Abstract
The occurrence and acuteness of liver cirrhosis were strongly associated with the hepatocarcinogenesis and the prognosis of hepatocellular carcinoma (HCC). This study compared the prognostic significance of non-invasive fibrosis panel containing 15 indices in hepatitis-B-associated HCC patients’ post-curative resection. Four hundred and five consecutive hepatitis-B-related HCC patients who went through curative hepatectomy were investigated retrospectively. The multivariate Cox proportional hazard model was used to evaluate independent prognostic factors for overall survival (OS). The accuracy in diagnosis of each non-invasive fibrosis index in cirrhosis detection was determined by the area under receiver operating characteristic (AUC) curve. Preoperative AST to platelet ratio index (APRI), Goteburg University Cirrhosis Index (GUCI), and King Score all exhibited a superior performance in diagnosis of cirrhosis detection with AUC > 0.7. APRI and Fibro-α Score were the risk factors that behaved independently in predicting the OS of HCC patients, with an hazard ratio (HR) value of 1.550 (P = 0.012) and 1.420 (P = 0.033), respectively. Preoperative APRI was a relatively accurate predictor of cirrhotic status and prognosis in HCC due to hepatitis-B among the 15 non-invasive fibrosis indices.
Impact statement
Non-invasive fibrosis indices, according to regular laboratory and clinical data, could be useful in assessing liver fibrosis in chronic hepatitis patients. However, the role of these biomarkers remains unclear in predicting the outcome of HBV-associated HCC in patients. This study was carried out retrospectively and included a relatively large sample size (n = 405) with a heterogeneous population of HBV infected patients and longer duration of prospective follow-up. Our study suggested that APRI and Fibro-α Scores are inversely correlated with overall survival in HBV-associated HCC patients. Meanwhile, GUCI, King Score, and APRI were highly correlated with cirrhosis status. Also, in subgroups of cirrhosis or non-cirrhosis, Fibro-α Scores could differentiate patients with good prognosis from those with poor outcome. This result would aid clinicians in acquiring preventive and therapeutic methods in patients with high risk.
Keywords: Liver cirrhosis, non-invasive fibrosis marker, hepatitis B virus, hepatocellular carcinoma, prognosis, cancer
Introduction
Worldwide, among cancer-associated death, hepatocellular carcinoma (HCC) ranks as the third leading cause and is the fifth most prevalent cancer. According to the statistics of global cancer, in 2012, nearly 782,500 new liver cancer cases and 745,500 deaths took place globally, out of which 50% of deaths were reported from China alone.1 Cirrhosis, especially that associated with hepatitis C virus (HCV) and hepatitis B virus (HBV) infections, is a prevalent and predominant risk factor for the development of HCC.2 Majority of Chinese HCC patients are diagnosed as positive for chronic HBV infection. Long-standing HBV infection frequently results in liver fibrosis and cirrhosis which can eventually lead to hepatocarcinogenesis. Moreover, previous studies also suggested that post curative resection, a manor risk factor for HCC recurrence and survival is the degree of fibrosis.3–5 Surgical resection is currently considered as a potential HCC treatment, but has unsatisfactory long-term survival because of high rates of recurrence and distant metastasis. These findings may implicate that the occurrence, as well as the intensity of liver cirrhosis associated strongly with the hepatocarcinogenesis and the prognosis of HCC.
The risk factors related to the postoperative outcomes have been extensively studied, including serum α-fetoprotein (AFP) levels, the number and size of tumors, vascular invasion, and the extent of functional reserve of the liver.6–9 The majority of these factors depend on the postoperative histopathological and biochemical examinations. Meanwhile, molecular markers for HCC prognosis have been widely studied, but these are not cost-effective investigations in healthcare practice. Non-invasive fibrosis biomarkers are now widely used to evaluate the prognosis of HCC, as they are economical and have enhanced diagnostic utility.
Regarding other investigations, the benchmark for the assessment of liver fibrosis is histopathology of liver biopsy, although it has limitations in clinical setup due to certain factors like life-threatening complications, invasive procedure, error in sampling, interobserver variations, high cost, and less than average tolerance.10–12 Of late, several studies have reported that indices based on non-invasive fibrosis, regular laboratory, and clinical data could be useful in evaluating liver fibrosis in chronic hepatitis patients. However, the function of these biomarkers in predicting the outcome of HBV-associated HCC in patients is still unclear.
Thus, this study evaluated the performance of 15 simple and non-invasive fibrosis-associated indices to predict cirrhosis occurrence and the prognosis of overall survival in hepatitis-B-related HCC patients’ post-curative resection.
Materials and methods
Study population
We retrospectively reviewed the patient records of 405 cases of histopathology-confirmed HCC, treated with radical hepatectomy from 2004 to 2010 in the Chinese People’s Liberation Army (PLA) General Hospital. The study adhered to current ethical guideline, the Helsinki Declaration standards and, with a prior approval of the protocol from PLA General Hospital, Research Ethics Committee.
The inclusion criteria were the cases that were: (1) HBV-positive; (2) Child-Pugh A or B; (3) without portal vein main trunk involvement and distant metastasis; (4) no anticancer therapy for HCC prior to surgery; (5) fully available data of laboratory, clinical, and follow-up tests; and (6) patient survived for a minimum of 30 days after operation. Moreover, patients who underwent palliative surgery, accompanied with other malignancies or less than three years’ follow-up time were not included.
