Abstract
The microglia are the resident immune cells in the central nerve system. In the various pathological conditions, prolonged activated microglia could deteriorate brain damage. The regulation of the microglia polarization should be considered in developing an intervention for ischemic stroke patients. Normobaric intermittent hypoxic training protects the brain from intensive ischemic stresses. This study examined the role of intermittent hypoxic training in the regulation of microglia polarization that occurs in the in vitro model of oxygen–glucose deprivation (OGD)–reoxygenation. EOC20 were assigned to the following groups; (1) Normoxia, (2) oxygen–glucose deprivation–reoxygenation, (3) intermittent hypoxic training, (4) oxygen–glucose deprivation–reoxygenation + intermittent hypoxic training; 24 h after the intermittent hypoxic training, microglia were harvested to perform the following experiments; cell viability (Calcein AM and LDH activity assay), quantification of proteins (Western blot), cytokine (ELISA), and reactive oxygen species (ROS) (H2DCFDA assays), phagocytic activity by using latex beads coated with FITC, and cell phenotype (immunocytochemistry and flow cytometric analysis, and immunoblot CD206 (M2)). One-way ANOVA with Tukey’s post hoc test was used for the statistical analysis. Oxygen–glucose deprivation/reoxygenation decreases cell viability to 50% of normoxia. Intermittent hypoxic training protects the microglia from oxygen–glucose deprivation/reoxygenation stress. Intermittent hypoxic training regulates the polarization of the microglial phenotype toward anti-inflammatory type M2 (vs. oxygen–glucose deprivation and reoxygenation). Intermittent hypoxic training increases phagocytic activity (about 12 folds) vs. normoxia. ROS in the oxygen–glucose deprivation/reoxygenation group is increased, but intermittent hypoxic training lowers the ROS generation by oxygen–glucose deprivation/reoxygenation. The protein content of the toll-like receptor (TLR2) was significantly elevated in the oxygen–glucose deprivation and reoxygenation group, and intermittent hypoxic training lowered to normoxia level. Anti-inflammatory cytokines, such as IL-10 and IL-4, were significantly increased in the intermittent hypoxic training groups. Due to the effect of intermittent hypoxic training on the microglia phenotype, intermittent hypoxic training could be considered as an effective intervention in the treatment or rehabilitation program for the ischemic stroke victims.
Impact statement
The effects of intermittent hypoxic training or conditioning on many pathological conditions have been widely investigated. One of the pathological conditions dealt with intermittent hypoxic training is ischemic stroke. Well-known mechanisms of intermittent hypoxia-induced protection are related to increased energy metabolism and the enhanced antioxidant effects. In the last decades, the role of microglia in the progress of ischemic stroke-related brain damage has been focused. The dual-edge function of microglia indicates that the microglia-mediated inflammatory response is definitely beneficial in the early stage of ischemic stroke, but long-term activation of microglia is rather detrimental during the recovery process. The effect of IHT on microglia polarization is not investigated. This study focused on whether IHT regulates the polarization of microglia without dampening its classic phagocytic function. This study will provide pivotal information regarding the effects of IHT on the long-term effects on the recovery process from ischemic stroke.
Keywords: Microglia, ischemic stroke, intermittent hypoxia training, inflammation, phagocytosis, oxygen–glucose deprivation
Introduction
Stroke is the fifth leading cause of death and is a major cause of long-term disability in the United States.1,2 On average, someone in the US is experiencing symptoms of a stroke every 40 s and this approximates the number of people affected by stroke to 795,000 (new or recurrent) each year.3 Data show that by 2030, 3.88% of the US population (older than 18 years of age) is projected to have had a stroke.4
There are two major types of stroke; (1) Hemorrhagic stroke which is characterized by a ruptured blood vessel that has already been weakened by aneurysms or arteriovenous malformations. (2) Ischemic stroke which is caused by obstructing a blood vessel in the brain, compromising its blood supply. According to a study, approximately 87% of strokes are diagnosed as ischemic strokes.3 When an ischemic stroke occurs, it triggers a cascade of inflammatory events that are characterized by two types of injury, the immediate infarct manifested by cell death through necrosis, and the peri-infarct penumbra that causes delayed programmed cell death.5 The intervention for ischemic stroke is still limited to the only FDA-approved treatment available, the recombinant tissue-plasminogen activator (rtPA).5 However, its therapeutic window is limited up to 4.5 h after the onset of the ischemic stroke, as recommended by the American Heart Association. The ischemic penumbra’s slower progression extends the treatment window for ischemic stroke.6 The microglia, a resident immune cell of the central nervous system (CNS), is a critical cell type during this stage.
