Abstract
CD8+ T cells are considered to be critical in tumor surveillance and elimination. Increased CD8+ T cell frequency and function is associated with better prognosis in cancer patients. Interleukin 10 is a cytokine with controversial roles in CD8+ T cell–mediated anti-tumor immunity. We therefore examined the interleukin 10 expression and consumption in CD8+ T cells harvested from the peripheral blood and resected tumors of gastric cancer patients of stages II–IV. We found that the gastric cancer patients presented significantly elevated frequencies of interleukin 10–expressing cells in both CD4+ and CD8+ T cells compared to healthy controls. But distinctive from the interleukin 10–expressing CD4+ T cells, which increased in frequency in advanced cancer, the interleukin 10–expressing CD8+ T cells did not increase with cancer stage in the peripheral blood and actually decreased with cancer stage in resected tumor. Interleukin 10 and interleukin 10 receptor expression was also enriched in interferon gamma–expressing activated CD8+ T cells. Compared to interleukin 10–nonexpressing CD8+ T cells, interleukin 10 receptor–expressing CD8+ T cells secreted significantly elevated interferon gamma levels. Treatment of anti-CD3/CD28-stimulated, purified CD8+ T cells with interleukin 10 alone could significantly enhance CD8+ T cell survival, an effect dependent on interleukin 10 receptor expression. Interleukin 10 also increased CD8+ T cell proliferation synergistically with interferon gamma but not alone. Analysis of downstream signal transducer and activator of transcription molecules showed that interleukin 10 treatment significantly increased the phosphorylation of signal transducer and activator of transcription 3 and signal transducer and activator of transcription 1 to lesser extent. Together, these results demonstrate that interleukin 10 possessed stimulatory roles in activated CD8+ T cells from gastric cancer patients.
Keywords: Interleukin 10, gastric cancer
Introduction
Interleukin 10 (IL-10) is generally characterized as an anti-inflammatory cytokine for its role in mediating tissue protection during autoimmune disorders and chronic inflammations.1,2 IL-10 treatment decreases the expression of major histocompatibility complex (MHC) class II and costimulatory molecules and promotes anti-inflammatory cytokine expression in antigen-presenting cells (APCs).3 These IL-10-induced changes in APCs can limit the magnitude of T cell responses and prevent excessive T cell responses. Exogenous IL-10 can also directly inhibit human CD4+ T cell responses in vitro, resulting in reduced cytokine expression and proliferation.4–6 These findings seem to suggest that in CD8+ T cells, IL-10 also exert an inhibitory role. However, several recent findings have demonstrated that IL-10 might promote tumor immunity, especially with regard to CD8+ T cell–mediated immune surveillance.7 In mice, Fujii et al.8 showed that IL-10 injection increased the number of CD8+ CD44hi CD122+ T cells and enhanced the antigen-specific proliferation. Mumm et al.9 demonstrated that host or exogenous pegylated IL-10 significantly increased the presence of CD8+ T cells in spontaneous and large transplanted tumors. IL-10 expression also correlated with the level of interferon gamma (IFN-γ), granzymes, and MHC expression in the microenvironment of human tumors. Emmerich et al.10 further demonstrated that IL-10 induced the expansion tumor-resident CD8+ T cells and activated their IFN-γ production. These results were corroborated by the related finding that IL-10 could act as a chemoattractant to CD8+ T cells,11 could activate cytotoxic T cell activity,12,13 and could increase the maturation of CD8+ T memory cells.14 Together, these studies suggest that IL-10 is an integral part of CD8+ T cell–mediated anti-tumor immunity.
The roles of IL-10 in CD8+ T cells in gastric cancer patients have not been extensively examined. In 2012, gastric cancer represented the fourth most common malignancy in the world and the second most common cause of cancer-related mortality.15 Chronic inflammation induced by Helicobacter pylori infection is considered to be the principle risk factor for cancer development, but precise details of the underlying inflammatory mechanisms are poorly understood.16 The prognosis of gastric cancer by conventional curative resection and adjuvant chemotherapy is poor, with high risk for recurrence in advanced tumors.17,18 Furthermore, many patients of advanced stages do not qualify for surgery. Better treatment options are urgently needed.
To examine the possibility of using IL-10 to promote CD8+ T cell–mediated anti-tumor immunity in gastric cancer, we investigated the role of IL-10 in circulating- and tumor-infiltrating CD8+ T cells in stages II–IV gastric cancer patients. Our results demonstrated that IL-10 expression was enriched in activated CD8+ T cells, enhanced CD8+ T cell survival by itself, and synergistically increased CD8+ T cell proliferation with IFN-γ. Interestingly, the frequency of IL-10-expressing CD8+ T cells was comparable among stages II–IV patients in peripheral blood but decreased from stage II to stage IV in tumor, suggesting a tumor-specific regulation of IL-10-expressing CD8+ T cells.