Collection of data and non-invasive fibrosis indices calculation
Demographics (including gender and age), routine assessments including a complete physical examination and laboratory investigations (serum level of α-fetoprotein (AFP), platelet count (PLT), alanine aminotransferase (ALT), albumin, gamma-glutamyl transferase (γ-GT), aspartate aminotransferase (AST), total bilirubin (TB), international normalized ratio (INR)), postoperative histopathological data (differentiation and number of nodules of tumor, maximal tumor diameter, liver cirrhosis, capsulation, micro-vascular tumor thrombus (MVTT), Child-Pugh classification, and the 7th TNM stage), abdominal ultrasound and magnetic resonance imaging (MRI) or computed tomography (CT) observations were obtained from electronic medical records.
Non-invasive fibrosis-associated indices, i.e. the AAR (aspartate aminotransferase to alanine aminotransferase), AARP (AAR-platelet score), AARPRI (AAR-to-platelet ratio index), API (age-platelet index), APRI (AST-to-platelet ratio index), FIB-4 Index (fibrosis index based on the four factors), CDS (cirrhosis discriminant score), Fibro-α Score, fibrosis index, fibro-quotient (Fibro Q), GUCI (Goteburg University Cirrhosis Index), King Score, P2/MS, platelet-AST-age (PLASA), and Pohl Scores were determined from preoperative biochemical and baseline demographic data by using the published formulas presented in Table 1.
Table 1.
The formulas of 15 non-invasive fibrosis indices included in the current study.
| Index | Calculation |
|---|---|
| AAR13 | AST/ALT |
| AARP14 | 1: AAR ≥1 or PLT<150 (×109/L) or else, the score = 0 |
| AARPRI15 | AAR/[PLT (×109/L)/150] |
| API16 | Age (years): <30 = 0; 30–39 = 1; 40–49 = 2; 50–59 = 3; 60–69 = 4; >70 = 5PLT (×109/L): 225 = 0; 200 – 224 = 1; 175 – 199 = 2; 150 – 174 = 3; 125 – 149 = 4; <125 = 5API is the sum of the above (possible value 0–10). |
| APRI17 | {[AST (U/L)/ULN]/PLT (×109/L)} × 100 |
| CDS18 | PLT (×109/L): >340 = 0; 280 – 339 = 1; 220 – 279 = 2; 160 – 219 = 3; 100–159 = 4;40–99 = 5; <40 = 6ALT/AST ratio: >1.7 = 0; 1.2–1.7 = 1; 0.6–1.19 = 2; <0.6 = 3INR: <1.1 = 0; 1.1–1.4 = 1; >1.4 = 2CDS is the sum of the above (possible value 0–11) |
| FIB-4 Index19 | Age (years) × AST/[PLT (109/L) × (ALT)1/2] |
| Fibro-α Score20 | 1.35 + AFP (IU/ml) × 0.009584 + AST/ALT × 0.243–PLT (×109/L) × 0.001624 |
| Fibrosis Index21 | 8–0.01× PLT (×109/L) –albumin (g/dL) |
| Firbro Q22 | [10 × Age (y) × AST (U/L) × INR]/[PLT (×109/L)× ALT (U/L)] |
| GUCI23 | Normalized AST × INR × 100/ PLT (×109/L) |
| King Score24 | Age (years) × AST (U/L) × INR/ PLT (×109/L) |
| P2/MS25 | PLT(×109/L)2/(monocyte fraction × segmented neutrophil fraction) |
| PLASA Score26 | 0.067 × Age (years) + 0.020 × AST(U/L)–0.004 × PLT(×109/L) – 3.028 |
| Pohl Score27 | 1: AAR ≥1 and PLT <150 (×109/L) or else, the score = 0 |
AAR: aspartate aminotransferase to alanine aminotransferase ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PLT: platelet count; AARP: AAR-platelet score; AARPRI: AAR-to-platelet ratio index; API: age-platelet index; APRI: AST to platelet ratio index; ULN: upper limit of normal; CDS: cirrhosis discriminant score; INR: international normalized ratio;FIB-4 Index: fibrosis index based on the four factors; AFP: alpha-fetoprotein; Fibro Q: fibro-quotient; GUCI: Goteburg University Cirrhosis Index; PLASA: platelet-AST-age.
Histopathological examination
Resected para-cancerous and tumor tissue were brought to the pathology department in our hospital after surgical resection. An experienced pathologist, blinded to the patients’ clinical and diagnostic data confirmed the diagnosis of liver cirrhosis and HCC.
Follow-up
After hepatectomy, all patients had routine follow-up examinations which consisted of a detailed physical evaluation, tests for liver function, and AFP, CT (ultrasound or dynamic contrast-enhanced), or MRI at 90- to 180-day intervals. The OS was determined from the date of surgery until the final follow-up visit or death. Death of patients due to HCC was classified as tumor-related death. The patients lost to follow-up were not included.
