Abstract
The commerce of illegal and counterfeit medicinal products on internet is a serious criminal problem. Drugs for erectile dysfunction such as phosphodiesterase type 5 inhibitor are the most commonly counterfeited medicines in Europe. The search of possible toxic chemical substances in seized products is needed. Moreover, the profiling of the material can be the source of relevant forensic information. For the first time a combined approach based on liquid chromatography (LC) coupled to high resolution mass spectrometry (HRMS) and instrumental neutron activation analysis (INAA) is proposed and tested, allowing characterisation of both authentic and illegal pharmaceuticals containing sildenafil seized in Italy. LC-HRMS allowed the detection and identification of unknown impurities not reported on labels in illegal products and the quantitation of the sildenafil. INAA showed to be suitable to provide both qualitative and quantitative information for forensic purposes on 23 elements, allowing discrimination between legal and illegal products.
Keywords: Illegal pharmaceutical products, Neutron activation analysis, High resolution mass spectrometry, Forensic analysis, Sildenafil
1. Introduction
The Internet plays a critical role in illegal trafficking, providing illegal products online from anywhere around the world, including counterfeit goods such as pharmaceuticals [1]. Broséus et al. has recently studied the darknet market and found that most of the sale proposals (63%) concern licit or illicit drugs but in this category there are also prescription drugs and medicines [2]. Illegal and counterfeit medicinal products on internet is a criminal problem that poses a serious threat to public health. This issue pushed the research to develop many analytical tools to allow forensic characterisation of such products [[3], [4], [5]]. Drugs for erectile dysfunction such as phosphodiesterase type 5 (PDE5) inhibitor medications belong to a special class of illegal pharmaceutical products sold on the Internet [[6], [7], [8]]. They are the most commonly counterfeited medicines in Europe and they were also found in dietary supplements [9,10]. The analysis of these products is important for two reasons: on one hand the search of possible toxic chemical substances is needed to protect public health, on the other hand the profiling of the material can support to infer about the source of illegal materials and other relevant forensic information. PDE5 inhibitor medications were analysed by high-performance liquid chromatography (HPLC) [11], liquid chromatography-mass spectrometry (LC-MS) [[12], [13], [14], [15]], nuclear magnetic resonance (NMR) [16,17], Fourier transformed infrared spectroscopy (FTIR) [18] and Raman micro-spectroscopy [19].
Organic analysis is not enough for forensic characterisation of illegal pharmaceutical products. Heavy metals can be found into pharmaceutical products as impurities, to be monitored because some metals are known to be toxic even in small doses. The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), the European Pharmacopoeia (EP) and the United States Pharmacopoeia (USP) have made regulations for inorganic impurities. Among the suitable methods are inductively coupled plasma atomic emission spectrometry (ICP-AES) and inductively coupled plasma atomic mass spectrometry (ICP-MS), which have the capability to carry out rapid and accurate multi-element analysis at and below ng/g levels, coupled with excellent performance characteristics such as wide elemental coverage, rapid analysis (all elements at once), wide analytical working range (up to 9 orders), simple spectra and high tolerance to matrix effect [20]. Since 1990s researchers studied heavy metals by atomic absorption spectrometry (AAS), inductively coupled plasma mass spectrometry (ICP-MS), instrumental neutron activation analysis (INAA), in illicit drugs such as heroin and MDMA to establish the profiling and infer about their origins. The classification of seized drugs by impurity profiling is able to provide useful forensic information to identify drug traffic routes, clandestine laboratories and methods of drug preparation. This study has been becoming a great tool for police activities [21]. AAS is still popular because allows reliable determination of metallic impurities but is not multi-element and flame AAS suffers of poor sensitivity. ICP-MS has multi-element capability and shows higher sensitivity, accuracy and precision compared with AAS.
Before the advent of ICP-MS, INAA was used for long time to analyse trace metal elements, not only into illicit drugs [22,23] but also to obtain important information on the concentration levels of several toxic elements (Hg, Cd, As, Se, Sb, U and Th) into radiopharmaceuticals (DTPA, HMPAO, DMSA) [24] and trace elements into drugs samples [25].
Wollein et al. [26] showed the development and validation of the methods used for the determination of 21 selected metals in 113 samples from drug products and their active pharmaceutical ingredients. To analyse metal residues, ICP-MS was used for the determination of Mn, Co, Ni, Mo, Ru, Rh, Pd, Cd, Sn, Sb, Ir, Pt, and Pb, V and Os; Cu, Fe, and Zn were analysed using an ICP optical emission spectroscopy (OES); Hg was analysed by cold vapour atomic absorbance spectroscopy (CV-AAS). Determination of As was carried out using a hydride generation (HG) AAS and Cr by graphite F-AAS. The advantage of ICP-MS was shown, as the majority of the selected elements could be quantified by using this technique with RSD lower than 4.5%. Afterwards, the ICP-MS was applied to quantitative analysis of heavy metal of 190 samples from 31 different excipients and 15 samples from eight drug substances provided through the International Pharmaceutical Excipient Council of the Americas [27]. In each run, 24 elements including Cd, Pb, As, Hg, Co, V, Ni, Tl, Au, Pd, Ir, Os, Rh, Ru, Se, Ag, Pt, Li, Sb, Ba, Mo, Cu, Sn, and Cr were measured, some at multiple isotopic masses to identify and correct for interferences. In the 2000s it has been proposed the ICP-MS how alternative method to the heavy metal test for pharmaceutical material [28,29], which was later tested on selected drugs such as dicydomine-HCl, ethambutol, pyrazinamide and furazolidone [30], and antihypertensive drugs [31]. In a review published in 2007 the ICP-MS resulted as the most used method to find the metal elements into drugs and pharmaceutical material [32] and more applications were published later about ICP-MS [33,34] and laser ablation (LA) ICP-MS [35] in applications meeting Pharmacopeia requirements for analysis of elemental impurities in pharmaceutical goods. If ICP-MS showed to be perfectly suitable for highly standardised products such as medicines but, when considering the forensic issue of illegal and counterfeit pharmaceutical products, INAA is expected to have some advantages. First of all it allows to avoid representative sub-sampling and sample preparation difficulties. Then as it is based on completely different physical principles, it can be considered as validation technique. Moreover, the degree of accuracy and metrological traceability of the values of the measurement can meet the highest international metrological requirements [21].
