Abstract
Fluorescence in situ hybridization (FISH) is a standard technique used in routine diagnostics of genetic aberrations. Thanks to simple FISH procedure is possible to recognize tumor-specific abnormality. Its applications are limited to designed probe type. Gene rearrangements e.g., ALK, ROS1 reflecting numerous translocational partners, deletions of critical regions e.g., 1p and 19q, gene fusions e.g., COL1A1-PDGFB, genomic imbalances e.g., 6p, 6q, 11q and amplifications e.g., HER2 are targets in personalized oncology. Confirmation of genetic marker is frequently a direct indication to start specific, targeted treatment. In other cases, detected aberration helps pathologists to better distinguish soft tissue sarcomas, or to state a final diagnosis. Our main goal is to show that applying FISH to formalin-fixed paraffin-embedded tissue sample (FFPE) enables assessing genomic status in the population of cells deriving from a primary tumor or metastasis. Although many more sophisticated techniques are available, like Real-Time PCR or new generation sequencing, FISH remains a commonly used method in many genetic laboratories.
Keywords: personalized medicine; targeted treatment; FISH; HER2; ALK; ROS1; personalized oncology; t(X,18); COL1A1-PDGFB
1. Introduction of In Situ hybridization
Screening by in situ hybridization plays a supportive role in personalized medicine. A wide range of recognized aberrations: rearrangements resulting from translocations, insertions or inversions, losses (deletions) and gains (e.g., amplification) can be evaluated mainly using fluorescence in situ hybridization (FISH). In certain cases, chromogenic in situ hybridization (CISH) is introduced into the molecular pathology and molecular oncology field. Both in situ hybridization methods are based on the same idea of annealing to the region of interest, detection, assessment and spatial localization [1]. The main difference between the methods is the method of genomic region labeling, either with biotin or digoxigenin (CISH) or with fluorescent tags (FISH), followed by an appropriate detection system. For probes labeled with biotin, streptavidin conjugated with horseradish peroxidase (HRP-streptavidin) is used for detection. For probes with attached digoxigenin, an anti-digoxigenin fluorescein primary antibody, followed by an HRP-conjugated anti-fluorescein secondary antibody are used, and signals are counted under a bright-field microscope [2,3]. FISH detection is direct and requires fluorescence microscopy. Testing of genetic markers often plays a pivotal role in making decisions concerning patients, from patient risk stratification to implementing the appropriate treatment. Both in situ techniques are used in this field. For example, compared with FISH, CISH has been shown to have a sensitivity of 97.5% and a specificity of 94% for detection of the HER-2/neu gene amplification [4]. The concordance rate between FISH and CISH results calculated in a group of 4460 patients diagnosed with breast cancer was at the level of 96%, showing CISH to be a comparable technique to FISH. Most sources are in agreement and report almost equal performance of FISH and CISH in gene amplification assays. However, CISH may show lower sensitivity for low-level amplifications due to the presence of polysomy of chromosome 17 [2]. Similar observations were presented for ALK (ALK receptor tyrosine kinase) rearrangements tested using FISH and CISH. Testing 449 samples indicated 19 and 18 ALK-positive samples, respectively, in a comparison of these two methods. CISH sensitivity was at a level of 94.4% and specificity at 100%. Although every diagnostic tool, not only based on hybridization, but also on protein expression, such as immunohistochemistry (IHC), has advantages and disadvantages, the FISH technique is currently stated as the reference method [5]. A detailed comparison of the three methods is presented in Table 1.
Table 1.
Comparison of immunohistochemistry (IHC), chromogenic in situ hybridization (CISH) and fluorescence in situ hybridization (FISH) techniques.
| IHC | CISH | FISH | |
|---|---|---|---|
| Concept of the Method | |||
| assessment of protein expression using antigen-specific antibodies | assessment of chromogenic effect in an enzymatic reaction | assessment of chromosomal aberration using a fluorescent probe | |
| Advantages | |||
| preparation | |||
| analysis | |||
| other |
|
|
|
| Disadvantages | |||
| preparation |
|
||
| analysis |
|
|
|
| other | - | - | |
| Examples of solid tumors with use of the method | |||
An alternative approach to in situ testing is the use of mRNA as a biomarker. The technique uses a single-stranded DNA probe complementary to the tested mRNA molecule. Standard protocol includes digestion, hybridization, detection using an anti-digoxigenin antibody and an HRP-labeled secondary antibody, and result visualization using a chromogen, e.g., DAB (3,3′-diaminobenzidine) and a bright-field microscope. mRNA ISH is faster, cheaper, easier in preparation and less toxic in comparison with the above mentioned ISH techniques. A ready-to-use kit can be used for the HER2 gene expression assessment in breast cancer on formalin-fixed paraffin-embedded (FFPE) samples. The main limitation, as in other techniques based on mRNA assessment, is the poor stability of ribonucleic acid [3,6].
2. Fluorescence In Situ Hybridization In Solid Tumors
Fluorescence in situ hybridization is a cytogenetic-molecular technique developed in the 1980s. The standard protocol of FISH carried out on formalin-fixed paraffin-embedded (FFPE) tissue begins with a selection of the representative population of tumor cells by a pathologist who marks a section for FISH analysis on a Hematoxylin and Eosin (H&E)-stained histopathological tissue sample. A crucial issue at this pre-analytical step is the percentage of tumor cells in the sample, since a low percentage may lead to an uninformative result of FISH scoring and the need to repeat the whole procedure, starting from the selection of a new FFPE section. In the following step, an unstained sliced histological sample undergoes a standard procedure of deparaffinization and rehydration, consisting of heating the slide in a cabinet pre-warmed to 60 °C and immersing the slide in a series of wells with xylene and absolute ethanol. Subsequently, incubation with a pretreatment solution is followed by digestion using a protease solution. Incubation time is optimized individually for every FISH probe protocol. This procedure enables removing chemicals used previously to provide the best conditions for maintaining cell integrity as well as DNA structure. The nucleic acid bereft of cross-links can easily bind with a complementary sequence of the probe, significantly improving the efficiency of hybridization. Some protocols require the use of hydrochloric acid (HCl) and additional washing in saline-sodium citrate (SSC). The FISH protocol includes the following steps: denaturation of cellular DNA of the sample and the probe into single strands and hybridization of the probe with a target nucleic sequence. Fast-working hybridization buffers shorten this step significantly from an overnight incubation to a few hours. The final steps of the procedure are post-hybridization washes in SSC solutions of enriched with non-ionic detergent (NP-40) which reduce unspecific signals of the unbound probe. The final analysis of the FISH slide involves detection using an epifluorescence microscope equipped with an adjusted set of filters [8,34,35,36,37].
New approaches to FISH preparation include automated systems in which the whole procedure may be performed by a device, e.g., Ventana Medical System (Tucson, AZ, USA), with a slight support from a laboratory technician. This approach spares time and eliminates exposure to harmful chemicals, such as xylene which is used in the manual procedure.
FISH results are obtained by counting hybridization signals of the probe in each cell. Every laboratory should define its own counting procedure including the number of analyzed cells, the percentage of re-scoring of cells by a second diagnostician, control slides, cut-off for an abnormal result. Although counting signals is mostly still performed in a manual way, there are automatic counting systems available as well. Such software uses algorithms programmed to search for objects with the required shape (cells) and the presence of fluorescence signals, which are recognized as bright dots and then counted. This approach is based on an analysis of photographs, taken by a diagnostician, of representative fields with neoplastic cells. Every field is verified, which includes exclusion of non-cell objects, correction of the inaccurately marked shape of cells and verification of recognized signals. The final result is presented as the abnormal cell percentage (as for break-apart probes, e.g., for genes ALK, ROS1, EWSR1) or ratio (for HER2/CEP17, 1p/1q, 19q/19p) [35,38].
Modern concept of FISH technique present microfluidic platforms which are dedicated to the analysis of circulating tumor cells (CTCs). The cells can be obtained from the patient blood sample and used as a tumor biomarker in diagnosing and metastasis prognosis. Cellular DNA applied on a microarray is put into microchannel what enables flow-based incubation with chemicals. In this manner kinetics of hybridization process between probe and target sequence is improved. This significantly enhances signal strength. Moreover, the method improved reproducibility and reduce labor time. The method has been tested for genes HER2, KMT2A (lysine methyltransferase 2A) and others. The way of receiving CTCs seems to be the biggest challenge of the microfluidic platform [35,39].