Microglia composes 10–20% of glial cells in the CNS and acts as its main immune defense.7 Microglia is an integral part of the innate immune system and plays a significant role in brain development and the prognosis of conditions including, but not limited to, multiple sclerosis, Alzheimer’s disease, and brain injury.8 Microglia activation is considered the hallmark of brain pathology.9 Disturbances promoted by diseases or even caused by injuries like ischemic stroke and other traumatic brain damages can stimulate the activation of microglia. Activated microglia becomes highly motile and migrates to the injured area to start engulfing cell debris and damaged cells.7 They also are involved in the inflammatory response by secreting cytokines. This is the M1 phenotype of the microglia.10 On the other hand, microglia can also be alternatively activated to express another phenotype called M2, which is known as anti-inflammatory microglia. M2 type of microglia induces the phagocytic function of the microglia but also promotes the limiting of the inflammation around the site of injury.10
Immediate physiological response to stress is to initiate an immune response inflammation. While the inflammatory response is an effective way to contain the damage at the site of injury, chronic inflammation can impede the repair process. Therefore, inflammation has to be in the checklist during the healing procedure. Therefore, we investigated whether IHT could regulate the microglia polarization from M1 to M2 from the stress caused by OGD and reoxygenation. Previously, it is known that IHT could protect the brain from various stress models, but the role of IHT in induction of M2 microglia phenotype was not investigated.
Materials and methods
Oxygen–glucose deprivation and reoxygenation
Mice microglia, EOC20, was purchased from American Type Culture Collection (ATCC, Old Town Manassas, VA). EOC20 cells (passage less than 20) were assigned to the following groups; Normoxia control, OGD–reoxygenation, Normobaric intermittent hypoxia conditioning (IHT), OGD+IHT. Treatment groups consisted of six independent trials. Cells were maintained with DMEM (VWR life science, Road Radnor, PA) containing high glucose, glutamate and pyruvate, and supplemented with 20% fetal bovine serum (FBS) and penicillin (10,000 units/mL) and streptomycin (10,000 μg/mL). EOC20 cells (10,000 cells per well) were seeded in the 96-well plate. To achieve an OGD state, DMEM with high glucose and pyruvate was replaced with DMEM (Gibco, Waltham, MA) without glucose and pyruvate. EOC20 was placed into the programmable hypoxia chamber (Biospherix, Parish, NY) (0.1% O2 and 5% CO2) for 90 min. Afterward, OGD cells were returned to the regular CO2 incubator, and at the beginning of reoxygenation, DMEM (VWR life science, Road Radnor, PA) + high glucose, glutamate, and pyruvate were added to the OGD-conditioned cells. EOC20 in IHT groups exposed to a three days IHT program consisting of five to eight daily, 5- to 10-min cycles of hypoxia (4–3.5% O2) with intervening 4-min reoxygenation.
Cell viability assay
Cell viability of EOC20 cells in each group was evaluated by using Calcein-AM assay and LDH activity in the DMEM after OGD–reoxygenation and three days of IHT. After 24 h of reoxygenation, LDH and Calcein-AM assay were performed. LDH activity was measured by using commercial LDH assay kit and spectrophotometric kinetic assay (Pointe Scieitific, Canton, MI). Cells were washed with phosphate-buffered saline (PBS, pH7.0) and incubated with Calcein-AM (1 μM, AnaSpec, Fremont, CA) in PBS for 14 min at 37°C. Fluorescent intensity which emitted by live cells was measured by using Spectra Max Plus 384 (Molecular Devices, Sunnyvale, CA) with 485/530 nm (excitation and emission). Cell viability was reported as percent viability compared to the normal control. Treatment groups consisted of six independent trials, each in sextuplicate. After spectrometric quantification, representative images were taken using an Olympus BX41 fluorescent microscope.
