Short abstract
Objective
To measure the effect of febuxostat on the serum levels of uric acid (sUA) and the proinflammatory cytokines interleukin (IL)-6, IL-17 and tumour necrosis factor-α (TNF-α) in Chinese Han patients with gout and hyperuricaemia.
Methods
This randomized, double-blind, placebo-controlled pilot study enrolled patients with gout and hyperuricaemia (sUA ≥ 8 mg/dl). Patients were randomized to receive either febuxostat 80 mg or placebo once daily for 24 weeks. The serum levels of sUA, IL-6, IL-17 and TNF-α were measured at weeks 0 (baseline), 2, 4, 8, 12, 16 and 24. Baseline clinical and demographic characteristics were recorded for all patients.
Results
A total of 156 patients were randomized: placebo group (n = 78) and febuxostat group (n = 78). The febuxostat group showed a significantly greater reduction in sUA compared with the placebo group. Serum uric acid concentration was reduced below 8 mg/dl in 46 of 61 patients (75.4%) by week 24. There were also reductions in the serum levels IL-6, IL-17 and TNF-α in the febuxostat group. In the febuxostat group, 10 of 78 patients (12.82%) discontinued treatment due to adverse drug reactions.
Conclusion
Febuxostat reduced the levels of sUA, TNF-α, IL-6 and IL-17, but there were some side-effects.
Keywords: Febuxostat, serum urate, hyperuricaemia, gout
Introduction
Gout is an inflammatory arthritis characterized by the deposition of monosodium urate crystals in the joints and other connective tissues, secondary to long-standing hyperuricaemia.1 To alleviate severe gout symptoms, the concentration of serum uric acid (sUA) must be reduced.2 Successful management of gout requires a sustained reduction in the concentration of sUA below a target of < 6.0 mg/dl, which leads over time to the dissolution of the urate crystals and alleviation of the gout symptoms.3
The majority of patients with gout are treated with the xanthine oxidase inhibitors allopurinol and febuxostat,4 which reduce the production of urate; and the uricosuric drugs probenecid, benzbromarone, sulfinpyrazone and lesinurad, which increase the excretion of sUA by inhibiting its reabsorption, to achieve a sustained reduction in sUA.5
The recommended dosage of febuxostat in Europe is 80 mg once daily.6 Its pharmacokinetics are not significantly altered in patients with moderate renal function or hepatic impairment.7 Almost all regular treatments administered to gout patients show side-effects and limitations.8 Some concerns about the safety of febuxostat have been expressed9,10 and the effects of febuxostat on inflammatory mediators are poorly understood.
The rate of gout has recently increased sharply in China.11 The aim of this randomized, double-blind, placebo-controlled pilot study was to observe the effect and tolerability of febuxostat in Chinese Han adults with gout and hyperuricaemia.
Patients and methods
Study design and patient population
This randomized, double-blind, placebo-controlled pilot study enrolled Chinese Han patients with gout and hyperuricaemia (a screening sUA ≥ 8 mg/dl) in the Department of Rheumatology, First Hospital of Jilin University, Changchun, Jilin Province, China between June 2014 and September 2015. Gout was diagnosed by the treating physician from the patient’s history and available laboratory data. The inclusion criteria were as follows: (i) observation follow-up of at least 24 weeks; (ii) age between 18 and 70 years. The exclusion criteria were as follows: (i) patients with a history of other autoimmune diseases; (ii) patients with nephropathy; (iii) patients with cancer; (iv) patients with haematopathy.
This study was conducted and approved by the Ethics Committee of the First Hospital of Jilin University (no. 2015-267) according to the ethical guidelines of the 1975 Declaration of Helsinki. All study participants provided written informed consent.
Randomization and treatment
The patients were randomized in a 1:1 ratio by a computer-generated randomization schedule to receive a single treatment of either 80 mg febuxostat dissolved in 200 ml water or placebo dissolved in 200 ml water once daily for 24 weeks. All study drugs were administered orally once daily in the morning after breakfast in the hospital. Patients were monitored for safety throughout the study by researchers. The treatment efficacy was compared between the placebo and febuxostat groups. In the febuxostat group, based on the sUA level at week 24, the patients were separated into three groups (incomplete treatment, effective treatment and ineffective treatment).