On the other hand, mass spectrometric techniques, mainly coupled to gas or liquid chromatography, are the golden standard for forensic characterisation of drugs and impurities. About sildenafil characterisation, a comparison among different analytical techniques demonstrated that UPLC-MS ingredients profile was the most reliable technique for distinguish authentic and unauthentic drugs [7].
The aim of the present research was to develop a novel approach based on LC coupled to high resolution mass spectrometry (HRMS) and INAA to characterise both the authentic and illegal pharmaceuticals containing sildenafil seized in Italy and to provide quantitative elemental data for forensic purposes. The specific role of INAA in the research was to obtain information on the presence of both toxic elements and trace elements allowing inferring about the possible common origin of confiscated material.
2. Materials and methods
2.1. Chemicals and reagents
Ultrapure water, acetonitrile, ammonium formate, formic acid, sildenafil were purchased from Sigma-Aldrich (Milan, Italy); methanol was obtained from Merck (Merck KGaA, Darmstadt, Germany).
The standards reference materials used for INAA were NIST-SRM 1633a and NIST-SRM 1547 from the National Institute of Standards and Technology (NIST).
2.2. LC/HRMS equipment
The LC/HRMS system was composed of a Thermo ULTIMATE 3000 equipped with a Thermo Acclaim RSLC 120 C18 analytical column (2.1 × 100 mm, 2.2 μm particle size) coupled to a Thermo single-stage Orbitrap (Exactive) MS system, interfaced with a HESI Ion Max source.
2.3. LC/HRMS conditions
Mobile phase A was ultrapure water with 0.1% formic acid/ammonium formate 5 mM, mobile phase B was methanol/acetonitrile 1:1 with 0.1% formic acid. The analytical column was maintained at 40 °C, and sample injection volume was 10 μL. The flow rate was set at 400μ L/min. The mobile phase gradient was as follows: 100% A for 1 min, gradient to 15% B in 4.1 min, to 50% B in 1.8 min and then to 100% B in 2.1 min and maintained for 3.5 min. A column re-equilibration was performed with a linear gradient to 100% A in 3.0 min and then maintained for 3.0 min. The HESI source was heated at 340 °C. The other following parameters were used: source current, 6 μA; sheath and auxiliary gas (both nitrogen) flow rates, 35 and 18 arbitrary units, respectively; capillary temperature, 275 °C, and capillary voltage 45 V. The data were acquired in full scan mode over a mass range of 110–650 m/z. The instrument was operating in positive ion mode with a resolving power of 100000 FWHM. Mass calibration was performed according to the guidelines provided by the instrument's supplier. The recommended calibration solution was made of MRFA (L-methionyl-arginyl-phenylalanyl-alanine acetate), caffeine and Ultramark® 1621 dissolved in methanol/water (1:1). The automatic calibration feature of the Exactive tune software was used for calibration. The mass scale was calibrated every 2 days over a mass range m/z 50–2000. Lock mass was employed during sample analyses to compensate any possible mass axis drifts. The diisodecyl phthalate ion 391.2843, generally present in a laboratory environment, was used as lock mass.
2.4. INAA equipment and conditions
INAA was performed using the standard techniques adopted in the Radiochemistry Laboratory of the Department of General Chemistry of the University of Pavia. Irradiation has been carried out in the Triga Mark II 250 kW research reactor of Pavia University at a thermal neutron flux of about 1·1012 n·cm−2·s−1 for 12 h. Induced radioactivity was measured by γ-ray spectrometry using a HPGe detector coupled to a computer assisted Ortec spectral analysis system. Counting started 3 days after the end of irradiation and was repeated after 6, 12 and 24 days.
2.5. Samples
15 samples of illegal products containing sildenafil sold through the net and confiscated by Carabinieri (see three of the illegal products analysed in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5) were analysed for characterisation, identification and quantification of the active compound [36]. The samples were then analysed by INAA, in order to obtain information on the presence of both toxic elements and trace elements and to allow inferring about the possible common origin of confiscated material.
Fig. 1.
Package of Kamagra Gold.
Fig. 2.
Kamagra Gold tablet.
Fig. 3.
Package of Golden Root.
Fig. 4.
Golden root capsule.
