Abstract
The phosphoinositide 3-kinase (PI3-K)/Akt signaling pathway is important in the regulation of cell proliferation through its production of phosphatidylinositol 3,4,5-triphosphate (PIP3). Activation of this pathway is frequently observed in human cancers, including non-small cell lung carcinoma. The PI3-K/Akt pathway is negatively regulated by the dual-specificity phosphatase and tensin homolog (PTEN) protein. PTEN acts as a direct antagonist of PI3-K by dephosphorylating PIP3. Studies have shown that PTEN phosphatase activity is inhibited by PREX2, a guanine nucleotide exchanger factor (GEF). Multiple studies revealed that CELF2, an RNA binding protein, cooperates synergistically with PTEN as a tumor suppressor in multiple cancers. However, the underlying mechanism as to how CELF2 enhances PTEN activity remains unclear. Here, we report that CELF2 interacts with PREX2 and reduces the association of PREX2 with PTEN. Consistent with this observation, PTEN phosphatase activity is upregulated with CELF2 overexpression. In addition, overexpression of CELF2 represses both Akt phosphorylation and cell proliferation only in the presence of PTEN. In an ex vivo study, CELF2 gene delivery could significantly inhibit patient-derived xenografts (PDX) tumor growth. To further investigate the clinical relevance of this finding, we analyzed 87 paired clinical lung adenocarcinoma samples and the results showed that CELF2 protein expression is downregulated in tumor tissues and associated with poor prognosis. The CELF2 gene is located on the chromosome 10p arm, a region frequently lost in human cancers, including breast invasive carcinoma, low-grade glioma and glioblastoma. Analysis of TCGA datasets showed that CELF2 expression is also associated with shorter patient survival time in all these cancers. Overall, our work suggests that CELF2 plays a novel role in PI3-K signaling by antagonizing the oncogenic effect of PREX2.
CELF2 plays a key role in PI3-K signaling by antagonizing the oncogenic effect of PREX2 and inhibits non-small cell lung cancer growth.
Introduction
Lung cancer remains the world’s leading cause of cancer death (1). Non-small cell lung cancer (NSCLC) is the most common form of lung cancer, with a 5-year overall survival rate of approximately 20% (2). NSCLC has a very low response rate to standard therapies, such as chemotherapy and radiotherapy (3). Therefore, a better understanding of the molecular mechanisms implicated in NSCLC development and progression could identify new therapeutic candidates to improve the clinical outcomes of NSCLC.
The phosphoinositide 3-kinase (PI3-K)/Akt signaling pathway is an important signal transduction pathway that regulates cell proliferation, growth, survival, apoptosis and motility (4). Aberrant changes in this signaling pathway could result in malignant transformation. In lung adenocarcinoma, PI3-K activation frequently occurs due to an activating mutation of the epithelial growth factor receptor and the GTPase, Ras (5,6). Once PI3-K is activated, the second messenger phosphatidylinositol 3,4,5-triphosphate (PIP3) is produced by phosphorylation of phosphatidylinositol 3,4,5-biphosphate (PIP2) (4). The phosphatase and tensin homolog (PTEN) protein, is a dual-specificity phosphatase of which the primary function is to negatively regulate PI3-K/Akt signaling by dephosphorylating PIP3 to PIP2. Inactive PTEN leads to accumulation of PIP3 and, consequently, increases PI-3K/Akt signaling, which promotes carcinogenesis (7). Recently, the PIP3-dependent Rac exchanger 2 (PREX2) was identified as a PTEN-interacting protein that attenuates PTEN phosphatase activity (8). PREX2 was originally identified as a guanine nucleotide exchange factor (GEF) for the small GTPase, Rac (9,10). PREX2 consists of two isoforms, PREX2a and PREX2b. PREX2b is a splice variant of PREX2a, which lacks the phosphatase domain at the C-terminal (10). Increasing evidence suggests that PREX2 plays an important role in cancer. Whole genome sequencing also revealed that PREX2 is one of the most frequently mutated GEFs in cancers including lung, pancreatic and skin (11–13). In addition, wild-type PREX2 is also overexpressed in various cancers, including breast, pancreatic and brain (8). All these changes in PREX2 have been implicated in various phases of carcinogenesis such as cell growth, cell motility and tumor formation (8,13,14). Although many studies have reported the oncogenic role of PREX2, novel functional and cellular regulatory characteristics of this protein have yet to be explored.
RNA-binding proteins (RBPs) are proteins that bind to double- or single-stranded RNA and regulate the expression of thousands of transcripts (15). Currently, several thousand RBPs have been identified and studies have shown that RBPs are ubiquitously expressed and evolutionarily conserved (16,17). However, their biological functions and mechanisms remain largely unknown. CELF2 is a RBP belonging to the CELF family of proteins (18). The CELF family contains six members, CELF1 to CELF6. CELF2 and CELF1 are founding members of the CELF family with protein sequences being 76% identical (19). Comprehensive genomic analysis showed that RBPs, including CELF2, are predominantly downregulated in tumors compared with normal tissues and play a crucial role in tumor development (20). Similar to other RBPs, CELF2 has been shown to regulate cancer-related transcripts, such as the inhibition of the translation of the MCL-1 and COX-2 transcripts (21,22). More recently, a high-throughput sequencing study emphasized the synergistic effect of CELF2 in preventing malignant progression in cooperation with PTEN (23). However, the underlying mechanism of CELF2 cooperation with PTEN leading to tumor suppression is still not understood.
In the present study, we found that CELF2 is downregulated in NSCLC and is associated with poor clinical outcome in patients. Furthermore, instead of mRNA regulation, we showed a non-canonical function of CELF2 binding to PREX2, which in turn, led to a reduction of the interaction of PREX2 with PTEN. We then demonstrated that CELF2 overexpression in NSCLC cancer cells with functional PTEN could antagonize Akt signaling and thus, substantially inhibit proliferation. Finally, we used adeno-associated virus (AAV) technology and found that CELF2 gene overexpression significantly suppressed tumor growth of NSCLC patient-derived xenografts (PDX). Overall, our findings demonstrate that CELF2 is a novel tumor suppressor and could be considered as a potential candidate for gene therapy in NSCLC.
Materials and methods
Chemicals and reagents
All chemicals and reagents were purchased from Sigma–Aldrich (St. Louis, MO) unless stated otherwise. Rosewell Park Memorial Institute (RPMI)-1640 medium, Dulbecco’s modified eagle medium and fetal bovine serum (FBS) were obtained from Biological Industries (Beit-Haemek, Israel). Gentamicin, antibacterial-antimycotic solution, trypsin- ethylenediaminetetraacetic acid (EDTA) and Opti-MEM were all from Life Technologies, Inc. (Grand Island, NY). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA) and mammalian protease cocktail inhibitors were from MedChem Express (Princeton, NJ).
Antibodies
Antibodies against CELF2 (Cat# 186430) and PREX2 (Cat# 121462) were purchased from Abcam (Cambridge, MA). The PTEN antibody (Cat# sc-7974) and the secondary antibodies against rabbit (Cat# sc-2004) and mouse (Cat# sc-2005) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other antibodies, including β-actin (Cat# 4970), glyceraldehyde 3-phosphate dehydrogenase (Cat# 2118), lamin A/C (Cat# 4777), Akt (Cat# 4691), p-Akt (Ser473; Cat# 4060), p-Akt (Thr308; Cat# 13038), p-PTEN (Ser380/Thr382/383; Cat# 9549), HA-Tag (Cat# 3724), Myc-Tag (Cat# 2278) and V5-Tag (Cat# 13202), were purchased from Cell Signaling Technology (Danvers, MA).