Blood sampling
Venous blood samples were collected after an overnight fast of at least 10 h and serum was separated after centrifugation at 1200 g force for 5 mins at 4°C in a Thermo Scientific™ MicroCL 17R Microcentrifuge (Thermo Fisher Scientific Inc., Rockford, IL, USA). The serum was stored at −80°C until analysis. Demographic and laboratory parameters, as well as medical history, were carefully recorded by experienced endocrinologists. The data included levels of sUA, fasting blood glucose, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), heart rate, respiration rate, blood pressure and body mass index (BMI).
Determination of serum cytokine levels
Using the serum samples described above, the serum levels of cytokines interleukin (IL)-6, IL-17 and tumour necrosis factor-α (TNF-α) were measured during the treatment at weeks 0 (baseline), 2, 4, 8, 12, 16 and 24. The serum cytokine levels were measured using commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). The minimum detectable concentrations were 2 pg/ml for IL-6, 1.6 pg/ml for IL-17 and 0.31 pg/ml for TNF-α. Intra- and interassay coefficients of variation for all ELISAs were < 9.8% and < 9.1%, respectively. Optical densities were calculated using a hybrid multi-mode microplate reader (BioTek ELx808 Absorbance Reader; BioTek, Winooski, VT, USA) with Gen5 Microplate Reader and Imager Software (BioTek). The standard curve was drawn by Curve Expert version 1.4 software (Hyams Development, Chattanooga, TN, USA).
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Data are presented as mean ± SD and n of patients (%). Differences between the two groups were compared using Student’s t-test. Correlations between variables were calculated by the least-squares method and are expressed as Pearson coefficient of variation. A P-value < 0.05 was considered statistically significant.
Results
This randomized, double-blind, placebo-controlled pilot study enrolled 156 Han Chinese patients that were randomized to one of two treatment groups: the febuxostat group (n = 78) or the placebo group (n = 78). Their baseline demographic and clinical characteristics are presented in Table 1. The mean ± SD ages of the febuxostat and placebo treatment groups were not significantly different (42.83 ± 11.65 and 43.33 ± 10.17 years, respectively). There were no significant differences between the two groups in terms of systolic blood pressure, diastolic blood pressure, sUA levels, TG levels, LDL-C levels, BMI, fasting blood glucose, heart rate and respiration rate.
Table 1.
Baseline demographic and clinical data of patients (n = 156) with gout and hyperuricaemia treated with either 80 mg febuxostat or placebo once daily for 24 weeks.
| Characteristic | Febuxostat group n = 78 |
Placebo group n = 78 |
|---|---|---|
| Age, years | 42.83 ± 11.65 | 43.33 ± 10.17 |
| Body mass index, kg/m2 | 26.5 ± 3.5 | 25.51 ± 2.77 |
| Serum uric acid, µmol/l | 593.43 ± 90.65 | 595.67 ± 87.64 |
| Systolic blood pressure, mmHg | 125.26 ± 4.51 | 129.25 ± 12.59 |
| Diastolic blood pressure, mmHg | 80.66 ± 5.34 | 76.08 ± 10.87 |
| Fasting blood glucose, mmol/l | 5.19 ± 0.54 | 4.94 ± 0.46 |
| Triglyceride, mmol/l | 2.65 ± 1.65 | 2.43 ± 1.63 |
| Low-density lipoprotein-cholesterol, mmol/l | 2.96 ± 0.72 | 3.11 ± 0.59 |
| Heart rate, beats/min | 75.89 ± 10.56 | 71.12 ± 5.15 |
| Respiration rate, breaths/min | 17.07 ± 0.65 | 16.75 ± 0.45 |
Data presented as mean ± SD.
No significant between-group differences (P ≥ 0.05); Student’s t-test.
The patients in the febuxostat group received 80 mg febuxostat once daily for 24 weeks. The target sUA level (6.7 mg/dl) was achieved at study end in 55.7% of patients (34 of 61 patients) that completed the entire treatment. After 24 weeks of treatment, 75.4% (46 of 61 patients) achieved an sUA level of 8 mg/dl, 41.0% (25 of 61 patients) achieved an sUA level of 6 mg/dl and 19.7% (12 of 61 patients) achieved an sUA level of 5 mg/dl (Table 2). The mean concentration of sUA had decreased by 30.1% after 24 weeks of treatment. In the placebo group, the sUA level did not decrease during treatment. The drug efficacy analysis showed significant differences between febuxostat and placebo (P < 0.01).
Table 2.