Fig. 5.
Cenforce tablet.
Many of the products were confiscated as blisters containing the tablets without boxes or package insert and no indication on the composition of the product, except for the sildenafil amount (generally claimed as 100 mg).
As reference, 10 Viagra® 25 mg, 1 sildenafil DOC 25 mg, 1 sildenafil TEVA 100 mg, and powder of pure sildenafil were analysed.
2.6. Sample preparation
For LC-HRMS characterisation, both pharmaceutical products and confiscated samples were grinded and homogenized in a mortar; 10 mg of the obtained powder were dissolved in 10 mL of methanol under sonication for 10 minutes and centrifuged. 10 μL of the surnatant were subsequently diluted in 1 mL of methanol and 10 μL directly injected in the LC-HRMS instrument. For LC-HRMS quantitation of active compound, samples were prepared and analysed as reported elsewhere [36]. For INAA all samples were grinded and homogenized in a mortar. Preliminarily aliquots of 200 mg were submitted to homogeneity tests. Variance analysis, taken as an evaluation of the sample variability due to sampling, provided evidence aliquots of 70 mg or greater are homogeneous and representative of the investigated materials.
3. Results
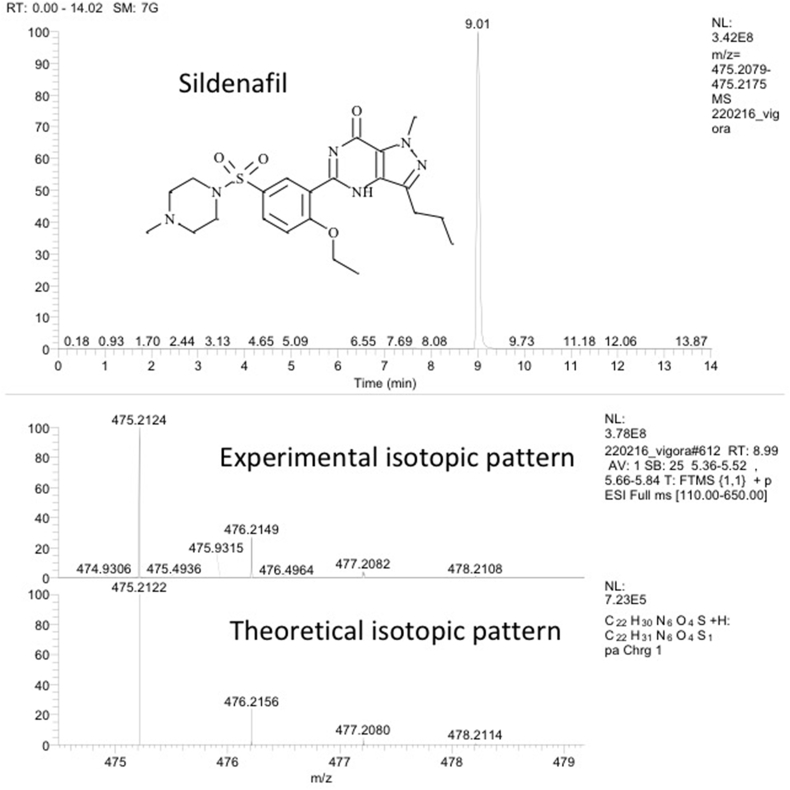
Sildenafil was identified and quantified in all the illegal products by LC/HRMS. Fig. 6 shows the HRMS spectrum with the accurate mass of an illegal sildenafil and the theoretical spectrum.
Fig. 6.
Extracted ion chromatogram of sildenafil (exact mass [M+H]+ = 475.2122) in an authentic sample (above plot), the related experimental isotopic pattern of sildenafil [M+H]+ ionic species (middle plot) and theoretical isotopic pattern of ionic species with the elemental composition C22H31N6O4S (bottom plot).
The amount of sildenafil determined in the tablets/capsules was always different from that declared, spanning from 36 to 221 mg per unit. In the product “golden root”, claimed to contain only natural extracts, both sildenafil and thiosildenafil were present. LC/HRMS analyses identified also the chemical substance aspartame (L-aspartil-l-phenylalanine methylester) in 5 samples. Other main impurities found were imidazosagatriazinone (5-(2-Ethoxyphenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7(6H)-one) in 6 of the 15 illegal products analysed and 5-chloroimidazosagatrizinone in 11 of the 15 illegal products analysed, as shown in Fig. 7, Fig. 8, Fig. 9. The analytical findings obtained by LC/HRMS are depicted in Table 1.
Fig. 7.
Extracted ion chromatogram of aspartame (exact mass [M+H]+ = 295.1288) in an authentic sample (above plot), the related experimental isotopic pattern of aspartame [M+H]+ ionic species (middle plot) and theoretical isotopic pattern of ionic species with the elemental composition C14H19N2O5 (bottom plot).
Fig. 8.
Extracted ion chromatogram of imidazosagatriazinone (exact mass [M+H]+ = 313.1659) in an authentic sample (above plot), the related experimental isotopic pattern of imidazosagatriazinone [M+H]+ ionic species (middle plot) and theoretical isotopic pattern of ionic species with the elemental composition C17H21N4O2 (bottom plot).
Fig. 9.
