Abstract
Background
Mesenchymal stem cells (MSCs) may be used to treat steroid-refractory graft versus host disease (GVHD). However, the effects of MSCs in haploidentical peripheral blood stem cell transplantation (haplo-PBSCT) have not been confirmed in randomized studies.
Methods
We conducted a randomized clinical study to investigate the effects of pre-infusion (1 × 106 cells/kg) MSCs on hematopoietic recovery, Epstein–Barr and cytomegalovirus infection, GVHD, and relapse in patients undergoing haplo-PBSCT. Fifty patients with acute leukemia or myelodysplastic syndrome were randomly divided into an MSC group administered 1 × 106 MSCs/kg 4 to 6 hours before infusion of peripheral stem cells and a control group without MSCs.
Results
Mean platelet engraftment time was significantly faster in the MSC compared with the control group (12.28 vs 13.29 days). The mean neutrophil engraftment time was comparable in both groups (10.76 ± 2.40 vs. 10.29 ± 1.72 days). Grade II or above acute GVHD was significantly decreased in the MSC compared with the control group (12% vs. 36%). There were no significant differences in relapse rate or overall survival between the groups.
Conclusion
These results suggest that pre-infusion single-dose MSCs promote platelet engraftment and decrease severe acute GVHD without increasing relapse rate.
Keywords: Mesenchymal stem cell, haploidentical peripheral blood stem cell transplantation, graft versus host disease, platelet engraftment, acute leukemia, myelodysplastic syndrome
Introduction
Hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for malignant and non-malignant hematological diseases. Haploidentical HSCT is an option for patients with no human leukocyte antigen (HLA)-matched siblings or unrelated donors. However, the major obstacle to haploidentical transplantation is the high incidence of graft failure and graft versus host disease (GVHD). Pluripotent mesenchymal stem cells (MSCs) have demonstrated immunomodulatory functions both in vitro and in vivo and could be used to treat steroid-refractory GVHD.1 Liu et al.2 reported that MSCs enhanced platelet recovery without increasing the recurrence of leukemia or influencing the incidence of acute GVHD in haploidentical bone marrow (BM) combined with PBSCT. Gao et al.3 reported that repeated infusion of MSCs inhibited chronic GVHD in HLA-haploidentical HSCT without increasing the relapse rate. However, no randomized controlled studies have been conducted to confirm the impact of pre-infusion single-dose MSCs on engraftment, GVHD, or relapse rate in patients undergoing haploidentical peripheral blood stem cell (PBSC) transplantation (haplo-PBSCT). We therefore conducted a phase II clinical study to assess the efficacy of a pre-infusion single dose of MSCs in patients with acute leukemia or myelodysplastic syndrome (MDS) undergoing haplo-PBSCT.
Subjects and methods
Study design and patients
This was an open-label, randomized phase II clinical study (ChiCTR-INR- 16008399, www.chictr.org.cn) that enrolled patients with acute leukemia or MDS treated at the First Affiliated Hospital of Xi’an Jiaotong University, China, between January 2016 and December 2018. The primary objective of the study was to determine the time to neutrophil and platelet engraftment and the incidence and severity of acute GVHD (Seattle criteria) in patients treated with a single-dose pre-infusion of MSCs prior to haplo-PBSCT. The secondary objectives were to evaluate the relapse rate and overall survival (OS) rate, and the incidence of Epstein–Barr virus (EBV) and cytomegalovirus (CMV) infection. Patients were eligible if they met the following criteria: acute leukemia or MDS with indications for allogeneic stem cell transplantation but with no HLA-matched sibling or unrelated donors; age < 60 years; and absence of uncontrolled infections and severe liver, renal, lung, or heart disease. The study protocol was approved by the First Affiliated Hospital of Xi’an Jiaotong University Ethics Committee (XJTU1AF2016LSL-020). All patients and donors, or their legal guardians, provided written informed consent in accordance with the Declaration of Helsinki.
Patients were divided randomly into an MSC group and a control group. The MSC group was administered a pre-infusion single dose of 1 × 106 MSCs/kg 4 to 6 hours before infusion of PBSCs.
Conditioning regimen
All patients were administered a modified BuCy2+ATG conditioning regimen as follows: cytosine arabinoside (4 g/m2/day intravenously (i.v.) ×2), busulfan (0.8 mg/kg i.v. in 12 doses), cyclophosphamide (1.8 g/m2/day i.v. for 2 days), and antihuman thymocyte immunoglobulin (2.5 mg/kg/day i.v. for 4 days).