The efficacy of febuxostat treatment for reducing elevated serum uric acid (sUA) in patients (n = 78) with gout and hyperuricaemia treated with 80 mg febuxostat once daily for 24 weeks compared with the placebo group (n = 78).
| Treatment | Duration, weeks |
n | Mean decrease of sUA,a % |
Proportion of patients to achieve this level of sUA |
||
|---|---|---|---|---|---|---|
| sUA 8 mg/dl n (%) |
sUA 6 mg/dl n (%) |
sUA 5 mg/dl n (%) |
||||
| Febuxostat80 mg | 2 | 76 | 37.9 | 71 (93.4) | 33 (43.4) | 14 (18.4) |
| 4 | 69 | 37.6 | 60 (87.0) | 35 (50.7) | 17 (24.6) | |
| 8 | 68 | 33.2 | 52 (76.5) | 22 (32.4) | 9 (13.2) | |
| 12 | 68 | 30.1 | 52 (76.5) | 27 (39.7) | 12 (17.6) | |
| 16 | 64 | 32.5 | 48 (75.0) | 25 (39.1) | 12 (18.8) | |
| 20 | 64 | 33.4 | 49 (76.6) | 25 (39.1) | 14 (21.9) | |
| 24 | 61 | 30.1 | 46 (75.4) | 25 (41.0) | 12 (19.7) | |
| Placebo | 2 | 77 | 0.7 | 2 (2.6) | 0 (0.0) | 0 (0.0) |
| 4 | 76 | 0.3 | 2 (2.6) | 0 (0.0) | 0 (0.0) | |
| 8 | 74 | –2.6 | 1 (1.4) | 0 (0.0) | 0 (0.0) | |
| 12 | 72 | –0.4 | 1 (1.4) | 0 (0.0) | 0 (0.0) | |
| 16 | 71 | 1.1 | 1 (1.4) | 0 (0.0) | 0 (0.0) | |
| 20 | 68 | –3.2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 24 | 68 | –4.5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
aA negative sign indicates a mean increase in sUA.
In the febuxostat group, 17 of 78 patients (21.79%) did not complete the treatment course. Of these, 10 of 78 patients (12.82%) discontinued treatment due to adverse drug reactions. The most common adverse drug reaction in the febuxostat group was liver function abnormalities (28 of 78 patients; 35.90%) (Table 3). A smaller proportion of patients experienced leukocytosis (four of 78 patients; 5.13%), polycythaemia (two of 78 patients; 2.56%), diarrhoea (one of 78 patients; 1.28%), headache (one of 78 patients; 1.28%), nausea (one of 78 patients; 1.28%), rash (one of 78 patients; 1.28%), sleep disorder (one of 78 patients; 1.28%) and thrombocytopaenia (one of 78 patients; 1.28%) (Table 3). In the placebo group, 10 of 78 patients (12.82%) did not complete the full treatment period due to personal wishes, although none of the control patients discontinued treatment due to adverse drug reactions.
Table 3.
The tolerability of febuxostat treatment in patients (n = 78) with gout and hyperuricaemia treated with 80 mg febuxostat once daily for 24 weeks.
| n | Patients that did not complete treatment | Patients that had effectivetreatmenta | Patients that had ineffectivetreatmenta | |
|---|---|---|---|---|
| Number of patients | 78 | 17 (21.79) | 34 (43.59) | 27 (34.62) |
| Side-effects | 38 (48.72) | 11 (14.10) | 14 (17.95) | 13 (16.67) |
| Liver dysfunction | 28 (35.90) | 10 (12.82) | 10 (12.82) | 8 (10.26) |
| Leukocytosis | 4 (5.13) | 2 (2.56) | 1 (1.28) | 1 (1.28) |
| Polycythaemia | 2 (2.56) | 1 (1.28) | 1 (1.28) | |
| Diarrhoea | 1 (1.28) | 1 (1.28) | ||
| Headache | 1 (1.28) | 1 (1.28) | ||
| Nausea | 1 (1.28) | 1 (1.28) | ||
| Rash | 1 (1.28) | 1 (1.28) | ||
| Sleep disorder | 1 (1.28) | 1 (1.28) | ||
| Thrombocytopaenia | 1 (1.28) | 1 (1.28) |
Data presented as n of patients (%).
aIf the patients completed the treatment period and the target sUA level (6.7 mg/dl) was achieved at study end then the febuxostat treatment was considered to have been effective (ET); if the sUA target was not achieved, then it was considered ineffective treatment (IET).
