Abstract
Progressive hemifacial atrophy (PHA) is mainly characterized by asymmetrical atrophy of craniofacial tissue; however, 10% to 30% of patients with PHA exhibit ocular manifestations. Here, we describe abnormal ocular findings in a Chinese patient with PHA. The patient was a 29-year-old Chinese man. Characteristic ocular findings in his affected eye included keratic precipitate, corneal endothelial degeneration, fundus tessellation, pupillary dilation, direct light reflex loss, and visual evoked potential alteration. Whole exosome sequencing revealed that the patient harbored a mutation in the CRB1 gene; this gene has been associated with various retinal dystrophies. During 10 years of follow-up, the patient’s ocular status remained stable. To the best of our knowledge, this is the first report of ocular manifestations of PHA in a Chinese patient, and the first report of a CRB1 mutation in a patient with PHA; these findings may inform future research regarding PHA.
Keywords: Gene mutation, progressive hemifacial atrophy, visual evoked potential, exosome sequencing, pupillary dilation, CRB1 mutation, fundus tessellation, keratic precipitate, corneal endothelial degeneration
Introduction
Progressive hemifacial atrophy (PHA) is an extremely rare disease that is also described as Parry–Romberg syndrome. As its name suggests, the disease is characterized by slowly progressive unilateral atrophy of craniofacial structures, including skin, subcutaneous fat, muscle tissue, cartilage, and bone.1 In the past 10 years, there have been several reports of patients with PHA who exhibit various ocular findings.2–4
The human CRB1 gene is located on the long arm of chromosome 1 (1q31.1) and encodes a transmembrane protein, the crumbs protein, which consists of 1406 amino acids. Mutations in the CRB1 gene have been associated with various phenotypes in patients with severe retinal dystrophies.5
At present, the pathogenesis of PHA remains unclear and an effective remedy is unavailable. Here, we describe the ocular findings in a patient with PHA. To facilitate analyses regarding the pathogenesis of PHA, we also performed whole exome sequencing of a blood sample from the patient.
Case report
Patient history
The case report was approved by the ethics committee of Tianjin Eye Hospital. Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A 29-year-old Chinese man with a history of progressive enophthalmos on the right side was referred to our ophthalmology clinic. The patient had developed alopecia at 5 years of age; when he was approximately 13 years old, his parents observed that his face appeared to be asymmetric. At that time, the patient did not experience headaches, dizziness, or psychiatric symptoms; however, the extent of hemifacial atrophy continued to worsen. When the patient was 15 years old, he developed alopecia in the right eyebrow and his hair became slightly gray. Fortunately, the atrophic condition of his right face subsequently stabilized.
Clinical manifestations
The patient’s blood pressure and blood glucose were normal. On examination, the patient exhibited obvious facial asymmetry, with noticeable enophthalmos and exotropia on the right side (Figure 1a). The right half of his forehead and cheek were slightly atrophic compared with the corresponding left halves. In addition, the patient exhibited two zones of alopecia areata (3 cm × 3 cm) (Figure 1b), including the right eyebrow; the cranium under the skin of these zones was palpably indented. Skin on the patient’s forehead and at the zones of alopecia appeared normal without pigmentation or shine.
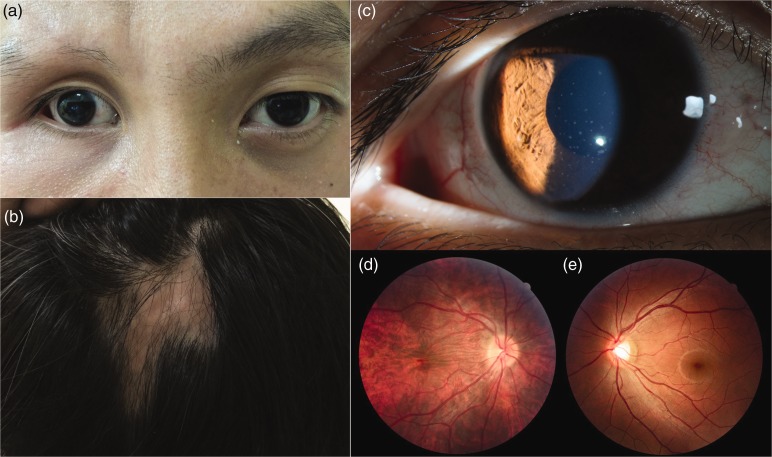
Figure 1.