Statistical analysis
SPSS (version 19.0) and MedCalc (version 13.1.2.0) statistical software were applied to assess the data. Categorical variables were described in terms of the frequencies, and the expression of continuous data was done as the median and range. Life tables were used to determine the cumulative OS rates. The Kaplan-Meier method was used for univariate survival analysis and differentiated by the Log-rank test. Significant variables in the univariate analysis were included into the multivariate analysis through the stepwise forward selection method. The independent risk factors for OS were assessed through multivariate Cox proportional hazard model. The accuracies in the diagnosis of all non-invasive fibrosis indexes and cut-off values were determined by the receiver operating characteristic (ROC) curve and the area under the ROC (AUROC) curve. To evaluate the sensitivity and specificity for the five-year OS by the ROC curve, the cut-off value that was the best and the closest to maximum sensitivity as well as specificity through MedCalc statistical software was noted and were used to classify into high- or low-risk groups. Briefly, an event was noted if death occurred within 60 months, and in the case of non-events, the patients survived more than 60 months; the patients who did not participate in follow-up were not included in the analysis.
The AUROC values for outcome prediction between different indices were contrasted as per the method suggested by McNeil and Hanley. Each statistical testing was two-tailed at a 5% level, and a P-value of less than 0.05 was deemed significant statistically.
Results
Baseline clinicopathologic features
The baseline features of all 405 HBV-HCC patients are listed in Table 3. This includes a total of 356 males (87.9%) and 49 female patients (12.1%) with 52 years median age (ranged from 22 to 78 years). The majority (96%) of patients were in Child-Pugh grade A, while only 16 (4%) patients were in grade B. On assessing tumor features, single tumor nodule was present in 345 (85.2%) patients, while 33 (8.1%) presented two or three, and 27 (6.7%) presented greater than three tumor nodules. The maximal tumor diameter median value was 4.8 cm (0.8–26). Inherent liver cirrhosis and microvascular tumor thrombus were found, respectively, in 323 (79.8%) and 21 (5.2%) patients. As per American Joint Committee on Cancer (AJCC) 7th edition, TNM staging system, 340 (84%) were stage I patients, 3 (7.4%) were in stage II, and 35 (8.6%) were at stage III of HCC. The types of fibrosis indices such as AAR, AARP, AARPRI, API, APRI, CDS, FIB-4 Index, Fibro-α Score, fibrosis index, fibro Q, GUCI, King Score, P2/MS, PLASA, and Pohl Score were calculated following the formula shown in Table 1 and presented in Table 2.
Table 3.
The diagnostic performance of 15 non-invasive fibrosis indices in the detection of cirrhosis in hepatitis-B-related HCC.
| Variable | Cut-off value | AUC | 95% CI | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|---|
| AAR | 0.6362 | 0.509 | 0.459 to 0.559 | 18.6 | 87.8 | 0.7908 |
| AARP | 0 | 0.592 | 0.543 to 0.640 | 69.7 | 48.8 | 0.0026 |
| AARPRI | 0.9245 | 0.622 | 0.572 to 0.669 | 50.2 | 72.0 | 0.0002 |
| API | 5 | 0.651 | 0.602 to 0.697 | 60.4 | 68.3 | <0.0001 |
| APRI | 0.4511 | 0.707 | 0.660 to 0.751 | 56.7 | 76.8 | <0.0001 |
| CDS | 5 | 0.661 | 0.613 to 0.707 | 58.8 | 63.4 | <0.0001 |
| FIB-4 Index | 1.5597 | 0.686 | 0.638 to 0.731 | 66.6 | 64.6 | <0.0001 |
| Fibro-α Score | 1.3548 | 0.528 | 0.478 to 0.578 | 83.2 | 29.3 | 0.4682 |
| Fibrosis Index | 2.25 | 0.692 | 0.645 to 0.737 | 57.0 | 75.6 | <0.0001 |
| Fibro Q | 3.1624 | 0.642 | 0.593 to 0.689 | 58.2 | 64.6 | <0.0001 |
| GUCI | 0.5105 | 0.722 | 0.676 to 0.765 | 55.7 | 80.5 | <0.0001 |
| King Score | 8.254 | 0.711 | 0.664 to 0.755 | 77.7 | 54.9 | <0.0001 |
| P2/MS | 78.6612 | 0.683 | 0.635 to 0.728 | 67.8 | 62.2 | <0.0001 |
| PLASA Score | 0.2671 | 0.616 | 0.567 to 0.664 | 87.3 | 35.4 | 0.0019 |
| Pohl Score | 0 | 0.566 | 0.516 to 0.615 | 24.1 | 89.0 | 0.0018 |
AAR: aspartate aminotransferase to alanine aminotransferase ratio; AST: aspartate aminotransferase; PLT: platelet count; AARP: AAR-platelet score; AARPRI: AAR-to-platelet ratio index; API: age-platelet index; APRI: AST to platelet ratio index; CDS: cirrhosis discriminant score; FIB-4 Index: fibrosis index based on the four factors; Fibro Q: fibro-quotient; GUCI: Goteburg University Cirrhosis Index; PLASA: platelet-AST-age; AUC: area under the receiver operating characteristic curve; CI: confidence interval.
Table 2.