3. Types of Probes Available in FISH Technique
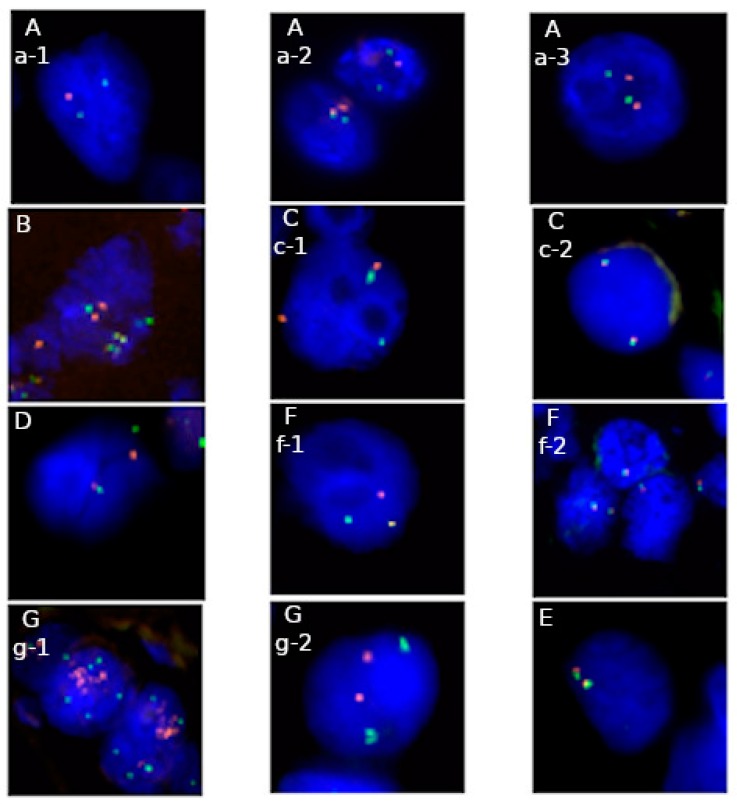
A significant issue in the FISH technique is the choice of the appropriate probe which determines the value of a test for a patient (Table 2). Every type of FISH probe enables detecting different chromosomal aberrations. Examples of practical use of a different type of FISH probes present Figure 1A–G. Locus-specific probes may be used to assess the number of extra copies of the target. Establishing the status of amplification requires two differently labeled loci, target and control regions. The decisive factor for amplification is the ratio parameter. It is a quotient of the total number of red-labeled tested gene signals divided by the total number of green-labeled control sequence signals. A minimum result of 2.0 confirms amplification [12,36].
Table 2.
Summary of FISH technique value in solid tumors.
| Type of Tumor | Diagnostic Value | Prognostic Value | Predictive Value | Available FISH Probe |
|---|---|---|---|---|
| Lung cancer ALK, ROS1 |
no | yes | yes crizotinib |
▪ Vysis ALK Break Apart FISH Probe Kit (Abbott Molecular) [7,40] ▪ Vysis 6q22 ROS1 Break Apart FISH Probe (Abbott Molecular) [41] ▪ ROS1 Break Apart FISH Probe (Empire Genomics) |
| Glioma co-deletion 1p19q |
yes | yes | No | ▪ Vysis LSI 1p36 SpectrumOrange/1q25 SpectrumGreen Probes and Vysis LSI 19q13 SpectrumOrange/19p13 SpectrumGreen Probes (Abbott Molecular) [13] |
| Breast cancer HER2 |
no | yes | yes trastuzumab, lapatinib |
▪ PathVysion HER-2 DNA Probe Kit (Abbott Molecular) [37] |
| Ovarian cancer CCNE1 |
no | yes | yes platinum-based agents |
▪ CCNE1/CEN19p FISH Probe (Abnova) [23] |
| Ewing sarcoma EWSR1 |
yes | yes | No | ▪ Vysis EWSR1 Break Apart FISH Probe Kit (Abbott Molecular) [14] |
| Synovial sarcoma SS18 |
yes | yes | No | ▪ Vysis SS18 Break Apart FISH Probe Kit (Abbot Molecular) [14] |
| Dermatofibrosarcoma protuberans COL1A1-PDGFB |
no | yes | yes TKI (imatinib) |
▪ SPEC COL1A1-PDGFB Dual Color Dual Fusion (ZytoLight) [42] |
Figure 1.
Applications of fluorescence in situ hybridization (FISH) in genetic diagnostics in solid tumors on FFPE material: A—1p/19q probe: a-1 deletion of 1p32 locus, a-2 normal signal pattern (cell on the left) and deletion of 19q13 locus (cell on the right), a-3 normal signal pattern (Abbott Molecular), B—dual fusion probe: fusions and normal signals pattern of COL1A1 and PDGFB loci (ZytoVision), C—break apart probe: c-1 rearrangement of ALK gene, c-2 normal signal pattern (Abbott Molecular), D—break apart probe: rearrangement of EWSR1 locus (Abbott Molecular), F—break apart probe: f-1 rearrangement of ROS1 gene, f-2 normal signal pattern (Empire Genomics), G—locus specific probe: g-1 amplification of HER2 locus, g-2 normal signal pattern (Abbott Molecular), E—break apart probe: normal signal pattern of SS18 locus (Abbott Molecular). Majority probes indicate region of interest in red color and control region—in green, excluding picture B, where red color indicates COL1A1 gene locus, green color—PDGFB gene locus, yellow color—fusion of COL1A1-PDGFB and PDGFB-COL1A1. Full names of available commercial probes are presented in Table 2.
Locus-specific probe can also be used to assess the loss of a chromosome sequence in a genome. As previously said, a probe consists of two differently marked regions, target and control locus. Deletion is reported when the number of cells with dominating control locus signals exceeds that of cells with dominating target locus signals. The cut-off should be calculated for each histological sample separately [12].
Different from the above-mentioned types of probes are those used for detecting chromosomal rearrangements. One of these probes, the dual-color probe, encompasses two gene targets. Due to the breaks within these targets and exchange between their halves, two fusions arise, which are the proof of translocation at the chromosome level. In the case of the split type probe, two contrast fluorescent dyes are complementary to two distant ends of the same gene, staying in close relation to the most frequent breakpoint the target [12].
4. Validation of FISH Method
Standards and guidelines for chromosome studies of lymph node- and solid tumor-acquired chromosomal abnormalities using FISH assays, developed for clinical laboratory geneticists on behalf of the American College of Medical Genetics and Genomics (ACMG) Laboratory Quality Assurance Committee, have not changed since their last revision in 2018 [43]. The result of the first counting assessment should be verified by a second diagnostician to assure reproducibility, increase the analytical reliability of FISH results and reduce the risk of errors [38]. Furthermore, automation of the in situ hybridization process and advanced software may significantly improve the quality of work. It has been estimated that the upper cut-off for normal results in a FISH test should be determined by calculating the 95% confidence level for the probe signal patterns detected in representative normal control samples [34]. As a good practice, laboratories should regularly participate in external quality assessment [44].
5. FISH Analysis in Solid Tumors
5.1. Lung Cancer
NSCLC (non-small cell lung cancer) is an example of a tumor with well-known genetic and epigenetic mechanisms of carcinogenesis. The Cancer Genome Atlas project has helped to identify the most common genomic alterations in NSCLC which include point mutations and structural rearrangements in protooncogenes, such as: EGFR (epidermal growth factor receptor), KRAS (KRAS proto-oncogene, GTPase), BRAF (B-Raf proto-oncogene serine/threonine kinase), MET (MET proto-oncogene, receptor tyrosine kinase), ALK, RET (ret proto-oncogene), ROS1 (ROS protooncogene 1, receptor tyrosine kinase), MYC (MYC proto-oncogene, bHLH transcription factor). For example, mutations in the EGFR gene occur in up to 15% of European patients [45] and in 9%–10,62% of Polish patients [46,47,48]. Moreover, rearrangements, within the most common ALK kinase, in general, account for around 3%–7% of cases if the most frequent partner, the EML4 (EMAP like 4) gene, is involved. This situation accounts for 2.7%–7.5% [49,50,51] and 2.82%–4.5% [40,52] of European and Polish patients, respectively.
5.2. ALK Gene
There are three ways of ALK activation. The first one, believed to be the original event in the NSCLC oncogenesis, involves numerous structural rearrangements of the short arm of chromosome 2, including gene locus 2p23. The inversion inv (2) (p21p23) results in gene fusion between the ALK and EML4 genes. For the EML4-ALK fusion, at least 15 different variants have been recognized. What is more, this chimeric transcript is only one of 22 possible fusion products [18,53]. Other potential translocational partners include the following genes: KLC1 (kinesin light chain 1) [54], KIF5B (kinesin family member 5B) [55], TFG (trafficking from ER to Golgi regulator) [56]. The breakpoint of the rearranged ALK gene lies in the kinase domain, frequently within exon 20 [53].
The second activating mechanism are gains of the ALK locus. Visually, in the FISH analysis, they are observed as extra copies of un-rearranged ALK loci. They differ from polysomy by the presence of at least 10 gene copies per cell in at least 10% of the analyzed cell population [16,40]. They are observed in a significant percentage of cells, ranging from 50% to 95%, in up to 63% of patient samples [16].
The third known activating mechanism are mutations. Mutations in the ALK gene, such as L1196M, modify the protein structure which prevents the protein from binding drug molecules. ALK mutations are responsible for TKI (tyrosine kinase inhibitors) treatment resistance in about 25%–30% of cases, while ALK amplification in 15% [18,53]. The last two mechanisms are observed in patients with secondary resistance to ALK-TKI, such as crizotinib. The co-existence of both of them is possible [18].
Detection of an ALK rearrangement or gain is possible with the FISH technique. The method is sufficient to detect numerous fusion variants thanks to the special structure of the probe—two differentially labeled short DNA sequences that flank breakpoints in the ALK gene. The short distance between these red and green parts of the probe yields a picture of overlapped signals or a fusion signal. The rearrangement is recognized when the distance between the 5′ and 3′ parts exceeds two diameters of the signal. This principle may sometimes cause uncertainty in the analysis, therefore passing the external quality control assessment is vital. The result is stated as truly positive when more than 25 cells out of 50 (50%) counted from at least four microscopic fields indicate the above-described separation of red and green signals in at least one gene fusion. When the abnormality is observed in 5–25 cells (10%–50%), the result is considered equivocal. Analysis done by a second analyst on 50 new cells qualifies the result as positive when the abnormality occurs in more than 15 cells (15%) out of the total 100 analyzed [40].