Clinical manifestations of progressive hemifacial atrophy in a Chinese patient. a) Enophthalmos and exotropia on the right side; b) two zones of alopecia areata; c) white, round, medium-sized keratic precipitates on the central to inferior cornea; d, e) atrophy of the posterior choroid in the right eye.
Ocular findings
A comprehensive ocular examination was performed, which included measurements of visual acuity and intraocular pressure, as well as slit lamp examination and fundus photography. The patient also underwent corneal endothelial analysis (specular microscopy), electroretinography, and visual evoked potential (VEP) assessment. The findings are described in Table 1. White, round, medium-sized keratic precipitates were found on the central to inferior cornea (Figure 1c). Funduscopy revealed atrophy of the posterior choroid in the right eye (i.e., fundus tessellation) (Figure 1d, e). In addition, the affected eye exhibited a dilated pupil (6–7 mm) with poor reaction to light. The abnormal pupillary light reflex suggested that the patient exhibited optic nerve abnormalities; these were investigated by electroretinography and VEP assessment. Delayed VEP latency was found in the affected eye; this was suggestive of demyelination in the visual pathway,6,7 and confirmed that the affected eye exhibited optic nerve abnormalities (Table 1). The patient confirmed that these ocular findings and the abovementioned bone and skin findings were not present in other members of his family.
Table 1.
Ocular findings in a Chinese patient with progressive hemifacial atrophy.
| Examination |
Result |
|
|---|---|---|
| Right eye | Left eye | |
| Clinical | Obvious enophthalmos | Normal |
| Visual acuity (logMAR) | 0.6 | 0.9 |
| Ocular inspection | Exotropia | Normal alignment |
| Intraocular pressure | 7.3 mmHg | 15.0 mmHg |
| Axial length | 22.35 mm | 25.45 mm |
| Slit lamp | Keratic precipitates, with otherwise clear cornea. Other anterior chamber structures were normal. | Normal |
| Specular microscopy | Teardrop-shaped endothelial corneal degeneration. Cell density = 2101.8/mm2 Hexagonality = 50% | Teardrop-shaped endothelial corneal degeneration. Cell density = 2605.8/mm2 Hexagonality = 59% |
| Fundus photography | Fundus tessellation and loss of foveal reflex | Normal |
| Electroretinography | Normal | Normal |
| Visual evoked potential | Normal P100 with delayed latency | Normal |
Abbreviation: logMAR, logarithm of the minimum angle of resolution.
Exome sequencing results
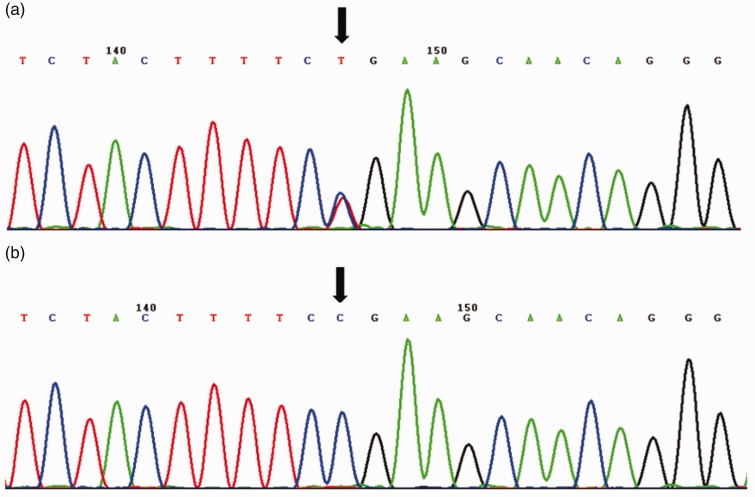
Whole exome sequencing was performed, using a 5-mL blood sample collected from the patient. Genomic DNA (1.5 µg) was extracted; exomes were then captured by Axeq Technologies, using a SureSelect Human All Exon kit, version 5 (Agilent, Santa Clara, CA, USA), in accordance with the manufacturer’s protocol. Each captured library was loaded onto a HiSeq 4000 sequencing platform (Illumina, San Diego, CA, USA) and 100-bp paired-end read were performed. All variants of interest were confirmed by Sanger sequencing. The PolyPhen-2 and SIFT software applications were used to predict the risks of amino acid changes due to missense mutations. Splice site mutations were predicted by Human Splicing Finder (http://www.umd.be/HSF3/HSF.htm). Whole exome sequencing revealed a pathogenic mutation in the CRB1 gene; this gene has been associated with retinal dystrophies. The gene is located in 1q31.3 (transcript no. NM_201253); the mutation was a missense C→T transition mutation in exon 6 (526th codon), which was not identified in normal controls in our gene database (Figure 2). This mutation led to production of a stop codon instead of an arginine, which interrupted mRNA transcription and caused reduction of protein synthesis.