Western blot analysis
Western blotting assay (n = 6) was used for the comparative analysis of the proteins. After 24 h of reoxygenation, proteins from the cells were extracted. Cells were washed with PBS twice and were lysed in the cell lysis buffer, containing Tri (20 mM), NaCl (100 mM), and EDTA (1 mM), freshly mixed with inhibitors of protease and phosphatase for 15 min at 4°C on a shaker followed by centrifugation at 110,000g for 20 min at 4°C. Supernatant of whole cell extract was saved at −80°C for later use. Protein concentration of the protein extract was measured by using Pearce BCA Protein assay kits (Thermo Scientific, Waltham, MA). Protein (20 μg/lane) was loaded and separated within SDS-PAGE electrophoresis and transferred to the nitrocellulose membrane. The following primary antibodies were used; Goat-antiTLR2 (1:100, R&D system, Minneapolis, MN), mouse-CD206 (1:500, Novus Biologicals, Littleton, CO), mouse anti-β-actin (1:1000, Santa Cruz Biotechnology, Dallas, TX). Primary antibodies were incubated overnight at 4°C. Mouse anti-goat (Santa Cruz Biotechnology, Dallas, TX) and goat anti-mouse (ImmunoReagent, Raleigh, NC) secondary antibodies conjugated with HRP were used at 4500:1 dilution for 1 h at room temperature. Protein content was quantified by densitometry (Ultraviolet Products, Upland, CA) and normalized to the β-actin density.
Reactive oxygen species measurements
Primarily superoxide generated from EOC20 was measured by using H2DCFDA (Invitrogen, Eugene, OR). Two different sets of experiments were parallelly conducted for image and quantification. After OGD and reoxygenation, EOC20 cells were washed with PBS and incubated with DCFDA (25 μmol/L) for 30 min at 37°C. After gentle washing, fluorescent images with DAPI nuclear stain were immediately obtained by using an Olympus BX41 fluorescent microscope. The intensity of fluorescence which is proportional to the ROS quantity was measured by using Spectra Max Plus 384 (Molecular Devices, Sunnyvale, CA) with 492/520 nm (excitation and emission). The florescence intensity was normalized to the average normoxia value.
Immunocytochemistry of M2 microglia
The phenotype of microglia in each group was determined by immunocytochemistry. EOC20 cells were cultured on a glass coverslip in DMEM containing 10% FBS and 1% streptomycin (10,000 μg/mL)–penicillin (10,000 units/mL). EOC20 monolayer was fixed with cold methanol and permeabilized with 1% Triton X. After blocking with goat serum (5%), mouse CD206 antibody (1:40, Novus Biologicals, Littleton, CO) was used as an M2 marker. Goat anti-mouse Alexa flour plus 555 (1:500, Invitrogen, Carlsbad, CA) was used for immunocytochemistry. Fluorescent images were immediately obtained by using an Olympus BX41 fluorescent microscope.
Cytokine quantification
IL-10 and IL-4 were measured by enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, MA). Sample preparation and quantification of cytokines were done by following the manufacture’s instruction. Cytokines’ determination was done in duplicate. Protein extractions and standards (100 μL) were placed to the antibody-coated plates and incubated for 2.5 h at room temperature. After thorough washing, biotinylated cytokine detecting antibody was added and incubated for 1 h at room temperature. HRP-streptavidin solution was added to each well and incubated for 45 min at room temperature. Then, one step TMB substrate reagent was added to each well and incubated for 30 min at room temperature in the dark. After adding a stop solution, each cytokine was measured by using Spectra Max Plus 384 (Molecular Devices, Sunnyvale, CA) at 450 nm immediately. Cytokine concentration was reported as unit per mg of protein.
Phagocytic activity of microglia
Phagocytic activity of microglia was compared between groups by using the Phagocytosis Assay kit (Cayman Chemical, Ann Arbor, MI). EOC20 cells were cultured less than 70% confluency; 2 h of each experimental treatment, latex beads-rabbit IgG-FITC complex were directly applied to prewarmed DMEM supplemented with 10% FBS and 1% streptomycin (10,000 μg/mL)–penicillin (10,000 units/mL). EOC20 cells displaying fluorescent, after 1 h incubation at 37°C, were counted by flow cytometric analysis using 2100 bio-analyzer (Agilent, Santa Clara, CA). For the fluorescence microscopy, duplicated experiments were performed, and images were taken by using an Olympus BX41 fluorescent microscope.