Materials and methods
Subjects and sample collection
In total, 30 gastric cancer patients and 10 age- and sex-matched healthy controls were recruited for this study. Patient and control demography and clinical information are summarized in Table 1. Staging was performed according to the 7th Union for International Cancer Control TNM system.19 Peripheral blood samples were obtained from all participants by venipuncture and processed by standard Ficoll-Hypaque gradient centrifugation to obtain peripheral blood mononuclear cells (PBMCs). Tumor samples were obtained from all gastric cancer patients who underwent surgical resection and immediately washed in Hank’s balanced salt solution (Thermo Fisher Scientific) supplemented with 5% fetal bovine serum (FBS; Gibco), 100 IU/mL penicillin, 100 µg/mL streptomycin, 2.5 µg/mL amphotericin B, and 100 µg/mL gentamicin (Sigma). The tissues were then minced and digested in an extracellular matrix degradation mix with dispase, pronase, and DNase (Sigma) at 37°C for 12 h.20,21 The cell suspension was filtered with a 70-µm strainer and centrifuged using the standard Ficoll-Hypaque method to obtain tumor-infiltrating lymphocytes (TILs). All participants provided written informed consent, and all protocols were approved by the ethics board of The 155 Central Hospital of PLA.
Table 1.
Demographic and clinical characteristics of study participants.
| Control | Cancer | p | |
|---|---|---|---|
| n | 10 | 30 | – |
| Female, n (%) | 3 (30) | 9 (30) | >0.05 |
| Age (years), range | 34–68 | 38–66 | >0.05 |
| Tumor stage, n (%) | |||
| II | – | 7 (23) | – |
| III | – | 10 (33) | – |
| IV | – | 13 (43) | – |
| Receiving surgical resection, n (% in each stage) | |||
| II | – | 7 (100) | – |
| III | – | 8 (80) | – |
| IV | – | 3 (23) | – |
Flow cytometry
Monoclonal antibodies specific to human CD3, CD4, CD8, IL-10, IFN-γ, IL-10 receptor (IL-10R), phosphorylated signal transducer and activator of transcription 1 (pSTAT1; Y701), pSTAT3 (Y705), and pSTAT5 (Y694) were purchased from eBioscience and BioLegend. For analyzing the frequency of intracellular IL-10-expressing cells, unfractionated PBMCs or TILs were stimulated with 5 ng/mL of phorbol myristate acetate (PMA) + 500 ng/mL of ionomycin for 5 h with brefeldin A (BD Biosciences) at 37°C and 5% CO2 before surface staining. For examining proliferation, cells were carboxyfluorescein succinimidyl ester (CFSE)-labeled by the CellTrace CFSE Cell Proliferation Kit (Thermo Fisher Scientific) following the provided instructions and then incubated in a 96-well flat bottom plate with 2 µg/mL plate-bound anti-CD3 (OKT3) and CD28 (CD28.2) for 72 h in 37°C and 5% CO2 before surface staining. For detecting apoptotic cells, the Annexin V–FITC Apoptosis Detection Kit (Abcam) was used following the provided instructions. In some experiments, recombinant human IL-10 and/or IFN-γ (R&D Systems) were added at 10 µg/mL at the beginning of incubation. Cells were then transferred to V-bottom plates and stained with surface markers. Cytofix/Cytoperm reagent and the Perm/Wash Buffer (BD Biosciences) were used for intracellular staining, following provided the instructions. Live cell sorting was performed in BD FACSAria. Other samples were acquired in BD LSR II.
CD8+ T cell purification
PBMCs or TILs were processed using the EasySep Human CD8+ T Cell Enrichment Kit (Stemcell Technologies) to obtain untouched pure CD8+ T cells, which were then used in enzyme-linked immunosorbent assay (ELISA) or stained with anti-human IL-10R for further live sorting in flow cytometry.
ELISA
Sorted CD8+ T cells were cultured at 2 × 104 cells per 200 µL of media in anti-CD3 and anti-CD28-bound 96-well flat bottom plate for 12 h in 37°C and 5% CO2. The cell cultures were then transferred to a 96-well V-bottom plate and centrifuged at 300g for 5 min. A volume of 100 µL supernatant was taken for ELISA measurement. The Human IFN-gamma and IL-10 Ready-SET-Go kits from eBioscience were used according to the provided instructions.