Identification of genetic abnormalities of the ALK gene (conducted in association with established therapies) is confirmed by the U.S. Food and Drugs Administration (FDA)-validated and approved tests, including FISH. The potential of ALK testing extends to searching for possible mechanisms of crizotinib resistance, which is believed to be related to not only mutations, but also fusion amplification, for example, EML4-ALK. Therefore, the diagnostics of this marker may be helpful in the development of next-generation ALK inhibitors [57].
5.3. ROS1 Gene
Another tumorigenesis pathway in NSCLC are rearrangements in the ROS1 gene. Disruptions of this gene have been identified for the first time in glioblastoma cell lines [57,58,59]. ROS1 rearrangements can play a role in targeted treatment as important as ALK-ones, although they represent only 0.9% adenocarcinomas and 2% of NSCLC cases [7,60]. The ROS1 gene is involved in translocations with other genes, located on many chromosomal loci. Currently, 14 different gene partners have been determined, for instance: EZR (ezrin) [61,62,63], GOPC (Golgi associated PDZ and coiled-coil motif-containing) [64], KDELR2 (KDEL endoplasmic reticulum protein retention receptor 2) [65], LRIG3 (leucine-rich repeats and immunoglobulin-like domains 3) [59], SDC4 (syndecan 4) [62,66], TPM3 (tropomyosin 3) [62], and others. In every such translocation, including rearrangement with the SLC34A2 (solute carrier family 34 member 2) gene and the most frequent one with the CD74 gene [56], the telomeric part of the ROS1 gene in transferred to the fusion partner gene or is deleted. Consequently, activated kinase enhances proliferation of tumor cells, while also protecting them from death [7,67].
Assessing the ROS1 gene status is possible thanks to FISH using a break-apart probe. Similarly to the ALK gene, a positive result is established if the separation of both differently labeled ends of the probe is greater than two diameters of a signal, or a deletion of the 5′ end of the gene is observed next to at least one non-rearranged signal. ROS1 gene rearrangement has to occur in at least 15% of the analyzed population of nuclei to assure that patient is ROS1 positive [7].
According to the newest recommendations released by the NCCN (National Comprehensive Cancer Network) issued in 2018, testing of ROS1 rearrangements is required prior to the use of TKI inhibitors, as it is in ALK-positive patients. Crizotinib used in metastatic and advanced NSCLC significantly improves response rate [68,69].
5.4. c-MET Gene
In lung cancer, as well as in other tumors such as kidney, stomach, colon and NSCLC, overexpression of the c-MET gene is observed. The c-MET protooncogene belongs to transmembrane proteins which, after binding a ligand–HGF (hepatocyte growth factor) for c-MET, activate the MAPK (mitogen-activated protein kinases) signaling pathway [70]. There are a few possible ways of activation of the gene, for instance: amplification, activating mutations, transcriptional regulations and auto- or paracrine regulation related with the ligand [71]. When extra copies of the gene locus and/or mutation of the gene arise, this signal becomes enhanced which leads to a stronger cell proliferation and blockade of apoptosis, and consequently promotes neoplastic progression. Each of these alterations results in a permanent phosphorylation of the protein and, consequently, its constitutive activation [72].
Gains of the c-MET locus (≥5 copies/nucleus) occur in NSCLC with a frequency of 8%–10% [73,74]. Recognition of abnormality type as copy number aberration versus amplification depends on the technique used. In MLPA (multiplex ligation probe amplification), gain of the c-MET locus occurs in 7% NSCLC, while amplification, regarded as ≥4 copies, in 5% of cases [75]. Assessing the number of c-MET gene copies in FISH is possible thanks to probes complementary to the tested gene locus and control region of the centromere of chromosome 7 [76]. Microscopic analysis of about 100 tumor cells confirms amplification if the ratio result for c-MET/centromere 7 is ≥2 or if more than four copies of the c-MET locus are observed [71,72]. According to other references, 80% of cells should present more than 6 copies of the gene and a ratio result exceeding 2 [76].
Overexpression of the c-MET gene has been associated with unfavorable responses to treatment, for example in the therapy of EGFR-positive patients with tumors resistant to TKI [77].
5.5. Gliomas
Gliomas represent the most frequent type of de novo brain tumor, with a frequency reaching 30% of all brain tumors, including 80% of all nervous system [78]. The four-degree tumor aggressiveness WHO classification was proposed for Classification of Tumors of the Central Nervous System in the 2007. The newest, 5th version of this classification, considers not only histological analysis, but also genetic aberrations. This approach has contributed to the classification of cases of astrocytic and oligodendroglial tumors into one category, as mutations in the IDH1/2 (isocitrate dehydrogenase (NADP (+)) 1/2) gene occur in both types. Further differentiation is based on unique genetic changes. Oligodendroglioma and anaplastic oligodendroglioma present the 1p/19q-codeletion, while astrocytoma presents mutations in the ATRX (ATRX chromatin remodeler) and TP53 (tumor protein p53) genes [79,80].
Codeletion of 1p/19q, explained as the presence of a chromosomal deletion of the short arm of chromosome 1 and the long arm of chromosome 19 in the same cell, was considered for the first time as an oncological marker in neuronal tumors in 1994 [81]. Coexistence of 1p and 19q deletions is a result of an unbalanced translocation t(1;19) (q10;q10). The aberration can be detected using routine cytogenetic methods based on tumor cell cultures and G-banding (Giemsa-banding), but more frequently FISH on FFPE is used [13,15]. The nature of the translocation can be tested using the chromosome 1 alpha-satellite (CEP1) and locus 19q12 probes [22]. In the routine cytogenetic–molecular diagnostics, a kit of two probes is used: 1p36/1q25 and 19p13/19q13. Each set requires analysis of at least 100 non-overlapping nuclei for each pair of chromosomes. Interpretation of results is based on standards established for neuroblastoma by the International Society of Pediatric Oncology (SIOP). The calculated percentage of cells harboring the deletion has to be higher than 50% and ratio lower than 0.75%–0.8% for 1p36/1q25 as well as for 19q13/19p13 [79,82]. Alternative methods of detection of the codeletion include CGH (comparative genome hybridization) or loss of heterozygosity (LOH) analysis based on microsatellite sequences [79]. In addition to 1p19q aberrations, extra unbalances might occur, for example, trisomies of chromosomes 11, 17, 21, 22, and monosomies of chromosomes 14, 15, 18 [78].
Codeletion of 1p/19q plays a decisive role in distinguishing between oligodendroglioma and astrocytoma, which is especially helpful in histologically difficult cases. The genetic marker has also a prognostic value [15,79].
5.6. Breast Cancer
Breast cancer (BC) is the most frequent type of tumor identified in women [83]. Moreover, it is one of the main mortality factors in the world in this group of patients, with a five-year survival rate ranging from 57.9%–82% in Europe, depending on the country [84], with 77.2% in Poland [85].
The ERBB2 (erb-b2 receptor tyrosine kinase) gene, frequently called HER2, is a proto-oncogene coding for a cell receptor involved in a few signal pathways responsible, e.g., for cell division and viability [83]. A significant abnormality of HER2 is the occurrence of extra copies of this gene defined as amplification. A consequence of this aberration is over-expression of the protein which is found in approximately 22%–28% of BCs [86,87,88,89] and 8.2%–53.4% of gastric cancers [20,90,91]. While routine analysis of the protein may be conducted using IHC, the number of gene copies is assessed using the gold standard FISH on FFPE samples or CISH [11,19,37]. Status of the HER2 gene is evaluated following the guidelines issued by the American Society of Clinical Oncology and the College of American Pathologists (ASCO-CAP), for the first time defined in 2007 [92]. According to the newest version of 2018, amplification is considered for HER2/CEP17 (centromere 17) ratios of ≥2.0, with at least 4 copies of the HER2 gene per cell. For ratios of <2 and at least 6 copies of the HER2 gene per cell, the final result is positive only for IHC3+ or when an additional analysis performed by a new observer on at least 20 cells confirms the same result (ratio of <2 and ≥6 HER2 signals/nucleus) for IHC2+. A similar procedure relates to equivocal results as well, still defined as a combination of a ratio of <2 and HER2/CEP17 ≥4 and <6. In this situation, IHC3+ results again to facilitate the final diagnosis as HER2-positive. However, for IHC2+ results, only a result of an extra analysis of at least 20 cells with a ratio of ≥2.0 and HER2/CEP17>6 is consistent with HER2-amplified status of the gene [93]. This borderline situation occurs in about 10%–17% of FISH analyses [19].
Although the use of two differently labeled probes for the HER2 locus and centromere 17 at the same time seems to be an optimal solution, results may not reflect only the amplification of HER2 in the analyzed population of tumor cells, but also co-amplification of a bigger part of chromosome 17. The polysomy event, defined as at least 3 copies of the centromere of chromosome 17 per cell, is observed in 12%–46% of breast cancer cases [30,94,95]. Moreover, it is frequent in IHC2+ cases [20]. They are available other probes for HER2 gene with a bigger distance between tested and control region, for instance, RARA (retinoic acid receptor alpha)—17q21.2, RAI1 (retinoid acid-induced 1)—17p11.2, TP53–17p13.1 [19].