Immunohistochemistry staining
The human lung adenocarcinoma tissue array was purchased from Shanghai Outdo Biotech Co. Ltd (Cat #HLug-Ade180Sur; Shanghai, China). The array was deparaffinized in xylene solution and rehydrated using gradient ethanol concentrations. Antigen retrieval was performed using sodium citrate and the slides were then incubated with H2O2 to block endogenous peroxidases. Thereafter, the primary antibodies, including CELF2 (1:100), phosphorylated (p)-Akt (1:100), p-PTEN (1:100) and PREX2 (1:100), were incubated at 4°C overnight and the signals were visualized by the indirect avidin biotin-enhanced horseradish peroxidase method according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). After development, all sections were observed by microscope (400×) and quantitative analysis was performed using the Image-Pro Premier software (v.9.0) program (Media Cybernetics, Rockville, MD).
Plasmid construction
The pLKO.1 (lentiviral backbone; Addgene plasmid #8453) was a gift from Bob Weinberg (24). The psPAX2 (a packaging vector) and pMD2.G (an envelope vector) were gifts from Didier Trono (Addgene plasmid #12259 and #12260). The PREX2 pcDNA3.1 V5/His was a gift from Ramon Parsons (Addgene plasmid #41555) (8). The open-reading frame of CELF2 (NM_001025076.2) was synthesized on the pcDNA4/HisMax A (Invitrogen, Fremont, CA) backbone at the BamHI/XhoI sites by Genewiz Inc. (Beijing, China). Using CELF2 pcDNA4/His as a DNA template, the CELF2 gene was then PCR-amplified and cloned into the SalI/BglII sites of the pCMV-HA vector (Clontech, Palo Alto, CA) and the BglII/XhoI sites of the pAAV-IRES-hrGFP vector (Stratagene, La Jolla, CA). The pET-46 Ek/LIC vector was obtained from Novagen (Darmstadt, Germany) and the CELF2 gene was inserted according to the manufacturer’s instructions. To construct the Myc-tagged expression vector of the PREX2 domains, the DNA sequences corresponding to DH (aa 23–214), PH (aa 245–365), DEP1 (aa 390–465), DEP2 (aa 489–566), PDZ1 (aa 591–673) and PDZ2 (aa 675–758) were amplified by PCR using specific primers and PREX2 pcDNA3.1 V5/His as a template. The PCR products were then digested and inserted into the XhoI/NotI sites of the pCMV-Myc vector. All the primers used for vector construction are listed in Supplementary Table 1, available at Carcinogenesis Online. The CELF2 shRNA sequences, C1 (5′-CCCAGAATGCACTGCACAATA-3′) and C2 (5′-GATCGGCATGAAACGCTTGAA-3′), were cloned into the pLKO.1 backbone at the AgeI and EcoRI restriction sites. The pLKO.1-puro Non-Target shRNA Control Plasmid DNA (Scramble) was purchased from Sigma–Aldrich. All the constructed vectors were confirmed by direct DNA sequencing (Genewiz Inc.). The sequences of all primers used for plasmid construction are also listed in Supplementary Table 1, available at Carcinogenesis Online.
Cell culture and transfection
All cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) except the gefitinib-resistant HCC827 cell line (HCC827GR), which was kindly provided by Dr Pasi A. Jänne (Harvard Medical School, Boston, MA). All cells were cytogenetically tested and authenticated before freezing. All cell culture conditions were created and maintained following ATCC’s instructions. All lung adenocarcinoma cells, including A549, H1299, H1975, HCC827, HCC827GR, H1650 and H460 cells lines, were cultured in RPMI-1640, whereas U87, U251 and HEK293T cells were cultured in Dulbecco’s modified eagle medium, supplemented with 10% (v/v) FBS, 2 mM glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2. Each vial of frozen cells was thawed and maintained in culture for 10–20 passages. A549 and H1299 cells stably expressing pcDNA4/His-CELF2 were established using Lipofectamine 2000 transfection reagent (Thermo Scientific, Fremont, CA) followed by Zeocin selection (200 µg/ml). All transient transfections were performed with Lipofectamine 2000 following the manufacturer’s protocol.
Lentivirus production and infection
The lentiviral vector was triple-transfected using Lipofectamine 2000 (Invitrogen) into HEK293T cells together with the psPAX2 (packaging vector) and pMD2.G (envelope vector) vectors. Viral supernatant fractions were harvested at 24 and 48 h. The pooled supernatant fractions were then filtered through a 0.45 µm PVDF filter and frozen at −80°C for later use. The appropriate cells were infected with the viral supernatant fraction together with 8 µg/ml polybrene (Millipore, Billerica, MA). After overnight infection, 7.5 mg/ml puromycin was subsequently used to select the stably transduced cells.
Western blot analysis
Cells were rinsed with ice-cold phosphate-buffered saline and disrupted in lysis buffer containing 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 100 mM NaCl, 5 mM MgCl2, 1% (v/v) Triton X-100, 5 mM NaF, 10% (v/v) glycerol, 0.5 (v/v) 2-mercaptoethanol, 0.1 mM Na3VO4 and protease cocktail inhibitors. After centrifugation at 12,000 × g for 15 min at 4°C, the protein concentration of the cleared cell lysates was determined using the bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rockford, IL), following the manufacturer’s instructions. Cell lysates were separated by 8–15% SDS-PAGE and transferred to Immobilion-P (Millipore) in 20 mM Tris-HCl (pH 8.0), containing 150 mM glycine and 20% (v/v) methanol. Membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 (TBS-T) and incubated with their specific primary antibodies. The membranes were then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody and visualized using the enhanced chemiluminescence reagent (Millipore).
Protein purification and bead pulldown
To purify the His6-CELF2 fusion protein, the pET46-Ek/LIC vector encoding His6-CELF2 was transformed into BL21 (DE3) cells and a single colony was picked. The cells were then grown in LB broth containing 100 mg/l ampicillin. When the absorbance at 600 nm (A600) reached 0.4, protein expression was induced by the addition of 0.1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) for 3 h at 37°C. Cells were disrupted and resuspended in 15 ml of buffer containing 50 mM Tris; pH 7.4, 500 mM NaCl, phenylmethylsulfonyl fluoride, 1 mM NaVO3, 1 mM EDTA and protease cocktail inhibitors. The cell lysate was vortexed, sonicated and the soluble-protein fraction was recovered by centrifugation for 1 h at 20,000 × g at 4°C. The lysate was added to 0.2 ml of 50% (v/v) Ni-NTA bead slurry that was pre-equilibrated in ice-cold lysis buffer. The mixture was incubated and rotated at 4°C overnight to enable sufficient binding of the His6-CELF2 protein to the Ni-NTA beads. The beads were then washed with buffer (50 mM Tris, pH 7.4, 500 mM NaCl, phenylmethylsulfonyl fluoride, 1 mM NaVO3, 1 mM EDTA, protease cocktail inhibitors, 100 mM imidazole) and centrifuged five times at 1200 rpm for 2 min each at 4°C. The purity of His6-CELF2 in the Ni-NTA bead slurry was determined by SDS-PAGE followed by Coomassie Blue staining. For pulldown of CELF2, 20 µl of CELF2 bead slurry was incubated with HCC827 cell lysates for 6 h rotating at 4°C. The beads were washed five times in lysis buffer and resuspended in 20 µl of 2× Laemmli sample buffer. Three independent samples were analyzed by SDS-PAGE and the bands were excised. The protein bands were digested overnight with sequencing grade trypsin. These digests were analyzed by microcapillary liquid chromatography/tandem mass spectrometry (LC-MS/MS) and the MS/MS spectra were searched against the reversed and concatenated Swiss-Prot protein database (25).