Statistical analysis
Data are expressed as mean ± SEM. Multi comparison between groups was accomplished by one-way analysis of variance combined with Tukey multi comparison test to identify statistically significant differences (SPSS data analysis software). Probability value < 0.05 was taken to indicate statistically significant effects.
Results
IHT protects the microglia from the OGD and reoxygenation stress
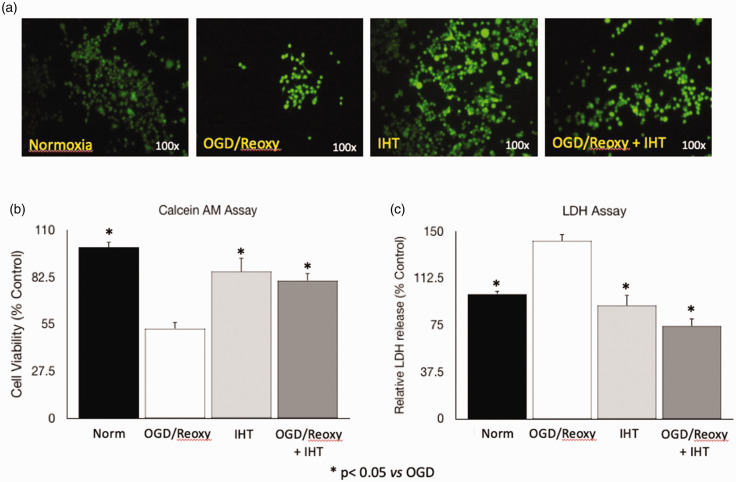
Similar to the other types of cells that suffer, microglia are damaged without sufficient oxygen and nutrition. Damaged microglia could be a trigger of ischemia-related brain damage. LDH activity assay in cell culture media, which indicates hypoxic stress level on the cells, and cell viability assay supports that IHT protects the microglia from the OGD and reoxygenation stress (Figure 1). OGD and reoxygenation decreased cell viability to 65% of normoxia (control). IHT itself does not change cell viability compared to the normoxia group. Normobaric IHT protects the microglia from the OGD–reoxygenation caused cell death (Figure 1(a) and (b)). LDH activity in the cell culture media shows the opposite pattern to the cell viability assay. LDH activity in the OGD–reoxygenation group is increased by approximately 40% when compared to the normoxia group (Figure 1(c)).
Figure 1.
IHT protects the microglia from the OGD and reoxygenation. (a, b) The Calcein-AM assay shows the protective effect of IHT against OGD and reoxygenation. (c) LDH assay supports that IHT protects the microglia by presenting that microglia exposed to the OGD and reoxygenation get stressed and increased LDH release into the culture media. *P < 0.05 vs. OGD/Reoxy. (A color version of this figure is available in the online journal.)
IHT induces the M2 phenotype of microglia under OGD and reoxygenation condition
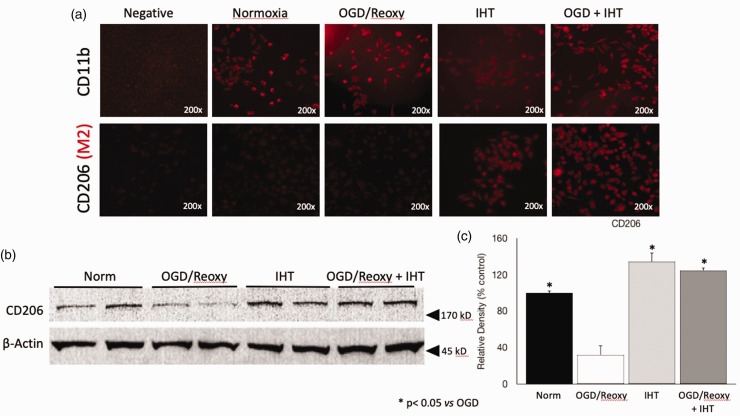
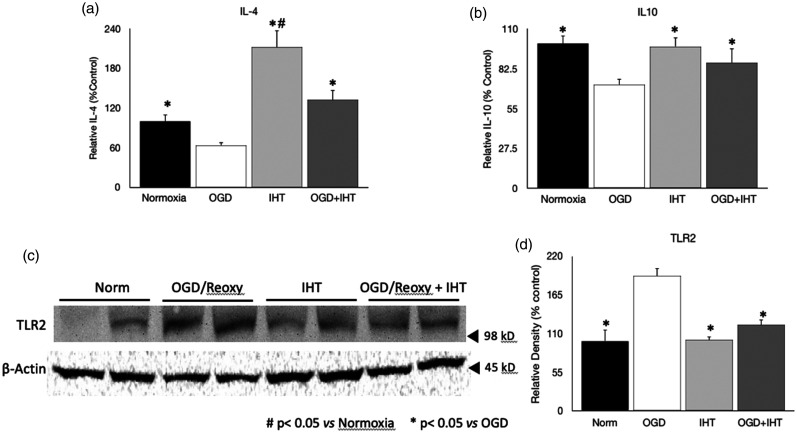
