Abstract
In this study, a family of porphyrins based on 5,10,15,20-Tetrakis(4-ethylphenyl)porphyrin (1, Ph) and six metallo-derivatives (Zn2+(2, Ph-Zn), Sn4+(3, Ph-Sn), Mn2+ (4, Ph-Mn), Ni2+ (5, Ph-Ni), Al3+ (6, Ph-Al), and V3+ (7, Ph-V)) were tested as photosensitizers for photodynamic therapy against Leishmania braziliensis and panamensis. The singlet oxygen quantum yield value (ΦΔ) for (1–7) was measured using 1,3-diphenylisobenzofuran (DPBF) as a singlet oxygen trapping agent and 5,10,15,20-(tetraphenyl)-porphyrin (H2TPP) as a reference standard; besides, parasite viability was estimated by the MTT assay. After metal insertion into the porphyrin core, the ΦΔ increased from 0.76–0.90 and cell viability changed considerably. The ΦΔ and metal type changed the cytotoxic activity. Finally, (2) showed both the highest ΦΔ (0.90) and the best photodynamic activity against the parasites studied (IC50 of 1.2 μM).
Keywords: porphyrin, metalloporphyrins, photodynamic therapy, Leishmania braziliensis, Leishmania panamensis, singlet oxygen
1. Introduction
Leishmania spp are extra and intracellular protozoan parasites that infect a variety of animals (e.g., dogs, rodents, reptiles). However, this zoonosis also affects human beings when they invade the habitat of both natural reservoirs and transmitting vectors thereof [1,2]. The parasite vectors are female hematophagous mosquitoes of the genera Phlebotomus and Lutzomyia [3,4]. The appearance of this disease in humans can be observed on the skin surface, in mucous membranes, and in some organs (liver and vessels). Among these, the cutaneous is the most frequent form of appearance. Those three clinical presentations have distribution in more than 100 countries in five continents [4], with an estimate of 350 million people at risk of suffering from it. Currently, there are 12 million people infected, with an annual incidence of 2 million people, with around 65,000 deaths reported per year [1,2,5,6]. This disease is considered a priority problem for public health around the world, with greater interest in poorest countries with high levels of malnutrition, economic and social inequality once they face the biggest impacts and incidence [7,8]. Currently, the pharmacological treatments against this disease (e.g., glucantime, miltefosine, pentamidine, isethionate, amphotericin B) have shown some degree of effectiveness against the parasites. However, due to their high toxicity and a wide range of adverse effects, such treatments are controlled and restricted [9,10,11,12,13,14,15,16]. Besides, given the resistance observed in recent years by Leishmania spp against all these therapeutic options [17], it is of utmost importance to search for new therapeutic alternatives that are more effective, less toxic, and both safer and more affordable for all vulnerable populations [18]. In this sense, due to their biological and photodynamic properties to produce reactive oxygen species (ROS), especially singlet oxygen when they are irradiated with visible light, porphyrin and metalloporphyrin derivatives have been used as an alternative tool in photodynamic therapy (PDT) against Leishmania spp [19,20]. Previous studies reveal that metals affect the stability of the porphyrin macrocycle and, therefore, metals can alter the photophysical properties of the sensitizer; while enhanced intersystem crossing to the triplet state might be expected, followed by metalation of porphyrins due to the heavy-atom effect. Complexes with diamagnetic metals (e.g., Zn) have higher singlet oxygen quantum yields, since diamagnetic metals promote intersystem crossing and have a long triplet lifetime [21,22,23,24]. This behavior is not reported for paramagnetic metals (e.g., Sn, Al, V, Mn). In the case of substituents of intermediate size into the porphyrin core, the triplet lifetimes are observed to decrease by up to two orders of magnitude. This is attributed to a distortion of the macrocycle symmetry when the substituents “squeeze through” upon the hindered rotation of the phenyl group [25,26]. Besides, amphiphilic groups can facilitate better delivery and accumulation of porphyrins in the cells. Several studies have shown that the efficiency in photoactivity increases when the number of carbon atoms in the side chains is increased [27]. The presence of a long alkyl chain was shown to be important for high PDT efficiency of the amphiphilic tripyridyl porphyrins. Lesar et al. showed that lipophilic moiety significantly improved PDT efficiency compared with the hydrophilic analog which lacked the long alkyl chain [28]. Literature data suggest that hydrophilic porphyrins linked to long hydrophobic chains are incorporated much easier into micelle formed by fatty substances. According to expectations, the attachment of alkyl chain to the porphyrin molecule considerably increased its hydrophobic properties [29]. Some reports suggest that when the substituent chain is increased, a greater affinity of the sensitizer to the membranes is allowed, that is why its photodynamic activity increases [30]. Ezzeddine et al. reported a progressive increase of lipophilicity from shorter hydrophilic (methyl) to longer amphiphilic (hexyl) alkyl chains which increased the phototoxicity of the Zn(II) N-alkylpyridylporphyrins [31]. However, the photophysical properties, such as fluorescence lifetime and quantum yield, of singlet oxygen do not change significantly [32,33]. Furthermore, β-substituted porphyrin systems have been evaluated against L. panamensis in the amastigote stage, showing IC50 values between 5.7 and 24.1 µM [34]. Besides, regarding these compounds, there have been reports of cellular viabilities <10% against L. major and L. braziliensis in the promastigote stage [35]. Other systems like the benzoporphyrins have shown suitable IC50 values (3.35 µM) against L. major [36]. Substantial improvements have been reported for these systems when metals are introduced into the macrocycle core. Gomes et al. reported improvement of activity against L. amazonensis for inclusion of Bi3+ and Sb5+, with IC50 values of 93.8 µM and 52.4 µM against L. amazonensis [37]. Moreover, the cytotoxic activity of these systems against L. braziliensis in the promastigote stage was improved after the inclusion of Zn2+ [38]. In a recent publication, our group reported in detail the photophysical and DFT results for (1–7). In that study, we proposed that (1–7) could be tested as sensitizers for photodynamic therapy [39]. In view of that, in the present study, our aim is to demonstrate the cytotoxic activity of (1–7) against L. braziliensis and L. panamensis.