FISH testing of the HER2 gene locus has a well-documented value for breast cancer patients. Personalized therapy can be used after assessing the HER2 gene using one of the FDA-approved methods IHC, CISH or FISH, compared. Treatments include inhibitors or humanized monoclonal antibodies (e.g., pertuzumab, trastuzumab). Results stating overexpression or amplification of HER2 are indications to start targeted treatment. In this case, personalized medicine contributes to the success of treatment, improves overall survival and extends the time to progression and metastasis. Moreover, thanks to focusing the therapy on HER2-positive patients, the general costs of breast cancer therapy are reduced [2,3,9,11,37,93,96,97].
5.7. Ovarian Cancer
Ovarian cancer is a heterogeneous type of tumor with multiple entities, the most typical of which are epithelial, germ cell and specialized stromal cell tumors. The first type, which reflects most of the cases, is divided into low-grade endometrioid cancer type I with gradual growth and beneficial prognosis and high-grade non-endometroid cancer type II with fast progression and adverse response to treatment. The leading form of the epithelial type is a very aggressive high-grade serous ovarian carcinoma (HGSOC) [24,98]. Its etiopathogenesis lies in mutations of the TP53 gene, believed to be a primary event in carcinogenesis, but also in mutations in genes such as BRCA1 (BRCA1 DNA repair associated) and BRCA2 (BRCA2 DNA repair associated), involved in the reliable mechanism of double-stranded breaks (DSBs) repair called homologous recombination (HR), and finally in copy number alterations, including extra copies of the CCNE1 (cyclin E1) gene [98]. The protein product of this gene, cyclin E, takes part in switching cell cycle between the G1 and S phases by activation of cyclin-dependent kinase 2 (Cdk2), phosphorylation of the Rb protein and E2f-1-dependent transcription [24]. Increased expression of CCNE1 stimulates uncontrolled replication of DNA, amplification of centrosome and enhances chromosomal instability [98,99].
Assessment of the copy number of the CCNE1 gene is based on FISH on FFPE using a locus-specific probe for a target gene located in 19q12 and a control region which is the centromere of the same chromosome (CEP19). At least 50 fully exposed nuclei undergo the analysis. A sample is considered as positive if the status of amplification understood as the CCNE1/CEP19 ratio of ≥2 occurs in more than 20% of tumor cells, or if ≥4 CCNE1 copies are presented in ≥40% of analyzed cells, which is regarded as high polysomy [23]. What is interesting, the profile of the amplified gene locus varies between cases of clear cell carcinoma and high-grade serous carcinoma. In the former, extra copies are easy to count which suggests amplification of a double minute (dm) type, while in the latter, signals do not have sharp contours, and frequently are impossible to precise number assessing like in homogeneously staining region (hsr) type. These differences suggest the occurrence of two ways in which extra copies of the CCNE1 gene are gained [23].
CCNE1 testing is valuable for several reasons. Firstly, as overexpression occurs in clear-cell carcinoma cases, the test may be helpful in differentiation from other ovarian tumors based on endometriosis. Secondly, gains of CCNE1 may be a predictor of short survival rate in clear-cell carcinoma. Thirdly, patients with identified gains of CCNE1 may receive target-based therapy [23]. That last reason seems to play an important role due to the availability of therapeutic options. The common use of cytotoxic agents based on platinum is responsible for insensitivity to treatment if CCNE1 amplification is present [24]. Searching for new molecules inhibiting CDKs, such as CDK2, may bring some benefits for patients with ovarian cancer [98].
5.8. Soft Tissues Sarcomas
Soft tissue sarcomas (STS) are uncommon cancers, with a frequency of 3.58–6.1/100,000 [100,101]. Their complex biology impedes the correct identification of tumor types in many cases. Therefore, both immunohistochemical and histological analyses are required. Although genetic testing is not the main diagnostic tool, it significantly supports the final diagnosis. The value of these tests has been approved in the WHO classification for many sarcomas in which crucial gene sequences have been identified, e.g., Ewing sarcoma (ES) [14].
At the molecular level, ES is characterized by rearrangements of the EWSR1 (EWS RNA binding protein 1) gene [102]. The gene has several translocational partners which belong to the ETS family of transcriptional factors, such as the FLI1 (Fli-1 proto-oncogene, ETS transcription factor) gene located in locus 11q24 [103], or the ERG (ETS transcription factor ERG) gene in locus 21q22. Translocations of the EWSR1 gene with its partners are responsible for carcinogenesis: changes in cellular metabolism and proliferation, as well as apoptosis blockade [32,104,105].
Another sarcoma—synovial type (SS)—is represented by abnormalities of the SS18 (SS18 subunit of BAF chromatin remodeling complex) gene, for example as a result of a t(X;18) (p11.2;q11.2) translocation. Gene partners such as SSX1 (SSX family member 1) and SSX2 (SSX family member 2) determine the histological subtype of SS: monophasic and biphasic, respectively. It has been observed that patients with the fusion SYT–SSX1 have a longer survival [105,106,107,108,109].
In both mentioned sarcomas, assessing the gene status is possible using a break-apart probe and the FISH technique. Thanks to the structure of the probe, the demanded picture should present separation of fusion signals in more than 14 out of 50 analyzed tumor cells for the EWRS1 gene and at least 10 cells for the SS18 gene [14].
FISH testing based on recognition of gene rearrangements or specific gene fusions allows distinguishing pathological diagnosis, which is especially helpful in poorly differentiated sarcomas, e.g., some SS tumors may resemble small round cells of EWS, but genetically have a rearrangement of SS18 gene. The correctness of diagnosis has a direct impact on the course of therapy and the patient’s prognosis [12,14,105].
Dermatofibrosarcoma protuberans (DFSP) is an uncommon tumor of mesenchymal tissues. In the newest WHO Classification of Soft Tissue Tumors issued in 2013, DFSP was assigned into the intermediate group of fibroblastic/myofibroblastic tumors. Recurrence of lesions is frequent and results in repeated surgeries. Metastases appear mostly in cases with a fibrosarcomatous component [110]. Diagnostics of this type of neoplasm is based on physical examination followed by morphological and immunohistochemical testing. However, since the discovery that a genetic abnormality underlies a great part of cases, genetic testing has become a helpful diagnostic procedure [26]. In 68%–96% of dermatofibrosarcoma protuberans cases, a translocation between chromosomes 17 and 22 occurs [26,33,111,112,113]. A result of this aberration is a fusion of the COL1A1 (collagen type I alpha 1 chain) gene with the PDGFB (platelet-derived growth factor subunit B) gene [114]. The fusion results primarily in an enhanced expression of PDGFB, but also in a strong activation of the receptor of this ligand PDGFRB and stimulation of proliferation of mesenchymal cells [115]. The abnormality can be detected with the FISH method using a dual-color dual-fusion probe. When overlapping of signals of both differently labeled loci is observed as a yellow signal, the result is considered positive on condition that it affects at least 10% of analyzed cells. Alternatively, the PDGFB locus might be tested with the use of split type probe. Separation of signals complementary to the tested locus in at least 10% of cells confirms the rearrangement of that locus [26,33].
Diagnosis of the fusion COL1A1–PDGFB plays a crucial role in future therapeutic decisions as it is an indication for starting treatment based on imatinib mesylate. The inhibitor can be used in inoperable, metastatic and recurrent cases of dermatofibrosarcoma and seems to be an option as first-line treatment as well [116].
6. Conclusions
Established in the 1980s, fluorescence in situ hybridization is a technique useful in detecting various abnormalities, such as fusion of genes and genomic gains and deletions. Owing to gradually implemented improvements, the method has been yielding crucial information to be used not only in more precise patient diagnostics, but also in matching best therapies [39]. Some excellent examples include genetic tests of the ALK and ROS1 genes performed in lung cancer required for TKI treatment. The ASCO recommendations of 2018 accept an IHC test of ALK in this neoplasia provided that the result is obviously positive or negative. For ROS1-positive and ALK-weak staining, the FISH method is proposed as decisive [117]. Other non-in situ methods, e.g., RT-PCR (reverse transcriptase-polymerase chain reaction) or NGS (next-generation sequencing) are also used, for instance for searching aberrations in lung cancer. The former method has a high sensitivity and specificity, but faces many issues related to isolation of ribonucleic acid derived from an FFPE sample or numerous gene partners. The latter seems superior as it requires a low amount of RNA, even from FFPE samples, and keeps sensitivity and specificity at the level of 100% [118]. Compared with the above mentioned IHC and FISH, high costs of chemistry and equipment and complex analysis and interpretation of results performed by a specialist in medical laboratory genetics still make NGS a possible supportive rather than routine technique [119]. To date, timeless and costless fluorescence in situ hybridization remains a gold standard, for example in NSCLC [31].