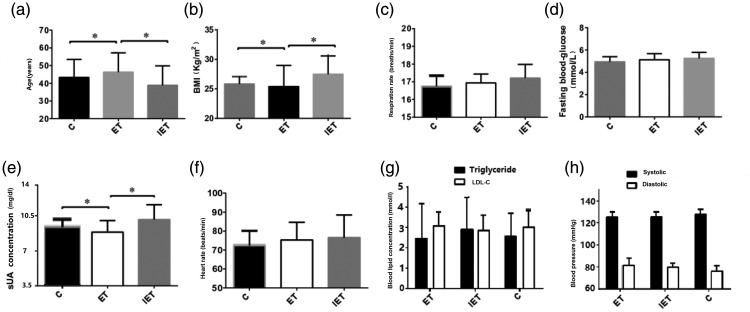
According to the completion of treatment and sUA levels, the patients treated with febuxostat were categorized into three groups: incomplete treatment, effective treatment (ET) and ineffective treatment (IET). The mean ± SD age of the IET group (38.89 ± 10.98 years) was significantly lower than that of the ET group (46.29 ± 10.89 years) (P < 0.05) (Figure 1). The mean ± SD BMI of the IET group (27.49 ± 3.07 kg/m2) was significantly higher than that of the ET group (25.40 ±3.58 kg/m2) (P < 0.05). There were no significant differences between the ET and IET groups in terms of fasting blood glucose, TG, LDL-C, systolic blood pressure, diastolic blood pressure, heart rate and respiration rate.
Figure 1.
Comparison of baseline clinical and demographic characteristics between patients (n = 78) with gout and hyperuricaemia treated with 80 mg febuxostat once daily for 24 weeks categorized at the end of treatment based on whether they experienced effective treatment (ET) or ineffective treatment (IET). These two groups of febuxostat-treated patients were also compared with the control group (c) (n = 78) that received placebo once daily for 24 weeks. (a) Age; (b) body mass index (BMI); (c) respiration rate; (d) fasting blood glucose; (e) serum uric acid (sUA); (f) heart rate; (g) triglyceride and low-density lipoprotein cholesterol (LDL-C); (h) systolic and diastolic blood pressure. Data presented as mean ± SD. *P < 0.05; Student’s t-test.
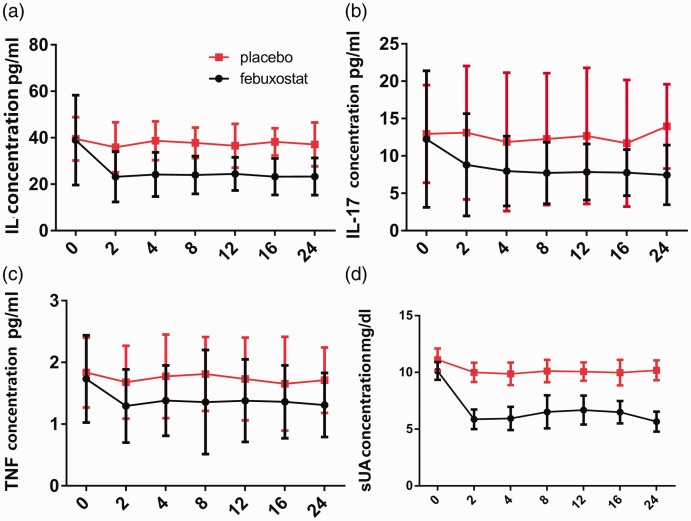
The serum levels of IL-6, IL-17 and TNF-α were measured during febuxostat treatment at weeks 0 (baseline), 2, 4, 8, 12, 16 and 24 (Figure 2). In the febuxostat group, the serum levels of IL-6, IL-17 and TNF-α were decreased by 38.25%, 39.86% and 21.91%, respectively, after 8 weeks of treatment (Table 4). The serum levels of IL-6, IL-17 and TNF-α remained lower than the placebo group from 8 to 24 weeks. The decrease in the serum levels of IL-6, IL-17 and TNF-α correlated with the decrease of sUA (Pearson correlation coefficient r > 0.8). There were minor changes in the serum levels of IL-6, IL-17 and TNF-α observed in the placebo group.
Figure 2.