Extracted ion chromatogram of 5-chloroimidazosagatriazinone (exact mass [M+H]+ = 347.1269) in an authentic sample (above plot), the related experimental isotopic pattern of 5-chloroimidazosagatriazinone [M+H]+ ionic species (middle plot) and theoretical isotopic pattern of ionic species with the elemental composition C17H20ClN4O2 (bottom plot).
Table 1.
Impurities found by LC-HRMS and quantitative results. * Authentic samples.
| Name of sample | 5-chloroimidazosagatrizinone | imidazosagatriazinone | aspartame | mg sildenafil per unit |
|---|---|---|---|---|
| 1 Aurogra | present | present | present | 221 |
| 2 Cockfoster | present | present | present | 136 |
| 3 Cenforce a | present | present | 167 | |
| 4 Cenforce b | present | present | 170 | |
| 5 Golden root | present | present | 10 + thiosildenafil | |
| 6 Kamagra a | 95 | |||
| 7 Kamagra b | present | 80 | ||
| 8 Kamagra c | 75 | |||
| 9 Kamagra oral jelly | 95 | |||
| 10 Silagra | present | present | 140 | |
| 11 Sildenafil citrate | present | 95 | ||
| 15 Sildigra | present | present | 60 | |
| 12 Vigora | present | present | 36 | |
| 13 Blue | 40 | |||
| 14 Kamagra gold | present | present | 71 | |
| *Viagra Pfizer 25 mg | absent | absent | absent | 24 |
| *Sildenafil DOC 25 mg | present | absent | absent | 24 |
| *Sildenafil TEVA 100 mg | absent | absent | absent | 101 |
| *Sildenafil powder | present | absent | absent |
The minimum detectable amounts (MDA) by INAA for the 23 elements monitored are reported in Table 2. Analytical results from authentic samples from the legal market are reported in Table 3. In the original Viagra® samples INAA allowed to measure concentrations above the MDAs only for 5 elements: Na, Ca, Br, La and Cl. Table 4 shows analytical results from the 15 illegal products seized in Italy by Carabinieri and analysed by INAA determining a set of the eleven elements (Na, Cl, K, Ca, Cr, V, Fe, Co, Zn, Br, La) whose signal was above MDAs values. Cenforce a and b refer to illegal products seized in different times, as Kamagra a, b and c. 15a, 15b, 15c and 15d Sildigra refer to four different pills belonging to the same seizure. In all the illegal samples analysed more than 5 elements were identified and quantified except for Cockfoster, Cenforce b, Kamagra b, Kamagra c, Kamagra oral jelly and Blue. A set of eleven elements (Na, Cl, K, Ca, Cr, V, Fe, Co, Zn, Br, La) was able to show significant differences by comparing authentic Viagra® and illegal products.
Table 2.
Minimum detectable amount (MDA) for the 23 elements monitored. Values are expressed in μg/g if not specified.
| Element | MDA | |
|---|---|---|
| 1 | Na | 0,005 (%) |
| 2 | Cl | 50 |
| 3 | K | 0,01 (%) |
| 4 | Ca | 0,01 (%) |
| 5 | Sc | 0,05 |
| 6 | V | 0.1 |
| 7 | Cr | 1 |
| 8 | Fe | 250 |
| 9 | Co | 0,5 |
| 10 | Zn | 10 |
| 11 | Br | 1 |
| 12 | Rb | 15 |
| 13 | Cs | 1 |
| 14 | Ba | 200 |
| 15 | La | 0,1 |
| 16 | Ce | 1 |
| 17 | Sm | 0,05 |
| 18 | Eu | 0,2 |
| 19 | Tb | 0,5 |
| 20 | Yb | 2 |
| 21 | Lu | 1 |
| 22 | Hf | 0,2 |
| 23 | Th | 0,2 |
Table 3.
INAA elemental analytical results of authentic Viagra®.
| Na (%) | Ca (%) | Br (μg/g) | La (μg/g) | Cl (μg/g) | |
|---|---|---|---|---|---|
| Viagra Pfizer 25 A | 0.26 | 1.1 | 0.40 | 0.17 | 94 |
| Viagra Pfizer 25 B | 0.27 | 1.2 | 0.52 | 0.18 | 98 |
| Viagra Pfizer 25 C | 0.27 | 1.4 | 0.48 | 0.20 | 102 |
| Viagra Pfizer 25 D | 0.26 | 1.3 | 0.38 | 0.14 | 90 |
| Viagra Pfizer 25 E | 0.27 | 1.4 | 0.48 | 0.21 | 107 |
| Viagra Pfizer 25 F | 0.27 | 1.3 | 0.37 | 0.22 | 94 |
| Viagra Pfizer 25 G | 0.28 | 1.3 | 0.41 | 0.19 | 100 |
| Viagra Pfizer 25 H | 0.28 | 1.4 | 0.50 | 0.20 | 106 |
| Viagra Pfizer 25 I | 0.28 | 1.1 | 0.50 | 0.18 | 105 |
| Arithmetic mean | 0.27 | 1.3 | 0.45 | 0.19 | 99.6 |
| Standard deviation | 0.01 | 0.1 | 0.06 | 0.02 | 6.0 |
| CV% | 2.88 | 9.4 | 13.0 | 12.7 | 6.0 |
Table 4.