Abbreviations
The following abbreviations are used in this manuscript:
| ACMG | the American College of Medical Genetics and Genomics |
| ASCO-CAP | the American Society of Clinical Oncology and the College of American Pathologists |
| BC | breast cancer |
| Cdk2 | cyclin-dependent kinase 2 |
| CGH | comparative genome hybridization |
| CISH | chromogenic in situ hybridization |
| CTC | circulating tumor cells |
| DAB | 3,3′-diaminobenzidine; |
| DFSP | dermatofibrosarcoma protuberans |
| DSBs | double-stranded breaks |
| EGFR | epidermal growth factor receptor |
| ES | Ewing sarcoma |
| EZR | Ezrin |
| FDA | U.S. Food and Drugs Administration |
| FFPE | formalin-fixed paraffin-embedded |
| FISH | fluorescence in situ hybridization |
| G-banding | Giemsa-banding |
| H&E | Hematoxylin and Eosin |
| HCl | hydrochloric acid |
| HGF | hepatocyte growth factor |
| HGSOC | high-grade serous ovarian carcinoma |
| HRP | horseradish peroxidase |
| HR | homologous recombination |
| hsr | homogeneously staining region |
| IHC | Immunohistochemistry |
| KLC1 | kinesin light chain 1 |
| LOH | loss of heterozygosity |
| MAPK | mitogen-activated protein kinases |
| MLPA | multiplex ligation probe amplification |
| mRNA | messenger ribonucleic acid |
| NCNN | the National Comprehensive Cancer Network; |
| NGS | next-generation sequencing |
| NSCLC | non-small cell lung cancer |
| PDGFB | platelet-derived growth factor beta |
| RT-PCR | reverse transcriptase-polymerase chain reaction |
| SDC4 | syndecan 4 |
| SIOP | the International Society of Pediatric Oncology |
| SS | synovial sarcoma |
| SSC | saline-sodium citrate |
| STS | soft tissue sarcomas |
| TKI | tyrosine kinase inhibitors |
| TP53 | tumor protein 53 |
| TPM3 | tropomyosin 3 |
Author Contributions
Conceptualization M.A.L., resources M.A.L. and J.K., writing—original draft preparation N.M.C., writing—review and editing M.A.L. and J.K.; visualization N.M.C., supervision M.A.L., funding acquisition J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funds for statutory research from the Ludwik Rydygier Collegium Medicum Nicolaus Copernicus University (UMK CM 2018 WL103).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Summersgil B., Clarc J., Shipley J. Fluorescence and Chromogenic in Situ Hybridization to Detect Genetic Aberrations in Formalin-Fixed Paraffin Embedded Material, Including Tissue Microarrays. Nat. Protoc. 2008;3:220–234. doi: 10.1038/nprot.2007.534. [DOI] [PubMed] [Google Scholar]
- 2.Rosa F.E., Santos R.M., Rogatto S.R., Domingues M.A.C. Chromogenic in Situ Hybridization Compared with Other Approaches to Evaluate HER2/Neu Status in Breast Carcinomas. Braz. J. Med. Biol. Res. 2013;46:207–216. doi: 10.1590/1414-431X20132483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furrer D., Sanschagrin F., Jacob S., Diorio C. Advantages and Disadvantages of Technologies for HER2 Testing in Breast Cancer Specimens: Table 1. Am. J. Clin. Pathol. 2015;144:686–703. doi: 10.1309/AJCPT41TCBUEVDQC. [DOI] [PubMed] [Google Scholar]
- 4.Sáez A., Andreu F.J., Baré M.L., Fernández S., Dinarés C., Rey M.J. HER-2 Gene Amplification by Chromogenic in Situ Hybridisation(CISH) Compared with Fluorescence in Situ Hybridisation(FISH) in Breast Cancer—A Study of Two Hundred Cases. Breast. 2006;15:519–527. doi: 10.1016/j.breast.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Kim H., Yoo S., Paik J., Xu X., Nitta H., Zhang W., Grogan T.M., Choon-Taek L., Sangonhoon J. Detection of ALK Gene Rearrangement in Non-Small Cell Lung Cancer: A Comparison of Fluorescence in Situ Hybridization and Chromogenic in Situ Hybridization with Correlation of ALK Protein Expression. J. Thorac. Oncol. 2011;6:1359–1366. doi: 10.1097/JTO.0b013e31821cfc73. [DOI] [PubMed] [Google Scholar]
- 6.Wang F., Flanagan J., Su N., Wang L.-C., Bui S., Nielson A., Wu X., Vo H.-T., Ma X.-J., Luo Y. RNAscope. J. Mol. Diagn. JMD. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers T.-M., Russell P.A., Wright G., Wainer Z., Pang J.-M., Henricksen L.A. Comparison of Methods in the Detection of ALK and ROS1 Rearrangements in Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2015;10:611–618. doi: 10.1097/JTO.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 8.Broholm H., Born P.W., Guterbaum D., Dyrbye H., Laursμen H. Detecting Chromosomal Alterations at 1p and 19q by FISH and DNA Fragment Analysis--a Comparative Study in Human Gliomas. Clin. Neuropathol. 2008;27:378–387. doi: 10.5414/NPP27378. [DOI] [PubMed] [Google Scholar]
- 9.Hicks D.G., Kulkarni S. HER2+ Breast Cancer: Review of Biologic Relevance and Optimal Use of Diagnostic Tools. Am. J. Clin. Pathol. 2008;129:263–273. doi: 10.1309/99AE032R9FM8WND1. [DOI] [PubMed] [Google Scholar]
- 10.Thunnissen E., Bubendorf L., Dietel M., Elmberger G., Kerr K., Lopez-Rios F., Moch H., Olszewski W., Pauwels P., Penault-Llorca F., et al. EML4-ALK Testing in Non-Small Cell Carcinomas of the Lung: A Review with Recommendations. Virchows Arch. 2012;461:245–257. doi: 10.1007/s00428-012-1281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulsen T.S., Espersen M.L.M., Kofoed V., Dabetic T., Høgdall E., Balslev E. Comparison of Fluorescence In Situ Hybridization and Chromogenic In Situ Hybridization for Low and High Throughput HER2 Genetic Testing. Int. J. Breast Cancer. 2013;2013 doi: 10.1155/2013/368731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugita S., Hasegawa T. Practical Use and Utility of Fluorescence in Situ Hybridization in the Pathological Diagnosis of Soft Tissue and Bone Tumors. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2017;22:601–612. doi: 10.1016/j.jos.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins R.B., Blair H., Ballman K.V., Giannini C., Arusell R.M., Law M. A t(1;19)(Q10;P10) Mediates the Combined Deletions of 1p and 19q and Predicts a Better Prognosis of Patients with Oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 14.Asif A., Mushtaq S., Hassan U., Akhtar N., Hussain M., Azam M., Qazi R. Fluorescence in Situ Hybridization(FISH) for Differential Diagnosis of Soft Tissue Sarcomas. Asian Pac. J. Cancer Prev. APJCP. 2018;19:655–660. doi: 10.22034/APJCP.2018.19.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polivka J., Polivka J., Repik T., Rohan V., Hes O., Topolcan O. Co-Deletion of 1p/19q as Prognostic and Predictive Biomarker for Patients in West Bohemia with Anaplastic Oligodendroglioma. Anticancer Res. 2016;36:471–476. [PubMed] [Google Scholar]
- 16.Salido M., Pijuan L., Martínez-Avilés L., Galván A.B., Cañadas I., Rovira A., Zanui M., Martínez A. Increased ALK Gene Copy Number and Amplification Are Frequent in Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2011;6:21–27. doi: 10.1097/JTO.0b013e3181fb7cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesoła M., Jeleń M. A Comparison of IHC and FISH Cytogenetic Methods in the Evaluation of HER2 Status in Breast Cancer. Adv. Clin. Exp. Med. 2015;24:899–904. doi: 10.17219/acem/27923. [DOI] [PubMed] [Google Scholar]
- 18.Duchemann B., Friboulet L., Besse B. Therapeutic Management of ALK+ Nonsmall Cell Lung Cancer Patients. Eur. Respir. J. 2015;46:230–242. doi: 10.1183/09031936.00236414. [DOI] [PubMed] [Google Scholar]
- 19.Agersborg S., Mixon C., Nguyen T., Aithal S., Sudarsanam S., Blocker F., Weiss L., Gasparini R. Immunohistochemistry and Alternative FISH Testing in Breast Cancer with HER2 Equivocal Amplification. Breast Cancer Res. Treat. 2018;170:321–328. doi: 10.1007/s10549-018-4755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halon A., Donizy P., Biecek P., Rudno-Rudzinska J., Kielan W., Matkowski R. HER-2 Expression in Immunohistochemistry Has No Prognostic Significance in Gastric Cancer Patients. Sci. World J. 2012;2012:1–6. doi: 10.1100/2012/941259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelpi E., Ambros I.M., Birner P., Luegmayr A., Drlicek M., Fischer I., Kleinert R., Maier H. Fluorescent in Situ Hybridization on Isolated Tumor Cell Nuclei: A Sensitive Method for 1p and 19q Deletion Analysis in Paraffin-Embedded Oligodendroglial Tumor Specimens. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2003;16:708–715. doi: 10.1097/01.MP.0000076981.90281.BF. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez F.J., Mota R.A., Scheithauer B.W., Giannini C., Blair H., New K.C., Wu K.J., Dickson D.W. Interphase Cytogenetics for 1p19q and t(1;19)(Q10;P10) May Distinguish Prognostically Relevant Subgroups in Extraventricular Neurocytoma. Brain Pathol. Zurich Switz. 2009;19:623–629. doi: 10.1111/j.1750-3639.2008.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayhan A., Kuhn E., Wu R.-C., Ogawa H., Bahadirli-Talbott A., Mao T.-L. CCNE1 Copy-Number Gain and Overexpression Identify Ovarian Clear Cell Carcinoma with a Poor Prognosis. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2017;30:297–303. doi: 10.1038/modpathol.2016.160. [DOI] [PubMed] [Google Scholar]