Co-immunoprecipitation
A full-length V5-PREX2 domain or Myc-tagged PREX2 domain was transfected into HEK293T cells together with HA-CELF2. Cells were washed with ice-cold phosphate-buffered saline and disrupted in lysis buffer (25 mM Tris, 250 mM NaCl, 0.1% Triton X-100, 10% glycerol, 1 mM NaVO3, 1 mM NaF, protease cocktail inhibitors pH 8.0). The cells were vortexed, sonicated and then centrifuged at 20,000 × g for 30 min at 4°C. The protein concentrations of the lysates were measured as above and protein concentration was equalized for all samples. The samples were then precleared with rabbit IgG and protein A/G Plus agarose for 1 h with rotation at 4°C. Finally, the samples were immunoprecipitated with 5 µl of anti-HA overnight followed by incubation with 20 µl protein A/G agarose beads for another 2 h at 4°C. The beads were then washed five times and resuspended in 2× Laemmli sample buffer. For endogenous coimmunoprecipitation, the same protocol (2.5 µg of CELF2 antibody) was followed except that the proteins were from HCC827 cancer cells and lung tissues of SCID mice.
PTEN lipid phosphatase activity
For measurement of in vitro PTEN lipid phosphatase activity, the malachite green phosphatase assay kit (Echelon Biosciences Inc., Salt Lake City, UT) was used following the manufacturer’s instructions. Briefly, cells were disrupted in lysis buffer and 750 µg of cell lysate were immunoprecipitated with 8 µl of anti-PTEN overnight at 4°C. The immunocomplex was then captured by incubation with 20 µl protein A/G agarose beads for another 2 h at 4°C. The beads were then washed three times and used for western blot and phosphatase assays. For the phosphatase assay, the beads were washed, resuspended in enzyme reaction buffer (ERB; 50 mM Tri-HCl, pH 8.0, 50 mM NaCl, 10 mM dithiothreitol and 10 mM MgCl2) and distributed in triplicates of 20 µl each in a 96-well flat-bottom plate. The reaction was initiated by adding 30 µl of ERB containing 5 µM dioctanoyl phosphatidylinositol 3,4,5-triphosphate. The reaction was then terminated with 100 µl malachite green reagent and free phosphate levels were measured at an absorbance at 620 nm after 15 min of incubation at room temperature. The PTEN activity was normalized to the corresponding parental cells (set as 100%).
Immunofluorescence
Cells (1 × 103) plated on gelatin-coated coverslips were fixed in 4% paraformaldehyde for 1 h, permeabilized with 0.1% Triton X-100, and blocked with 10% goat serum. PTEN and PREX2 antibodies were each applied at a 1:100 dilution. Appropriate fluorescence-conjugated secondary antibodies were used at 1:200 after washing. Coverslips were counterstained with 4′,6-diamidino-2-phenylindole and mounted with DAKO® fluorescent mounting medium (Dako Corporation, Carpinteria, CA). The stained cells were visualized using a Nikon confocal microscope A1R+ and Nis-Elements software. Each image is a single Z section taken at the same cellular level.
Nuclear and cytoplasmic fractionation
Subcellular fractionation was performed using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL) following the manufacturer’s instructions. The extracts were stored at −80°C prior to western blot analysis. The purity of nuclear and cytoplasmic fractions was verified using lamin A/C and β-tubulin antibodies.
Cell proliferation assay
Cells (3000 per well) were plated in a 96-well plate in at least triplicate for each experiment. Cells were allowed to proliferate for 72 h and then 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 0.3 mg/ml) was added and cells incubated for 1 h at 37°C. The reaction was terminated by the addition of 100 µl dimethyl sulfoxide. The optical density of MTT formazan formation was read at 490 nm on a microplate reader. Absorbance values were normalized as percentage of respective parental cells (set at 100%).
Anchorage-independent cell growth assay
Cells (8 × 103) were suspended in 1 ml RPMI-1640/10% FBS/0.33% agar and plated on 3 ml solidified RPMI-1640/10% FBS/0.5% agar in each well of six-well plates in triplicate and cultured for 3 weeks. Images of each well from five independent fields were captured by a microscope and by using the Image-Pro Plus software program. Colony numbers greater than 200 pixels were quantified using the Image J software program (NIH).
AAV-mediated CELF2 overexpression in an NSCLC patient-derived xenograft model
This study was performed according to guidelines approved by the Zhengzhou University Institutional Animal Care and Use Committee. The AAVs were packaged by co-transfecting the pAAV-IRES-hrGFP, pHelper and pAAV-RC plasmids into HEK293T cells according to the manufacturer’s protocol. Cells were harvested at 72 h post-transfection and disrupted by freezing and thawing three times. The AAVs were then precipitated and purified as previously described (26). AAV particles (5 × 1012) were diluted in 10 ml of the Defined Keratinocyte-Serum Free Medium (27). The PDX tissues were infected with the AAV virus in medium and maintained at 37°C in a humidified atmosphere with 5% CO2 for 16 h before inoculating into SCID mice. Tumor measurements were performed twice a week and tumor volume was calculated based on the formula: length × width2 × 0.5. At the end of the experiment, mice were killed prior to removal of the tumors for further analysis.
Statistical analysis
Each experiment was performed three times independently. All quantitative data are expressed as mean values ± standard error of the mean and significance between groups was determined by using either the Student’s t-test or one-way analysis of variance unless otherwise indicated. Asterisks indicate (*, **, ***) P values of < 0.05, < 0.01 and < 0.001, respectively. Survival curves were plotted using the Kaplan–Meier method and compared using a log-rank test.
Results
CELF2 expression is downregulated in lung adenocarcinoma tissues and is associated with poor prognosis
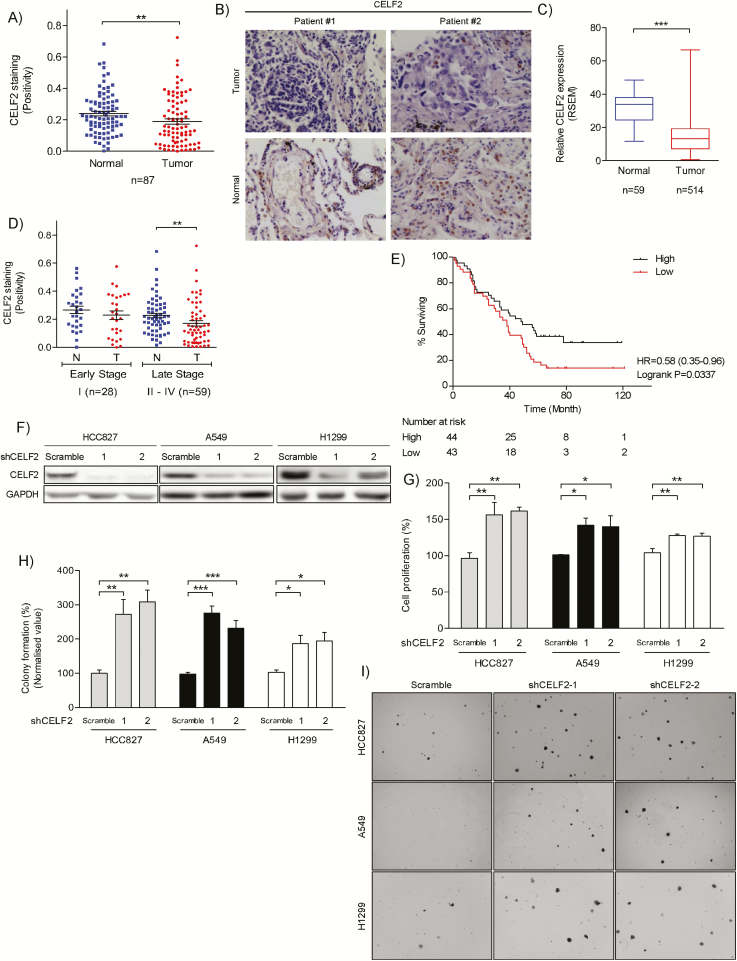
We first analyzed CELF2 expression in 87 paired lung adenocarcinoma specimens. Immunohistochemical analysis demonstrated that CELF2 expression was significantly downregulated in lung adenocarcinoma compared to paired non-tumor adjacent tissues (Figure 1A and B), which is consistent with the results of the TCGA sample analysis (LUAD; Figure 1C). Next, we examined the relationship between CELF2 expression and pathological stages of NSCLC. Results revealed no significant difference in CELF2 expression between NSCLC tissues and non-tumor adjacent tissues in patients with early-stage disease. However, CELF2 expression was significantly lower in tumor tissues compared with non-tumor adjacent tissues as NSCLC progresses to later stages (Figure 1D). We then used the Kaplan–Meier plot to assess the relationship between CELF2 expression and overall survival. Results clearly indicated that higher CELF2 expression was associated with significantly longer survival time in patients with NSCLC compared to those with lower CELF2 expression (Log-rank P = 0.037, HR = 0.58; Figure 1E). Because CELF2 expression is downregulated in NSCLC tissues, we hypothesized that CELF2 downregulation might be responsible for uncontrolled cancer cell growth. Therefore, to examine the function of CELF2 in cell proliferation, we used two different shCELF2 sequences, C1 and C2, to generate CELF2 knockdown HCC827, A549 and H1299 lung cancer cells (Figure 1F). Our results showed that silencing of CELF2 significantly promoted both cell proliferation (Figure 1G) and anchorage-independent cell growth (Figure 1H and I). These findings suggest that CELF2 downregulation plays a significant role in NSCLC progression.