Statistical analysis
Data normality was determined by D’Agostino–Pearson test. Parametric or nonparametric tests were then applied accordingly. For comparison between two groups, t test with Welch’s correction or Mann–Whitney U test was applied. For multiple groups, one-way or two-way analysis of variance (ANOVA) with multiple comparisons post test was applied. All statistical analysis was performed in GraphPad PRISM. p < 0.05 was considered to be statistically significant.
Results
Characteristics of study participants
A total of 30 patients diagnosed of gastric cancer with stages II–IV were recruited in this study. PBMC samples were harvested before treatment as well as after curative resection (n = 18). Furthermore, the TILs were harvested from resected tumors. In total, 10 healthy subjects were recruited as controls. The demographic and clinical characteristics of all participants are summarized in Table 1.
IL-10 and IL-10R expression in CD4+ and CD8+ T cells in PBMCs
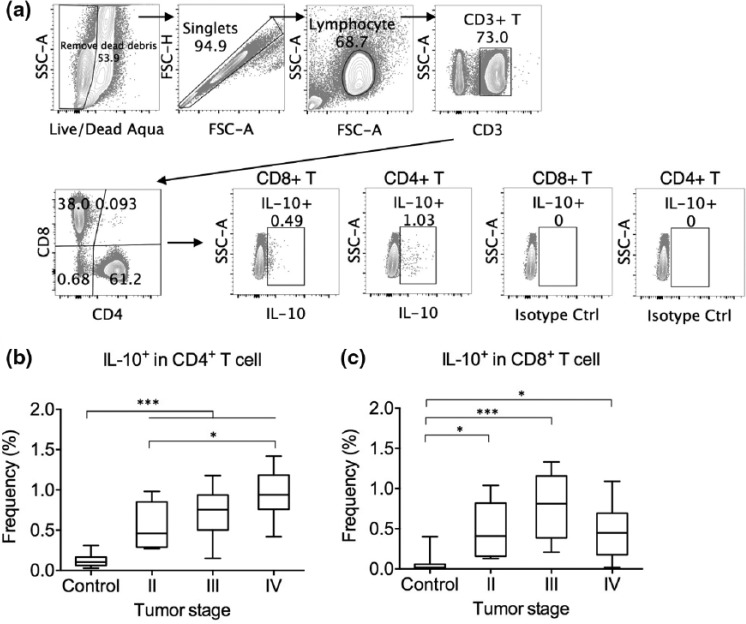
We first examined the intracellular IL-10 by circulating CD4+ and CD8+ T cells (Figure 1(a)). Although almost all leukocyte subtypes are capable of IL-10 expression, in healthy individuals, few CD4+ and CD8+ T cells in the PBMCs expressed IL-10 constitutively (Figure 1(b)).1 In contrast, significantly more CD4+ T cells in gastric cancer individuals expressed IL-10 (Figure 1(b)). Interestingly, stage IV gastric cancer patients presented higher frequencies of IL-10-expressing CD4+ T cells than stage II patients. The gastric cancer patients also presented significantly elevated frequencies of IL-10-expressing CD8+ T cells than controls (Figure 1(c)), with no statistical difference found between different stages.
Figure 1.
IL-10-expressing CD4+ and CD8+ T cells in gastric cancer patients and controls. (a) Representative gating of IL-10-expressing cells in CD4+ and CD8+ T cells. PBMCs were stimulated with PMA + ionomycin for 5 h with brefeldin A before intracellular staining. Gating standard was set using an isotype control antibody. (b) Frequency of IL-10-expressing cells as a percentage in CD4+ T cells in controls and gastric cancer patients of stages II–IV. (c) Frequency of IL-10-expressing cells as a percentage in CD8+ T cells in controls and gastric cancer patients of stages II–IV (*p < 0.05; ***p < 0.001).