The function of IHT on the regulating microglia phenotype was tested by using immunocytochemistry. CD11b, a marker of microglia, is used to confirm the characteristic of EOC20 cells (Figure 2(a)). The CD206 expression on the cell was only significantly increased in the IHT and OGD–reoxygenation + IHT groups (Figure 2(a)). A significantly increased CD206 content in the IHT group also support that IHT increased M2 phenotype of microglia (Figure 2(b) and (c)). CD 206 content in the Western blot was about four times greater than OGD and reoxygenation group (Figure 2(b) and (c)). The involvement of the anti-inflammatory cytokines in the polarization of microglia to the M2 phenotype is well reported. Intermittent hypoxic training significantly increased IL-4 concentration compared to the other groups (Figure 3(a)). On the other hand, IL-4 in the OGD–reoxygenation group was significantly decreased compared to the normoxia group, but IHT followed by OGD–reoxygenation increased the IL-4 back to the normoxia control level (Figure 3(a)). IHT also mitigated the reduction of IL-10, anti-inflammatory cytokine, by OGD–reoxygenation (Figure 3(b)). Toll-like receptor 2 (TLR2), a representative pattern recognition receptor (PRR), was significantly increased in the microglia after OGD–reoxygenation, and IHT reduced the TLR2 of microglia exposed to the OGD–reoxygenation back to the level in the normoxia control group (Figure 3(d)).
Figure 2.
IHT derives microglia toward the anti-inflammatory M2 phenotype. (a) The characteristic of EOC20 cell is confirmed by detecting CD11b which is a microglia marker. CD206, M2 marker, is noticeably increased on the microglia treated with IHT. (b,c) Western blot data clearly show that CD206 protein content in the microglia is significantly increased by IHT compared to the microglia in the OGD and reoxygenation group. *P < 0.05 vs. OGD/Reoxy. (A color version of this figure is available in the online journal.)
Figure 3.
Anti-inflammatory cytokines and toll-like receptor 2 content. (a) IHT increased the IL-4 contents compared to other groups including OGD and control. (b) IL-10 is not increased compared to the Normoxia but increased compared to the OGD group. (c,d) Toll-like receptor 2 is significantly increased in the OGD and reoxygenation group. #P < 0.05 vs. Normoxia *P < 0.05 vs. OGD/Reoxy.