Figure 1.
Downregulation of CELF2 promotes lung adenocarcinoma and is associated with poor prognosis. (A) Immunohistochemical analysis of CELF2 protein expression in normal and lung adenocarcinoma tissues (n = 87). The integrated optical density (IOD) was evaluated using the Image-Pro premier software offline (v9.0) program. (B) Representative images of CELF2 expression in clinical samples described in A. (C) The expression level of CELF2 mRNA in lung adenocarcinomas from the TCGA sample set is compared between primary tumors (n = 514) and solid normal tissues (n = 59). (D) Box-and-whiskers plot showing CELF2 protein expression in normal and cancer tissues in early-stage (I) and late stage (II-IV) lung adenocarcinoma in our sample set. (E) Kaplan–Meier estimate of lung adenocarcinoma survival time based on CELF2 protein expression of 87 patients in our sample set. (F) Western blot analysis of CELF2 expression in HCC827 (left), A549 (middle) and H1299 (right) lung adenocarcinoma cells transduced with shCELF2 or scramble (as a control). (G) Knockdown of CELF2 promotes cell proliferation and (H) anchorage-independent cell growth. Results were normalized to corresponding parental cells. (I) Representative images from anchorage-independent growth assay.
CELF2 is a novel PREX2-interacting protein that inhibits the interaction between PREX2 and PTEN
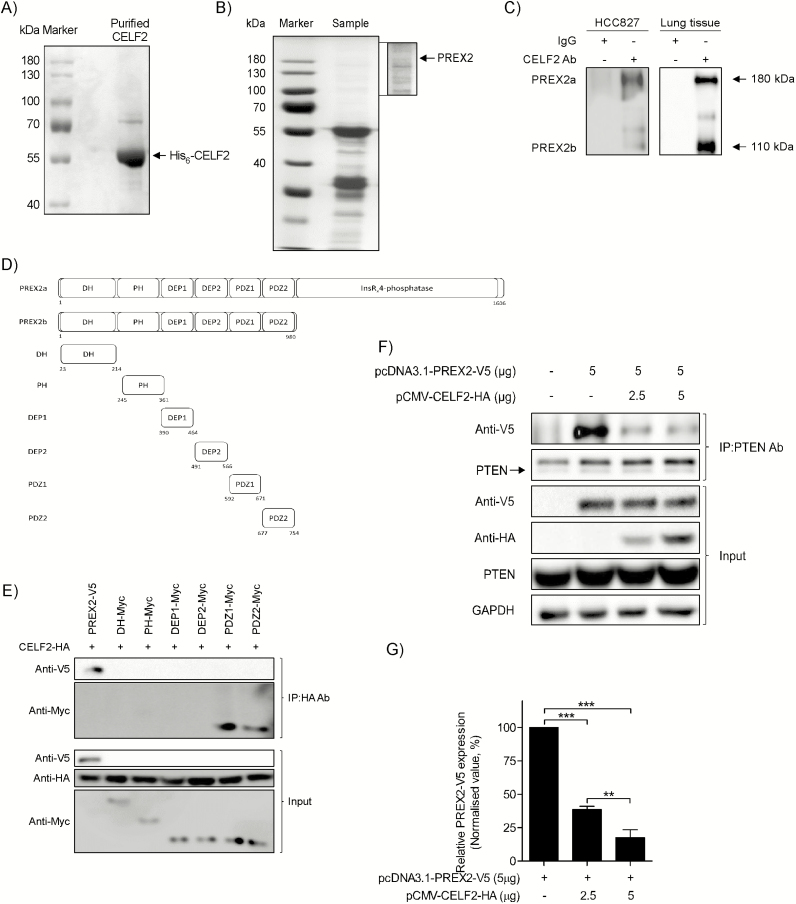
To elucidate the cellular function of CELF2, we identified CELF2-interacting proteins by affinity purification. The His6-CELF2 protein was first purified from Escherichia coli by using Ni-NTA agarose beads. The purified His6-CELF2 protein was separated by SDS-PAGE and stained with Coomassie Blue (Figure 2A). Next, pulldown experiments to detect the His6-CELF2 protein were performed using the HCC827 cell line. Mass spectrometry analyses revealed a total of 47 proteins and identified PREX2 as a CELF2-interacting partner. The silver-stained gel is shown in Figure 2B. Next, the endogenous interaction of PREX2 and CELF2 was confirmed using co-immunoprecipitation in the HCC827 cell line and in mouse lung tissues (Figure 2C). The CELF2 antibody precipitated both the PREX2a and PREX2b isoforms. To identify the domain of PREX2 involved in its association with CELF2, we constructed a series of Myc-tagged domains of PREX2 and the schematic diagram is shown as Figure 2D. Co-immunoprecipitation experiments using HA-tagged CELF2 revealed that both the PDZ1 and PDZ2 domains of PREX2 interacted with CELF2 (Figure 2E). Increasing evidence suggests that PREX2 is an oncogene that inhibits PTEN function. Having identified an interaction between CELF2 and PREX2, we investigated whether CELF2 binding with PRX2 could affect the association of PREX2 and PTEN. A PREX2-V5 plasmid was transfected into HEK293T in the presence of increasing plasmid concentrations of CELF2-HA (0–5 µg). Following immunoprecipitation of PTEN, transfected PREX2-V5 was co-immunoprecipitated and quantified by Western blotting. Increasing levels of CELF2 expression reduced the association between PREX2 and PTEN (Figure 2F and G), suggesting that increasing amounts of CELF2 disrupted the PREX2 and PTEN interaction.
Figure 2.
CELF2 interacts with the PDZ domains of PREX2 and reduces the PTEN-PREX2 interaction. (A) Coomassie stain of purified His6-CELF2 purified from BL21 (DE3) cells by using Ni-NTA agarose beads. (B) Silver staining of affinity-purified CELF2-interacting proteins. HCC827 cell lysates were incubated with His6-CELF2 for 6 h at 4°C. All CELF2-interacting proteins were separated by SDS-PAGE and identified by LC-MS/MS. PREX2 proteins are indicated by arrows. (C) Co-immunoprecipitation of endogenous CELF2 and PREX2 in HCC827 NSCLC cells (left) and lung tissues from SCID mice (right). HCC827 cell lysates and protein extracts from mouse lung tissues were immunoprecipitated with anti-CELF2 or control IgG. The immunoprecipitated complex was detected by Western blotting with anti-PREX2. (D) Schematic representation of N-terminally Myc-tagged PREX2 domains of Myc-tagged constructs. (E) CELF2 interacts with the PDZ1 and PDZ2 domains of PREX2. The HA-CELF2 and V5-PREX2 or Myc-PREX2 domains were co-transfected into HEK293T cells and cell lysates were harvested after 48 h. HA immunoprecipitations and whole cell lysates were immunoblotted with anti-Myc and anti-V5. (F) Concentration-dependent disruption of PREX2-PTEN complexes by CELF2. 5µg V5-PREX2 plasmid was co-transfected with increasing plasmid concentrations of HA-CELF2 (0–5 µg) into HEK293T cells. Following immunoprecipitation of PTEN, Western blot analysis was performed to detect the levels of V5-PREX2 co-immunoprecipitated with PTEN. Immunoblots of transfected HA-CELF2, V5-PREX2 and PTEN are also shown. (G) The ratio of immunoprecipitated V5-PREX2 to PTEN was quantified from 3 independent experiments.