2. Results and Discussion
2.1. Singlet Oxygen Quantum Yield
The efficient interaction of the photosensitizer triplet state with the molecular oxygen ground state may result in generation of singlet oxygen [40]. In order to determine ΦΔ, DPBF was used as a singlet oxygen trapping agent and H2TPP as a reference standard. The generation of singlet oxygen by (1–7) is evidenced by chemical trapping of singlet oxygen by DPBF, and the ΦΔ values of the compounds are listed in Table 1. The results indicate that (Ph-Zn, Ph-Mn, Ph-Al, Ph-V) had a quantum yield higher than (1, Ph). This difference could be related to an increase of relaxation of excited states in macromolecule; moreover, the insertion of these metals inside the ring generated more stability for the generation of singlet oxygen [25,41,42].
Table 1.
Singlet oxygen quantum yield (ΦΔ) and fluorescence quantum yield (Φf) for (1–7).
| Compound | ε (M−1cm−1 × 104) | Φf | ΦΔ |
|---|---|---|---|
| 1, Ph | 5.0 | 0.11 ± 0.02 | 0.76 ± 0.09 |
| 2, Ph-Zn | 2.5 | 0.11 ± 0.03 | 0.90 ± 0.03 |
| 3, Ph-Sn | 6.0 | 0.32 ± 0.02 | 0.76 ± 0.05 |
| 4, Ph-Mn | 4.0 | 0.17 ± 0.02 | 0.83 ± 0.03 |
| 5, Ph-Ni | 7.0 | 0.08 ± 0.02 | 0.68 ± 0.02 |
| 6, Ph-Al | 3.4 | 0.010 ± 0.005 | 0.84 ± 0.04 |
| 7, Ph-V | 3.1 | 0.0020 ± 0.0005 | 0.86 ± 0.01 |
In general, the ΦΔ of (1–7) were lower for the paramagnetic metals than for the diamagnetic ones, and this is in line with previous studies, which showed that porphyrins containing paramagnetic ions were very poor photosensitizers [24,26]. It is possible that the introduction of low energy charge-transfer states associated with disruption of the planarity of the macrocyclic ring system provides alternative non-radiative deactivation channels. Finally, since ΦΔ values as low as 0.11 are known for porphyrins derivatives in clinical trials, such as Lutetium Texaphyrin [43], and because singlet oxygen has been implicated as an intermediary species leading to cell death following photoexcitation sensitizers agents in photodynamic therapy [24], the results shown in Table 1 indicate that (1–7) are suitable as potential materials for photodynamic therapy.
2.2. Antileishmanicidal Activity
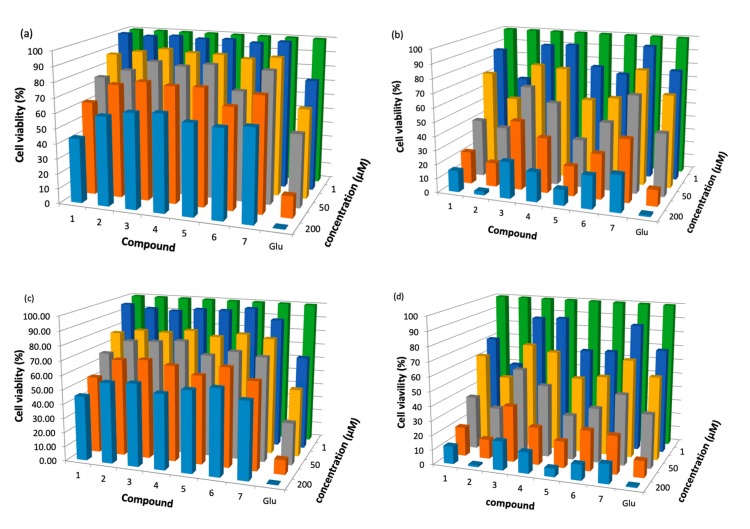
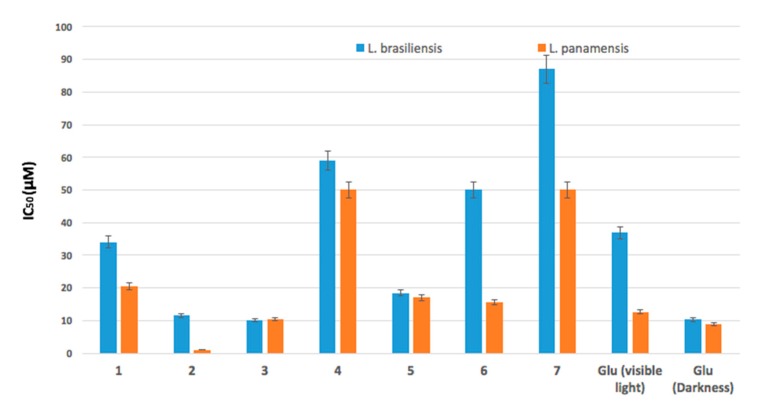
Several compounds have already used sensitizers against Leshmania species [44,45]. However, the search for new substances is an important topic in this research field. Compounds (1–7) were studied in the promastigotes stage of L. panamensis and L. braziliensis, with viability assessed by the MTT assay. Figure 1 shows in detail the viability (%) results of L. braziliensis and L. panamensis with incubation periods of 24 h in the presence of (1–7) both in the dark and under visible irradiation. The results show that (1–7) had the ability to effectively inhibit the parasites. In addition, a decrease in the viability of the parasites was observed with the increase in the concentrations of the treatment. Figure 1a,c show that, under light irradiation, the viability of (1–7) was similar to the viability of the Glucantime control for all ranges of concentration. These results are relevant, it verifies the potential of the (1–7) as sensitizers for PDT. Furthermore, the inhibitory activity was lower without light irradiation for (1–7), and this is due to the interaction of light with endogenous biomolecules [46]. When 200 μM of the compounds were used, (2, Ph-Zn) had the highest inhibitory activity against both