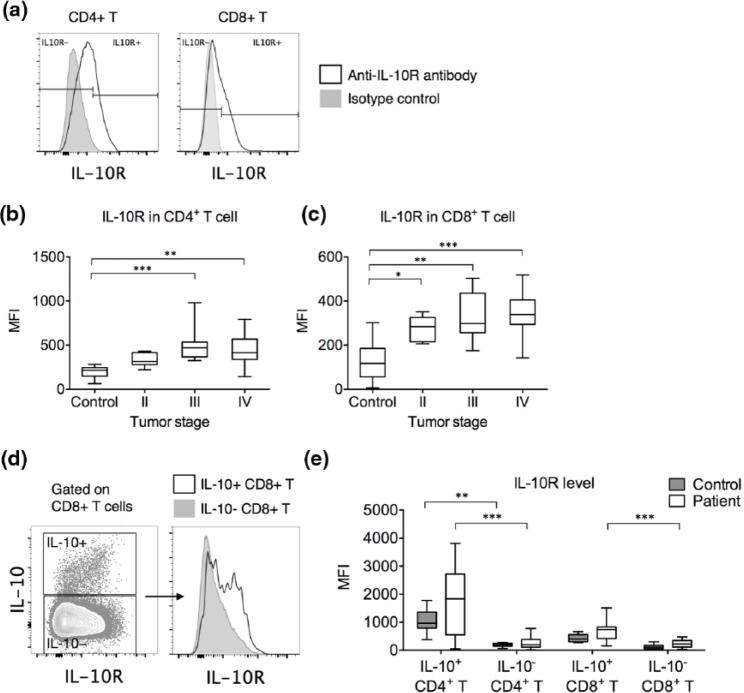
We next examined whether these CD4+ and CD8+ T cells could act as recipients of IL-10 by examining the expression of IL-10R (Figure 2(a)). Compared to those in healthy controls, the circulating CD4+ T cells from stages III and IV patients and the circulating CD8+ T cells from stages II and IV patients presented elevated IL-10R expression (Figure 2(b) and (c)). In gastric cancer patients, IL-10-expressing CD4+ and CD8+ T cells expressed significantly higher IL-10R than IL-10-nonexpressing CD4+ and CD8+ T cells (Figure 2(d) and (e)), suggesting that the IL-10-expressing T cells were better equipped at consuming IL-10. In healthy controls, only IL-10-expressing CD4+ T cells presented higher IL-10R expression than IL-10-nonexpressing CD4+ T cells.
Figure 2.
IL-10R expression in CD4+ and CD8+ T cells in gastric cancer patients and controls. (a) Representative gating of IL-10R-expressing cells in CD4+ and CD8+ T cells. (b) Frequency of IL-10R-expressing cells as a percentage in CD4+ T cells in controls and gastric cancer patients of stages II–IV. (c) Frequency of IL-10R-expressing cells as a percentage in CD8+ T cells in controls and gastric cancer patients of stages II–IV. (d) Representative IL-10R expression by IL-10+ CD8+ T cells versus IL-10− CD8+ T cells (left: IL-10 vs IL-10R contour plot in CD8+ T cells and right: histogram comparison of IL-10R expression by IL-10+ (black unfilled) vs IL-10− (gray filled) CD8+ T cells). (e) Differences in IL-10R expression level between IL-10+ and IL-10− cells in CD4+ and CD8+ T cells from healthy controls and gastric cancer patients (*p < 0.05; **p < 0.01; ***p < 0.001).
Together, these results demonstrated that compared to healthy controls, gastric cancer patients presented a significantly increased proportion of circulating CD4+ and CD8+ T cells that could act as producers and recipients of IL-10.
IL-10 expression marked a group of activated CD8+ T cells
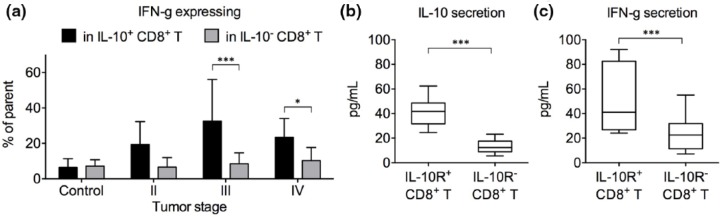
IL-10 expression by CD4+ T cells was principally described in regulatory T cell subsets, such as Foxp3+ Treg cells and Foxp3− Tr1 cells, as an essential regulatory cytokine.22,23 The role of IL-10 in CD8+ T cells is more controversial. We therefore focused on the effect of IL-10 production and consumption in CD8+ T cells. Intracellular IFN-γ expression is frequently used as a marker of CD8+ T cell activation. Interestingly, in stages III and IV patients, the frequency of IFN-γ-expressing cells was significantly higher in IL-10-expressing CD8+ T cells than in IL-10-nonexpressing CD8+ T cells, suggesting that IL-10 marked a group of activated CD8+ T cells (Figure 3(a)). No such result was observed in healthy controls or stage II patients. To confirm this finding, we utilized the previous finding that IL-10-expressing T cells expressed higher IL-10R than IL-10-nonexpressing T cells and enriched IL-10-expressing CD8+ T cells by IL-10R sorting. The IL-10R+ and IL-10R− CD8+ T cells were then cultured at the same cell density separately for 12 h. Higher IL-10 secretion by IL-10R+ CD8+ T cells was confirmed by ELISA (Figure 3(b)). IL-10R+ CD8+ T cells secreted significantly higher levels of IFN-γ than IL-10R− CD8+ T cells (Figure 3(c)).