IHT enhances the phagocytic activity of microglia and reduced ROS generation
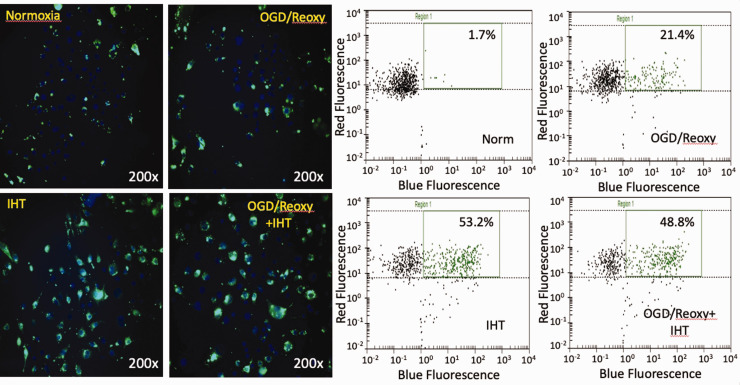
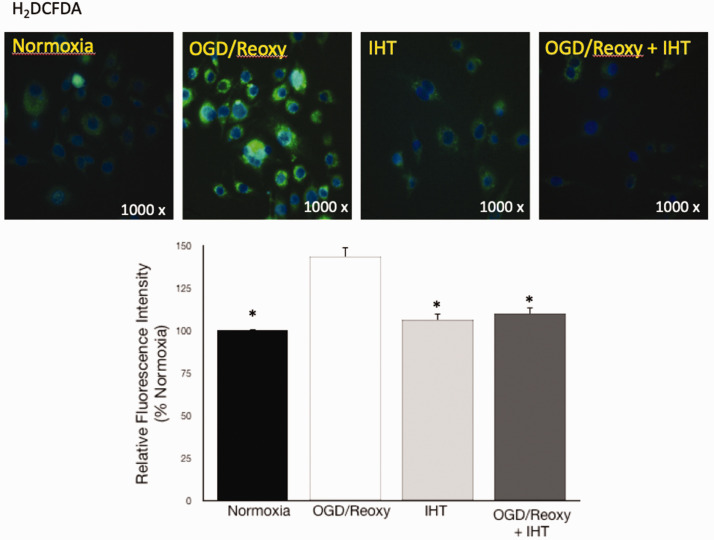
OGD–reoxygenation-activated microglia increased phagocytic activity vs. normoxia group, 21.4%, and 1.7%, respectively (Figure 4); 53.2% of microglia in the IHT without the OGD–reoxygenation group detected as positive in phagocytic activity. IHT-enhanced phagocytic activity was maintained even after OGD–reoxygenation stress (Figure 4). ROS generation was monitored by using the ROS indicator, H2DCFDA. It is previously reported that IHT enhances endogenous redox potential. In this study, we were able to recapitulate it by monitoring the ROS generation (Figure 5).
Figure 4.
Phagocytic activity of microglia enhanced by IHT. The phagocytic activity of microglia activated by OGD and reoxygenation is enhanced compared to the unstressed control group. IHT increases the phagocytic activity of microglia greater than the OGD–reoxygenation group. (A color version of this figure is available in the online journal.)
Figure 5.
IHT reduced ROS generation from microglia. ROS generation in the OGD and reoxygenation activated-microglia was dampened by IHT. The bar graph represents a quantitative analysis of the H2DCFDA spectrophotometry assay. *P < 0.05 vs. OGD/Reoxy. (A color version of this figure is available in the online journal.)
Discussion
Cerebral hypoxia, accompanied by glucose deprivation and subsequent reoxygenation stress is a significant component of injuries pertaining to the brain. Conditions like, but not limited to, traumatic brain injury, heart attack, cerebral edema, obstructive sleep apnea, and ischemic stroke can initiate a proteolytic cascade of reaction that is directly associated with neuronal apoptosis.11 When the OGD/reoxygenation stress occurs, it induces inflammatory reaction which is triggered by activation of microglia leading to eventual neuronal cell death.
In the previous studies of our laboratory as well as of other laboratories, IHT-induced cellular protection has been reported. IHT stabilizes the α-subunit of the hypoxia-inducible factor (HIF) and activates HIF gene regulatory mechanism.12 Erythropoietin (EPO) is a pivotal cytokine/hormone of IHT-induced cellular protection. The biological function of EPO is the erythropoiesis and inducing the activation of anti-apoptotic kinases, such as Erk and Akt.13 Additionally, activated HIF increases glycolytic enzymes involved in energy metabolism and enhances the redox potential of the cells.14 Overall, IHT balances between energy demand and supply so that ROS generation is diminished. Although numerous studies have reported a series of potential cellular protective mechanisms against ischemia and reperfusion stresses, it is evidently not satisfactory to explain IHT-induced cerebral protective mechanisms. A piece of missing puzzles might be associated with protecting microglia and regulating its polarization. We demonstrated by using calcein-AM and media LDH activity assay that IHT protects the microglia from the OGD–reoxygenation stress. LDH, a cytoplasmic enzyme, is increased under hypoxic conditions and released to the extracellular environment when the cell membrane is compromised. The LDH activity in the extracellular media in the OGD–reoxygenation group was increased because of severely damaged microglia, but IHT can reduce the extracellular LDH activity by protecting the microglia from intense OGD–reoxygenation stress. This supports the result of increased viability after IHT treatment of OGD-exposed cells, indicating that IHT enhances a healing procedure after an injury. Decreased microglia in the core of brain parenchyma in the process of ischemic stroke results in increased peripheral neutrophil accumulation.15 Therefore, protecting microglia from ischemia and reperfusion injury is an important factor to be considered in developing an intervention for ischemic stroke patients. Furthermore, previous study tested the effect of microglia in the rodent ischemic stroke model. Selective deletion of microglia results in about 60% increased neuronal death in the ischemic stroke model.16
Abnormally increased ROS has been reported as a principal constituent in various pathophysiological conditions. An excessive amount of ROS deteriorates cellular homeostasis by damaging protein, lipids, and even genetic nucleic acids. On the other hand, moderately increased ROS could serve as a regulator in signaling pathway17 and in the transcription for cytoprotective proteins synthesis .18 For example, under hypoxia, the rate of ROS generation is increased which results in HIF-1α stabilized. HIF-induced antioxidants are able to maintain the ROS level. However, once ROS exceeds the point where HIF can control, HIF is also inactivated and eventually causes cell death.14,19 ROS is increased with activated microglia and by the cells affected by activated microglia.20 In here, the antioxidant function of IHT is demonstrated. Increased ROS with OGD and reoxygenation was dampened by IHT.