Demographics of 405 patients included in the study.
| Variables | N = 405 | Univariate analysisP value |
Multivariate analysis |
|
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | |||
| Gender (male/female) | 356/49 | 0.067 | ||
| Age (years) | 52 (22–78) | 0.769 | ||
| Child-Pugh grade (A/B) | 389/16 | 0.005 | 2.548 (1.374–4.726) | 0.003 |
| ALT (U/L) | 33.5 (6–344.5) | 0.002 | ||
| AST (U/L) | 30.4 (12.8–355.7) | 0.000 | ||
| γ-GT (U/L) | 52.7 (8.6–1588.60) | 0.000 | ||
| Total bilirubin (Umol/L) | 15.1 (4.4–248.9) | 0.060 | ||
| INR | 1.11 (0.83–1.58) | 0.927 | ||
| Albumin (g/L) | 40.9 (23.5–52.6) | 0.056 | ||
| PLT (109/L) | 160 (40–466) | 0.160 | ||
| AFP (IU/mL) | 51.89 (0.61–24,200) | 0.001 | ||
| Tumor number (1/2–3/>3) | 345/33/27 | 0.000 | ||
| Maximal tumor diameter (cm) | 4.8 (0.8–26) | 0.000 | 1.482 (1.263–1.738) | 0.000 |
| Cirrhosis (yes/no) | 323/82 | 0.914 | ||
| Capsulation (yes/no) | 101/301 | 0.429 | ||
| MVTT (yes/no) | 21/384 | 0.012 | ||
| TNM stage (I/II/III) | 340/30/35 | 0.000 | 1.655 (1.348–2.032) | 0.000 |
| AAR | 0.92 (0.35–6.09) | 0.106 | ||
| AARP (0/1) | 138/267 | 0.942 | ||
| AARPRI | 0.85 (0.22–7.75) | 0.540 | ||
| API | 6 (0–10) | 0.498 | ||
| APRI | 0.4511 (0.11–3.23) | 0.000 | 1.550 (1.101–2.183) | 0.012 |
| CDS | 6 (1–9) | 0.161 | ||
| FIB-4 Index | 1.80 (0.49–12.98) | 0.014 | ||
| Fibro-α Score | 1.83 (1.02–233.25) | 0.000 | 1.420 (1.030–1.960) | 0.033 |
| Fibrosis Index | 2.26 (0.25–4.42) | 0.263 | ||
| Firbro Q | 3.4 (0.73–40.55) | 0.247 | ||
| GUCI | 0.50 (0.12–4.04) | 0.001 | ||
| King Score | 11.61 (2.65–99.13) | 0.002 | ||
| P2/MS | 63.50 (4.13–506.9) | 0.193 | ||
| PLASA Score | 0.52 (–1.74–5.26) | 0.006 | ||
| Pohl Score (0/1) | 318/87 | 0.351 | ||
ALT: alanine aminotransferase; AST: aspartate aminotransferase; γ-GT: γ-glutamyl transpeptidase; INR: international normalized ratio; PLT: platelet count; AFP: alpha-fetoprotein; MVTT: micro-vascular tumor thrombus; AAR: aspartate aminotransferase to alanine aminotransferase ratio; AARP: AAR-platelet score; AARPRI: AAR-to-platelet ratio index; API: age-platelet index; APRI: AST to platelet ratio index; CDS: cirrhosis discriminant score; FIB-4 Index: fibrosis index based on the four factors; Fibro Q: fibro-quotient; GUCI: Goteburg University Cirrhosis Index; PLASA: platelet-AST-age; CI: confidence interval.
Diagnostic performance in predicting hepatic cirrhosis
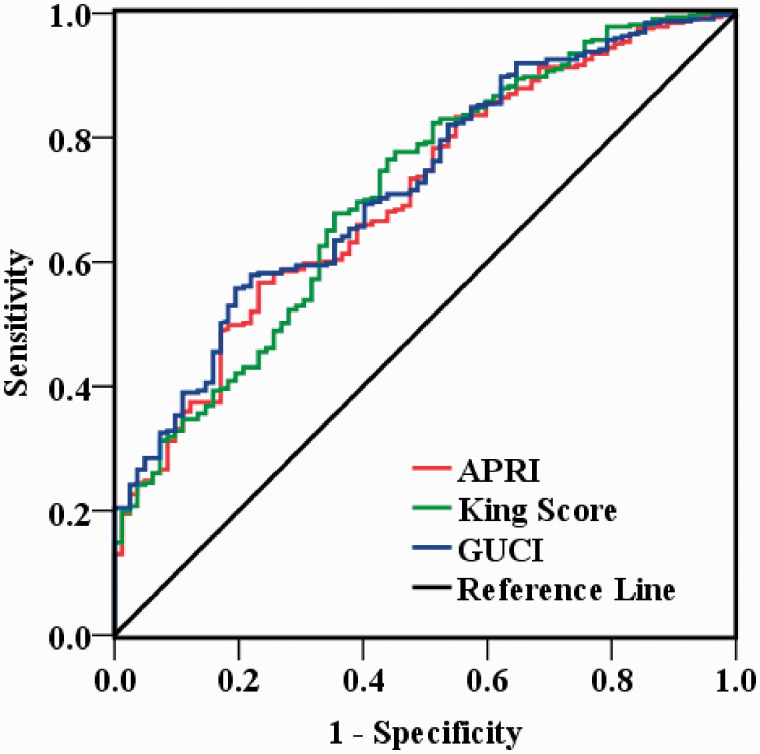
The accuracies in the diagnosis of 15 non-invasive fibrosis indices for predicting cirrhosis in HCC patients are presented in Table 3. Among these indices, GUCI, King Score, and APRI had a superior diagnostic performance for the detection of cirrhosis with AUC > 0.7 in patients of HCC on whom curative resection was done. To be specific, the AUC values of GUCI, King Score, and APRI were 0.722 (95% CI, 0.676–0.765) with 55.7% sensitivity and 80.5% specificity, 0.711 (95% CI, 0.664 to 0.755) with 77.7% sensitivity, and 54.9% specificity, 0.707 (95% CI, 0.660 to 0.751) with 56.7% sensitivity and 76.8% specificity, respectively. The ROC curves of GUCI, King Score, and APRI are shown in Figure 1.