INAA elemental analytical results for illegal samples.
| Name of sample | Na (%) | Cl (μg/g) | K (%) | Ca (%) | Cr (μg/g) | V (μg/g) | Fe (μg/g) | Co (μg/g) | Zn (μg/g) | Br (μg/g) | La (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Aurogra | 0.11 | 193 | – | 0.40 | – | 0.17 | 310 | 0.64 | – | 1.9 | – |
| 2 Cockfoster | 0.12 | 810 | – | 0.30 | – | 0.29 | – | 0.52 | – | – | – |
| 3 Cenforce a | 0.71 | 565 | 1.1 | – | 2.3 | 1.1 | 304 | – | – | 4.8 | 0.71 |
| 4 Cenforce b | 0.16 | – | 0.05 | 0.20 | 1.8 | – | – | – | – | 1.9 | – |
| 5 Golden root | 0.03 | 664 | 0.19 | – | 1.0 | 0.63 | 313 | – | – | – | 0.33 |
| 6 Kamagra a | 0.21 | 1553 | – | 0.30 | 2.6 | 0.24 | 271 | 2.0 | – | 6.5 | – |
| 7 Kamagra b | 0.29 | 2908 | – | 0.30 | – | – | – | – | – | 4.4 | – |
| 8 Kamagra c | 0.31 | 2059 | – | 0.30 | – | 0.32 | – | – | – | 4.8 | – |
| 9 Kamagra oral jelly | 0.24 | 2610 | 0.44 | – | – | – | – | – | – | – | – |
| 10 Silagra | 0.03 | 248 | – | 1.7 | 1.2 | 0.22 | – | 2.1 | – | – | – |
| 11 Sildenafil citrate | 0.05 | 270 | – | 0.40 | – | 0.45 | – | – | 5633 | – | 0.12 |
| 12 Vigora | 0.09 | 555 | – | 2.5 | 2.7 | 8.6 | 528 | 0.5 | – | – | 0.26 |
| 13 Blue | 0.04 | – | 0.04 | – | 1.5 | – | – | – | – | 3.7 | – |
| 14 Kamagra gold | 0.33 | – | – | 0.30 | – | – | 500 | 0.80 | 17 | 5.8 | – |
| 15a Sildigra | 0.26 | 366 | – | 0.50 | 3.8 | 0.31 | 285 | 0.88 | 237 | 5.9 | – |
| 15b Sildigra | 0.21 | 307 | – | 0.50 | 3.4 | – | 282 | 0.57 | 238 | 10 | – |
| 15c Sildigra | 0.27 | 327 | – | 0.40 | 4.6 | – | 322 | 1.2 | 127 | 8.4 | – |
| 15d Sildigra | 0.15 | 190 | – | 0.20 | 1.8 | 0.11 | – | 1.2 | 134 | 5.1 | – |
4. Discussion
The combination of the two specific analytical techniques allowed the characterisation of illegal medicaments not only by identification and quantitation of the active compound sildenafil but by identification of other chemical substances and elements of interest. One interesting compound identified by LC/HRMS in 5 samples was aspartame. In 2013 the European Food Safety Authority (EFSA) conducted a comprehensive review of the evidence about aspartame and concluded that it was safe for human consumption, including pregnant women and children, but EFSA also reported that the acceptable daily intake recommendations did not apply to people with phenylketonuria (PKU), a rare genetic disorder where the body cannot break down phenylalanine [37]. Only the product “Kamagra gold”, containing aspartame, had its packaging reporting the ingredients, where the presence of aspartame was anyway not reported. Other products had not packaging or package insert, and the blister didn't report the presence of this substance. Another chemical substance found by LC/HRMS in 6 samples was imidazosagatriazinone, a possible by-product. A Warning Signal for Globally Harmonized System (GHS) Hazard is reported for this substance, as can provoke skin, eye and respiratory tract irritation [38]. A third chemical substance found by LC/HRMS in 11 samples was chloro-imidazosagatriazinone. No toxicity data was found for this substance. It has to be noted that chloro-imidazosagatriazinone was also present in Sildenafil DOC and in sildenafil pure powder, used for galenic preparations. These by-products are probably due to the poor manufacturing processes, in which a poor purification (or no purification at all) is performed after the synthesis of sildenafil. The presence of one or more of these compounds can be hence be presumptive of illegal sildenafil. Another interesting result about the illegal products, resulting in possible danger for health, is the unpredictable amount of active principle present. In fact, it varies from 36 mg, to 220 mg, over twice the declared dose, which was always 100 mg, with inherent health risks for the users. It is interesting to consider that some of the authors already reported sildenafil in illegal products associated with new psychoactive substances (NPS) in Italy [39].
INAA analyses allowed to show that in most cases the illegal products contain more than the 5 elements present in authentic Viagra®. Cockfoster contained V and Co, while Br and La were absent. Cenforce b contained K and Cr, while Cl and La were absent. Kamagra c contained V, while La was absent. Kamagra oral jelly contained K, while Ca, Br and La were absent. Blue contained K and Cr, while Cl, Ca and La were absent. Qualitative differences can be examined by considering the samples 15a, 15b, 15c and 15d Sildigra, referring to four different pills belonging to the same seizure. In Kamagra b the only qualitative feature which differentiate the sample from the authentic Viagra® was the absence of La and therefore we examined quantitative values. In this case a t-test allowed to demonstrate significant differences (p < 0,05) for Cl, Ca and Br compared to authentic Viagra®.