- 24.Noske A., Brandt S., Valtcheva N., Wagner U., Zhong Q., Bellini E., Fink D., Obermann E.C., Moch H., Wild P.J. Detection of CCNE1/URI (19q12) Amplification by in Situ Hybridisation Is Common in High Grade and Type II Endometrial Cancer. Oncotarget. 2017;8:14794–14805. doi: 10.18632/oncotarget.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornick J.L. Novel Uses of Immunohistochemistry in the Diagnosis and Classification of Soft Tissue Tumors. Mod. Pathol. 2014;27:S47–S63. doi: 10.1038/modpathol.2013.177. [DOI] [PubMed] [Google Scholar]
- 26.Karanian M., Pérot G., Coindre J.-M., Chibon F., Pedeutour F., Neuville A. Fluorescence in Situ Hybridization Analysis Is a Helpful Test for the Diagnosis of Dermatofibrosarcoma Protuberans. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2015;28:230–237. doi: 10.1038/modpathol.2014.97. [DOI] [PubMed] [Google Scholar]
- 27.Schildhaus H.-U., Deml K.-F., Schmitz K., Meiboom M., Binot E., Hauke S., Merkelbach-Bruse S., Büttner R. Chromogenic in Situ Hybridization Is a Reliable Assay for Detection of ALK Rearrangements in Adenocarcinomas of the Lung. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2013;26:1468–1477. doi: 10.1038/modpathol.2013.95. [DOI] [PubMed] [Google Scholar]
- 28.Shia J., Klimstra D.S., Li A.R., Qin J., Saltz L., Teruya-Feldstein J., Akram M., Chung K.Y., Yao D., Paty P.B., et al. Epidermal Growth Factor Receptor Expression and Gene Amplification in Colorectal Carcinoma: An Immunohistochemical and Chromogenic in Situ Hybridization Study. Mod. Pathol. 2005;18:1350–1356. doi: 10.1038/modpathol.3800417. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai A., Motoi T., Tsuji K., Imamura T., Fukusato T. Detection of SYT and EWS Gene Rearrangements by Dual-Color Break-Apart CISH in Liquid-Based Cytology Samples of Synovial Sarcoma and Ewing Sarcoma/Primitive Neuroectodermal Tumor. Am. J. Clin. Pathol. 2010;134:323–331. doi: 10.1309/AJCPTLSM15XKPDDU. [DOI] [PubMed] [Google Scholar]
- 30.Ciesielski M., Szajewski M., Pęksa R., Lewandowska M.A., Zieliński J., Walczak J., Szefel J., Kruszewski W.J. The Relationship between HER2 Overexpression and Angiogenesis in Gastric Cancer. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000012854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shackelford R.E., Vora M., Mayhall K., Cotelingam J. ALK-Rearrangements and Testing Methods in Non-Small Cell Lung Cancer: A Review. Genes Cancer. 2014;5:1–14. doi: 10.18632/genesandcancer.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamberi G., Cocchi S., Benini S., Magagnoli G., Morandi L., Kreshak J. Molecular Diagnosis in Ewing Family Tumors. J. Mol. Diagn. JMD. 2011;13:313–324. doi: 10.1016/j.jmoldx.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel K.U., Szabo S.S., Hernandez V.S., Prieto V.G., Abruzzo L.V., Lazar A.J.F., López-Terrada D. Dermatofibrosarcoma Protuberans COL1A1-PDGFB Fusion Is Identified in Virtually All Dermatofibrosarcoma Protuberans Cases When Investigated by Newly Developed Multiplex Reverse Transcription Polymerase Chain Reaction and Fluorescence in Situ Hybridization Assays. Hum. Pathol. 2008;39:184–193. doi: 10.1016/j.humpath.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Wan T.S.K. Cancer Cytogenetics: Methodology Revisited. Ann. Lab. Med. 2014;34:413. doi: 10.3343/alm.2014.34.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber D., Voith von Voithenberg L., Kaigala G.V. Fluorescence in Situ Hybridization (FISH): History, Limitations and What to Expect from Micro-Scale FISH? Micro Nano Eng. 2018;1:15–24. doi: 10.1016/j.mne.2018.10.006. [DOI] [Google Scholar]
- 36.Chen A.Y.-Y., Chen A. Fluorescence In Situ Hybridization. J. Investig. Dermatol. 2013;133:1–4. doi: 10.1038/jid.2013.120. [DOI] [PubMed] [Google Scholar]
- 37.Bogdanovska-Todorovska M., Petrushevska G., Janevska V., Spasevska L., Kostadinova-Kunovska S. Standardization and Optimization of Fluorescence in Situ Hybridization(FISH) for HER-2 Assessment in Breast Cancer: A Single Center Experience. Bosn. J. Basic Med. Sci. 2018;18:132–140. doi: 10.17305/bjbms.2018.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hastings R.J., Bown N., Tibiletti M.G., Debiec-Rychter M., Vanni R., Espinet B. Guidelines for Cytogenetic Investigations in Tumours. Eur. J. Hum. Genet. 2016;24:6–13. doi: 10.1038/ejhg.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato K. Microdevice in Cellular Pathology: Microfluidic Platforms for Fluorescence in Situ Hybridization and Analysis of Circulating Tumor Cells. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2015;31:867–873. doi: 10.2116/analsci.31.867. [DOI] [PubMed] [Google Scholar]
- 40.Wojas-Krawczyk K., Krawczyk P.A., Ramlau R.A., Szumiło J., Kozielski J., Kalinka-Warzocha E. The Analysis of ALK Gene Rearrangement by Fluorescence in Situ Hybridization in Non-Small Cell Lung Cancer Patients. Contemp. Oncol. Poznan Pol. 2013;17:484–492. doi: 10.5114/wo.2013.38758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H.R., Lim S.M., Kim H.J., Hwang S.K., Park J.K., Shin E. The Frequency and Impact of ROS1 Rearrangement on Clinical Outcomes in Never Smokers with Lung Adenocarcinoma. Ann. Oncol. 2013;24:2364–2370. doi: 10.1093/annonc/mdt220. [DOI] [PubMed] [Google Scholar]
- 42.Walluks K., Chen Y., Woelfel C., Yang L., Cui T., Seliger C. Molecular and Clinicopathological Analysis of Dermatofibrosarcoma Protuberans. Pathol. Res. Pract. 2013;209:30–35. doi: 10.1016/j.prp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Cooley L.D., Morton C.C., Sanger W.G., Saxe D.F., Mikhail F.M. Section E6.5–6.8 of the ACMG Technical Standards and Guidelines: Chromosome Studies of Lymph Node and Solid Tumor–Acquired Chromosomal Abnormalities. Genet. Med. 2016;18:643–648. doi: 10.1038/gim.2016.51. [DOI] [PubMed] [Google Scholar]
- 44.Hastings R., Howell R., Bricarelli F.D., Kristoffersson U., Cavani S. European Cytogeneticists Association (E.C.A.). A Common European Framework for Quality Assessment for Constitutional, Acquired and Molecular Cytogenetic Investigations. General Guidelines and Quality Assurance for Cytogenetics. Newsletter. 2012;29:7–25. [Google Scholar]
- 45.Midha A., Dearden S., McCormack R. EGFR Mutation Incidence in Non-Small-Cell Lung Cancer of Adenocarcinoma Histology: A Systematic Review and Global Map by Ethnicity (MutMapII) Am. J. Cancer Res. 2015;5:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 46.Krawczyk P., Ramlau R., Chorostowska-Wynimko J., Powrózek T., Lewandowska M.A., Limon J., Wasąg B., Pankowski J. The Efficacy of EGFR Gene Mutation Testing in Various Samples from Non-Small Cell Lung Cancer Patients: A Multicenter Retrospective Study. J. Cancer Res. Clin. Oncol. 2015;141:61–68. doi: 10.1007/s00432-014-1789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewandowska M.A., Jóźwicki W., Jochymski C., Kowalewski J. Application of PCR Methods to Evaluate EGFR, KRAS and BRAF Mutations in a Small Number of Tumor Cells in Cytological Material from Lung Cancer Patients. Oncol. Rep. 2013;30:1045–1052. doi: 10.3892/or.2013.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szumera-Ciećkiewicz A., Olszewski W.T., Tysarowski A., Kowalski D.M., Głogowski M., Krzakowski M. EGFR Mutation Testing on Cytological and Histological Samples in Non-Small Cell Lung Cancer: A Polish, Single Institution Study and Systematic Review of European Incidence. Int. J. Clin. Exp. Pathol. 2013;6:2800–2812. [PMC free article] [PubMed] [Google Scholar]