Microglia, resident macrophage of the CNS, has a significant role in sustaining the homeostasis of the CNS by having dual-edge functions in the inflammation; enhancing or mitigating inflammation. The microglia’s first response to the occlusion of a vessel in the brain is to promote inflammation. This is an innate response to promote damage containment at the site of injury. Although this is an effective way of starting the healing process, the pro-inflammatory process alone is not enough to prevent further damage. The prolonged microglial inflammatory response in the CNS can induce secondary damage in the tissue and hinder neuronal regeneration.3,21,22 Disturbances in the CNS could activate microglia and likely polarize to the M1 phenotype. Microglia in the form of prolonged active inflammation slows the recovery of the CNS from previous stress conditions that caused it. Therefore, the timing and balancing of the polarization of microglia are a critical factor in minimizing tissue injuries and enhancing recovery from ischemic conditions. The effect of IHT on the polarization of microglia toward the immune suppressive M2 phenotype was examined in this study. Activation of TLR2 results in increased transcription of various pro-inflammatory mediators which could be deriving factor for the polarization of microglia.23 It is reported that the activation of TLR2 by the TLR2 agonist, GT1b, induces M1 microglia activation.24 IL-10 is a potent anti-inflammatory cytokine and counteracts the damage brought upon by an injury.25 It is well supported that increased anti-inflammatory IL-10 is contributing to the polarization of microglia to the M2 phenotype and loss of IL10 increases M1 phenotype.26,27 IL-4 is another potent anti-inflammatory cytokine synthesized by microglia.28 IL-4 is known to drive the microglia to its M2 phenotype.28 This study proposes that IHT-induced cytokine profile modifications result in an increased M2 phenotype of microglia. It also results in the augmentation of phagocytosis-mediated tissue cleanup which can enhance functional recovery.7,28
As mentioned earlier, the phagocytic function of microglia is an essential physiologic and immunologic function and a significant component of repair and regeneration of the CNS after injury.11 It is undoubtedly observed that the activated microglia possess the enhanced phagocytic function compared to the microglia in the resting state. We also demonstrated that the phagocytic activity of the microglia increased after being exposed to OGD–reoxygenation stress. Interestingly enough, the OGD-exposed cells that were subsequently treated with IHT displayed an even higher phagocytic activity. The increased phagocytic activity promoted without increasing ROS by IHT can be beneficial to ischemic stroke patients, especially during the early recovery phase.
In summary, the results of this study support that IHT induces the M2 microglia phenotype in the in vitro OGD–reoxygenation stress model. This finding could be beneficial in the long-term recovery of patients who suffered from ischemic stroke. Possibly, IHT protects the microglia from the ischemic stroke and subsequent reperfusion so that more microglia gets involved in the early healing procedure. Importantly, 24 h after the ischemic stroke occurs, IHT also increases the anti-inflammatory phenotype of microglia. This regulatory function of IHT in the induction of M2 microglia will dampen the CNS injury that is attributed to the prolonged inflammatory responses observed in the ischemic stroke patients. The IHT-induced M2 microglia maintains the phagocytic activity of activated microglia and mitigates ROS generation. Therefore, IHT can be considered as an efficient intervention that could be adapted in the current rehabilitation programs.