L. braziliensis and L. panamensis, even the cell viability of (2, Ph-Zn) was the same as that of the Glucantime control. According to Table 1, (Ph-Zn) had the highest ΦΔ (0.90), then under visible irradiation, the amount of singlet oxygen available to attack the leishmania parasite is larger and the cytotoxic effect could be bigger. The IC50 value (concentration that inhibited cell growth by 50%) was determined, and the results are shown in Figure 2. In all cases of the tests, the activation of sensitizers by irradiation ensures lower IC50 values. In the absence of light, the cytotoxic activity against the parasite was lower; the IC50 for (1–7) was higher than 200 μM in the dark; all compounds required light activation—these results are in line with other reports [20,47]. Compounds (1–7) showed high toxicity against the parasites under light irradiation, and (1, 3–7) had IC50 similar of larger than the positive control against both parasites; only (Ph-Zn) had lower IC50 (1.2 μM) comparing to the positive control under irradiation (12.7 μM) and in the dark (8.0 μM) against L. panamensis. This result is associated with the biggest ΦΔ value of (2). Table 2 lists the IC50 values for compounds (1–7) under visible irradiation and without irradiation (in the dark).
Figure 1.
Parasite viability percentage results for compounds (1–7) with incubation periods of 24 h: against L. braziliensis (a) in the dark, (b) under light irradiation; against L. panamensis (c) in the dark; (d) under light irradiation.
Figure 2.
IC50 values for photoinactivation of L. braziliensis and L. panamensis promastigotes after 24 h of incubation in the presence of (1–7) and the positive control under light irradiation.
Table 2.
IC50 values for photoinactivation of L. braziliensis and L. panamensis promastigotes in the dark and under visible irradiation.
| Compound | L. braziliensis | L. panamensis | ||
|---|---|---|---|---|
| IC50 (μM) Under Irradiation |
IC50 (μM) in the Dark |
IC50 (μM) under Irradiation |
IC50 (μM) in the Dark |
|
| 1, Ph | 34.1 ± 1.8 | 117 | 20.6 ± 1.3 | 105 |
| 2, Ph-Zn | 11.6 ± 1.0 | >200 | 1.2 ± 0.2 | >200 |
| 3, Ph-Sn | 10.1 ± 0.7 | >200 | 10.4 ± 0.8 | >200 |
| 4, Ph-Mn | 59.0 ± 2.5 | >200 | 50.0 ± 1.3 | >200 |
| 5, Ph-Ni | 18.4 ± 2.5 | >200 | 17.0 ± 1.0 | >200 |
| 6, Ph-Al | 50 ± 1.2 | >200 | 15.6 ± 0.9 | >200 |
| 7, Ph-V | 87 ± 3.5 | >200 | 50.0 ± 2.1 | >200 |
| Glucantime | -- | 10.3 ± 0.9 | -- | 8.8 ± 0.8 |
Besides, Figure 2 indicates that (1–7) were more effective against L. panamensis than against L. braziliensis. This result could be associated with the multi-resistance mechanism reported for L. braziliensis [48,49,50,51]. The parasite inhibition mechanism is unknown and there is no clear report in the literature [52]. However, after compounds (1–7) were irradiated with visible light (see Table 1), singlet oxygen was generated—this oxidant species could generate substantial damage to parasites at the cellular membrane level and even irreparable damage to vital proteins or DNA that induce death [52,53,54]. Our results suggest that singlet oxygen could be a reason for inactivation of the parasite. It is clear that those compounds operate efficiently under visible light; in the dark the damage to the parasites was not comparable to that of the positive control. Finally, these results are relevant and show the potential of (1–7) as sensitizers for PDT, which indicate that (Ph-Zn) is the best candidate for PDT applications.