Figure 3.
Expression of IFN-γ by IL-10+ versus IL-10+ CD8+ T cells. (a) The frequencies of IFN-γ-expressing cells in IL-10+ versus IL-10− CD8+ T cells in healthy control individuals and gastric cancer patients of stages II–IV. PBMCs were stimulated with PMA + ionomycin for 5 h with brefeldin A before intracellular staining (mean ± standard deviation). (b and c) IL-10R+ and IL-10R− CD8+ T cells from gastric cancer patients were sorted using the gate setting in Figure 2(a) and cultured separately at 105 cells per mL with plate-bound anti-CD3/CD28. Supernatant was harvested after 12 h. (b) Secreted IL-10 level and (c) secreted IFN-γ level were measured by ELISA (*p < 0.05; ***p < 0.001).
IL-10 enhanced CD8+ T cell survival alone and promoted proliferation in combination with IFN-γ
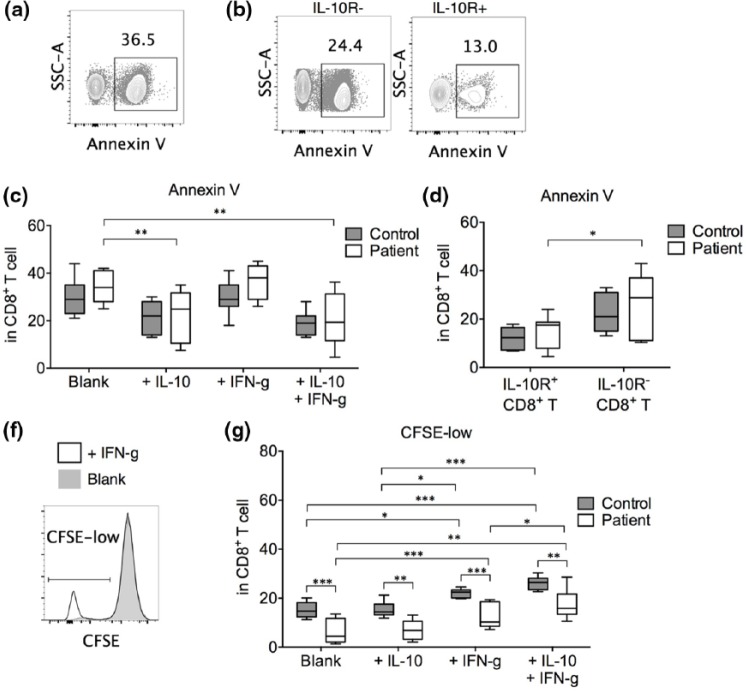
To examine the role of IL-10 consumption in CD8+ T cells, we cultured anti-CD3/CD28-stimulated CD8+ T cells from healthy controls and gastric cancer patients in the presence of exogenous IL-10 and/or IFN-γ. In gastric cancer patients, IL-10 alone or IL-10 + IFN-γ significantly suppressed CD8+ T cell apoptosis, while IFN-γ alone did not have an effect (Figure 4(c)). Presence of IFN-γ did not provide an additional survival benefit. In IL-10-treated CD8+ T cells, the IL-10R+ fraction presented significantly better survival than the IL-10R− fraction (Figure 4(b) and (d)). In control individuals, no statistical differences in cell survival between the treatments were found. Together, these results suggested that IL-10 alone could improve the survival of activated CD8+ T cells.
Figure 4.
Apoptosis and proliferation by CD8+ T cells after treatment with IL-10 and IFN-γ. Purified CD8+ T cells were incubated at 2 × 105 cells per mL in the presence of plate-bound anti-CD3/CD28 (2 µg/mL each) for 72 h without additional cytokine (blank) or with 10 µg/mL IL-10 and/or IFN-γ. In some cases, the CD8+ T cells were pre-labeled with CFSE before incubation. (a) Representative gating of apoptotic cells (Annexin V+) in blank-treated CD8+ T cells. (b) Representative Annexin V binding in IL-10-treated IL-10R+ versus IL-10R− CD8+ T cells in the same gastric cancer individual. (c) Summary of the percentage of apoptotic cells in CD8+ T cells cultured under various conditions. (d) Comparison between IL-10R+ and IL-10R− CD8+ T cells in the percentage of apoptotic cells under IL-10 treatment. (f) Representative CFSE-low gating in blank-treated versus IFN-γ-treated CD8+ T cells. (g) Percentage of proliferating cells (CFSE-low) in CD8+ T cells under various conditions (n = 7 for all experiments; *p < 0.05; **p < 0.01; ***p < 0.001).