- 49.Perner S., Wagner P.L., Demichelis F., Mehra R., Lafargue C.J., Moss B.J. EML4-ALK Fusion Lung Cancer: A Rare Acquired Event. Neoplasia N. Y. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S., Watanabe H. Identification of the Transforming EML4-ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 51.Martelli M.P., Sozzi G., Hernandez L., Pettirossi V., Navarro A., Conte D. EML4-ALK Rearrangement in Non-Small Cell Lung Cancer and Non-Tumor Lung Tissues. Am. J. Pathol. 2009;174:661–670. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grenda A., Jarosz B., Krawczyk P., Kucharczyk T., Wojas-Krawczyk K., Reszka K. Discrepancies between ALK Protein Disruption and Occurrence of ALK Gene Rearrangement in Polish NSCLC Patients. J. Thorac. Dis. 2018;10:4994–5009. doi: 10.21037/jtd.2018.07.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallberg B., Palmer R.H. The Role of the ALK Receptor in Cancer Biology. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27(Suppl 3):iii4–iii15. doi: 10.1093/annonc/mdw301. [DOI] [PubMed] [Google Scholar]
- 54.Togashi Y., Soda M., Sakata S., Sugawara E., Hatano S., Asaka R. KLC1-ALK: A Novel Fusion in Lung Cancer Identified Using a Formalin-Fixed Paraffin-Embedded Tissue Only. PLoS ONE. 2012;7:e31323. doi: 10.1371/journal.pone.0031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeuchi K., Choi Y.L., Togashi Y., Soda M., Hatano S., Inamura K., Takada S., Ueno T. KIF5B-ALK, a Novel Fusion Oncokinase Identified by an Immunohistochemistry-Based Diagnostic System for ALK-Positive Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:3143–3149. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 56.Rikova K., Guo A., Zeng Q., Possemato A., Yu J., Haack H., Nardone J., Lee K. Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Birchmeier C., Sharma S., Wigler M. Expression and Rearrangement of the ROS1 Gene in Human Glioblastoma Cells. Proc. Natl. Acad. Sci. USA. 1987;84:9270–9274. doi: 10.1073/pnas.84.24.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birchmeier C., O’Neill K., Riggs M., Wigler M. Characterization of ROS1 CDNA from a Human Glioblastoma Cell Line. Proc. Natl. Acad. Sci. USA. 1990;87:4799–4803. doi: 10.1073/pnas.87.12.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S., Birchmeier C., Nikawa J., O’Neill K., Rodgers L., Wigler M. Characterization of the Ros1-Gene Products Expressed in Human Glioblastoma Cell Lines. Oncogene Res. 1989;5:91–100. [PubMed] [Google Scholar]
- 60.Bergethon K., Shaw A.T., Ou S.-H.I., Katayama R., Lovly C.M., McDonald N.T., Massion P.P., Siwak-Tapp C. ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arai Y., Totoki Y., Takahashi H., Nakamura H., Hama N., Kohno T., Tsuta K., Yoshida A. Mouse Model for ROS1-Rearranged Lung Cancer. PLoS ONE. 2013;8:e56010. doi: 10.1371/journal.pone.0056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeuchi K., Soda M., Togashi Y., Suzuki R., Sakata S., Hatano S., Asaka R., Hamanaka W. RET, ROS1 and ALK Fusions in Lung Cancer. Nat. Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida A., Kohno T., Tsuta K., Wakai S., Arai Y., Shimada Y., Asamura H., Furuta K. ROS1-Rearranged Lung Cancer: A Clinicopathologic and Molecular Study of 15 Surgical Cases. Am. J. Surg. Pathol. 2013;37:554–562. doi: 10.1097/PAS.0b013e3182758fe6. [DOI] [PubMed] [Google Scholar]
- 64.Rimkunas V.M., Crosby K.E., Li D., Hu Y., Kelly M.E., Gu T.-L., Mack J.S., Silver M.R. Analysis of Receptor Tyrosine Kinase ROS1-Positive Tumors in Non-Small Cell Lung Cancer: Identification of a FIG-ROS1 Fusion. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 65.Govindan R., Ding L., Griffith M., Subramanian J., Dees N.D., Kanchi K.L., Maher C.A. Genomic Landscape of Non-Small Cell Lung Cancer in Smokers and Never-Smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies K.D., Le A.T., Theodoro M.F., Skokan M.C., Aisner D.L., Berge E.M. Identifying and Targeting ROS1 Gene Fusions in Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sehgal K., Patell R., Rangachari D., Costa D.B. Targeting ROS1 Rearrangements in Non-Small Cell Lung Cancer with Crizotinib and Other Kinase Inhibitors. Transl. Cancer Res. 2018;7(Suppl 7):S779–S786. doi: 10.21037/tcr.2018.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ettinger D.S., Aisner D.L., Wood D.E., Akerley W., Bauman J., Chang J.Y., Chirieac L.R., D’Amico T.A. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 5.2018. J. Natl. Compr. Canc. Netw. 2018;16:807–821. doi: 10.6004/jnccn.2018.0062. [DOI] [PubMed] [Google Scholar]
- 69.Gainor J.F., Shaw A.T. Novel Targets in Non-Small Cell Lung Cancer: ROS1 and RET Fusions. Oncologist. 2013;18:865–875. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bottaro D.P., Rubin J.S., Faletto D.L., Chan A.M., Kmiecik T.E., Vande Woude G.F., Aaronson S.A. Identification of the Hepatocyte Growth Factor Receptor as the C-Met Proto-Oncogene Product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 71.Virzì A.R., Gentile A., Benvenuti S., Comoglio P.M. Reviving Oncogenic Addiction to MET Bypassed by BRAF(G469A) Mutation. Proc. Natl. Acad. Sci. USA. 2018;115:10058–10063. doi: 10.1073/pnas.1721147115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M., Liang L., Lei X., Multani A., Meric-Bernstam F., Tripathy D., Wu Y., Chen H. Evaluation of CMET Aberration by Immunohistochemistry and Fluorescence in Situ Hybridization (FISH) in Triple Negative Breast Cancers. Ann. Diagn. Pathol. 2018;35:69–76. doi: 10.1016/j.anndiagpath.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Dziadziuszko R., Wynes M.W., Singh S., Asuncion B.R., Ranger-Moore J., Konopa K., Rzyman W., Szostakiewicz B. Correlation between MET Gene Copy Number by Silver in Situ Hybridization and Protein Expression by Immunohistochemistry in Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2012;7:340–347. doi: 10.1097/JTO.0b013e318240ca0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang L., Chen H., Tang Z., Kalhor N., Liu C.-H., Yao H., Hu S., Lin P. MET Amplification Assessed Using Optimized FISH Reporting Criteria Predicts Early Distant Metastasis in Patients with Non-Small Cell Lung Cancer. Oncotarget. 2018;9:12959–12970. doi: 10.18632/oncotarget.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewandowska M.A., Czubak K., Klonowska K., Jozwicki W., Kowalewski J., Kozlowski P. The Use of a Two-Tiered Testing Strategy for the Simultaneous Detection of Small EGFR Mutations and EGFR Amplification in Lung Cancer. PLoS ONE. 2015;10:e0117983. doi: 10.1371/journal.pone.0117983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rochigneux P., Thomassin-Piana J., Laibe S., Brunelle S., Salem N., Escudier B., Vassal G., Gravis G. Long-Term Efficacy of Crizotinib in a Metastatic Papillary Renal Carcinoma with MET Amplification: A Case Report and Literature Review. BMC Cancer. 2018;18:1159. doi: 10.1186/s12885-018-5049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O., Lindeman N., Gale C.-M. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 78.Paxton C.N., Rowe L.R., South S.T. Observations of the Genomic Landscape beyond 1p19q Deletions and EGFR Amplification in Glioma. Mol. Cytogenet. 2015;8:60. doi: 10.1186/s13039-015-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S.-H., Won J., Kim S.-I., Lee Y., Park C.-K., Kim S.-K., Choi S.-H. Molecular Testing of Brain Tumor. J. Pathol. Transl. Med. 2017;51:205–223. doi: 10.4132/jptm.2017.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol.(Berl.) 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 81.Reifenberger J., Reifenberger G., Liu L., James C.D., Wechsler W., Collins V.P. Molecular Genetic Analysis of Oligodendroglial Tumors Shows Preferential Allelic Deletions on 19q and 1p. Am. J. Pathol. 1994;145:1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 82.Iwadate Y., Matsutani T., Shinozaki N., Saeki N. Anaplastic Oligodendroglial Tumors Harboring 1p/19q Deletion Can Be Successfully Treated without Radiotherapy. Anticancer Res. 2011;31:4475–4479. [PubMed] [Google Scholar]
- 83.Shah R., Rosso K., Nathanson S.D. Pathogenesis, Prevention, Diagnosis and Treatment of Breast Cancer. World J. Clin. Oncol. 2014;5:283–298. doi: 10.5306/wjco.v5.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coleman M.P., Quaresma M., Berrino F., Lutz J.-M., De Angelis R., Capocaccia R., Baili P., Rachet B. Cancer Survival in Five Continents: A Worldwide Population-Based Study(CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 85.Wojciechowska U., Czaderny K., Ciuba A., OLasek P., Didkowska J. Nowotwory Złośliwe w Polsce w 2016 Roku. Centrum Onkologii Instytut Im. Marii Skłodowskiej-Curie; Warszawa, Poland: 2018. [Google Scholar]
- 86.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/Neu Oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 87.Bofin A.M., Ytterhus B., Martin C., O’Leary J.J., Hagmar B.M. Detection and Quantitation of HER-2 Gene Amplification and Protein Expression in Breast Carcinoma. Am. J. Clin. Pathol. 2004;122:110–119. doi: 10.1309/8A2DJFT07NE6EWHE. [DOI] [PubMed] [Google Scholar]