Overexpression of CELF2 enhances PTEN activity and decreases PI3-K/Akt signaling
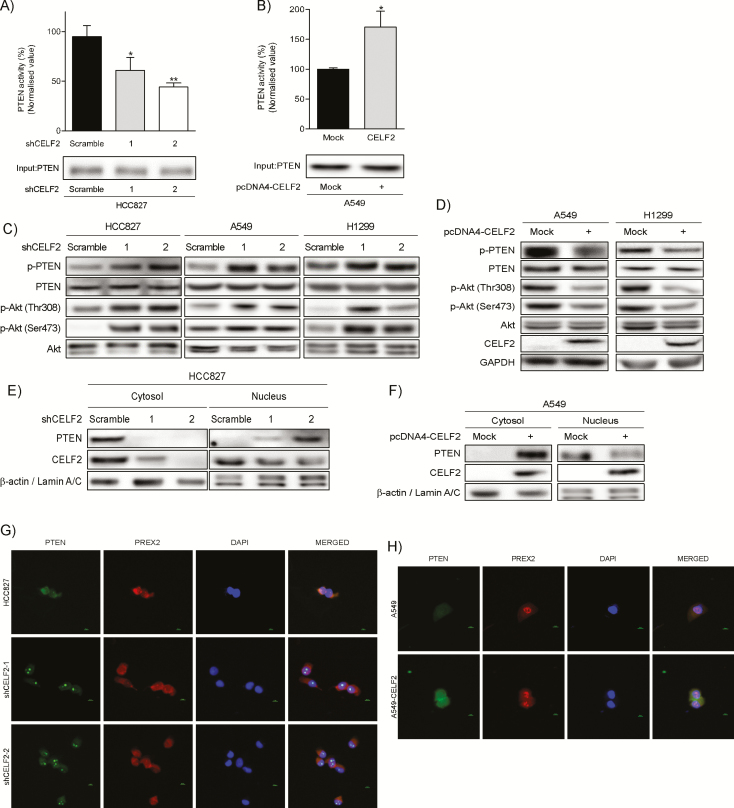
The above results prompted us to examine the effect of CELF2 on PTEN activity. We thus measured the phosphatase activity of PTEN that was immunoprecipitated from cell lysates with either knockdown or overexpression of CELF2. Our results show that PTEN activity was significantly decreased upon silencing of CELF2 (Figure 3A), whereas stable overexpression of CELF2 increased PTEN activity (Figure 3B). To further examine the effect of CELF2 on PIP3 signaling, we analyzed the phosphorylation status of Akt in NSCLC cells with overexpression or knockdown of CELF2. Immunoblotting results showed that both PTEN and Akt phosphorylation were upregulated in CELF2 knockdown cells (Figure 3C), whereas phosphorylation was downregulated with CELF2 overexpressing cells (Figure 3D). Because cytoplasmic PTEN mainly contributes to its phosphatase activity, we next investigated whether PTEN subcellular localization is affected by CELF2 expression (28). Western blot analysis of cytosolic and nuclear fractions showed that PTEN is mainly localized in the nucleus in CELF2 knockdown cells (Figure 3E), whereas PTEN is mainly located in the cytoplasm in CELF2 overexpressing cells (Figure 3F). Immunofluorescence images also confirmed these findings in cells with knockdown (Figure 3G) and overexpression (Figure 3H and Supplementary Figure 1, available at Carcinogenesis Online) of CELF2. Interestingly, we also observed that PTEN was localized in the nucleolus of CELF2 knockdown cells. To further validate this finding in clinical samples, we analyzed protein levels of non-phosphorylated PTEN (non-phos-PTEN) and phosphorylated (p-) Akt (Ser473) in 87 tumor tissues and examined their relationship to CELF2 expression. Correlation analysis revealed that expression of non-phos-PTEN level was directly proportional to CELF2 level (Supplementary Figure 2A, available at Carcinogenesis Online), whereas expression of p-Akt (Ser473) was inversely proportional to CELF2 level (Supplementary Figure 2B, available at Carcinogenesis Online). Not surprisingly, non-phos PTEN also showed a negative correlation to p-Akt (Ser473) (Supplementary Figure 2C, available at Carcinogenesis Online). Taken together, these results indicated that CELF2 expression is associated with PTEN phosphatase activity to attenuate PI3-K/Akt signaling.
Figure 3.
CELF2 expression affects PTEN activity and PI3-K/Akt signaling. An in vitro PTEN phosphatase assay in CELF2 knockdown (A) and overexpressing (B) lung cancer cells. Cell lysates were harvested and incubated overnight with a PTEN antibody conjugated with agarose beads. Phosphatase assays were performed on immunoprecipitated protein complexes and the phosphatase activity was normalized as a percentage of parental cell activity (set as 100%). (C) Levels of phosphorylated PTEN and Akt in CELF2 knockdown cells were analyzed by Western blotting. (D) Level of CELF2 expression, phosphorylated PTEN and Akt in CELF2 overexpressing cells. Subcellular localization of PTEN in CELF2 knockdown (E) and overexpressing (F) cells was analyzed by Western blotting. Immunofluorescence of PTEN and PREX2 in CELF2 knockdown (G) and overexpressing (H) cells. PTEN was stained with a mouse monoclonal antibody and Alexa Fluor-secondary antibody. PREX2 was stained with a rabbit monoclonal antibody and Alexa Fluor-secondary antibody and nuclei were stained with 4′,6-diamidino-2-phenylindole.
PTEN is an essential partner with CELF2 acting as a tumor suppressor in lung adenocarcinoma
We next evaluated whether CELF2 overexpression could inhibit proliferation of lung cancer cells. We found that overexpression of CELF2 substantially repressed both anchorage-independent growth (Figure 4A and B) and proliferation (Figure 4C) of both A549 and H1299 lung cancer cells. We then investigated whether CELF2 could act as a tumor suppressor without PTEN. First, we analyzed PTEN expression in various NSCLC and glioblastoma (GBM) cell lines. Western blot analysis showed that H1650 NSCLC and U251 GBM cells did not express the PTEN protein (Figure 4D), which is consistent with the literature (29,30). Stable overexpression of CELF2 in the H1650 and U251 cell lines had no effect on proliferation (Figure 4E). In addition, western blotting revealed that phosphorylation of Akt in each cell line was not affected by CELF2 overexpression (Figure 4F). To further confirm that the reduction of Akt signaling is not due to the direct interaction between CELF2 and the PDZ domains of PREX2, we transiently transfected the PDZ1 or PDZ2 domain into HEK293T, HCC827 and A549 cells. Our results show that neither the PDZ1 nor the PDZ2 domain could increase Akt phosphorylation (Figure 4G). These data suggest that CELF2 functions as a tumor suppressor only in the presence of a functional PTEN protein.
Figure 4.