Figure 2.
Identification of a CRB1 mutation in a Chinese patient with progressive hemifacial atrophy. a) Portion of CRB1 sequence in the patient (arrow indicates mutation); b) portion of CRB1 sequence in normal controls (arrow indicates absence of mutation in normal controls).
Diagnosis and treatment
There are no standardized criteria for the diagnosis of PHA; diagnosis is mainly based on clinical manifestations.8 Our patient presented with typical manifestations of progressive and self-limited dysplasia, which caused unilateral craniofacial atrophy. In addition, he exhibited noticeable enophthalmos and ocular abnormalities in the absence of trauma; these findings were indicative of PHA. The number of corneal endothelial cells was slightly reduced in the affected eye, compared with the number in the contralateral eye. The affected eye exhibited keratic precipitates, an abnormal pupillary light reflex, as well as delayed VEP latency; these findings were suggestive of optic nerve abnormalities associated with PHA. Moreover, the patient exhibited a mutation in the CRB1 gene; this gene has been associated with various retinal dystrophies. Notably, the patient’s condition has remained stable without treatment for more than 10 years.
Discussion
PHA is a rare clinical entity. To the best of our knowledge, this is the first report of a CRB1 mutation in a patient with PHA. Prior studies suggested that CRB1 mutations are associated with retinal pigmentation disorders or atrophy, which have been described as “CRB1 mutation-related ophthalmologic findings.”9,10 To enhance our understanding of PHA, we conducted an extensive literature review regarding ocular manifestations of the disease. We found that some patients with PHA have exhibited manifestations similar to those of patients with CRB1 mutation-related ophthalmologic findings, such as chorioretinal lesions,11,12 retinal pigment changes,13–15 chorioretinal atrophy.16 In our patient, some ocular abnormalities were consistent with CRB1 mutation-related ophthalmologic findings, including chorioretinal atrophy, abnormal pupillary light reflex, and delayed VEP latency. Taken together, the current and prior findings are suggestive of a relationship between CRB1 mutations and PHA.
PHA typically manifests in two stages. Hemifacial atrophy progresses over a period of 2 to 10 years, which is regarded as the progressing phase; subsequently, the condition generally stabilizes, which is regarded as the stable phase.17,18 The medical history of our patient was therefore consistent with the epidemiological and clinical characteristics of the disease. Notably, differential diagnoses for PHA include other forms of juvenile localized scleroderma, first and second branchial arch syndromes, other craniofacial dysplasias, and enophthalmos caused by eye trauma.19–21 Multiple clinicians have attempted to distinguish between PHA and linear scleroderma, also known as morphea en coup de sabre, based on clinical and histopathologic criteria.8 Proposed key manifestations for distinguishing linear scleroderma from PHA include cutaneous sclerosis, hyperpigmentation, and alopecia.21 Notably, there are many overlapping features between linear scleroderma and PHA; they might coexist in a single patient.1,22 In general, patients with PHA exhibit skin and craniofacial tissue atrophy below the forehead.23 Our patient had typical atrophy and ocular manifestations. In addition, his skin was not hyperpigmented and sclerosed. Therefore, although some manifestations were suggestive of linear scleroderma (i.e., alopecia and forehead atrophy), we made a diagnosis of PHA, based on his overall clinical characteristics and medical history. Notably, because the patient had not been born with facial abnormalities and denied previous trauma, congenital and trauma-related diagnoses were also excluded.