The proliferation of CD8+ T cells was examined by CFSE staining (Figure 4(f)). We found that in gastric cancer patients, compared to blank (no additional cytokine) anti-CD3/CD28-stimulated CD8+ T cells, addition of IL-10 did not significantly elevate proliferation while addition of IFN-γ did (Figure 4(g)). Interestingly, addition of IL-10 in the presence of IFN-γ significantly improved CD8+ T cell proliferation compared to IFN-γ alone, suggesting a synergistic effect of IL-10 and IFN-γ in CD8+ T cell proliferation. IL-10 possibly increased the number of proliferating cells by enhancing the survival of these activated CD8+ T cells. In healthy controls, we also observed a proliferation increase in IFN-γ- and IL-10 + IFN-γ-treated CD8+ T cells. Notably, regardless of cytokine treatment, the CD8+ T cells from healthy controls demonstrated significantly elevated proliferation than the CD8+ T cells from gastric cancer patients.
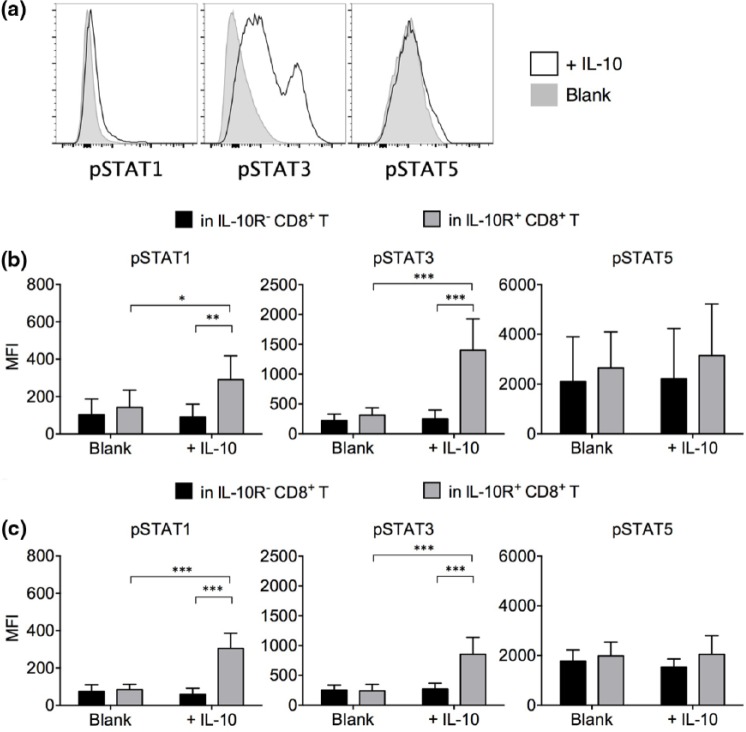
IL-10 treatment promoted the phosphorylation of STAT1 and STAT3 in CD8+ T cells
The IL-10 downstream signaling events were then examined. IL-10 activates the IL-10R-associated kinases, Janus kinase 1 (Jak1) and tyrosine kinase 2 (Tyk2), which mediate subsequent phosphorylation of STAT1, STAT3, and STAT5. Phosphorylation of STAT3 is critical to IL-10-mediated functions in macrophages.24 To explain the IL-10-mediated effects in CD8+ T cells, we performed flow cytometry to detect the phosphorylation of STAT molecules in IL-10-treated CD8+ T cells (Figure 5(a)). We found that in IL-10R+ CD8+ T cells from gastric cancer patients, IL-10 treatment significantly elevated the phosphorylation of STAT1 and STAT3 (Figure 5(b)). The IL-10R− CD8+ T cells did not react to IL-10 treatment, as expected. No significant change in STAT5 phosphorylation was observed. In IL-10R+ and IL-10R− CD8+ T cells from healthy individuals, a similar STAT phosphorylation pattern was observed (Figure 5(c)).
Figure 5.
Phosphorylation pattern in STATs after IL-10 treatment. (a) Representative pSTAT1, pSTAT3, and pSTAT5 staining pattern in IL-10-treated versus blank-treated IL-10R+ CD8+ T cells. (b) Summary of pSTAT1, pSTAT3, and pSTAT5 expression levels in blank and IL-10-treated IL-10R− versus IL-10R+ CD8+ T cells (n = 7 gastric cancer patients). (c) Summary of pSTAT1, pSTAT3, and pSTAT5 expression levels in blank and IL-10-treated IL-10R− versus IL-10R+ CD8+ T cells (n = 7 healthy controls; mean ± standard deviation; *p < 0.05; **p < 0.01; ***p < 0.001).