Figure 1.
The diagnostic efficiency of GUCI, King Score, and APRI in predicting cirrhosis in Hepatitis-B-associated HCC patients’ post-curative resection. The AUC values of GUCI, King Score, and APRI were 0.722, 0.711 and 0.707, respectively. (A color version of this figure is available in the online journal.)
Univariate analysis of prognostic factors
During a 60.7-month median follow-up (ranged from 36 to 117.8 months), 167 out of 405 (41.2%) patients died after curative hepatic resection. The rates of OS of one, three and five years were 81%, 64%, and 56%, respectively; the median OS was 96.23 months.
The univariate analysis of non-invasive indices showed that APRI (P = 0.000), FIB-4 Index (P = 0.014), Fibro-α Score (P = 0.000), GUCI (P = 0.001), King Score (P = 0.002), and PLASA Score (P = 0.006) correlated with OS (Table 2). The clinicopathologic and biochemical parameters were also subjected to univariate analysis. As shown in Table 2, Child-Pugh grade (P = 0.005), serum AFP (P = 0.001), ALT (P = 0.002), AST (P = 0.000), γ-GT (P = 0.000), tumor number (P = 0.000), maximum size of tumor (P = 0.000), MVTT (P = 0.012), and TNM classification (P = 0.000) were the associated risk factors in patients for survival in HBV-HCC post-hepatectomy.
Multivariate analysis of the prognostic value of non-invasive fibrosis indices
Significant factors in the univariate analysis were included into a multivariate Cox proportional hazards model as covariates by forward stepwise selection. Multivariate analysis show that Child-Pugh classification (HR: 2.548; 95%CI: 1.374–4.726, P = 0.005), maximum tumor size (HR, 1.482; 95%CI, 1.263–1.738, P = 0.000), TNM stage (HR, 1.655; 95%CI, 1.348–2.032, P = 0.000) were the independent prognostic predictors of OS (Table 2). However, APRI (HR, 1.550; 95%CI, 1.101–2.183, P = 0.012), Fibro-α Score (HR, 1.420; 95%CI, 1.030–1.960, P = 0.033) were the only independent indices to predict OS among the 15 non-invasive fibrosis indices analyzed.
Subgroup analysis according to cirrhosis status
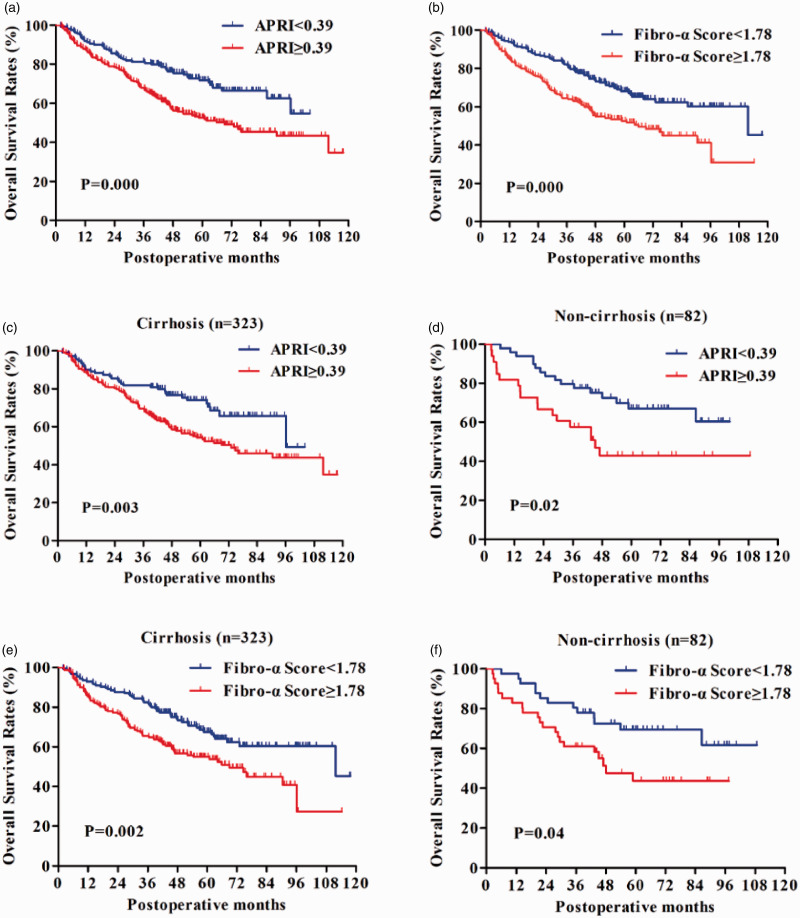
The prognostic values of APRI and Fibro-α Score were further assessed by grouping the patients according to the cirrhosis status. Figure 2(a) shows a significant variation in two groups in terms of OS of APRI <0.39 and ≥0.39 (P = 0.003 and P = 0.002), respectively.