- 88.Slamon D.J., Godolphin W., Jones L.A., Holt J.A., Wong S.G., Keith D.E., Levin W.J., Stuart S.G. Studies of the HER-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 89.Owens M.A., Horten B.C., Da Silva M.M. HER2 Amplification Ratios by Fluorescence in Situ Hybridization and Correlation with Immunohistochemistry in a Cohort of 6556 Breast Cancer Tissues. Clin. Breast Cancer. 2004;5:63–69. doi: 10.3816/CBC.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 90.Barros-Silva J.D., Leitão D., Afonso L., Vieira J., Dinis-Ribeiro M., Fragoso M., Bento M.J., Santos L. Association of ERBB2 Gene Status with Histopathological Parameters and Disease-Specific Survival in Gastric Carcinoma Patients. Br. J. Cancer. 2009;100:487–493. doi: 10.1038/sj.bjc.6604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abrahao-Machado L.F., Scapulatempo-Neto C. HER2 Testing in Gastric Cancer: An Update. World J. Gastroenterol. 2016;22:4619–4625. doi: 10.3748/wjg.v22.i19.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolff A.C., Hammond M.E.H., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., Dowsett M., Fitzgibbons P.L. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 93.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., Bilous M., Ellis I.O. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 94.Vranic S., Teruya B., Repertinger S., Ulmer P., Hagenkord J., Gatalica Z. Assessment of HER2 Gene Status in Breast Carcinomas with Polysomy of Chromosome 17. Cancer. 2011;117:48–53. doi: 10.1002/cncr.25580. [DOI] [PubMed] [Google Scholar]
- 95.Vanden Bempt I., Van Loo P., Drijkoningen M., Neven P., Smeets A., Christiaens M.-R., Paridaens R., De Wolf-Peeters C. Polysomy 17 in Breast Cancer: Clinicopathologic Significance and Impact on HER-2 Testing. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26:4869–4874. doi: 10.1200/JCO.2007.13.4296. [DOI] [PubMed] [Google Scholar]
- 96.Dietel M. Molecular Pathology: A Requirement for Precision Medicine in Cancer. Oncol. Res. Treat. 2016;39:804–810. doi: 10.1159/000453085. [DOI] [PubMed] [Google Scholar]
- 97.Rakha E.A., Pinder S.E., Bartlett J.M.S., Ibrahim M., Starczynski J., Carder P.J., Provenzano E., Hanby A. Updated UK Recommendations for HER2 Assessment in Breast Cancer. J. Clin. Pathol. 2015;68:93–99. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kroeger P.T., Drapkin R. Pathogenesis and Heterogeneity of Ovarian Cancer. Curr. Opin. Obstet. Gynecol. 2017;29:26–34. doi: 10.1097/GCO.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Etemadmoghadam D., Weir B.A., Au-Yeung G., Alsop K., Mitchell G., George J., Australian Ovarian Cancer Study Group. Davis S., D’Andrea A.D., Simpson K., et al. Synthetic Lethality between CCNE1 Amplification and Loss of BRCA1. Proc. Natl. Acad. Sci. USA. 2013;110:19489–19494. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saltus C.W., Calingaert B., Candrilli S., Lorenzo M., D’yachkova Y., Otto T., Wagner U., Kaye J.A. Epidemiology of Adult Soft-Tissue Sarcomas in Germany. Sarcoma. 2018;2018:5671926. doi: 10.1155/2018/5671926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mastrangelo G., Coindre J.-M., Ducimetière F., Dei Tos A.P., Fadda E., Blay J.-Y., Buja A., Fedeli U. Incidence of Soft Tissue Sarcoma and beyond: A Population-Based Prospective Study in 3 European Regions. Cancer. 2012;118:5339–5348. doi: 10.1002/cncr.27555. [DOI] [PubMed] [Google Scholar]
- 102.Ewing J. Diffuse Endothelioma of Bone. CA. Cancer J. Clin. 1972;22:95–98. doi: 10.3322/canjclin.22.2.95. [DOI] [PubMed] [Google Scholar]
- 103.Zucman J., Delattre O., Desmaze C., Plougastel B., Joubert I., Melot T. Cloning and Characterization of the Ewing’s Sarcoma and Peripheral Neuroepithelioma t(11;22) Translocation Breakpoints. Genes. Chromosomes Cancer. 1992;5:271–277. doi: 10.1002/gcc.2870050402. [DOI] [PubMed] [Google Scholar]
- 104.Dunn T., Praissman L., Hagag N., Viola M.V. ERG Gene Is Translocated in an Ewing’s Sarcoma Cell Line. Cancer Genet. Cytogenet. 1994;76:19–22. doi: 10.1016/0165-4608(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 105.Hoang N.T., Acevedo L.A., Mann M.J., Tolani B. A Review of Soft-Tissue Sarcomas: Translation of Biological Advances into Treatment Measures. [(accessed on 8 February 2020)]; doi: 10.2147/CMAR.S159641. Available online: https://www.dovepress.com/a-review-of-soft-tissue-sarcomas-translation-of-biological-advances-in-peer-reviewed-fulltext-article-CMAR. [DOI] [PMC free article] [PubMed]
- 106.Limon J., Dal Cin P., Sandberg A.A. Translocations Involving the X Chromosome in Solid Tumors: Presentation of Two Sarcomas with t(X;18)(Q13;P11) Cancer Genet. Cytogenet. 1986;23:87–91. doi: 10.1016/0165-4608(86)90152-4. [DOI] [PubMed] [Google Scholar]
- 107.Turc-Carel C., Dal Cin P., Limon J., Li F., Sandberg A.A. Translocation X;18 in Synovial Sarcoma. Cancer Genet. Cytogenet. 1986;23:93. doi: 10.1016/0165-4608(86)90153-6. [DOI] [PubMed] [Google Scholar]
- 108.Turc-Carel C., Dal Cin P., Limon J., Rao U., Li F.P., Corson J.M., Zimmerman R., Parry D.M. Involvement of Chromosome X in Primary Cytogenetic Change in Human Neoplasia: Nonrandom Translocation in Synovial Sarcoma. Proc. Natl. Acad. Sci. USA. 1987;84:1981–1985. doi: 10.1073/pnas.84.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iliszko M., Rys J., Wozniak A., Chosia M., Sciot R., Debiec-Rychter M., Limon J. Complex Tumor-Specific t(X;18) in Seven Synovial Sarcoma Tumors. Cancer Genet. Cytogenet. 2009;189:118–121. doi: 10.1016/j.cancergencyto.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 110.Fletcher C.D., Bridge J.A., Hogendoorn P.C., Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- 111.Takahira T., Oda Y., Tamiya S., Higaki K., Yamamoto H., Kobayashi C., Izumi T., Tateishi N., at el. Detection of COL1A1-PDGFB Fusion Transcripts and PDGFB/PDGFRB MRNA Expression in Dermatofibrosarcoma Protuberans. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2007;20:668–675. doi: 10.1038/modpathol.3800783. [DOI] [PubMed] [Google Scholar]
- 112.Segura S., Salgado R., Toll A., Martín-Ezquerra G., Yébenes M., Sáez A., Solé F., Barranco C. Identification of t(17;22)(Q22;Q13) (COL1A1/PDGFB) in Dermatofibrosarcoma Protuberans by Fluorescence in Situ Hybridization in Paraffin-Embedded Tissue Microarrays. Hum. Pathol. 2011;42:176–184. doi: 10.1016/j.humpath.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 113.Salgado R., Llombart B., M Pujol R., Fernández-Serra A., Sanmartín O., Toll A., Rubio L., Segura S., López-Guerrero J.A. Molecular Diagnosis of Dermatofibrosarcoma Protuberans: A Comparison between Reverse Transcriptase-Polymerase Chain Reaction and Fluorescence in Situ Hybridization Methodologies. Genes. Chromosomes Cancer. 2011;50:510–517. doi: 10.1002/gcc.20874. [DOI] [PubMed] [Google Scholar]
- 114.Simon M.P., Pedeutour F., Sirvent N., Grosgeorge J., Minoletti F., Coindre J.M., Terrier-Lacombe M.J. Deregulation of the Platelet-Derived Growth Factor B-Chain Gene via Fusion with Collagen Gene COL1A1 in Dermatofibrosarcoma Protuberans and Giant-Cell Fibroblastoma. Nat. Genet. 1997;15:95–98. doi: 10.1038/ng0197-95. [DOI] [PubMed] [Google Scholar]
- 115.Noujaim J., Thway K., Fisher C., Jones R.L. Dermatofibrosarcoma Protuberans: From Translocation to Targeted Therapy. Cancer Biol. Med. 2015;12:375–384. doi: 10.7497/j.issn.2095-3941.2015.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rutkowski P., Wozniak A., Switaj T. Advances in Molecular Characterization and Targeted Therapy in Dermatofibrosarcoma Protuberans. Sarcoma. 2011;2011 doi: 10.1155/2011/959132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kalemkerian G.P., Narula N., Kennedy E.B., Biermann W.A., Donington J., Leighl N.B., Lew M., Pantelas J. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:911–919. doi: 10.1200/JCO.2017.76.7293. [DOI] [PubMed] [Google Scholar]
- 118.Bubendorf L., Büttner R., Al-Dayel F., Dietel M., Elmberger G., Kerr K. Testing for ROS1 in Non-Small Cell Lung Cancer: A Review with Recommendations. Virchows Arch. Int. J. Pathol. 2016;469:489–503. doi: 10.1007/s00428-016-2000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dagogo-Jack I., Shaw A.T. Screening for ALK Rearrangements in Lung Cancer: Time for a New Generation of Diagnostics? Oncologist. 2016;21:662–663. doi: 10.1634/theoncologist.2016-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]