IL-10-expressing CD8+ T cell frequency was differentially regulated in different stages
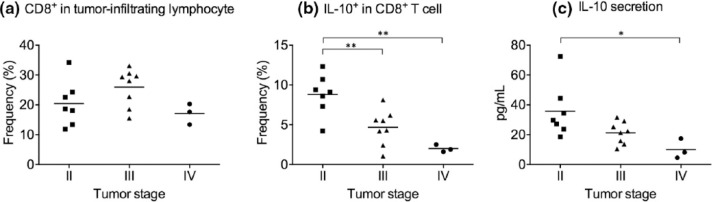
We next investigated the IL-10 expression in tumor-infiltrating CD8+ T cells, which made up from 11.8% to 34.2% of TILs in our cohort (Figure 6(a)). The experiment was performed using tumor samples from seven stage II, eight stage III, and three stage IV subjects in this cohort because not all subjects qualified for curative resection. Interestingly, the frequency of IL-10-expressing cells was significantly higher in tumor-infiltrating CD8+ T cells from stage II patients than from stage III and stage IV patients (Figure 6(b)), a trend not observed in peripheral blood (Figure 1(c)). Supernatant ELISA performed on tumor-infiltrating CD8+ T cells after 12 h incubation in vitro confirmed the flow cytometry findings (Figure 6(c)).
Figure 6.
IL-10 expression by tumor-infiltrating CD8+ T cells in gastric cancer patients. (a) Frequency of CD8+ T cells in tumor-infiltrating lymphocytes in resected gastric tumors. (b) Frequency of IL-10-expressing cells in tumor-infiltrating CD8+ T cells from resected gastric tumors. Tumor-infiltrating lymphocytes were stimulated with PMA + ionomycin for 5 h with brefeldin A before intracellular staining. (c) Isolated CD8+ T cells were cultured at 105 cells per mL with plate-bound anti-CD3/CD28. Supernatant was harvested after 12 h, and secreted IL-10 level was measured by ELISA (*p < 0.05; **p < 0.01).
Discussion
In this study, we demonstrated several novel findings regarding IL-10 expression and consumption in gastric cancer patients. We first discovered that the IL-10 expression pattern was different between CD4+ and CD8+ T cells. Compared to healthy controls, gastric cancer patients demonstrated upregulated levels of IL-10 expression in both CD4+ and CD8+ T cells from PBMCs. In circulating CD4+ T cells, the frequency of IL-10-expressing cells was higher in stage IV than in stage II patients. Such a trend was not observed in circulating CD8+ T cells. The frequency of IL-10-expressing tumor-infiltrating CD8+ T cells in stage III and stage IV patients, however, was significantly lower than that in stage II patients. At present, the underlying mechanism inducing these differences among patients at different cancer stages is not entirely clear. One possible explanation is that since IL-10 marked a group of activated IFN-γ-expressing CD8+ T cells, the expression of these two cytokines was possibly associated with each other. Reduction in IL-10 expression in later stage gastric cancer patients could be related to reduction in CD8+ T cell activation.
We found here that IL-10 expression was associated with activated CD8+ T cells and that exogenous IL-10 in vitro enhanced CD8+ T cell survival by itself and proliferation together with exogenous IFN-γ. These immunostimulatory effects were in agreement with some previous findings but in disagreement with others. Trandem et al.25 in mouse coronavirus-induced acute encephalitis demonstrated that IL-10-expressing CD8+ T cells were more activated than IL-10-nonexpressing CD8+ T cells and presented greater secretion of proinflammatory cytokines, chemokines, and cytotoxic molecules. Other studies have demonstrated that IL-10 expressed by CD4+ T cells and APCs, such as M2 macrophages and regulatory B cells, could suppress proinflammatory cytokine production by CD8+ T cells.26 One crucial difference between their studies and ours is that the APCs were present in the coculture with CD8+ T cells in their studies, while we used plate-bound anti-CD3/CD28 to stimulate purified CD8+ T cells. Therefore, one possible mechanism that resulted in these contrasting observations is that IL-10 possibly exerted inhibitory effects through modulating the functions of APCs, such as by reducing MHC class II expression and downregulating costimulatory molecules, but did not directly downplay CD8+ T cell activation in the absence of APCs. These discrepancies also suggest that the pleiotropic roles of IL-10 might present clinical difficulties if IL-10 is used as a therapeutic. Indeed, previous studies have shown that higher M2 macrophage (low IL-12, high IL-10) frequency was associated with worse gastric cancer prognosis.27
We also demonstrated that IL-10 treatment strongly elevated the phosphorylation of STAT3 and somewhat increased the phosphorylation of STAT1. Interestingly, the activation of STAT3 and STAT1 pathways can result in opposing effects in proliferation and immune regulation. STAT3 promotes the proliferation and survival of many cell types, including tumor cells. STAT1; however, promotes innate and adaptive immune responses and activates proapoptiotic and anti-proliferative genes. Exactly how STAT3/STAT1 activation resulted in the IL-10-mediated effects in CD8+ T cells is currently unclear. Concurrent activation of STAT1 and STAT3 can also be mediated by IL-21 and IL-27, which were known to mediate anti-tumor effects. Therefore, the combined effects of STAT1 and STAT3 activation in CD8+ T cell–mediated anti-tumor immunity need further investigation.