Donor stem cell mobilization and collection
Donors were ranked according to the HLA-matched loci (more matching loci preferred), age (younger age preferred), sex (male preferred), and health status (good health status preferred). High-resolution techniques were used to define class I and II HLA antigens. All donors were relatives of the transplant recipients and all donor-specific antibodies were negative (Table 1). PBSCs were mobilized by treatment with granulocyte colony-stimulating factor (G-CSF) (10 µg/kg/day and mononuclear cells were separated using an Optia II system (Terumo BCT, CO, USA).
Table 1.
Patient characteristics.
| MSC group | Control group | |
|---|---|---|
| Sex, male/female | 15/10 | 18/7 |
| Age, years, median (range) | 27 (13–58) | 33 (10–53) |
| Diagnosis of disease | ||
| ALL | 13 | 10 |
| AML | 8 | 15 |
| MDS | 4 | 0 |
| Disease status before HSCT | ||
| CR1 | 22 | 20 |
| CR2 | 1 | 3 |
| NR | 2 | 2 |
| Relatives of donors | ||
| Parent | 8 | 7 |
| Offspring | 2 | 5 |
| Sibling | 15 | 13 |
| HLA-matching locus | ||
| 5/10 | 24 | 19 |
| 6/10 | 1 | 1 |
| 7/10 | 0 | 3 |
| 8/10 | 0 | 2 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; myelodysplastic syndrome; HSCT, hematopoietic stem cell transplantation; CR, complete remission; NR, no remission; HLA, human leukocyte antigen.
Preparation of MSCs
MSCs were obtained from third-party cord sources and were prepared by the Shandong Cord Blood Bank. The criteria for the clinical use of the MSCs were as follows: spindle shape morphology, cell surface expression of CD44, CD106, and CD105, and no expression of CD34, CD45, CD31, and HLA-DR receptors. The cells were thawed approximately 24 hours prior to the scheduled infusion, and examined closely to confirm that they were viable and not contaminated. The target dose for MSC infusion was 1 × 106 cells/kg body weight.
GVHD prophylaxis
Cyclosporine A 3 mg/kg/day was administered from days –10 to 6 months post-transplantation to prevent GVHD. The dose of cyclosporine was adjusted according to the concentration and tapered starting at day +90 and discontinued on day +180 in the absence of GVHD. Methotrexate was administered on days +1 +3, +6, and +11 post-transplantation at 15, 10, 10, and 10 mg/m2, respectively. Oral mycophenolate mofetil was administered at 1 g/day from day −1 to day +30 and tapered to 0.5 g/day from day +31 to day +45.
Engraftment criteria
Myeloid engraftment was defined as the time at which the neutrophil count remained ≥0.5 × 109 cells/L for 3 consecutive days (the first day was used to calculate the engraftment time). The first day that the patients did not have a platelet transfusion and a platelet count of ≥20 × 109 cells/L for 7 consecutive days was defined as the platelet engraftment time.
EBV and CMV infection
CMV and EBV were screened by polymerase chain reaction weekly after neutrophil recovery, bi-weekly until day +100, and monthly thereafter. Acyclovir was administered for CMV prophylaxis or ganciclovir for pre-emptive treatment pre-transplantation. Pre-emptive therapy was started when CMV or EBV antigenemia was detected and then withdrawn after at least two negative tests.
Evaluation parameters and follow-up
The evaluation parameters included recovery time, hematopoiesis, CMV and EBV infection, and the severity of acute and chronic GVHD. The safety parameters included a physical examination, assessment of vital signs, blood biochemical tests, a whole blood count, and electrocardiogram on days 1, 3, 7, and 14 post-infusion. All the patients were followed up until June 2019. Disease relapse rate, progression-free survival (PFS), and OS were also observed.
Statistical analysis
Engraftment times were compare by Mann–Whitney tests and baseline characteristics, GVHD occurrence rate, and relapse rate were compared by χ2 tests. OS was estimated by Kaplan–Meier analysis. The data were analyzed using SPSS for Windows, Version 19.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Patient characteristics
Fifty-four patients were screened at the First Affiliated Hospital of Xi’an Jiaotong University between January 2016 and December 2018. Two refused to participate and two discontinued treatment during the study (Figure 1). Fifty patients therefore participated in this study and were randomized between the MSC and control group (n = 25 each). All patients enrolled were followed up. The characteristics of the patients are listed in Table 1. The two groups were comparable in terms of the sex, age, disease category, and disease status.
