Abstract
Cancer or uncontrolled cell proliferation is a major health issue worldwide and is the second leading cause of deaths globally. The high mortality rate and toxicity associated with cancer chemotherapy or radiation therapy have encouraged the investigation of complementary and alternative treatment methods, such as plant-based drugs. Moreover, over 60% of the anti-cancer drugs are molecules derived from plants or their synthetic derivatives. Therefore, in the present review, an attempt has been made to summarize the cytotoxic plants available in the Indian subcontinent along with a description of their bio-active components. The review covers 99 plants of 57 families as well as over 110 isolated bioactive cytotoxic compounds, amongst which at least 20 are new compounds. Among the reported phytoconstituents, artemisinin, lupeol, curcumin, and quercetin are under clinical trials, while brazilin, catechin, ursolic acid, β-sitosterol, and myricetin are under pharmacokinetic development. However, for the remaining compounds, there is little or no information available. Therefore, further investigations are warranted on these subcontinent medicinal plants as an important source of novel cytotoxic agents.
Keywords: anticancer, cytotoxic, Indian subcontinent plants, bioactive compounds
1. Introduction
Cancer is a severe metabolic disorder and the leading cause of death worldwide [1]. It involves unrestrained proliferation of normal cells, caused by genetic alterations and instabilities, resulting in the generation of malignant cells and initiation of metastasis or tissue invasion. The genetic alterations include mutation of tumor suppressor genes (NF1, NF2, p53 etc.), DNA repair genes (tool box for DNA and p21, p22, p27, p51, p53), oncogenes (MYC, RAS, Bcl-2, RAF), and genes involved in cell growth and metabolism [2]. These mutations are caused by internal factors, such as alteration in hormonal balance and immune system as well as by external factors, such as radiation and pesticide exposure, tobacco smoking, and ingestion of carcinogenic chemicals or metals [1]. The incidence and geographic distribution of cancer are also related to parameters such as gender, age, race, genetic predisposition, and exposure to environmental carcinogens including azo dyes, aflatoxins, petrol, and mutagenic agents [3].
Since time immemorial, drugs of natural origin have formed the basis of traditional medicine in different cultures and in present times, medicinal plants occupy the most important position as the source of new drug molecules [4]. Plants and their phytoconstituents have been indispensable in treating diverse forms of diseases, including cancer, bacterial infection, and inflammation [5,6].
Chemopreventive components of plant origin, that are known to target specific cancerous genes or malignant cells, interrupt carcinogenesis and prevent tumor growth, are currently being investigated extensively [7]. For the treatment of over 200 cancer types, only about 140 approved anticancer drugs are available, and there is a lack of effective therapies for most of the tumors [8,9]. Apart from chemotherapeutic drugs, other cancer treatment strategies include radiotherapy, immunotherapy, hormonal therapy, or surgical methods. However, all these strategies are associated with severe side effects, such as damage to normal body cells, disruption of natural metabolic functions, alteration of hormonal balance or immune system etc. and in case of metastatic cancer, chemotherapy is often the only available therapeutic option [8].
Therefore, there is a need to search for alternative and safe drugs and/or methods for the treatment of cancer. Despite the advent of modern drug discovery technologies, such as computer-aided drug design and combinatorial chemistry, isolating novel components from natural products are extremely vital [10]. This is because of the secondary metabolism in plants that naturally evolves in response to the diverse environmental conditions, thus suggesting that a sophisticated natural version of combinatorial chemistry is continually being carried out in plants [11]. The evolution of secondary metabolism in plants has led to the generation of secondary metabolites based on the plants’ requirements, and thus suggest the existence of a remarkable variety and quantity of natural products [12].
Previous studies have demonstrated that cytotoxic phytochemicals either induce apoptosis and necrosis or obstruct variety of cell-signaling pathways, thereby, leading to cell death or cell cycle arrest [13]. Tumor cells possess several molecular mechanisms to alleviate apoptosis and apoptosis suppression, in turn, plays a crucial role in cancer progression. Hence, apoptosis or necrosis induction by cytotoxic agents can be a radical therapeutic approach towards cancer chemotherapy [14].
Considering that over 60% of the antineoplastic drugs originate directly or indirectly (semi-synthetic origin) from plants and most of them are cytotoxic phytochemicals, it is crucial to screen the diverse natural plant resources for new and updated cytotoxic compounds. The Indian subcontinent, known as “the botanical garden of the world”, is of great prominence in this regard as it is one of the 12 regions in the world known to have plants with novel-biomolecules [15,16]. Moreover, approximately 70% of cancer deaths occur in low and middle-income countries like India, Bangladesh, Sri Lanka, Nepal, and Pakistan [8]. Hence, the current investigation was conducted to search for novel cytotoxic plants and active biomolecules in the Indian subcontinent (India, Bangladesh, Sri Lanka, Nepal, and Pakistan) with the hope of supporting new and alternative treatment strategies for cancer chemotherapy.