5. Conclusion
The research based on LC/HRMS and INAA to characterise both authentic and illegal pharmaceuticals containing sildenafil seized in Italy showed that the two techniques effectively provided complementary information. LC/HRMS not only allowed identification and quantitation of sildenafil in all the products but was used to search possible toxic chemical substances and other compounds that could be useful to infer about the origin of the product. Aspartame and two impurities of possible forensic interest were identified. Cheaper analytical methods such as LC/DAD can be used to provide the concentration of the active principle in the tablet but LC/HRMS was preferred to other cheaper analytical approaches because it allowed the identification of unknown molecules based on exact mass.
INAA did not identified elements particularly toxic but always found significant differences between legal and illegal products on the market.
The presence of unexpected inorganic elements in counterfeit products can be assumed to derive from poor management of the production line and a lack of adequate quality controls. In this case the use of the INAA technique allows a qualitative investigation suitable to identify these problems that afflict the entire matrix and certainly not due to the preparation of the sample.
We consider that the use of such powerful analytical techniques is expensive but the identification of toxic threats is the primary aim of the method we propose. A similar approach has been recently proposed to study amoxicillin drugs in Ghana, using both LC and proton induced X-ray emission (PIXE) [40].
Future studies with more samples are needed to allow inferring about the common origin of confiscated material, possibly with samples having different known geographical origin. The results of our approach demonstrated to allow effective discrimination of illegal samples compared to authentic pharmaceutical products. The profiling approach has been successfully studied to infer about the source of several illegal products, including cocaine, ecstasy pills, hashish, heroin, opium [21,[41], [42], [43], [44]]. The results of this pilot study, based on 23 elements monitored by INAA plus LC/HRMS analysis, is expected to support further development to obtain forensic characterisation of other illegal products, including new psychoactive substances, from different countries.
Conflict of interest
Author declare no conflict of interest.
Acknowledgements
The work was carried out in the framework of the project “Smart-Stop”, financed by the Department of Antidrug Policies of the Italian Presidency of the Council of Ministers. It was also developed under the IAEA Coordinated Research Project on “Enhancing Nuclear Analytical Techniques to Meet the Needs of Forensic Sciences” (F11021). Finally, we thank the Italian “Comando Carabinieri per la Tutela della Salute - Sezione Operativa Centrale” that performed the drugs seizures.
References
- 1.EUROPOL . 2018. Internet Organised Crime Threat Assessment (IOCTA), the Hague, The Netherlands. [Google Scholar]
- 2.Broséus J., Rhumorbarbe D., Morelato M., Staehli L., Rossy Q. A geographical analysis of trafficking on a popular darknet market. Forensic Sci. Int. 2017;277:88–102. doi: 10.1016/J.FORSCIINT.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Dégardin K., Roggo Y., Margot P. Understanding and fighting the medicine counterfeit market. J. Pharm. Biomed. Anal. 2014;87:167–175. doi: 10.1016/j.jpba.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Sacré P.-Y., Deconinck E., Daszykowski M., Courselle P., Vancauwenberghe R., Chiap P., Crommen J., De Beer J.O. Impurity fingerprints for the identification of counterfeit medicines—a feasibility study. Anal. Chim. Acta. 2011;701:224–231. doi: 10.1016/J.ACA.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Anzanello M.J., Ortiz R.S., Limbergerb R.P., Mayorga P. A multivariate-based wavenumber selection method for classifying medicines into authentic or counterfeit classes. J. Pharm. Biomed. Anal. 2013;83:209–214. doi: 10.1016/J.JPBA.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Jung C.R., Ortiz R.S., Limberger R., Mayorga P. A new methodology for detection of counterfeit Viagra® and Cialis® tablets by image processing and statistical analysis. Forensic Sci. Int. 2012;216:92–96. doi: 10.1016/J.FORSCIINT.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Anzanello M.J., Ortiz R.S., Limberger R.P., Mariotti K. A framework for selecting analytical techniques in profiling authentic and counterfeit Viagra and Cialis. Forensic Sci. Int. 2014;235:1–7. doi: 10.1016/J.FORSCIINT.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Campbell N., Clark J.P., Stecher V.J., Goldstein I. Internet-ordered Viagra (Sildenafil Citrate) is rarely genuine. J. Sex. Med. 2012;9:2943–2951. doi: 10.1111/J.1743-6109.2012.02877.X. [DOI] [PubMed] [Google Scholar]