CELF2 suppresses NSCLC cell growth in the presence of PTEN. (A) Proliferation and (B) anchorage-independent growth assays in NSCLC cells with CELF2 overexpression (left, A549; right, H1299). Results were normalized to corresponding parental cells. (C) Representative images from the anchorage-independent growth assay. (D) Expression of PTEN in lung adenocarcinoma and glioblastoma cell lines. (E) Stable overexpression of CELF2 in PTEN null (left, H1650) and mutant PTEN (right, U251) cells has no effect on proliferation. (F) Western blot analysis of pAkt (Ser473) and CELF2 protein levels in H1650 (left) and U251 (right) cells overexpressing CELF2. Total Akt and glyceraldehyde 3-phosphate dehydrogenase were used as loading controls. (G) Western blot analysis of Akt signaling in HCC827 (left), A549 (middle) and 293T (right) cells overexpressing the PDZ1 or PDZ2 domain.
AAV-mediated CELF2 overexpression represses the growth of NSCLC PDX tumors
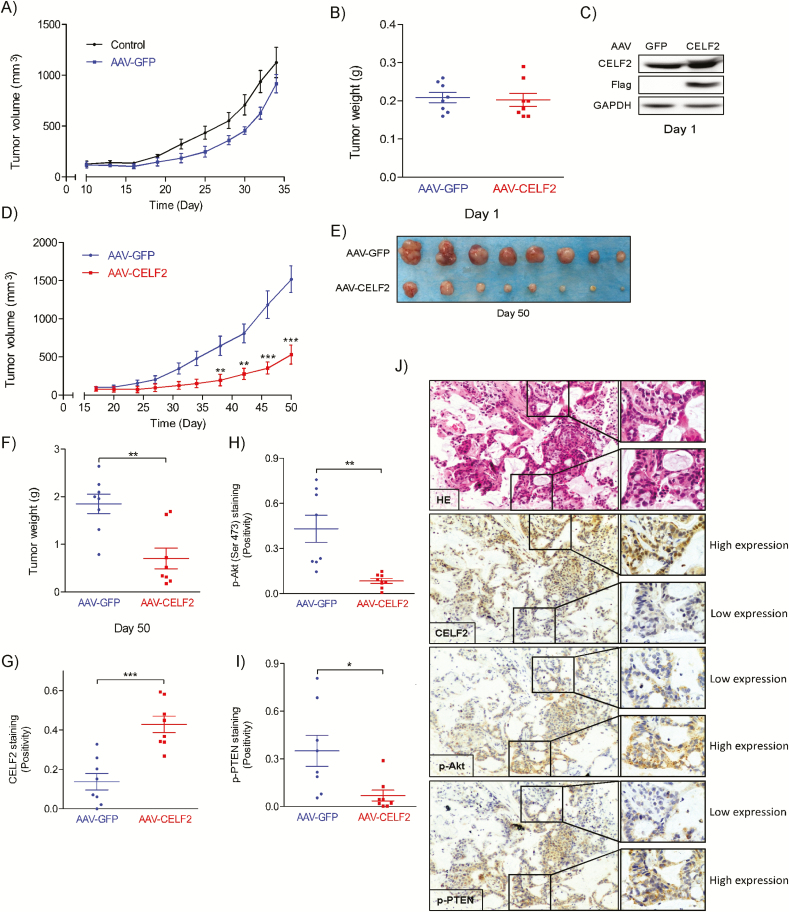
To further investigate the effect of CELF2 on NSCLC progression ex vivo and to determine its clinical relevance, we conducted ex vivo experiments using AAV to deliver CELF2 DNA to NSCLC PDX tumor tissues. The PDX information is summarized in Supplementary Table 2, available at Carcinogenesis Online. Due to the limited number of PDX tissues for each passage, we first examined whether AAV infection would have an impact on the growth of PDX tumors. Results showed that AAV infection alone did not have a significant effect on PDX tumor growth compared to control tumors (Figure 5A). Next, the PDX tissues were infected for 16 h with either AAV-GFP (as a control) or AAV-CELF2. The weight of PDX tissues between the two groups was comparable (Figure 5B) and AAV delivery of CELF2 was confirmed by western blotting (Figure 5C) at Day 1. Mice were killed at Day 50 and results show that CELF2 overexpression delivered by AAV markedly reduced NSCLC PDX tumor volume (Figure 5D and E) and weight (Figure 5F). Immunohistochemical analysis of harvested PDX tumors was then conducted to evaluate the expression level of CELF2 (Figure 5G), p-Akt (Ser473) (Figure 5H), p-PTEN (Figure 5I) and PREX2 (Supplementary Figure 3A, available at Carcinogenesis Online). Our results show that there was no significant difference in PREX2 level among two experimental groups. In the AAV-CELF2-infected group, CELF2 expression was significantly increased, whereas both p-Akt (Ser473) and p-PTEN expression were significantly decreased compared to the AAV-GFP-infected group. In addition, immunohistochemical analysis from the same cross-sectional area revealed that CELF2 expression was inversely proportional to p-Akt (Ser473) and p-PTEN expression (Figure 5J) with comparable PREX2 expression (Supplementary Figure 3B, available at Carcinogenesis Online). These results provide strong evidence showing that CELF2 could be a promising clinical candidate for NSCLC gene therapy.
Figure 5.
AAV-mediated CELF2 protein expression inhibits tumor growth in a PDX mouse model. (A) Tumor growth kinetics over 36 days of uninfected or AAV-GFP-infected PDX. (B) Tumor weight at Day 1. (C) Western blot analysis of CELF2 protein expression in AAV-GFP- and AAV-CELF2-infected PDX tissues at Day 1. (D) Tumor growth kinetics over 50 days of AAV-GFP- (as control) or AAV-CELF2-infected PDXs. Tumor size was measured twice a week and calculated based on the formula: length × width2 × 0.5. (E) Tumor size and (F) tumor weight at Day 50. (G) CELF2, (H) pAkt (Ser473) and (I) p-PTEN expression and HE staining in harvested xenograft tissues were assessed by immunohistochemistry. The integrated optical density was evaluated using the Image-Pro premier software offline (v9.0) program. (J) Representative photographs of immunohistochemistry analysis for each antibody are shown.
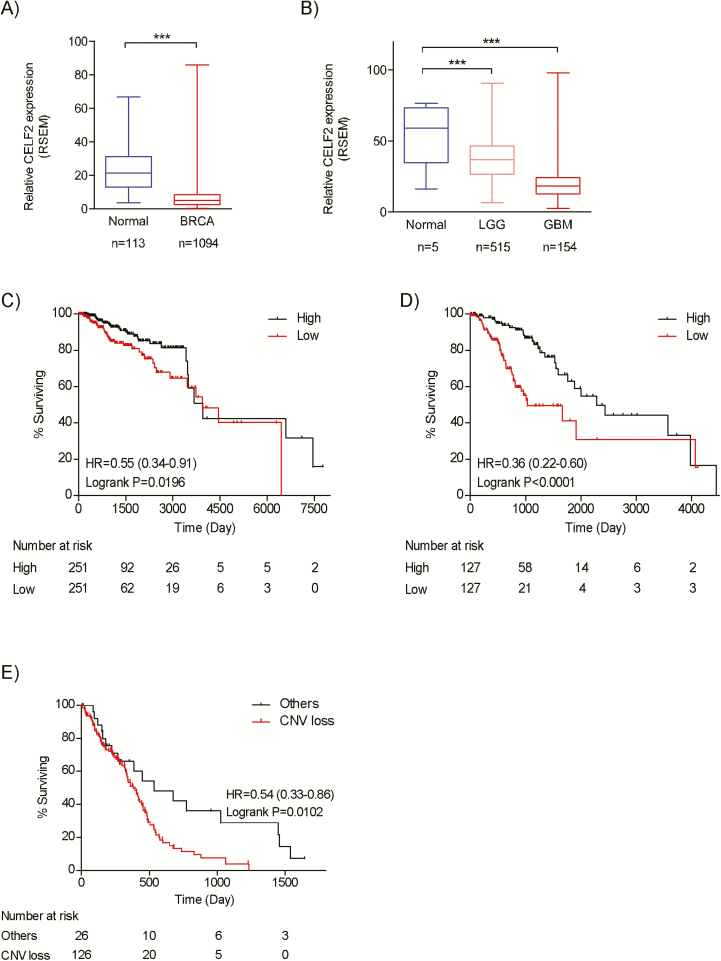
CELF2 expression is downregulated in multiple cancers and associated with poor clinical outcomes
The CELF2 gene is located on chromosome 10, a region frequently lost in various cancers. Analysis of the TCGA database showed that copy number variation loss of the 10p arm occurs in 16% of breast invasive carcinoma (BRCA), 16% of low-grade glioma (LGG), and 83% of GBM tissues (Supplementary Table 3, available at Carcinogenesis Online). In addition, CELF2 expression is significantly downregulated in all these cancers (Figure 6A and B). Next, we analyzed the impact of CELF2 expression on patient survival time in these datasets. By comparing the top and bottom quartiles, our results clearly indicated that the overall survival time of patients with higher CELF2 expression is significantly longer than patients with low CELF2 expression in both BRCA (Log-rank P = 0.0196, hazard ratio [HR] = 0.55) and LGG (Log-rank P < 0.0001, HR = 0.36) datasets, but not in the GBM dataset (Figure 6C and D). However, the Kaplan–Meier plot of the copy number variation of the CELF2 gene in GBM showed that patients with copy number loss of CELF2 had a significantly shorter survival time (Log-rank P = 0.0002, HR = 0.67; Figure 6E).