Authors’ contributions
MGR designed the experiment and analyzed the data. GT and MGR participated in conducting experiments and interpretation of the data and wrote and review the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Myoung-Gwi Ryou https://orcid.org/0000-0002-4494-7007
References
- 1.Kochanek KD, Murphy SL, Xu J, Arias E. Mortality in the United States. NCHS Data Brief 2013; 2014:1–8 [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler Cr, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2016. Update: a report from the American Heart Association. Circulation 2016; 133:e38–e360 [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease stroke statistics-2017. Update: a report from the American Heart Association. Circulation 2017; 135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 2013; 44:2361–75 [DOI] [PubMed] [Google Scholar]
- 5.Guruswamy R, ElAli A. Complex roles of microglial cells in ischemic stroke pathobiology: new insights and future directions. Int J Mol Sci 2017; 18:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke 2011; 42:S7–S11 [DOI] [PubMed] [Google Scholar]
- 7.Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 2014; 49:1422–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz KM, Nelson LH. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol 2018; 9:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007; 14:1189–97 [DOI] [PubMed] [Google Scholar]
- 10.Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol 2013; 2013:746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson BJ, Sanchez-Covarrubias L, Slosky LM, Zhang Y, Laracuente ML, Ronaldson PT. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab 2014; 34:699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan A, Arnold BM, Caine S, Toosi BM, Verge VMK, Muir GD. Acute intermittent hypoxia and rehabilitative training following cervical spinal injury alters neuronal hypoxia- and plasticity-associated protein expression. PLoS One 2018; 13:e0197486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nekoui A, Blaise G. Erythropoietin and nonhematopoietic effects. Am J Med Sci 2017; 353:76–81 [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Jiang L, Zhang H, Shimoda LA, DeBerardinis RJ, Semenza GL. Analysis of hypoxia-induced metabolic reprogramming. Meth Enzymol 2014; 542:425–55 [DOI] [PubMed] [Google Scholar]
- 15.Otxoa-de-Amezaga A, Miro-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruiz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, Marquez-Kisinousky L, Denes A, Gunzer M, Planas AM. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol 2019; 137:321–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, Csaszar E, Fekete R, West BL, Katona G, Rozsa B, Denes A. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 2016; 7:11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu G, Liu Z. Nuclear factor erythroid 2-Related factor 2 (Nrf2) mediates neuroprotection in traumatic brain injury at least in part by inactivating microglia. Med Sci Monit 2016; 22:2161–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 2013; 53:401–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriquez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000; 275:25130–8 [DOI] [PubMed] [Google Scholar]
- 20.Rawlinson C, Jenkins S, Thei L, Dallas ML, Chen R. Post-ischaemic immunological response in the brain: targeting microglia in ischaemic stroke therapy. Brain Sci 2020; 10:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amor S, Woodroofe MN. Innate and adaptive immune responses in neurodegeneration and repair. Immunology 2014; 141:287–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM. Inflammation in neurodegenerative diseases – an update. Immunology 2014; 142:151–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao K, Zhao YF. Aging modulates microglia phenotypes in neuroinflammation of MPTP-PD mice. Exp Gerontol 2018; 111:86–93 [DOI] [PubMed] [Google Scholar]
- 24.Lim H, Lee J, You B, Oh JH, Mok HJ, Kim YS, Yoon BE, Kim BG, Back SK, Park JS, Kim KP, Schnaar RL, Lee SJ. GT1b functions as a novel endogenous agonist of toll-like receptor 2 inducing neuropathic pain. EMBO J 2020; 39:e102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 2012; 32:23–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laffer B, Bauer D, Wasmuth S, Busch M, Jallivand TV, Thanos S, Meyer Z, Horste G, Loser K, Langmann T, Heiligenhaus A, Kasper M. Loss of IL-10 promotes differentiation of microglia to a M1 phenotype. Front Cell Neurosci 2019; 13:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellenbrand DJ, Reichl KA, Travis BJ, Filipp ME, Khalil AS, Pulito DJ, Gavigan AV, Maginot ER, Arnold MT, Adler AG, Murphy WL, Hanna AS. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. J Neuroinflamm 2019; 16:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Z, Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci 2015; 35:11281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]