Figure 2.
The impact of APRI and Fibro-α Score on OS in 405 hepatitis-B-associated HCC patients’ post-curative resection. Plotting of survival curves was done by Kaplan-Meier methods along with log-rank test comparison. (a) Overall survival (OS) rates stratified by APRI using 0.38 as the cut-off value. Higher APRI resulted in poorer overall survival rates. (b) The rates of OS stratified by Fibro-α Score using a cut-off value of 1.78. Patients with a Fibro-α Score <1.78 had longer overall survival than those with Fibro-α Score ≥1.78. (c, d) The APRI <0.38 group exhibited a statistically better prognosis in cases with or without cirrhosis than the ≥0.38 group. (e) and (f) In patients with or without cirrhosis, a significant difference in OS between Fibro-α Score <1.78 and the ≥1.78 groups was observed. (A color version of this figure is available in the online journal.)
As shown in Figure 2(b), patients with a Fibro-α Score of <1.78, the OS, respectively, were significantly improved than the ≥ 1.78 group (P = 0.002 and P = 0.04). In short, APRI and Fibro-α Score were both able to segregate the patients with different OS rates.
Discussion
Hepatitis virus infections and/or heavy alcohol consumption are the critical milestones for the global occurrence of cirrhosis and HCC. In China, it is predicted greater than 80% cases of HCCs arise from acute HBV infection-related liver cirrhosis.28 Chronic liver injury, necroinflammation, hepatocellular regeneration, advanced fibrosis, cirrhosis, and malignant transformation of proliferating hepatocyte might involve in the process of hepatocarcinogenesis.29 Until now, liver biopsy was deemed as the benchmark in assessing hepatic fibrosis,30 but its clinical use is limited due to certain drawbacks. Over- or underestimation of fibrosis can be possible due to the sampling errors and subjectivity in interpretation.12,31 Also, it is an expansive and invasive process which may lead to complications including pain, bleeding, and injury to the bile duct, or perforation of the abdominal viscera. These disadvantages may directly or indirectly lead to the patient’s poor compliance or follow-up. Consequently, alternative non-invasive processes to predict fibrosis have been an attractive field of research. To date, at least 30 different kinds of non-invasive fibrosis panels have been established in chronic liver disease patients, various etiologies including virus infection (HBV and/or HCV), alcohol, non-alcoholic fatty liver disease. However, most non-invasive indices were originally proposed for the use in HCV-infected patients; the application of indices to the cohort of HBV patients remains controversial.32,33 And more importantly, these markers are used less frequently in patients with HBV-related HCC. Despite showing that advanced fibrosis or cirrhosis causes a significant effect on HCC patients’ prognosis following surgical resection,5,34 the performance of these non-invasive indices associated with cirrhosis in the prediction of survival has not been elaborately studied. Therefore, we finally selected 15 fibrosis indices, including AA,13 AARP,14 AARPRI,15 API,16 APRI,17 CDS,18 FIB-4 Index,19 Fibro-α Score,20 fibrosis index,21 fibro Q,22 GUCI,23 King Score,24 P2/MS,25 PLASA Score,26 and Pohl Score27 which were derived to start with from assessment of HCV-associated fibrosis, routinely available clinical parameters, and easily monitored with repeated calculations to evaluate their diagnostic performance for the prediction of cirrhosis as well as their role in the patient prognosis with HBV-HCC post-curative resection.
Recently, Pang et al.35 investigated the prognostic significance of 12 cirrhosis-related non-invasive HCC models. They demonstrated that GUCI and APRI could predict cirrhosis status and prognosis in patients with HCC at significantly accurate level. Our findings identified preoperative APRI and Fibro-α Score as factors that acted independently in predicting the outcome of HBV-HCC patients at a long-term post-hepatectomy, besides Child-Pugh classification, size of tumor, and TNM stage findings, which were also consistent with previous studies. GUCI, King Score, and APRI had a superior diagnostic performance in detecting cirrhosis with AUC > 0.7. According to the consensus, APRI and GUCI are non-invasive indices that are directly correlated with cirrhosis status in HCC patients after hepatectomy, but APRI is also a self-sufficient risk factor for HCC patient survival. The differences in our study populations seem to provide an explanation to reconcile the discrepancies. Our study enrolled HBV-related HCC patients undergoing curative hepatectomy, but other studies investigated the patients without a single etiology. Additionally, we analyzed 15 non-invasive cirrhosis-related indices which were inconsistent in previous studies.
APRI was reported and used to predict cirrhosis and acute fibrosis in chronic hepatitis C cases.17 The utility of APRI is enhanced because it is determined using only two routine laboratory parameters that are tested in each patient. The APRI reveals significant performance in predicting fibrosis associated with HCV, but its role in predicting the fibrosis stage of HBV patients is controversial.36,37 In our study, the ROC of APRI was 0.707 which is not consistent with previous studies. It could be due to our different inclusion criteria. The current study showed a good APRI discriminating potential to predict patient OS with HBV-associated HCC going through curative resection, concurrent with other studies.38,39 Hung et al.38 reported that APRI ≤ 0.47 indicates higher survival and lower recurrence rate in solitary small HBV-related HCC patients who underwent resection. Later, another study further confirmed that APRI correlates with poor prognosis in hepatitis B-induced HCC patients.39 In comparison with the two studies, the strengths of our study include a relatively large sample size (n = 405) with a heterogeneous population of HBV infection patients and a longer duration of prospective follow-up.