The serum levels of cytokines interleukin (IL)-6, IL-17 and tumour necrosis factor-α (TNF-α) and serum uric acid (sUA) were measured in patients (n = 78) during febuxostat treatment at weeks 0 (baseline), 2, 4, 8, 12, 16 and 24 and compared with the placebo group (n = 78).
Table 4.
The effect of febuxostat on the mean decrease of cytokine levels in patients (n = 78) with gout and hyperuricaemia treated with 80 mg febuxostat once daily for 24 weeks.
| Cytokine | Group | Baseline level, pg/ml |
Week 2, pg/ml |
Percentage change from baseline to week 2 |
Week 4, pg/ml |
Percentage change from baseline to week 4 |
Week 8, pg/ml |
Percentage change from baseline to week 8 (%) |
|---|---|---|---|---|---|---|---|---|
| Interleukin-6 | Control | 39.57 | 36.23 | –8.44 | 40.07 | +1.26 | 39.44 | –0.33 |
| Febuxostat | 38.98 | 23.17 | –40.56 | 24.21 | –37.89 | 24.07 | –38.25 | |
| Interleukin-17 | Control | 12.66 | 13.17 | +4.03 | 12.23 | –3.40 | 12.46 | –1.58 |
| Febuxostat | 12.52 | 7.43 | –40.65 | 7.53 | –39.86 | 7.53 | –39.86 | |
| Tumour necrosis factor-α |
Control | 1.83 | 1.71 | –6.56 | 1.79 | –2.19 | 1.88 | +2.73 |
| Febuxostat | 1.78 | 1.33 | –25.28 | 1.42 | –20.22 | 1.39 | –21.91 |
Discussion
This current randomized, double-blind, placebo-controlled pilot study evaluated the effect of 80 mg febuxostat once daily for the treatment of gout and hyperuricaemia and the results demonstrated that sUA was reduced by 37.9% within 2 weeks of starting treatment.
Patients with gout usually have higher blood pressure12 and TG and LDL-C levels than a healthy individual.13–17 After 24 weeks of treatment in the current study, the mean sUA had decreased by 30.1%, which demonstrated that the efficacy was better than that shown in a previous study.18 The possible reasons for this disparity might be the small sample size and the fact that all of the patients were from the Chinese Han population in the current study.
Elderly patients with gout have distinct clinical features when compared with middle-aged patients.19 This current study demonstrated that age and BMI appeared to be associated with the effectiveness of the febuxostat treatment in terms of reducing sUA to the target level. Patients in the IET group had a higher mean BMI and a lower mean age compared with the ET group.
Neutrophils and macrophages are the major inflammatory cells involved in the pathological processes leading to gout.20 Proinflammatory cytokines and chemokines such as IL-6 and TNF-α can lead to a gouty inflammatory cascade.21 IL-17 is also an important proinflammatory cytokine, which is involved in the regulation of gouty inflammation.22 In patients with gout, the levels of proinflammatory cytokines increase during the development of gout and lead to exacerbation of the adverse symptoms of gout.23 The current results demonstrated a reduction in the levels of TNF-α, IL-6 and IL-17 over the 24-week course of febuxostat treatment in line with the reductions seen in sUA. The mechanisms remain unclear and require further study.
This current study had a number of limitations. First, the study only involved a small group of patients. Secondly, the study duration was short. Thirdly, there was only one dosage of febuxostat treatment used. Finally, although there were some side-effects reported, the main focus of the study was the therapeutic effects of febuxostat. This present pilot study has provided the basis for further research into the therapeutic potential of febuxostat in patients with hyperuricaemia and gout.
In conclusion, 80 mg febuxostat once daily for 24 weeks reduced both the levels of sUA and the levels of the proinflammatory cytokines TNF-α, IL-6, and IL-17 in Han Chinese patients with gout and hyperuricaemia, with some patients experiencing side-effects. Further studies with a larger sample size, as well as investigations into the underlying mechanisms of febuxostat action, are required in the future.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by funding from The National Natural Science Foundation of China (no. 81501343), The Bethune Plan Project of Jilin University (no. 2015410) and The Jilin Scientific and Technological Development Programme (no. 20170520010JH; no. 20150101152JC).
