DOI] [PubMed] [Google Scholar]
- 36.Topal S., Demir E., Atakul G. The effect of tracheotomy on ventilator-associated pneumonia rate in children. Int J Pediatr Otorhinolaryngol. 2020 doi: 10.1016/j.ijporl.2020.109898. Inpress. [DOI] [PubMed] [Google Scholar]
- 37.Schneider G.T., Christensen N., Doerr T.D. Early tracheotomy in elderly patients results in less ventilator-associated pneumonia. Otolaryngol Head Neck Surg. 2009;140:250–255. doi: 10.1016/j.otohns.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Yeh S.J., Huang K.Y., Wang T.G. Dysphagia screening decreases pneumonia in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci. 2011;306:38–41. doi: 10.1016/j.jns.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Lin Wen-Chih, Hsu Ya-Fang, Ho Chung-Han. One-year risk of pneumonia and mortality in patients with poststroke dysphagia: a nationwide population-based study. J Stroke Cerebrovascular Disea. 2018;27:1311–1317. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Alexander Kutz Tristan Struja, Eckart Andreas. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2019 doi: 10.1016/j.amjmed.2019.10.031. In press. [DOI] [PubMed] [Google Scholar]
- 41.Yang Yongshi, Peng Fujun, Wang Runsheng. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. Inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai Chih-Cheng, Shih Tzu-Ping, Ko Wen-Chien. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. Inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Aziz Tarek Mohamed Abd, Stockand James D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infection. Genetics Evolut. 2020 doi: 10.1016/j.meegid.2020.104327. Inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merchant S., Ye G., Tabak Y.P. Incremental clinical and economic burden of suspected respiratory infections due to multi-drug-resistant Pseudomonas aeruginosa in the United States. J Hosp Infect. 2019;103:134–141. doi: 10.1016/j.jhin.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Virasakdi Chongsuvivatwong Kachornsakdi Silpapojakul Sarunyou Chusri Clinical characteristics and outcomes of community and hospital-acquired Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. 2019;52:796–806. doi: 10.1016/j.jmii.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Kumar Nagen, Santosh Debata, Mohapatra Debi Prasad. Extensively drug-resistant and pandrug-resistant Gram-negative bacteria in a tertiary-care hospital in Eastern India: A 4-year retrospective study. J Global Antimicrob Resist. 2018;15:246–249. doi: 10.1016/j.jgar.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Chatzopoulou M., Reynolds L. Role of antimicrobial restrictions in bacterial resistance control: a systematic literature review. J Hosp Infect. 2020;104:125–136. doi: 10.1016/j.jhin.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Schaffer K., Lynch B.L. Can guidelines for the control of multi-drug-resistant Gram-negative organisms be put into practice? A national survey of guideline compliance and comparison of available guidelines. J Hosp Infect. 2019;102:1–7. doi: 10.1016/j.jhin.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Roudayna Diab Kiarash Ghazvini Bahman Khameneh Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathogenesis. 2016;95:32–42. doi: 10.1016/j.micpath.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Torok M.E., Chantratita N., Peacock S.J. Bacterial gene loss as a mechanism for gain of antimicrobial resistance. Curr Opin Microbiol. 2012;15:583–587. doi: 10.1016/j.mib.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolivet-Gougeon A., Bonnaure-Mallet M. Biofilms as a mechanism of bacterial resistance. Drug Discov Today Technol. 2014;11:49–56. doi: 10.1016/j.ddtec.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Alisia Mancini Letizia Di Pierangelo Bellio Inhibition of the transcriptional repressor LexA: Withstanding drug resistance by inhibiting the bacterial mechanisms of adaptation to antimicrobials. Life Sci. 2020;241:116–117. doi: 10.1016/j.lfs.2019.117116. [DOI] [PubMed] [Google Scholar]
- 53.Anuj S.A., Gajera H.P., Hirpara D.G. Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria. Life Sci. 2019;230:178–187. doi: 10.1016/j.lfs.2019.05.072. [DOI] [PubMed] [Google Scholar]