Figure 6.
CELF2 expression is downregulated in multiple cancers and associated with poor clinical outcomes. (A and B) The expression level of CELF2 mRNA in BRCA, LGG and GBM from the TCGA sample set is compared between primary tumors and solid normal tissues. Kaplan–Meier survival curves relative to CELF2 expression were estimated between the upper and lower quartiles in (C) BRCA and (D) LGG sample sets. (E) Kaplan–Meier plot for GBM patients with copy number loss of the CELF2 gene compared to wild type.
Discussion
In this study, we have shown that CELF2 is a novel suppressor of NSCLC. We showed that CELF2 expression is downregulated in NSCLC tissues compared to corresponding non-tumor tissues and that CELF2 expression is associated with patient survival time. In addition, we observed comparable CELF2 expression between tumor and non-tumor tissues in early-stage NSCLC, whereas CELF2 expression is significantly lower in tumor tissues as the disease progresses into later stages. To further determine the impact of CELF2 downregulation on cancer cell growth, we used lentivirus-based shRNA delivery to knock down CELF2 in lung cancer cells. Results show that knockdown of CELF2 significantly increases proliferation of lung cancer cells. These data indicate that downregulation of CELF2 promotes NSCLC progression and might act as a prognostic biomarker for NSCLC.
In an attempt to elucidate cellular and molecular mechanisms of CELF2 in carcinogenesis, we successfully identified PREX2 by pulldown assay, as a novel CELF2-interacting protein. The structural domains of PREX2 comprise a catalytic Dbl homology and pleckstrin homology (DH-PH) domain, two DEP domains, two PDZ domains, and an inositol polyphosphate-4 phosphatase (IP4P) domain (10). Our results show that CELF2 interacts with either the PREX2a or PREX2b isoform. PREX2b is a splice variant of PREX2a that lacks the IP4P phosphatase domain at the C-terminal, which suggests that the IP4P domain is not required in its interaction with CELF2. PREX2a is a GEF that regulates the activity of Rac when both PI3-K and G-coupled receptor signaling are activated (31). However, increasing evidence shows that another major function of PREX2 is the inhibition of PTEN activity through the direct interaction between its DH-PH catalytic domain and PTEN (8,32). Therefore, we investigated whether CELF2 could interact with PREX2 through the same domains as PTEN. Immunoprecipitation data showed that CELF2 interacts with both the PDZ1 and PDZ2 domains of PREX2, instead of the DH and PH domains. Multiple reports have shown that the PDZ domains are necessary for a number of scaffolding proteins to initiate PI3-K/Akt signaling (33–35). Therefore, determining whether the inhibition of PI3-K/Akt signaling is due to the association between CELF2 and PDZ domains of PREX2 is essential. However, we did not observe any changes in PI3-K/Akt signaling when PDZ domains were overexpressed in NSCLC cancer cells. Although CELF2 and PTEN interact with different domains of PREX2, we observed a reduced interaction between PREX2 and PTEN with increased concentrations of CELF2. Thus, we next examined whether CELF2 affected PTEN activity. In most mammalian cells, PTEN is mainly found in both the cytosol and nucleus, but with divergent roles in the different areas (36). Cytosolic PTEN is a well-known negative regulator of the PI3-K/Akt signaling pathway, whereas nuclear PTEN mainly regulates chromosome stability (28,36–38). Our results demonstrate that CELF2 expression and PTEN activity are directly proportional both experimentally and clinically. This is further supported by evidence showing that overexpression of CELF2 leads to an increasing level of cytosolic PTEN, while knockdown of CELF2 caused nuclear and nucleolar accumulation of PTEN. Although we have shown here that CELF2 expression affects the subcellular localization of PTEN, the underlying regulatory mechanism is yet to be determined in future studies. In addition, structural information of PREX2 could also provide a better prediction on how CELF2 reduces the association of PREX2 and PTEN. Nevertheless, these findings suggest that CELF2 could be an endogenous competitive inhibitor to reduce the interaction between PREX2 and PTEN and thus, enhance the PTEN lipid phosphatase activity.
The PTEN/PI3-K/Akt pathway regulates cancer cell proliferation, and changes in this pathway are associated with poor clinical outcome (4,39–41). Here, we showed that overexpression of CELF2 inhibits NSCLC cell proliferation and anchorage-independent growth, as well as PI3-K/Akt signaling. Recently, de la Rosa et al. showed that CELF2 is a PTEN-cooperating tumor suppressor, although the detailed mechanism of action was not investigated (23). In addition, evidence shows that PTEN is necessary for PREX2 to mediate its oncogenic function on Akt phosphorylation and cell proliferation (8). This is consistent with our results showing that the tumor suppressive effect of CELF2 is dependent on intact and functional PTEN expression. This finding is particularly significant in that CELF2 could be a potential clinical agent, because PTEN is not frequently mutated in NSCLC (42–44). To further translate our findings clinically, we used AAV to deliver the CELF2 gene to a NSCLC PDX tumor model as gene therapy. Our results clearly show that AAV-mediated CELF2 overexpression could effectively reduce PDX tumor size. Immunohistochemistry analysis also supported the finding that CELF2 expression is inversely associated with PTEN and Akt phosphorylation. These data strongly indicate that CELF2 could be a potential therapeutic target for NSCLC therapy. Importantly, we could not identify any relationship of CELF2 level to PREX2 and PTEN expression (Supplementary Figure 4, available at Carcinogenesis Online) and NSCLC oncogenic drivers such as Kras and egfr mutations (Supplementary Figure 5, available at Carcinogenesis Online). This suggests that CELF2 could be an independent therapeutic agent regardless to these molecular characteristics. However, a larger pool of NSCLC PDX models with different molecular characteristic should be used to determine the therapeutic potential of CELF2 in future studies.
These results could also be extended to other types of cancer. The CELF2 gene is located on chromosome 10p arm, which is a region frequently lost in multiple cancers, including BRCA, LGG and GBM. From the TCGA database, CELF2 expression is significantly downregulated in these tumor tissues compared to normal tissues. Interestingly, PREX2 expression is upregulated in these tumors (8). The Kaplan–Meier plot also showed that CELF2 mRNA levels in BRCA, LGG and GBM patients are associated with patient overall survival time, indicating that CELF2 might serve as a potential biomarker for prognostic evaluation. In future studies, we will study the underlying mechanism of the downregulation of CELF2 in these tumors. Collectively, our data strongly support our hypothesis that CELF2 functions as a tumor suppressor by antagonizing the oncogenic effect of PREX2 on PTEN.