Presumably, no previous study has assessed the prognostic value of Fibro-α Score in HBV-related HCC. Our study suggested that Fibro-α Score is another independent factor that influenced the OS rate of HCC patients after hepatectomy. Also, in subgroups of cirrhosis or non-cirrhosis, Fibro-α Scores were able to differentiate patients with good prognosis from those with poor outcome. This result would guide clinicians to take up preventive and therapeutic methods for the high-risk patients. The Fibro-α Score was first proposed for the prediction of acute liver fibrosis.20 The formula of the Fibro-α Score integrates AFP with AST/ALT and PLT. The researchers had thought that the elevated AFP levels were also detected in chronic hepatitis C cases and labeled it as the most efficient marker among others in acute liver fibrosis patients. Our findings were not consistent with the other AFP study which showed its superior efficacy in detecting cirrhosis in HCC patients compared to the other non-invasive indices.
In contrast with the APRI and Fibro-α Score, the status of liver cirrhosis was not associated with postoperative survival rates in the current study. Meanwhile, subgroup analysis indicates that APRI and Fibro-α Score were significantly affecting the OS independently in HCC patients whether cirrhosis was present or not. The results suggested that the HCC outcomes may more significantly correlate with cirrhosis severity, potentially indicated more by these non-invasive indices than cirrhosis itself. Certainly, this discrepancy between the non-invasive assessment and the histopathological assessment of the resected specimen observed in our study might be explained in effective ways. The histopathological examination of liver fibrosis was based on non-cancerous liver tissue at the periphery of HCC and may not reflect each time the degree of entire liver fibrosis. Usually, fibrosis can be overestimated due to direct extrapolation from the surgical sample.40 Furthermore, Child-Pugh classification which affects OS more may have abrogated the influence of cirrhosis on OS. These explanations also suggest that the non-invasive indices may indicate fibrosis severity of the remaining liver more effectively than histopathological assessment.
It is beyond doubt that there are certain limitations in our study. Several non-invasive indices such as Fibro Test, APGA, PAPAS, Forns index, Lok’s model, Hepascore, Fibros Spect, Fibro meters, and the European liver fibrosis index were not evaluated in our study. Some of them are based on rare tests, which are not readily available in clinical practice and is also costly. Moreover, the formula is also complicated and not suitable for clinical use. All the patients were ethnically Chinese and restricted to those infected with HBV. It is important to confirm our findings in patients with HCC due to other etiologies. Further, the study was conducted only at one institution. Therefore, there is a need of a larger-scale, multicenter and prospective study to validate the results.
To conclude, the results demonstrated that APRI and Fibro-α Scores inversely correlate with patients OS with HBV-related HCC. Meanwhile, GUCI, King Score, and APRI had a superior diagnostic performance for cirrhosis status. Therefore, APRI can likely act as a surrogate index in evaluating hepatic cirrhosis and OS prediction for HBV-related HCC patients after curative hepatectomy.
Authors’ contributions
ZTT, YSS contributed equally to this work. ZTT and YSS wrote the article; LJ and BL collected data; ZTT, YSS and LJ analyzed the patient data; the article was approved by BL approved and the final version of the article was approved by all authors.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The study conformed with current ethical guidelines and the standards of the Helsinki Declaration. Prior approval to the protocol was acquired from the Research Ethics Committee, PLA General Hospital.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108 [DOI] [PubMed] [Google Scholar]
- 2.Cabibbo G, Craxi A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 2010; 14:352–5 [PubMed] [Google Scholar]
- 3.Gassmann P, Spieker T, Haier J, Schmidt F, Mardin WA, Senninger N. Prognostic impact of underlying liver fibrosis and cirrhosis after curative resection of hepatocellular carcinoma. World J Surg 2010; 34:2442–51 [DOI] [PubMed] [Google Scholar]
- 4.Ko S, Kanehiro H, Hisanaga M, Nagao M, Ikeda N, Nakajima Y. Liver fibrosis increases the risk of intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Br J Surg 2002; 89:57–62 [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, Nagorney DM, Belghiti J, Ng IO, Yamaoka Y, Lauwers GY, Vauthey JN. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg 2005; 140:450–457; discussion 457–458 [DOI] [PubMed] [Google Scholar]
- 6.Kow AW, Kwon CH, Song S, Shin M, Kim JM, Joh JW. Risk factors of peritoneal recurrence and outcome of resected peritoneal recurrence after liver resection in hepatocellular carcinoma: review of 1222 cases of hepatectomy in a tertiary institution. Ann Surg Oncol 2012; 19:2246–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grazi GL, Cescon M, Ravaioli M, Ercolani G, Gardini A, Del Gaudio M, Vetrone G, Cavallari A. Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long-term survival and tumour recurrence in a single Centre. Aliment Pharmacol Ther 2003; 17: 119–29 [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Risk factors of poor prognosis and portal vein tumor thrombosis after curative resection of solitary hepatocellular carcinoma. Hbpd Int 2013; 12:68–73 [DOI] [PubMed] [Google Scholar]