The cause of PHA has not been fully elucidated. Enlenburg et al. emphasized that PHA was not an inherited disease as early as 1871 (as mentioned in a relatively recent review24). Indeed, our literature review suggested that most instances of PHA involve sporadic disease,8 as in our patient. Whole exome sequencing revealed a CRB1 gene mutation in our patient; mutations in CRB1 are associated with many retinal abnormalities mainly including retinitis pigmentosa, Leber’s congenital amaurosis, and pigmented paravenous chorioretinal atrophy.5 In our patient, the affected eye exhibited fundus tessellation, which suggested atrophy of the choroid and retinal pigment epithelium. Furthermore, the patient’s affected eye exhibited an abnormal pupillary light reflex and delayed VEP latency. These ocular abnormalities might have been associated with the CRB1 mutation. In addition, patients with PHA may exhibit many ocular abnormalities, including retinal pigmentation disorder diseases and chorioretinal atrophy.13 To the best of our knowledge, there is no known association between CRB1 mutations and PHA; this is the first such report. Because of the rarity of PHA, we have only encountered a single affected patient. Further reports are necessary to more clearly elucidate the relationship between PHA and CRB1 mutations. In addition, there have been few case reports regarding examination of VEP in patients with PHA. In general, VEP examinations are performed in patients who might exhibit optic nerve functional deficits.6 Notably, PHA may be related to nerve dysfunction.25 Therefore, we suggest that examinations of VEP should be performed in patients with PHA who exhibit ocular manifestations, particularly those with fundus abnormalities. Finally, treatment for PHA can be challenging. Because its pathogenesis has not been clearly elucidated, the most common therapies include immunosuppressive treatment, fat grafting, and prosthetic rehabilitation through operation.8
In summary, our patient exhibited typical clinical features of PHA. Although many patients with PHA have been described, this is the first such report of a Chinese patient with PHA who exhibited ocular manifestations. Moreover, to the best of our knowledge, this is the first report of a CRB1 mutation in a patient with PHA. Although our results do not strongly suggest that CRB1 is a causative pathogenic gene in patients with PHA, further research is needed to clarify the role of CRB1 in the etiology of PHA.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81170828, 81670837), Tianjin Science & Technology Foundation (15JCZDJC35300), and Tianjin Health and Family Planning Communication Foundation (14KG133).
ORCID iD
References
- 1.Sommer A, Gambichler T, Bacharach-Buhles M, et al. Clinical and serological characteristics of progressive facial hemiatrophy: a case series of 12 patients. J Am Acad Dermatol 2006; 54: 227–233. [DOI] [PubMed] [Google Scholar]
- 2.Kini TA, Prakash VS, Puthalath S, et al. Progressive hemifacial atrophy with ciliary body atrophy and ocular hypotony. Indian J Ophthalmol 2015; 63: 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott CR, Hasbani MJ, Levada AJ, et al. Ocular motor dysfunction in Parry-Romberg syndrome: four cases. J Pediatr Ophthalmol Strabismus 2011; 48 Online: e63–e66. [DOI] [PubMed] [Google Scholar]
- 4.Fea AM, Aragno V, Briamonte C, et al. Parry Romberg syndrome with a wide range of ocular manifestations: a case report. BMC Ophthalmol 2015; 15: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinga B, Isabelle A, Saddek MSD, et al. CRB1 mutations in inherited retinal dystrophies. Hum Mutat 2012; 33: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You Y, Klistorner A, Thie J, et al. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci 2011; 52: 6911–6918. [DOI] [PubMed] [Google Scholar]