3. Materials and Methods
3.1. Synthesis
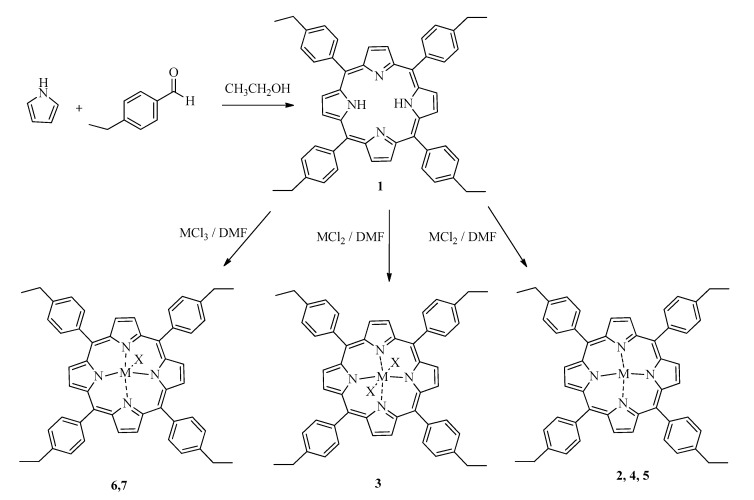
All reagents were supplied by Aldrich. We synthesized porphyrin according to Alder and Cols method [55], which relies on stirring aldehyde and pyrrole in propionic acid for 6 h at room temperature and an oxygen atmosphere (see Scheme 1) [39]:
Scheme 1.
Chemical synthesis of: (1) 5,10,15,20-Tetrakis(4-ethylphenyl)porphyrin and metal-derivatives, (2) Zn (II), (3) Sn (IV), (4) Mn (III), (5) Ni (II), (6) Al (III), (7) V (III). Into scheme, X means Cl−.
5,10,15,20-tetrakis(4-ethylphenyl)porphyrin (1): A mixture of pyrrole (8 mmol) and 4-ethylbenzaldehyde (8 mmol) in of propionic acid (60 mL) was stirred by 6 h at room temperature in an open container. The product was extracted from the reaction medium after addition of methanol (40 mL). We obtained 0.820 g of a bright purple powder that was purified through column chromatography using silica gel (2.5 × 24 cm) as stationary phase, and petroleum ether:ethyl acetate 5:1 (rf = 0.66). Yield: 0.680 g, 46%; melting point > 300 °C; UV-Vis (ethyl acetate) λ (nm): 415, 512, 547, 590, 646; FT-IR-ATR (cm−1): N-H (3312.97), Csp3-H (2960.44), C=C (1685.54), C=N (1180.23), C-N (1020.47); 1 H RMN (400 MHz, CDCl3) δ (ppm): 1.57 (12 H, t, J = 7.6 Hz, -CH2CH3), 3.02 (8 H, q, J = 7.6 Hz, -CH2CH3), 7.60 (8 H, d, J = 7.9 Hz, 3-HAr), 8.16 (8 H, d, J = 7.9 Hz, 4-HAr), 8.90 (8 H, s, Py); 13 C RMN (100 MHz, CDCl3) δ(ppm): 15.56 (-CH2CH3 × 4), 28.96 (-CH2CH3 × 4), 120.21 (2-CAr × 8), 126.23 (1-CAr × 4), 131.28 (Cβ-Py × 8), 134.59 (3-CAr × 8), 139.63 (Cα-Py × 8), 143.62 (4-CAr × 4); MS (ESI-IT), m/z: 727.2 [M + H]+; Anal. Elem. Calc. for C52H46N4 (%): C (85.91), H (6.39), N (7.71), Anal. Elem. Found. (%) C52H46N4, C (85.95), H (6.34), N (7.71).
Compound (2–7) were synthesized by mixing (1) with the metal chloride salt for each metal in DMF. The mixture was stirred for 6 h at room temperature. Then, the reaction mixture was cooled in ice-water bath; the formed precipitate was filtered and dried at room temperature; (2–7) were purified through column chromatography with silica gel (2.5 × 24 cm), petroleum ether:ethyl acetate (PE:EA) was used as mobile phase. Details of the spectroscopic characterization are listed in supplementary materials.
3.2. Singlet Oxygen Quantum Yield
The ΦΔ values of (1–7) were determined in air using the relative method with 1,3- diphenylisobenzofuran (DPBF) as a singlet oxygen trapping agent and 5,10,15,20-(tetraphenyl)-porphyrin (H2TPP) as a reference standard. The tests consisted of preparing a 1 × 10−9 M solution of each compound in Dimethylformamide (DMF) by triplicate, and calculations were determined according to Equation (1) [56,57,58,59]:
| (1) |
where Φ∆st is the singlet oxygen quantum yield of standard H2TPP in DMF (0.64), W y Wst are the DPBF photobleaching rates in the presence of complex (1 and 2) and standard porphyrin, respectively. Data for Singlet Oxygen Quantum Yield calculation are provided in supplementary materials.