Figure 1.
Flowchart of patients. MSC, mesenchymal stem cell.
Hematopoietic recovery
The median number (range) of mononuclear cells infused in the MSCs and control groups were 10.63 (5.95–16.04) × 109 and 10.13 (6.91–14.34) × 108 cells/kg, respectively, and the median number of CD34-positive cells infused were 9.38 (3.18–17.43) × 106 and 9.23 (3.81–27.07) × 109 cells/kg, respectively. G-CSF (10 µg/kg subcutaneously per day) was administered from day +4 after transplantation until the white blood cells were engrafted. The mean times to neutrophil engraftment in the MSC and control groups were 10.76 ± 2.40 and 10.29 ± 1.72 days, respectively. There was no significant difference between the numbers of infused mononuclear or CD34-positive cells or the neutrophil recovery time between the two groups. The mean platelet recovery time was 12.28 ± 2.75 days in the MSC group and 13.29 ± 4.79 days in the control group. There platelet engraftment time was significantly shorter in the MSC group (P < 0.05).
Incidence and severity of GVHD
Grade I acute GVHD occurred in 15 cases in the MSC group, including 13 cases of skin and two cases of gastrointestinal acute GVHD, grade II occurred in three cases, but there were no cases of grade III or above acute GVHD. Grade I acute GVHD occurred in eight cases in the control group, grade II in four cases, and grade III and above in five cases (Figure 2). The overall incidence of acute GVHD was 72% in the MSC group and 68% in the control group, but the difference was not significant. The incidence of severe acute GVHD (grade III or above) was significantly higher in the control group compared with the MSC group (P < 0.05).
Figure 2.
Acute graft versus host disease in the two groups. MSC, mesenchymal stem cell; aGVHD, acute graft versus host disease.
EBV and CMV infection
In this study, all patients and donors were CMV seropositive (CMV-specific immunoglobulin G-positive) before HSCT. No patient developed EBV or CMV viremia pre‐HSCT. Eighteen patients in the MSC group experienced CMV reactivation and developed CMV viremia without CMV disease post-HSCT and recovered after the administration of antiviral drugs. One patient in the MSC group had EBV viremia. Twenty-three patients in the control group experienced CMV reactivation and none had CMV disease; all recovered after the administration of antiviral treatment. Four patients in the control group developed EBV viremia. One patient in the control group developed EBV‐associated post‐transplantation lymphoproliferative disorder, failed to achieve complete remission after rituximab therapy, and subsequently died.
Relapse and survival rates
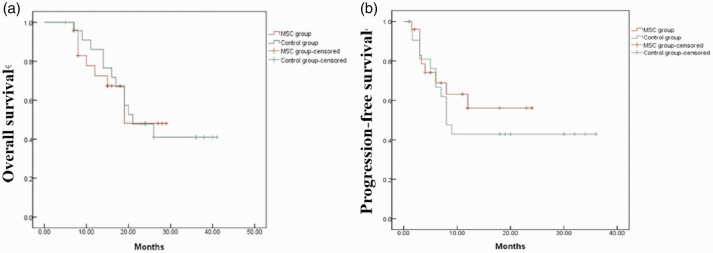
All patients were followed-up until June 2019. Seven patients in the control group and nine patients in the MSC group experience molecular or hematological relapse. Four patients in the MSC group died as a result of relapse. Twelve patients in the control group died, including eight due to disease relapse and three due to severe acute GVHD. The 1- and 2-year OS rates in the MSC group were 67.4% and 48.1%, respectively and the equivalent rates in the control group were 52.6% and 41%, respectively.
The 1- and 2-year PFS rates in the MSC group were 68.9% and 56.1%, respectively, and the equivalent rates in the control group were 42.9% and 42.9%, respectively (Figure 3). The relapse rate was 36% in the control group and 28% in the MSC group. There was no significant difference in OS, PFS, or relapse rate between the two groups.
Figure 3.
Overall (a) and progression-free survival (b) in the two groups. MSC, mesenchymal stem cell.
Safety
We monitored the safety profiles of the patients throughout the course of the study. None of the enrolled patients developed fever, hypotension, hypertension, pulmonary embolism, or other serious complications directly related to MSC infusion. No electrocardiogram abnormalities were observed either before or after cell infusion, and there were no significant changes in biochemical parameters from pre- to post-infusion. No patients have presented with any side effects related to MSC infusion to date.