- 9.Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol 2002; 81:195–202 [DOI] [PubMed] [Google Scholar]
- 10.Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep 2004; 6:30–6 [DOI] [PubMed] [Google Scholar]
- 11.Castera L, Negre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology 1999; 30:1529–30 [DOI] [PubMed] [Google Scholar]
- 12.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38:1449–57 [DOI] [PubMed] [Google Scholar]
- 13.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988; 95:734–9 [DOI] [PubMed] [Google Scholar]
- 14.Lee IC, Chan CC, Huang YH, Huo TI, Chu CJ, Lai CR, Lee PC, Su CW, Hung HH, Wu JC, Lin HC, Lee SD. Comparative analysis of noninvasive models to predict early liver fibrosis in hepatitis B e antigen-negative chronic hepatitis B. J Clin Gastroenterol 2011; 45:278–85 [DOI] [PubMed] [Google Scholar]
- 15.Tseng PL, Wang JH, Hung CH, Tung HD, Chen TM, Huang WS, Liu SL, Hu TH, Lee CM, Lu SN. Comparisons of noninvasive indices based on daily practice parameters for predicting liver cirrhosis in chronic hepatitis B and hepatitis C patients in hospital and community populations. Kaohsiung J Med Sci 2013; 29:385–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR cooperative study groups. J Viral Hepat 1997; 4:199–208 [DOI] [PubMed] [Google Scholar]
- 17.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26 [DOI] [PubMed] [Google Scholar]
- 18.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1997; 92:1302–4 [PubMed] [Google Scholar]
- 19.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, MSS, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25 [DOI] [PubMed] [Google Scholar]
- 20.Omran MM, Farid K, Emran TM, Attallah AA. Fibro-alpha score as a simple and useful non-invasive test for predicting significant liver fibrosis in chronic hepatitis C patients. Arab J Gastroenterol 2011; 12:74–9 [DOI] [PubMed] [Google Scholar]
- 21.Ohta T, Sakaguchi K, Fujiwara A, Fujioka S, Iwasaki Y, Makino Y, Araki Y, Shiratori Y. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama 2006; 60:77–84 [DOI] [PubMed] [Google Scholar]
- 22.Hsieh YY, Tung SY, Lee IL, Lee K, Shen CH, Wei KL, Chang TS, Chuang CS, Wu CS, Lin YH. FibroQ: an easy and useful noninvasive test for predicting liver fibrosis in patients with chronic viral hepatitis. Chang Gung Med J 2009; 32:614–22 [PubMed] [Google Scholar]
- 23.Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–72 [DOI] [PubMed] [Google Scholar]
- 24.Cross TJ, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King’s score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 2009; 21:730–8 [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Yoon JH, Lee CH, Myung SJ, Keam B, Kim BH, Chung GE, Kim W, Kim YJ, Jang JJ, Lee HS. Complete blood count reflects the degree of oesophageal varices and liver fibrosis in virus-related chronic liver disease patients. J Viral Hepat 2009; 16:444–52 [DOI] [PubMed] [Google Scholar]
- 26.Shehab H, Elattar I, Elbaz T, Mohey M, Esmat G. CUFA algorithm: assessment of liver fibrosis using routine laboratory data. J Viral Hepat 2014; 21:956–64 [DOI] [PubMed] [Google Scholar]
- 27.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol 2001; 96:3142–6 [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Lin Y, Wang YP, Chen YX, Shi B, Lu J, Xie WF. Hepatitis B virus DNA in patients with hepatitis B-related liver cirrhosis with or without hepatocellular carcinomas: a matched case-control study. J Dig Dis 2009; 10:138–44 [DOI] [PubMed] [Google Scholar]
- 29.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–2 [DOI] [PubMed] [Google Scholar]
- 30.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology 2002; 36:S152–160 [DOI] [PubMed] [Google Scholar]
- 31.Bedossa P. Assessment of hepatitis C: non-invasive fibrosis markers and/or liver biopsy. Liver Int 2009; 29:19–22 [DOI] [PubMed] [Google Scholar]
- 32.Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, Paik YH, Lee KS, Park YN, Han KH. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010; 30:546–53 [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, Huang HJ. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT. J Viral Hepat 2013; 20:e3–10 [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Fiel MI, Blank S, Luan W, Kadri H, Kim KW, Manizate F, Rosenblatt AG, Labow DM, Schwartz ME, Hiotis SP. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br J Cancer 2013; 109:573–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang Q, Zhang JY, Xu XS, Song SD, Chen W, Zhou YY, Miao RC, Qu K, Liu SS, Dong YF, Liu C. The prognostic values of 12 cirrhosis-relative noninvasive models in patients with hepatocellular carcinoma. Scand J Clin Lab Invest 2015; 75:73–84 [DOI] [PubMed] [Google Scholar]
- 36.Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading Meta-analysis. BMC Gastroenterol 2012; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis 2008; 40:267–74 [DOI] [PubMed] [Google Scholar]
- 38.Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, Huo TI, Lee PC, Kao WY, Lee SD, Wu JC. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int 2010; 4:691–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, Liang LJ, Guo P, Hao Y, Peng BG. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol 2014; 21:3802–9 [DOI] [PubMed] [Google Scholar]
- 40.Jung HY, Kim SH, Jing J, Gwak JM, Han CJ, Jang JJ, Lee KB. The histologic cut-off point for adjacent and remote non-neoplastic liver parenchyma of hepatocellular carcinoma in chronic hepatitis B patients. Korean J Pathol 2012; 46:349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]