3.3. Fluorescence Quantum Yield
The comparative method was used to determine fluorescence quantum yield. Fluorescein dissolved in water was standard, and sensitizers were dissolved in ethyl acetate. The fluorescence quantum yield values were determined by taking the maximum of the Soret band as the excitation wavelength (range 420–750 nm; slit = 2 nm). Quantum fluorescence yield was calculated with the following equation [37,42,60,61]:
| (2) |
where Fx and Fest correspond to the area under the curve in the fluorescence emission spectrum for compounds (1), (2) and standard. Ax and Aest correspond to absorbance at excitation wavelength for compounds (1), (2) and standard; ɳx and ɳest correspond to the refraction index for solvents (ɳethyl acetate = 1.3724 and ɳwater = 1.33336). Data for Singlet Oxygen Quantum Yield calculation are provided in supplementary materials.
3.4. Parasites
Leishmania panamensis (M2903) and Leishmania braziliensis (UA140) were used in the in vitro study. The parasites were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 1% glutamine and 4% antibiotics (200 U penicillin/200 μg Amikacin) under incubation conditions of 5% CO2. The metacyclic promastigotes in the infectious stage were isolated from stationary cultures of 5 days using a uniform procedure based on a modified density gradient purification.
3.5. Parasite Viability
Parasite viability was estimated by the MTT assay, converting a yellow tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into an insoluble product (formazan); the amount of formed formazan depends on the number of viable parasites present [58,59,62]. The antileishmanicidal activity was studied at different concentrations in the presence and absence of light. The irradiation source was Omnilux lamps (EL10000AG), with a range of λemission lamp = 420 nm–450 nm for using light intensity 80 J·cm−2. All the measurements of the optical densities were taken in microplates of 96 U-bottom wells, using the Multiskan Sky ThermoScientific equipment. Standard deviation was obtained from 12 independent experiments—these were correlated with a percentage variation coefficient <5%. We applied an ANOVA test to determine the differences or similarities between treatments and the positive control. In addition, a post hoc analysis was performed using Tukey statistics. Finally, differences were considered to be significant when p < 0.05.
4. Conclusions
Porphyrins (1–7) showed suitable singlet oxygen quantum yields, which induced inhibition of the L. braziliensis and L. panamensis growth when the compounds were irradiated with a visible light source. The non-irradiated treatments generated little or no inhibitory response of the parasites. All the results indicate that (1–7) have suitable properties to be used in photodynamic therapy. All the compounds showed better cytotoxic against L. panamensis than against L. braziliensis. Compound (2) was the best photosensitizer of all the compounds included in this study, as it showed a larger ΦΔ value (0.90) and a better IC50 value compared to that of the positive control. Therefore, compound (2) is the best candidate to be tested in photodynamic application against L. braziliensis.
Acknowledgments
F. Espitia would like to thank COLCIENCIAS (programa de apoyo Doctoral en la convocatoria 727-2015) for the grants. W. Vallejo and C. Diaz thank to Universidad del Atlántico (RES. No 002047 – 10/12/2018. COD CB 22 TGI 2018).
Supplementary Materials
The following materials are available online, FTIR, Florescence, UV-Vis, singlet oxygen plots data and the synthesis details.
Author Contributions
Conceptualization, C.D.-U., W.V., and F.E.-A.; methodology, C.D.-U., W.V., F.E.-A., D.G.-C., A.R.R.B.; software, F.E.-A., D.G.-C., A.R.R.B.; validation, C.D.-U., W.V., F.E.-A., D.G.-C., A.R.R.B.; formal analysis, C.D.-U., W.V., F.E.-A., D.G.-C., A.R.R.B; investigation, C.D.-U., W.V., F.E.-A., D.G.-C., A.R.R.B.; resources, C.D.-U., W.V., F.E.-A., D.G.-C., A.R.R.B.; data curation, C.D.-U., W.V., F.E.-A.; writing—original draft preparation, C.D.-U., W.V., F.E.-A.; writing—review and editing, C.D.-U., W.V., F.E.-A., D.G.-C., A.R.R.B.; project administration, C.D.-U., W.V., and D.G.-C.; funding acquisition, C.D.-U., W.V., F.E.-A., D.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by COLCIENCIAS for the grants. W. Vallejo and C. Diaz thank to Universidad del Atlántico (RES. No 002047 – 10/12/2018. COD CB 22 TGI 2018).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the freeze-dried powders are available from the authors.