- 7.Castoldi V, Marenna S, d’Isa R, et al. Non-invasive visual evoked potentials to assess optic nerve involvement in the dark agouti rat model of experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Brain Pathol 2020; 30: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolkachjov SN, Patel NG, Tollefson MM. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis 2015; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamosh A, Scott AF, Amberger J, et al. Online Mendelian Inheritance in Man (OMIM). Hum Mutat 2000; 15: 57–61. [DOI] [PubMed] [Google Scholar]
- 10.Khan KN, Robson A, Mahroo OAR, et al. A clinical and molecular characterisation of CRB1-associated maculopathy. Eur J Hum Genet 2018; 26: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph G, Haritoglou C, Kalpadakis P, et al. Hemifacial atrophy (Parry-Romberg syndrome) with papillitis, retinal alterations, and restriction of motility. J AAPOS 2002; 6: 126–129. [DOI] [PubMed] [Google Scholar]
- 12.Detilleux JM, Zanen J. Progressive facial hemiatrophy (Parry-Romberg disease) with chorioretinal lesions. Bull Soc Belge Ophtalmol 1970; 156: 608–618. [PubMed] [Google Scholar]
- 13.Miller MT, Spencer MA. Progressive hemifacial atrophy. A natural history study. Trans Am Ophthalmol Soc 1995; 93: 203–217. [PMC free article] [PubMed] [Google Scholar]
- 14.Theodossiadis PG, Grigoropoulos VG, Emfietzoglou I, et al. Parry-Romberg syndrome studied by optical coherence tomography. Ophthalmic Surg Lasers Imaging 2008; 39: 78–80. [DOI] [PubMed] [Google Scholar]
- 15.Moura RA. Progressive facial hemiatrophia. Report of a case showing ocular and neuro-ophthalmologic changes. Am J Ophthalmol 1963; 55: 635–639. [PubMed] [Google Scholar]
- 16.Kawazoe M, Hirata A, Okinami S. Fluorescein and indocyanine green angiographic findings in progressive hemifacial atrophy. J Pediatr Ophthalmol Strabismus 2009; 46: 56–58. [DOI] [PubMed] [Google Scholar]
- 17.Rogers BO. Progressive facial hemiatrophy (Romberg’s disease): A review of 772 cases. J Proc 3d Int Cong Plast Surg. Excerpta Medica ICS. 1964; 66: 681–689. [Google Scholar]
- 18.Roddi R, Riggio E, Gilbert PM, et al. Progressive hemifacial atrophy: historical review and actual features related to a new classification of facial hypoplasia and atrophy. Eur J Plast Surg 1994; 17: 178–183. [Google Scholar]
- 19.Iñigo F, Jimenez-Murat Y, Arroyo O, et al. Restoration of facial contour in Romberg’s disease and hemifacial microsomia: experience with 118 cases. Microsurgery 1999; 20: 167. [DOI] [PubMed] [Google Scholar]
- 20.Fry JA, Alvarellos A, Fink CW, et al. Intracranial findings in progressive facial hemiatrophy. J Rheumatol 1992; 19: 956–958. [PubMed] [Google Scholar]
- 21.Orozco-Covarrubias L, Guzman-Meza A, Ridaura-Sanz C, et al. Scleroderma ‘en coup de sabre’ and progressive facial hemiatrophy. Is it possible to differentiate them? J Eur Acad Dermatol Venereol 2002; 16: 361–366. [DOI] [PubMed] [Google Scholar]
- 22.Jun JH, Kim HY, Jung HJ, et al. Parry-Romberg Syndrome with en coup de sabre. Ann Dermatol 2011; 23: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duymaz A, Karabekmez FE, Keskin M, et al. Parry-Romberg syndrome: facial atrophy and its relationship with other regions of the body. Ann Plast Surg 2009; 63: 457–461. [DOI] [PubMed] [Google Scholar]
- 24.Terenzi V, Leonardi A, Covelli E, et al. Parry-Romberg syndrome. Plast Reconstr Surg 2005; 116: 97e–102e. [DOI] [PubMed] [Google Scholar]
- 25.Tebloev IK, Karlov VA, Gemonov VV. Trophic disorders in ganglionitis of the superior cervical sympathetic ganglion under experimental and clinical conditions. Zh Nevropatol Psikhiatr Im S S Korsakova 1976; 76: 199–203. [in Russian] [PubMed] [Google Scholar]