Discussion
Allogeneic HSCT is a potentially curative treatment for patients with hematological malignancies and non-malignant diseases. HLA-haploidentical HSCT (haplo-HSCT) can be used for patients who lack HLA-matched donors and/or are in urgent need of transplantation. However, haplo-HSCT has been associated with high incidences of GVHD, graft rejection, and infection.4–6 GVHD is the most common complication of haplo-HSCT and numerous strategies have been used to try and prevention it, including T cell depletion and the use of immunosuppressive drugs or BM grafts. The higher content of T cells in PBSC compared with BM grafts means that the incidence of GVHD is higher in PBSC than in BM or BM combined with PBSC transplantation, while PBSC transplantation is associated with shorter neutrophil and platelet recovery times.7 The pain and risks involved in donor BM collection mean that individuals are increasingly willing to undergo PBST transplantation rather than BM transplantation, and reducing GVHD thus presents an urgent problem.
MSCs have demonstrated immunomodulatory effects in vitro and in vivo.8,9 MSCs can regulate T and B lymphocyte proliferation, activation, and maturation to induce regulatory T lymphocyte formation. Improved engraftment of hematopoietic stem cells (HSCs) and decreased risk of GVHD after co-transplantation of ex vivo-expanded human MSCs and HSCs were shown in preclinical animal studies.9 MSCs have also been used to treat steroid-refractory acute GVHD; however, the use of MSCs to prevent GVHD remains a topic of debate.10–12
Tisato et al.13 reported that the systemic administration of umbilical cord blood (UCB)-MSCs markedly reduced human T-cell proliferation and associated tissue damage, and significantly improved survival in preclinical studies; however, the effect could only be achieved if UCB-MSCs were administered in multiple doses. Liu et al.3 previously reported that the pre-infusion of MSCs obtained from the BM of a healthy donor (3–5 × 105/kg) within 24 hours before infusion of HSCs enhanced platelet recovery without increasing the recurrence of leukemia in haploidentical BM combined with PBSCT but had no impact on the incidence of acute GVHD. However, Liu et al.14 reported that co-transplantation of MSCs from a healthy donor (3.2–4.1 × 106/kg) 6 hours before stem cell transfusion followed by a second dose at day +14 could reduce the risk of graft failure and severe acute and chronic GVHD in haplo-HSCT for severe aplastic anemia, but was associated with a relatively higher incidence of EBV (31.8%) and CMV (65.9%) infection. Kuzmina et al.15 reported that i.v. injection of 1 × 106/kg MSCs at the precise moment of blood cell reconstitution induced a significant three-fold decrease in acute GVHD and improved OS compared with the standard-prophylaxis group. These findings indicate that the time and dose of MSC infusion are important in determining their preventive effect on GVHD. Gao et al.4 reported that the repeated infusion of MSCs inhibited chronic GVHD in HLA-haploidentical HSCT without increasing the relapse rates. In contrast, the current study, a pre-infusion single dose of MSCs (1 × 106/kg) 4 to 6 hours before infusion of PBSCs decreased severe acute GVHD, which was inconsistent with Liu et al.’s report. This apparent discrepancy may due to the dose of MSCs being higher and being infused 4 to 6 hours before infusion of HSCs in our study, leading to a greater benefit of MSCs in terms of the prevention of GVHD. This also suggests that high-dose or multiple infusions may improve the effects of MSCs. The timing of MSC infusion is also important, and infusion before stem cell transfusion and precisely before hematopoietic recovery may reduce the risk of severe GVHD.
Whether co-infusion of MSCs increases the recurrence of leukemia and incidence of infection remains controversial. Schmidt et al.16 and Zheng et al.17 found that MSCs could phagocytize Aspergillus spores without affecting phagocytosis in vitro, and could even have an antiseptic effect. Dotoli et al.18 reported on the use of MSCs to treat three patients with steroid-resistant acute GVHD, of whom one died of leukemia relapse and one of viral encephalitis. Jurado et al.19 used MSCs to treat chronic GVHD in two patients with severe bacterial infection and one with a viral infection, none of whom relapsed. Peng et al.20 reported that 20 patients received MSCs for the treatment of chronic GVHD, among whom three died of leukemia relapse and two of pulmonary fungal infection. Kang et al.21 found no significant difference in disease relapse rates between children with hematologic malignancies treated with MSCs and the control group; however, CMV reactivation was significantly increased in the MSC group. However, Gao et al.4 reported relapse rates in the MSC and control groups of 29.0% and 30.6%, respectively, with mortality rates due to infection of 4.8% and 8.1%, respectively, with no significant differences in terms of relapse or infection between the two groups. In the current study, the recurrence rate and incidence of infection did not increase after a single pre-infusion. Considering that the activity of MSCs in vivo lasts 2 weeks, the immune suppression and regulation time of a single infusion was shorter, with no significant effect on long-term disease recurrence or infection.