References
- 1.WHO Leishmaniasis. [(accessed on 12 February 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- 2.Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M., WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khademvatan S., Salmanzadeh S., Foroutan-Rad M., Bigdeli S., Hedayati-Rad F., Saki J., Heydari-Gorji E. Spatial distribution and epidemiological features of cutaneous leishmaniasis in southwest of Iran. Alex. J. Med. 2017;53:93–98. doi: 10.1016/j.ajme.2016.03.001. [DOI] [Google Scholar]
- 4.Steverding D. The history of leishmaniasis. Parasit. Vectors. 2017;10:82. doi: 10.1186/s13071-017-2028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reithinger R., Dujardin J.-C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 6.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Patiño-Londoño S.Y., Salazar L.M., Acero C.T., Bernal I.D.V. Aspectos socioepidemiológicos y culturales de la leishmaniasis cutánea concepciones, actitudes y prácticas en las poblaciones de Tierralta y Valencia, (Córdoba, Colombia) Salud Colect. 2017;13:123–138. doi: 10.18294/sc.2017.1079. [DOI] [PubMed] [Google Scholar]
- 8.Gore Saravia N., Nicholls R.S. Leishmaniasis: Un reto para la salud pública que exige concertación de voluntades y esfuerzos. Biomédica. 2012;26:5. doi: 10.7705/biomedica.v26i1.1493. [DOI] [PubMed] [Google Scholar]
- 9.Singh S., Sivakumar R. Challenges and new discoveries in the treatment of leishmaniasis. J. Infect. Chemother. 2004;10:307–315. doi: 10.1007/s10156-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 10.Lockwood D., Moore E. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010;2:151. doi: 10.4103/0974-777X.62883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuon F.F., Amato V.S., Graf M.E., Siqueira A.M., Nicodemo A.C., Neto V.A. Treatment of New World cutaneous leishmaniasis - a systematic review with a meta-analysis. Int. J. Dermatol. 2008;47:109–124. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- 12.Monge-Maillo B., López-Vélez R. Therapeutic Options for Old World Cutaneous Leishmaniasis and New World Cutaneous and Mucocutaneous Leishmaniasis. Drugs. 2013;73:1889–1920. doi: 10.1007/s40265-013-0132-1. [DOI] [PubMed] [Google Scholar]
- 13.Araujo-Melo M.H., Meneses A.M., Schubach A.O., Moreira J.S., Conceição-Silva F., Salgueiro M.M., Pimentel M.I.F., Araújo-Silva M., Oliveira R.V.C., Carmo C.N., et al. Risk factors associated with dizziness during treatment of mucosal leishmaniasis with meglumine antimoniate: 16-year retrospective study of cases from Rio de Janeiro, Brazil. J. Laryngol. Otol. 2010;124:1056–1060. doi: 10.1017/S0022215110001325. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira L.F., Schubach A.O., Martins M.M., Passos S.L., Oliveira R.V., Marzochi M.C., Andrade C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011;118:87–96. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Berman J.D. Chemotherapy for leishmaniasis: Biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- 16.Heruti R.J., Sharabi Y., Arbel Y., Shochat T., Swartzon M., Brenner G., Justo D. ORIGINAL RESEARCH—EPIDEMIOLOGY: The Prevalence of Erectile Dysfunction Among Hypertensive and Prehypertensive Men Aged 25–40 Years. J. Sex. Med. 2007;4:596–601. doi: 10.1111/j.1743-6109.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 17.Olliaro P.L., Guerin P.J., Gerstl S., Haaskjold A.A., Rottingen J.-A., Sundar S. Treatment options for visceral leishmaniasis: A systematic review of clinical studies done in India, 1980–2004. Lancet Infect. Dis. 2005;5:763–774. doi: 10.1016/S1473-3099(05)70296-6. [DOI] [PubMed] [Google Scholar]
- 18.Renslo A.R., McKerrow J.H. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 19.Al-Qahtani A., Alkahtani S., Kolli B., Tripathi P., Dutta S., Al-Kahtane A.A., Jiang X.J., Ng D.K.P., Chang K.P. Aminophthalocyanine-mediated photodynamic inactivation of Leishmania tropica. Antimicrob. Agents Chemother. 2016;60:2003–2011. doi: 10.1128/AAC.01879-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peloi L.S., Biondo C.E.G., Kimura E., Politi M.J., Lonardoni M.V.C., Aristides S.M.A., Dorea R.C.C., Hioka N., Silveira T.G.V. Photodynamic therapy for American cutaneous leishmaniasis: The efficacy of methylene blue in hamsters experimentally infected with Leishmania (Leishmania) amazonensis. Exp. Parasitol. 2011;128:353–356. doi: 10.1016/j.exppara.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Jeong H.-G., Choi M.-S. Design and Properties of Porphyrin-based Singlet Oxygen Generator. Isr. J. Chem. 2016;56:110–118. doi: 10.1002/ijch.201500026. [DOI] [Google Scholar]
- 22.Josefsen L.B., Boyle R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met. Based Drugs. 2008;2008 doi: 10.1155/2008/276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skwor T.A., Klemm S., Zhang H., Schardt B., Blaszczyk S., Bork M.A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus and Escherichia coli: A metalloporphyrin comparison. J. Photochem. Photobiol. B Biol. 2016;165:51–57. doi: 10.1016/j.jphotobiol.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Mathai S., Smith T.A., Ghiggino K.P. Singlet oxygen quantum yields of potential porphyrin-based photosensitisers for photodynamic therapy. Photochem. Photobiol. Sci. 2007;6:995–1002. doi: 10.1039/b705853e. [DOI] [PubMed] [Google Scholar]