- 9.Jack A. Counterfeit medicines. Bitter pills. BMJ. 2007;335:1120–1121. doi: 10.1136/bmj.39412.431655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kee C.-L., Ge X., Gilard V., Malet-Martino M., Low M.-Y. A review of synthetic phosphodiesterase type 5 inhibitors (PDE-5i) found as adulterants in dietary supplements. J. Pharm. Biomed. Anal. 2018;147:250–277. doi: 10.1016/J.JPBA.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Park S., Lee J.G., Roh S.H., Kim G., Kwon C.H., Park H.R., Kwon K.S., Kim D., Kwon S.W. Determination of PDE-5 inhibitors and appetite suppressants in adulterated dietary supplements using LC/PDA and LC/MS. Food Addit. Contam. Part B. 2012;5:29–32. doi: 10.1080/19393210.2012.656706. [DOI] [PubMed] [Google Scholar]
- 12.Lee S., Ji D., Park M., Chung K.H. Development of a comprehensive spectral library of sildenafil and related active analogues using LC–QTOF–MS and its application for screening counterfeit pharmaceuticals. Forensic Sci. Int. 2015;257:182–188. doi: 10.1016/J.FORSCIINT.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Song F., El-Demerdash A., Lee S.-J.S.H. Screening for multiple phosphodiesterase type 5 inhibitor drugs in dietary supplement materials by flow injection mass spectrometry and their quantification by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012;70:40–46. doi: 10.1016/J.JPBA.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Patel D.N., Li L., Kee C.-L., Ge X., Low M.-Y., Koh H.-L. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges. J. Pharm. Biomed. Anal. 2014;87:176–190. doi: 10.1016/J.JPBA.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz R.S., Mariotti K., Holzschuh M.H., Romão W., Limberger R., Mayorga P. Profiling counterfeit cialis, Viagra and analogs by UPLC-MS. Forensic Sci. Int. 2013;229:13–20. doi: 10.1016/j.forsciint.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Mustazza C., Borioni A., Rodomonte A.L., Bartolomei M., Antoniella E., Di Martino P., Valvo L., Sestili I., Costantini E., Gaudiano M.C. Characterization of Sildenafil analogs by MS/MS and NMR: a guidance for detection and structure elucidation of phosphodiesterase-5 inhibitors. J. Pharm. Biomed. Anal. 2014;96:170–186. doi: 10.1016/J.JPBA.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Monakhova Y.B., Kuballa T., Löbell-Behrends S., Maixner S., Kohl-Himmelseher M., Ruge W., Lachenmeier D.W. Standardless 1H NMR determination of pharmacologically active substances in dietary supplements and medicines that have been illegally traded over the Internet. Drug Test. Anal. 2012;5:400–411. doi: 10.1002/dta.1367. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz R.S., de Cássia Mariotti K., Fank B., Limberger R.P., Anzanello M.J., Mayorga P. Counterfeit Cialis and Viagra fingerprinting by ATR-FTIR spectroscopy with chemometry: can the same pharmaceutical powder mixture be used to falsify two medicines? Forensic Sci. Int. 2013;226:282–289. doi: 10.1016/j.forsciint.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Sacré P.-Y., Deconinck E., Saerens L., De Beer T., Courselle P., Vancauwenberghe R., Chiap P., Crommen J., De Beer J.O. vol 56. 2011. pp. 454–461. (Detection of Counterfeit Viagra® by Raman Microspectroscopy Imaging and Multivariate Analysis). [DOI] [PubMed] [Google Scholar]
- 20.Balaram V. Recent advances in the determination of elemental impurities in pharmaceuticals – status, challenges and moving frontiers. TrAC Trends Anal. Chem. 2016;80:83–95. doi: 10.1016/J.TRAC.2016.02.001. [DOI] [Google Scholar]
- 21.Bode P., Romanò S., Romolo F.S. 2017. Large Sample Neutron Activation Analysis Avoids Representative Sub-sampling and Sample Preparation Difficulties: an Added Value for Forensic Analysis. [DOI] [Google Scholar]
- 22.Fakhar F.E., Moalemi S., Rachiti M.L., Oliaiy P., Esmaeili N., Shokouhi F., Ghods H., Tahani V. Qualitative and Quantitative study of trace element in drugs (OPIUM, HASHISH, ECSTASY PILL) by PIXE and NAA. Int. J. PIXE. 2012;22:241–248. doi: 10.1142/S0129083512400335. [DOI] [Google Scholar]
- 23.Zhang Z.Y., Yang J.H., Ouyang H., Li Z.J., Chai Z.F., Zhu J., Zhao J.Z., Yu Z.S., Wang J. Study of trace impurities in heroin by neutron activation analysis. J. Radioanal. Nucl. Chem. 2004;262:295–297. doi: 10.1023/B:JRNC.0000040888.85552.5d. [DOI] [Google Scholar]
- 24.Capote G., Ribeiro S., Arribére M.A., Hernández A. Determination of elemental levels in radiopharmaceuticals by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 2001;249:657–661. doi: 10.1023/A:1013275005161. [DOI] [Google Scholar]
- 25.Tuckerman M.M., Bate L.C., Leddicotte G.W. Determination of trace elements in drugs by neutron activation analysis. J. Pharm. Sci. 1964;53:983–984. doi: 10.1002/JPS.2600530841. [DOI] [PubMed] [Google Scholar]