Conclusion
In conclusion, our study revealed a functional role of CELF2 in NSCLC. We also showed for the first time that CELF2 is downregulated in patient NSCLC tissues and that loss of CELF2 expression might be an important mechanism that cancer cells adopt to gain advantages in cell growth through PI3-K/Akt signaling. As a PREX2-interacting partner, CELF2 reduces the binding between PREX2 and PTEN. With overexpression of CELF2 in vitro, we observed enhanced PTEN phosphatase activity, reduced PI3-K/Akt signaling, and inhibition of NSCLC cell growth. Most importantly, consistent with the in vitro results, we successfully repressed tumor growth in a NSCLC PDX model by using AAV-mediated CELF2 gene expression. Overall, these data indicate that CELF2 gene therapy might be effective in treatment of NSCLC clinically, although further studies using larger cohorts of patients will be necessary.
Funding
This work was supported by Division of Cancer Prevention, National Cancer Institute CA-187027 and CA-196639.
Conflict of Interest Statement: None declared.
Author contributions
Z.D. and Y.T.Y. designed research; Y.T.Y., S.F., S.Y., S.Y., W.N., M.W., and L.Z. performed experiments; T.L. and B.L. designed and performed animal experiments. Y.T.Y., X.L., and Z.D. analyzed the data; and Y.T.Y. and A.M.B. prepared the manuscript.
Supplementary Material
Glossary
Abbreviations
- AAV
adeno-associated virus
- BRCA
breast invasive carcinoma
- EDTA
ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- GBM
glioblastoma
- GEF
guanine nucleotide exchanger factor
- IPTG
isopropyl-β-D-1-thiogalactopyranoside
- LGG
low-grade glioma
- NSCLC
non-small cell lung cancer
- PDX
patient-derived xenografts
- PI3-K
phosphoinositide 3-kinase
- PIP3
phosphatidylinositol 3,4,5-triphosphate
- PTEN
phosphatase and tensin homolog
- RBP
RNA-binding protein
- RPMI
Rosewell Park Memorial Institute
References
- 1. Siegel R.L. et al. (2018) Cancer statistics, 2018. CA. Cancer J. Clin., 68, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Miller K.D. et al. (2016) Cancer treatment and survivorship statistics, 2016. CA. Cancer J. Clin., 66, 271–289. [DOI] [PubMed] [Google Scholar]
- 3. Rigas J.R. et al. (2005) Current perspectives on treatment strategies for locally advanced, unresectable stage III non-small cell lung cancer. Lung Cancer, 50 (suppl. 2), S17–S24. [PubMed] [Google Scholar]
- 4. Vivanco I. et al. (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer, 2, 489–501. [DOI] [PubMed] [Google Scholar]
- 5. Engelman J.A. et al. (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med., 14, 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pacold M.E. et al. (2000) Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell, 103, 931–943. [DOI] [PubMed] [Google Scholar]
- 7. Zhang S. et al. (2010) PI(3)king apart PTEN’s role in cancer. Clin. Cancer Res., 16, 4325–4330. [DOI] [PubMed] [Google Scholar]
- 8. Fine B. et al. (2009) Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science, 325, 1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donald S. et al. (2004) P-Rex2, a new guanine-nucleotide exchange factor for Rac. FEBS Lett., 572, 172–176. [DOI] [PubMed] [Google Scholar]
- 10. Rosenfeldt H. et al. (2004) P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett., 572, 167–171. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki A. et al. (2013) Identification and characterization of cancer mutations in Japanese lung adenocarcinoma without sequencing of normal tissue counterparts. PLoS One, 8, e73484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waddell N. et al.; Australian Pancreatic Cancer Genome Initiative (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature, 518, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berger M.F. et al. (2012) Melanoma genome sequencing reveals frequent PREX2 mutations. Nature, 485, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mense S.M. et al. (2015) PTEN inhibits PREX2-catalyzed activation of RAC1 to restrain tumor cell invasion. Sci. Signal., 8, ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glisovic T. et al. (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett., 582, 1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castello A. et al. (2012) Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell, 149, 1393–1406. [DOI] [PubMed] [Google Scholar]
- 17. Gerstberger S. et al. (2014) A census of human RNA-binding proteins. Nat. Rev. Genet., 15, 829–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ladd A.N. et al. (2001) The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol. Cell. Biol., 21, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dasgupta T. et al. (2012) The importance of CELF control: molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins. Wiley Interdiscip. Rev. RNA, 3, 104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z.L. et al. (2018) Comprehensive genomic characterization of RNA-binding proteins across human cancers. Cell Rep., 22, 286–298. [DOI] [PubMed] [Google Scholar]
- 21. Subramaniam D. et al. (2008) Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am. J. Physiol. Gastrointest. Liver Physiol., 294, G1025–G1032. [DOI] [PubMed] [Google Scholar]
- 22. Mukhopadhyay D. et al. (2003) Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol. Cell, 11, 113–126. [DOI] [PubMed] [Google Scholar]
- 23. de la Rosa J. et al. (2017) A single-copy Sleeping Beauty transposon mutagenesis screen identifies new PTEN-cooperating tumor suppressor genes. Nat Genet, 49, 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart S.A. et al. (2003) Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA, 9, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boutet E. et al. (2007) UniProtKB/Swiss-Prot. Methods Mol. Biol., 406, 89–112. [DOI] [PubMed] [Google Scholar]
- 26. Ayuso E. et al. (2010) High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther., 17, 503–510. [DOI] [PubMed] [Google Scholar]
- 27. Zhang F. et al. (2018) TGFβ1-induced down-regulation of microRNA-138 contributes to epithelial-mesenchymal transition in primary lung cancer cells. Biochem. Biophys. Res. Commun., 496, 1169–1175. [DOI] [PubMed] [Google Scholar]
- 28. Lee J.O. et al. (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell, 99, 323–334. [DOI] [PubMed] [Google Scholar]
- 29. Sos M.L. et al. (2009) PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res., 69, 3256–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park C.M. et al. (2006) Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res., 66, 8511–8519. [DOI] [PubMed] [Google Scholar]
- 31. Welch H.C. (2015) Regulation and function of P-Rex family Rac-GEFs. Small GTPases, 6, 49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hodakoski C. et al. (2014) Regulation of PTEN inhibition by the pleckstrin homology domain of P-REX2 during insulin signaling and glucose homeostasis. Proc. Natl. Acad. Sci. USA, 111, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James C.D. et al. (2016) Viral interactions with PDZ domain-containing proteins-an oncogenic trait? Pathogens, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frese K.K. et al. (2003) Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene, 22, 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L. et al. (2015) The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer, 14, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Planchon S.M. et al. (2008) The nuclear affairs of PTEN. J. Cell Sci., 121(Pt 3), 249–253. [DOI] [PubMed] [Google Scholar]
- 37. Shen W.H. et al. (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell, 128, 157–170. [DOI] [PubMed] [Google Scholar]
- 38. Bassi C. et al. (2013) Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science, 341, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takada M. et al. (2013) Alterations of the genes involved in the PI3K and estrogen-receptor pathways influence outcome in human epidermal growth factor receptor 2-positive and hormone receptor-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. BMC Cancer, 13, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu L.Z. et al. (2007) AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res., 67, 6325–6332. [DOI] [PubMed] [Google Scholar]
- 41. Faried L.S. et al. (2008) Expression of an activated mammalian target of rapamycin in adenocarcinoma of the cervix: a potential biomarker and molecular target therapy. Mol. Carcinog., 47, 446–457. [DOI] [PubMed] [Google Scholar]
- 42. Yokomizo A. et al. (1998) PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene, 17, 475–479. [DOI] [PubMed] [Google Scholar]
- 43. Teng D.H. et al. (1997) MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res., 57, 5221–5225. [PubMed] [Google Scholar]
- 44. Han S.Y. et al. (2000) Functional evaluation of PTEN missense mutations using in vitro phosphoinositide phosphatase assay. Cancer Res., 60, 3147–3151. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.