- 25.Ormond A.B., Freeman H.S. Effects of substituents on the photophysical properties of symmetrical porphyrins. Dye Pigment. 2013;96:440–448. doi: 10.1016/j.dyepig.2012.09.011. [DOI] [Google Scholar]
- 26.Cauzzo G., Gennari C., Jori G., Spikes J.D. The effect of chemical structure on the photosensitizing efficiencies of porphyrins. Photochem. Photobiol. 1977;25:389–395. doi: 10.1111/j.1751-1097.1977.tb07358.x. [DOI] [PubMed] [Google Scholar]
- 27.Lavi A., Weitman H., Holmes R.T., Smith K.M., Ehrenberg B. The depth of porphyrin in a membrane and the membrane’s physical properties affect the photosensitizing efficiency. Biophys. J. 2002;82:2101–2110. doi: 10.1016/S0006-3495(02)75557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesar A., Begić G., Malatesti N., Gobin I. Innovative approach in Legionella water treatment with photodynamic cationic amphiphilic porphyrin. Water Sci. Technol. Water Supply. 2019;19:1473–1479. doi: 10.2166/ws.2019.012. [DOI] [Google Scholar]
- 29.Rojkiewicz M., Kuś P., Kozub P., Kempa M. The synthesis of new potential photosensitizers. Dye Pigment. 2013;99:627–635. doi: 10.1016/j.dyepig.2013.06.029. [DOI] [Google Scholar]
- 30.Thomas M., Craik J.D., Tovmasyan A., Batinic-Haberle I., Benov L.T. Amphiphilic cationic Zn-porphyrins with high photodynamic antimicrobial activity. Future Microbiol. 2015;10:709–724. doi: 10.2217/fmb.14.148. [DOI] [PubMed] [Google Scholar]
- 31.Ezzeddine R., Al-Banaw A., Tovmasyan A., Craik J.D., Batinic-Haberle I., Benov L.T. Effect of molecular characteristics on cellular uptake, subcellular localization, and phototoxicity of Zn(2) N-Alkylpyridylporphyrins. J. Biol. Chem. 2013;288:36579–36588. doi: 10.1074/jbc.M113.511642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosomizu K., Oodoi M., Umeyama T., Matano Y., Yoshida K., Isoda S., Isosomppi M., Tkachenko N.V., Lemmetyinen H., Imahori H. Substituent effects of porphyrins on structures and photophysical properties of amphiphilic porphyrin aggregates. J. Phys. Chem. B. 2008;112:16517–16524. doi: 10.1021/jp807991k. [DOI] [PubMed] [Google Scholar]
- 33.Stasheuski A.S., Galievsky V.A., Knyukshto V.N., Ghazaryan R.K., Gyulkhandanyan A.G., Gyulkhandanyan G.V., Dzhagarov B.M. Water-Soluble Pyridyl Porphyrins with Amphiphilic N-Substituents: Fluorescent Properties and Photosensitized Formation of Singlet Oxygen. J. Appl. Spectrosc. 2014;80:813–823. doi: 10.1007/s10812-014-9849-1. [DOI] [Google Scholar]
- 34.Gardner D.M., Taylor V.M., Cedeño D.L., Padhee S., Robledo S.M., Jones M.A., Lash T.D., Vélez I.D. Association of Acenaphthoporphyrins with Liposomes for the Photodynamic Treatment of Leishmaniasis. Photochem. Photobiol. 2010;86:645–652. doi: 10.1111/j.1751-1097.2010.00705.x. [DOI] [PubMed] [Google Scholar]
- 35.Pinto J.G., Pereira A.H.C., de Oliveira M.A., Kurachi C., Raniero L.J., Ferreira-Strixino J. Chlorin E6 phototoxicity in L. major and L. braziliensis promastigotes—In vitro study. Photodiagn. Photodyn. Ther. 2016;15:19–24. doi: 10.1016/j.pdpdt.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Bristow C.-A., Hudson R., Paget T.A., Boyle R.W. Potential of cationic porphyrins for photodynamic treatment of cutaneous Leishmaniasis. Photodiagn. Photodyn. Ther. 2006;3:162–167. doi: 10.1016/j.pdpdt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Gomes M.L., DeFreitas-Silva G., dos Reis P.G., Melo M.N., Frézard F., Demicheli C., Idemori Y.M. Synthesis and characterization of bismuth(III) and antimony(V) porphyrins: High antileishmanial activity against antimony-resistant parasite. JBIC J. Biol. Inorg. Chem. 2015;20:771–779. doi: 10.1007/s00775-015-1264-4. [DOI] [PubMed] [Google Scholar]
- 38.Andrade C.G., Figueiredo R.C.B.Q., Ribeiro K.R.C., Souza L.I.O., Sarmento-Neto J.F., Rebouças J.S., Santos B.S., Ribeiro M.S., Carvalho L.B., Fontes A. Photodynamic effect of zinc porphyrin on the promastigote and amastigote forms of Leishmania braziliensis. Photochem. Photobiol. Sci. 2018;17:482–490. doi: 10.1039/C7PP00458C. [DOI] [PubMed] [Google Scholar]
- 39.Espitia-Almeida F., Díaz-Uribe C., Vallejo W., Gómez-Camargo D., Romero-Bohorquez A.R., Schott E., Zarate X. Synthesis and Characterization of 5,10,15,20-Tetrakis(4-ethylphenyl)porphyrin and (Zn2+, Mn2+, Sn2+, Ni2+, Al3+, V3+)-Derivatives: Photophysical and DFT study. ChemistrySelect. 2019;4:6290–6294. doi: 10.1002/slct.201900948. [DOI] [Google Scholar]
- 40.Dube E., Nwaji N., Oluwole D.O., Mack J., Nyokong T. Investigation of photophysicochemical properties of zinc phthalocyanines conjugated to metallic nanoparticles. J. Photochem. Photobiol. A Chem. 2017;349:148–161. doi: 10.1016/j.jphotochem.2017.09.020. [DOI] [Google Scholar]