This study had several limitations. First, we did not monitor changes in immune cell subsets or cytokine levels after a single infusion of MSCs. In addition, the number of cases was small, and further studies with larger patient cohorts are needed to verify these results. Nevertheless, this study showed that a single pre-infusion dose of MSCs could promote platelet engraftment and decrease severe acute GVHD without relapse in patients undergoing haploidentical peripheral blood stem cell transplantation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the NSFC (grant no. 81600179) the Natural Science Foundation of Shaan Xi Province (grant no. 2019–JM564) and the clinical research project of first affiliated hospital of xi''an jiaotong university (XJTU1AF-CRF-2015-014).
ORCID iD
Xiaoning Wang https://orcid.org/0000-0002-2472-4076
References
- 1.Resnick IB, Barkats C, Shapira MY, et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res 2013; 3: 225–238. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K, Chen Y, Zeng Y, et al. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev 2011; 20: 1679–1685. [DOI] [PubMed] [Google Scholar]
- 3.Gao L, Zhang Y, Hu B, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol 2016; 34: 2843–2850. [DOI] [PubMed] [Google Scholar]
- 4.Khan MA, Bashir Q, Chaudhry QU, et al. Review of haploidentical hematopoietic cell transplantation. J Glob Oncol 2018; 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 2014; 371: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atilla E, Atilla PA, Bozdağ SC, et al. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 2017; 45: 403–411. [DOI] [PubMed] [Google Scholar]
- 7.Chang YJ, Zhao XY, Huang XJ. Strategies for enhancing and preserving anti-leukemia effects without aggravating graft-versus-host disease. Front Immunol 2018; 9: 3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Luca L, Trino S, Laurenzana I, et al. Mesenchymal stem cell derived extracellular vesicles: a role in hematopoietic transplantation? Int J Mol Sci 2017; 18: 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grégoire C, Ritacco C, Hannon M, et al. Comparison of mesenchymal stromal cells from different origins for the treatment of graft-vs.-host-disease in a humanized mouse model. Front Immunol 2019; 10: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallekleiv M, Larun L, Bruserud Ø, et al. Co-transplantation of multipotent mesenchymal stromal cells in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Cytotherapy 2016; 18: 172–185. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Wang C, Yin J, et al. Efficacy of mesenchymal stem cell therapy for steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. PLoS One 2015; 10: e0136991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii S, Miura Y, Fujishiro A, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells 2018; 36: 434–445. [DOI] [PubMed] [Google Scholar]
- 13.Tisato V, Naresh K, Girdlestone J, et al. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia 2007, 21: 1992–1999. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Zhang Y, Xiao H, et al. Cotransplantation of bone marrow derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone Marrow Transplant 2017; 52: 704–710. [DOI] [PubMed] [Google Scholar]
- 15.Kuzmina LA, Petinati NA, Shipounova IN, et al. Analysis of multipotent mesenchymal stromal cells used for acute graft-versus host disease prophylaxis. Eur J Haematol 2016; 96: 425–434. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt S, Tramsen L, Schneider A, et al. Impact of human mesenchymal stromal cells on antifungal host response against Aspergillus fumigatus. Oncotarget 2017; 8: 95495–95503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng G, Huang R, Qiu G, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res 2018; 374: 1–15. [DOI] [PubMed] [Google Scholar]
- 18.Stoma I, Karpov I, Krivenko S, et al. Mesenchymal stem cells transplantation in hematological patients with acute graft-versus host disease: characteristics and risk factors for infectious complications. Ann Hematol 2018; 97: 885–891. [DOI] [PubMed] [Google Scholar]
- 19.Jurado M, De La Mata C, Ruiz-García A, et al. Adipose tissue derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: a phase I/II study. Cytotherapy 2017; 19: 927–936. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y, Chen X, Liu Q, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 2015; 29: 636–646. [DOI] [PubMed] [Google Scholar]
- 21.Kang HZ, Zheng XL, Wang ZD, et al. Efficacy and safety of co-transplantation of haploidentical-SC with umbilical cord mesenchymal stem cell in children with hematologic malignancies. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017; 25: 1151–1157. [DOI] [PubMed] [Google Scholar]