ORCID iD
Ling Zhao https://orcid.org/0000-0002-1180-451X
References
- 1.Borghi C. Gout and CV disease: it is time to move on. Int J Cardiol 2018; 270: 311. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya T, Sasaki T, Ohashi T. Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: a randomized, double-blinded, controlled phase 2b study. Clin Rheumatol 2017; 36: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Z, Yeh LT, Wallach K, et al. In Vitro and In Vivo Interaction Studies Between Lesinurad, a Selective Urate Reabsorption Inhibitor, and Major Liver or Kidney Transporters. Clin Drug Investig 2016; 36: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghi C, Perez-Ruiz F. Urate lowering therapies in the treatment of gout: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 2016; 20: 983–992. [PubMed] [Google Scholar]
- 5.Robinson PC, Dalbeth N. Febuxostat for the treatment of hyperuricaemia in gout. Expert Opin Pharmacother 2018; 19: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 6.Beard SM, von Scheele BG, Nuki G, et al. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ 2014; 15: 453–463. [DOI] [PubMed] [Google Scholar]
- 7.Reinders MK, Jansen TL. Management of hyperuricemia in gout: focus on febuxostat. Clin Interv Aging 2010; 5: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker RJ, Mark PB, Patel RK, et al. Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol 2017; 18: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Liu K, Sun Q, et al. Efficacy and safety of febuxostat for treating hyperuricemia in patients with chronic kidney disease and in renal transplant recipients: a systematic review and meta-analysis. Exp Ther Med 2018; 16: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tojimbara T, Nakajima I, Yashima J, et al. Efficacy and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in kidney transplant recipients. Transplant Proc 2014; 46: 511–513. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Yan S, Li C, et al. Risk factors for gout developed from hyperuricemia in China: a five-year prospective cohort study. Rheumatol Int 2013; 33: 705–710. [DOI] [PubMed] [Google Scholar]
- 12.Dang WT, Xie WG, Zhou JG. Expression of PYCARD gene transcript variant mRNA in peripheral blood mononuclear cells of primary gout patients with different Chinese medicine syndromes. Chin J Integr Med 2018; 24: 24–31. [DOI] [PubMed] [Google Scholar]
- 13.Teng GG, Leung YY, Ang LW, et al. Gout and risk of knee replacement for severe knee osteoarthritis in the Singapore Chinese Health Study. Osteoarthritis Cartilage 2017; 25: 1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagayama D, Yamaguchi T, Saiki A, et al. High serum uric acid is associated with increased cardio-ankle vascular index (CAVI) in healthy Japanese subjects: a cross-sectional study. Atherosclerosis 2015; 239: 163–168. [DOI] [PubMed] [Google Scholar]
- 15.Leiba A, Vinker S, Dinour D, et al. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. J Am Soc Hypertens 2015; 9: 600–609. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi Z, Moriwaki Y, Takahashi S, et al. Oxidized low-density lipoprotein autoantibodies in patients with primary gout: effect of urate-lowering therapy. Clin Chim Acta 2004; 339: 117–122. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi Z, Yamamoto T, Moriwaki Y, et al. Decreased activities of lipoprotein lipase and hepatic triglyceride lipase in patients with gout. Metabolism 2001; 50: 952–954. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuboshi S, Yamada H, Nagai K, et al. Switching from allopurinol to febuxostat: efficacy and tolerability in hemodialysis patients. J Pharm Health Care Sci 2015; 1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascart T, Lancrenon S, Lanz S, et al. GOSPEL 2 – Colchicine for the treatment of gout flares in France – a GOSPEL survey subgroup analysis. Doses used in common practices regardless of renal impairment and age. Joint Bone Spine 2016; 83: 687–693. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Chen R, Zhang X, et al. Role of immune microenvironmental factors for improving the IPI-related risk stratification of aggressive B cell lymphoma. Biomed Environ Sci 2017; 30: 492–500. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Wang Q, Deng W, et al. Anti-hyperuricemic and anti-gouty arthritis activities of polysaccharide purified from Lonicera japonica in model rats. Int J Biol Macromol 2019; 123: 801–809. [DOI] [PubMed] [Google Scholar]
- 22.Raucci F, Iqbal AJ, Saviano A, et al. IL-17A neutralizing antibody regulates monosodium urate crystal-induced gouty inflammation. Pharmacol Res 2019; 147: 104351. [DOI] [PubMed] [Google Scholar]
- 23.Punzi L, Scanu A, Ramonda R, et al. Gout as autoinflammatory disease: new mechanisms for more appropriated treatment targets. Autoimmun Rev 2012; 12: 66–71. [DOI] [PubMed] [Google Scholar]