- 26.Wollein U., Bauer B., Habernegg R., Schramek N. Potential metal impurities in active pharmaceutical substances and finished medicinal products – a market surveillance study. Eur. J. Pharm. Sci. 2015;77:100–105. doi: 10.1016/J.EJPS.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Li G., Schoneker D., Ulman K.L., Sturm J.J., Thackery L.M., Kauffman J.F. Elemental impurities in pharmaceutical excipients. J. Pharm. Sci. 2015;104:4197–4206. doi: 10.1002/JPS.24650. [DOI] [PubMed] [Google Scholar]
- 28.Wang T., Wu J., Hartman R., Jia X., Egan R.S. A multi-element ICP-MS survey method as an alternative to the heavy metals limit test for pharmaceutical materials. J. Pharm. Biomed. Anal. 2000;23:867–890. doi: 10.1016/S0731-7085(00)00361-7. [DOI] [PubMed] [Google Scholar]
- 29.Lewen N., Mathew S., Schenkenberger M., Raglione T. A rapid ICP-MS screen for heavy metals in pharmaceutical compounds. J. Pharm. Biomed. Anal. 2004;35:739–752. doi: 10.1016/J.JPBA.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Murty A.S.R., Kulshresta U.C., Rao T.N., Talluri M.V.N. Determination of heavy metals in selected drug substances by inductively coupled plasma–mass spectrometry. Indian J. Chem. Technol. 2005;12:229–231. [Google Scholar]
- 31.Silveira J.N., Pereira Lara P.C., Batista Dias M., Gomes Matos J.M., Da Silva J.C., Nascentes N.C., Ciminelli V., Borba da Silva J.B. Determination of As, Bi, Cd, Co, Cr, Ga, In, Mn, Ni, Pb, Sb, Se, Sn, Te, Tl, and V in antihypertensive drugs by inductively coupled plasma mass spectrometry. Atom. Spectros. Norwalk Connectut. 2007;28:1–7. [Google Scholar]
- 32.Nageswara Rao R., Kumar Talluri M.V.N. An overview of recent applications of inductively coupled plasma-mass spectrometry (ICP-MS) in determination of inorganic impurities in drugs and pharmaceuticals. J. Pharm. Biomed. Anal. 2007;43:1–13. doi: 10.1016/J.JPBA.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Van Hoecke K., Catry C., Vanhaecke F. Optimization of sample preparation and a quadrupole ICP-MS measurement protocol for the determination of elemental impurities in pharmaceutical substances in compliance with USP guidelines. J. Anal. At. Spectrom. 2012;27:1909–1919. doi: 10.1039/C2JA30128H. [DOI] [Google Scholar]
- 34.Fischer L., Zipfel B., Koellensperger G., Kovac J., Bilz S., Kunkel A., Venzago C., Hann S. Flow injection combined with ICP-MS for accurate high throughput analysis of elemental impurities in pharmaceutical products according to USP. J. Pharm. Biomed. Anal. 2014;95:121–129. doi: 10.1016/J.JPBA.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Rudovica V., Viksna A., Actins A. Application of LA-ICP-MS as a rapid tool for analysis of elemental impurities in active pharmaceutical ingredients. J. Pharm. Biomed. Anal. 2014;91:119–122. doi: 10.1016/j.jpba.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Strano-Rossi S., Odoardi S., Castrignanò E., Serpelloni G., Chiarotti M. Liquid chromatography–high resolution mass spectrometry (LC–HRMS) determination of stimulants, anorectic drugs and phosphodiesterase 5 inhibitors (PDE5I) in food supplements. J. Pharm. Biomed. Anal. 2015;106:144–152. doi: 10.1016/J.JPBA.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 37.EFSA panel on food additives and nutrient sources added to food (ANS). Scientific opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J. 2013;11:3496. doi: 10.2903/j.efsa.2013.3496. [DOI] [Google Scholar]
- 38.National Center for Biotechnology Information PubChem Compound Database; Imidazosagatriazinone, CID=899745. https://pubchem.ncbi.nlm.nih.gov/compound/899745
- 39.Odoardi S., Romolo F.S., Strano-Rossi S. A snapshot on NPS in Italy: distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci. Int. 2016;265:116–120. doi: 10.1016/J.FORSCIINT.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 40.Tandoh J., Bamford S., Wahab A., Nuviadenu C., Forson A., Ahiamadjie H., Banini G., Quashigah G., Sackey H., Gazoya D. Poster Presented at the Joint ICTP-IAEA Advanced Workshop on Enhancing Accelerator-Based Analytical Techniques for Forensic Science. Abdus Salam International Centre for Theoretical Physics (ICTP); Italy: 20-24 May 2019. Inorganic profiling of amoxicillin drugs in Ghana using PIXE technique. [Google Scholar]
- 41.Dams R., Benijts T., Lambert W.E., Massart D.L., De Leenheer A.P. Review: heroin impurity profiling: trends throughout a decade of experimenting. Forensic Sci. Int. 2001;123:81–88. doi: 10.1016/s0379-0738(01)00541-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z.Y., Yang J.H., Ouyang H., Li Z.J., Chai Z.F., Zhu J., Zhao J.Z., Yu Z.S., Wang J. Study of trace impurities in heroin by neutron activation analysis. J. Radioanal. Nucl. Chem. 2004;262:295–297. [Google Scholar]
- 43.Liu C., Hua Z., Bai Y., Liu Yao. Profiling and classification of illicit heroin by ICP-MS analysis of inorganic elements. Forensic Sci. Int. 2014;239:37–43. doi: 10.1016/j.forsciint.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., Hua Z., Meng X. Profiling of Illicit Cocaine Seized in China by ICP-MS analysis of inorganic elements. Forensic Sci. Int. 2017;276:77–84. doi: 10.1016/j.forsciint.2017.04.014. [DOI] [PubMed] [Google Scholar]