There were also several limitations in this study. First, only patients who were qualified to undergo tumor resection were included in the analyses of tumor-infiltrating CD8+ T cells, which limited the number of patients being included. Second, the amount of TILs was limited; as a result, functional analyses of IL-10 were performed using CD4+ and CD8+ T cells from PBMCs, which might not behave in the same fashion as tumor-infiltrating CD4+ and CD8+ T cells. Third, it is yet unclear whether IL-10 in vivo could promote anti-tumor CD8+ T cell responses in gastric cancer patients and whether IL-10-treated CD8+ T cells were more proficient at eliminating gastric cancer. These issues need to be further examined before incorporating IL-10 in tumor immunotherapies.
Acknowledgments
J.X. and M.X. contributed equally to this work.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Western Medicine Leader Program of Shanghai Municipal Science and Technology Commission (15411962300).
References
- 1. Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29: 71–109. [DOI] [PubMed] [Google Scholar]
- 2. Pestka S, Krause CD, Sarkar D, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004; 22: 929–979. [DOI] [PubMed] [Google Scholar]
- 3. Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 4. de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol 1993; 150: 4754–4765. [PubMed] [Google Scholar]
- 5. Del Prete G, De Carli M, Almerigogna F, et al. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol 1993; 150: 353–360. [PubMed] [Google Scholar]
- 6. Groux H, Bigler M, de Vries JE, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med 1996; 184: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dennis KL, Blatner NR, Gounari F, et al. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol 2013; 25: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujii S, Shimizu K, Shimizu T, et al. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood 2001; 98: 2143–2151. [DOI] [PubMed] [Google Scholar]
- 9. Mumm JB, Emmerich J, Zhang X, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 2011; 20: 781–796. [DOI] [PubMed] [Google Scholar]
- 10. Emmerich J, Mumm JB, Chan IH, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 2012; 72: 3570–3581. [DOI] [PubMed] [Google Scholar]
- 11. Jinquan T, Larsen CG, Gesser B, et al. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol 1993; 151: 4545–4551. [PubMed] [Google Scholar]
- 12. Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol 1991; 147: 528–534. [PubMed] [Google Scholar]
- 13. Santin AD, Hermonat PL, Ravaggi A, et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol 2000; 74: 4729–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laidlaw BJ, Cui W, Amezquita RA, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol 2015; 16: 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 16. Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest 2007; 117: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grillo F, Fassan M, Sarocchi F, et al. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol 2016; 22: 5879–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87: 236–242. [DOI] [PubMed] [Google Scholar]
- 19. Kwon SJ. Evaluation of the 7th UICC TNM staging system of gastric cancer. J Gastric Cancer 2011; 11: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park S, Woo Y, Kim H, et al. In vitro adenosine triphosphate based chemotherapy response assay in gastric cancer. J Gastric Cancer 2010; 10: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee JY, Son T, Cheong JH, et al. Association between chemotherapy-response assays and subsets of tumor-infiltrating lymphocytes in gastric cancer: a pilot study. J Gastric Cancer 2015; 15: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roncarolo MG, Gregori S, Battaglia M, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006; 212: 28–50. [DOI] [PubMed] [Google Scholar]
- 23. Petrelli A, Tresoldi E, Mfarrej BG, et al. Generation of donor-specific T regulatory type 1 cells from patients on dialysis for cell therapy after kidney transplantation. Transplantation 2015; 99: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 24. Benkhart EM, Siedlar M, Wedel A, et al. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol 2000; 165: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 25. Trandem K, Zhao J, Fleming E, et al. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J Immunol 2011; 186: 3642–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Haan JM, Kraal G, Bevan MJ. Cutting edge: lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol 2007; 178: 5429–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pantano F, Berti P, Guida FM, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med 2013; 17: 1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]