- 41.Zoltan T., Vargas F., López V., Chávez V., Rivas C., Ramírez Á.H. Influence of charge and metal coordination of meso-substituted porphyrins on bacterial photoinactivation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;135:747–756. doi: 10.1016/j.saa.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 42.Guillaumot D., Issawi M., Da Silva A., Leroy-Lhez S., Sol V., Riou C. Synergistic enhancement of tolerance mechanisms in response to photoactivation of cationic tetra (N-methylpyridyl) porphyrins in tomato plantlets. JPB. 2016;156:69–78. doi: 10.1016/j.jphotobiol.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Bonnett R. Chemical Aspects of Photodynamic Therapy. CRC Press; Boca Raton, FL, USA: 2014. [Google Scholar]
- 44.Pummer A., Knüttel H., Hiller K.-A., Buchalla W., Cieplik F., Maisch T. Antimicrobial efficacy of irradiation with visible light on oral bacteria in vitro: A systematic review. Future Med. Chem. 2017;9:1557–1574. doi: 10.4155/fmc-2017-0051. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro A.P.D., Andrade M.C., Bagnato V.S., Vergani C.E., Primo F.L., Tedesco A.C., Pavarina A.C. Antimicrobial photodynamic therapy against pathogenic bacterial suspensions and biofilms using chloro-aluminum phthalocyanine encapsulated in nanoemulsions. Lasers Med. Sci. 2015;30:549–559. doi: 10.1007/s10103-013-1354-x. [DOI] [PubMed] [Google Scholar]
- 46.Song D., Lindoso J.A.L., Oyafuso L.K., Kanashiro E.H.Y., Cardoso J.L., Uchoa A.F., Tardivo J.P., Baptista M.S. Photodynamic Therapy Using Methylene Blue to Treat Cutaneous Leishmaniasis. Photomed. Laser Surg. 2011;29:711–715. doi: 10.1089/pho.2010.2915. [DOI] [PubMed] [Google Scholar]
- 47.Hernández I.P., Montanari J., Valdivieso W., Morilla M.J., Romero E.L., Escobar P. In vitro phototoxicity of ultradeformable liposomes containing chloroaluminum phthalocyanine against New World Leishmania species. J. Photochem. Photobiol. B Biol. 2012;117:157–163. doi: 10.1016/j.jphotobiol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Piñero J.E., Jiménez I.A., Valladares B., Ravelo Á.G. Advances in leishmaniasis chemotherapy and new relevant patents. Expert Opin. Ther. Pat. 2004;14:1113–1123. doi: 10.1517/13543776.14.8.1113. [DOI] [Google Scholar]
- 49.Chakravarty J., Sundar S. Drug Resistance in Leishmaniasis. J. Glob. Infect. Dis. 2010;2:167. doi: 10.4103/0974-777X.62887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croft S.L., Sundar S., Fairlamb A.H. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouellette M., Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol. Today. 1993;9:150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- 52.Skovsen E., Snyder J.W., Lambert J.D., Ogilby P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chesm. B. 2005;109:8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 53.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 2nd ed. Oxford University Press; New York, NY, USA: 2015. [Google Scholar]
- 54.Breitenbach T., Kuimova M.K., Gbur P., Hatz S., Schack N.B., Pedersen B.W., Lambert J.D.C., Poulsen L., Ogilby P.R. Photosensitized production of singlet oxygen: Spatially-resolved optical studies in single cells. Photochem. Photobiol. Sci. 2009;8:442–452. doi: 10.1039/B809049A. [DOI] [PubMed] [Google Scholar]
- 55.Adler A.D., Longo F.R., Shergalis W. Mechanistic Investigations of Porphyrin Syntheses. I. Preliminary Studies on ms-Tetraphenylporphin. J. Am. Chem. Soc. 1964;86:3145–3149. doi: 10.1021/ja01069a035. [DOI] [Google Scholar]
- 56.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 57.Taylor V.M., Muñoz D.L., Cedeño D.L., Vélez I.D., Jones M.A., Robledo S.M. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol. 2010;126:471–475. doi: 10.1016/j.exppara.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 58.Akilov O.E., Kosaka S., O’Riordan K., Hasan T. Parasiticidal effect of δ-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp. Dermatol. 2007;16:651–660. doi: 10.1111/j.1600-0625.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 59.Kiderlen A.F., Kaye P.M. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J. Immunol. Methods. 1990;127:11–18. doi: 10.1016/0022-1759(90)90334-R. [DOI] [PubMed] [Google Scholar]
- 60.Durmuş M., Nyokong T. Photophysicochemical and fluorescence quenching studies of benzyloxyphenoxy-substituted zinc phthalocyanines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008;69:1170–1177. doi: 10.1016/j.saa.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 61.Khodov I.A., Nikiforov M.Y., Alper G.A., Mamardashvili G.M., Mamardashvili N.Z., Koifman O.I. Synthesis and spectroscopic characterization of Ru(II) and Sn(IV)-porphyrins supramolecular complexes. J. Mol. Struct. 2015;1081:426–430. doi: 10.1016/j.molstruc.2014.10.070. [DOI] [Google Scholar]
- 62.Moreira M.E.C., Del Portillo H.A., Milder R.V., Balanco J.M.F., Barcinski M.A. Heat shock induction of apoptosis in promastigotes of the unicellular organismLeishmania (Leishmania) amazonensis. J. Cell. Physiol. 1996;167:305–313. doi: 10.1002/(SICI)1097-4652(199605)167:23.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.