2. Methodology
2.1. Search Strategy
An extensive literature search was performed using several platforms such as SciFinder, PubMed, and Google Scholar. The search terms used were “anticancer,” “antitumor,” “cytotoxic,” “plants,” “bioactive compounds,” and “Indian subcontinent”. We focused only on reports in English due to language barrier, time constraints, and translation-related high costs. The obtained bioactive compounds were further evaluated using the database on the Developmental Therapeutic Program (DTP) of the U.S. National Cancer Institute (NCI). In addition, information on in vivo studies as well as reports on pharmacokinetic and clinical trials were included in the review.
2.2. Selection of Studies for Inclusion in the Review
The following types of studies were included in this review: (a) in vitro studies on cancer cell lines, (b) in vivo animal studies, (c) studies that indicated the concentrations or doses effective against cancer cells or tumors, and (d) studies that reported the mechanisms of action associated with different compounds. The focus of this review was on potential cytotoxic compounds available in the Indian subcontinent.
2.3. Data Extraction
The studies were reviewed with respect to the following information: effects on different cell lines and testing methods, isolated compounds as well as effective compounds, observations, results, suggested mechanisms of action, concentrations tested, and molecular mechanism involved. The chemical structures of the reported bioactive compounds were drawn using the ChemDraw software (version 12.0, PerkinElmer Inc., Waltham, MA, USA).
3. Cytotoxic Plants of Indian Subcontinent
In this review, we focused on cytotoxic potential and bioactive compounds obtained from the plants in the Indian subcontinent. A total of 99 cytotoxic plants from 57 families were reviewed and compiled in the current study. The plants were classified according to their families. The bioactive compounds, their respective IC50 values (concentration required to inhibit 50% population), and mechanisms of cytotoxicity were listed in Table 1.
Table 1.
Reported cytotoxic family of plants as well as their effectiveness against particular cell lines with quantification and isolated bioactive components.
| Family | Genus and Species | Parts Used | Study Methods | Cell Line | IC50 (μg/mL) | Bioactive Compounds | IC50 (μg/mL) for Bioactive Compound | Mechanism of Cytotoxicity | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | Barleria grandiflora | Leaves | In vivo DAL induced ascitic tumor model in mice | DLA, A-549 and Vero | - | - | - | Upsurge in ascitic fluid | [17,18] |
| Actinidiaceae | Saurauja roxburghii | Leaves | MTT assay | A431 and C6 rat glioma | - | - | - | Interaction with caspases 3 and 9 topoisomerase I and p21WAF1 cell-cycle regulator | [20,21] |
| Amaryllidaceae | Narcissus tazetta | Aerial parts, Flowers | MTT assay | MCF-7 and Hep-2 | - | - | - | - | [22] |
| Anacardiaceae | Cotinus coggygria | Leaves | MTT assay | HeLa and Vero | 293 for HeLa | - | - | - | [23] |
| Lannea coromandelica | Barks, Leaves | MTT assay | AGS, HT-29, MCF-7 and MDA-MB-231 | 90, 520, 270 and 160, respectively | 2-Palmitoylglycerol, myricadiol, pyrogallol, isovanillin etc. | - | Apoptosis and DNA fragmentation | [24,25,26] | |
| Annonaceae | Annona muricata | Leaves | MTT assay | EACC, MDA and SKBR3 | 335, 248 and 202, respectively | - | - | - | [27] |
| Annona squamosa | Flowers | MTT assay | MCF-7 and Vero | 6.9 and 75, respectively | Eupafolin, apigenin, rhamnetin etc. | - | - | [28] | |
| Apocynaceae | Tabernaemontana divaricata | Flowers | MTT assay | HL-60, SMMC-7721, A-549, MCF-7 and SW480, NIH3T3 and HeLa | 35.3 and >100 for NIH3T3 and HeLa, respectively | Ervachinine, cononitarine, conofoline, conophylline and cisplatin | Ranging between 0.17 to 1.49 for conophylline | - | [29,30] |
| Araceae | Luffa cylindrica | Aerial Parts | MTT assay | MCF-7 and Hep-2, BT-474, MDA-MB-231 and HepG2 |
329.2 | Kaempferide, eriodictyol 7-O-glucoside and apigenin 7-O-glucouronide | - | Interaction with caspases 3 and 8 | [22,31,32] |
| Aristolochiaceae | Aristolochia ringens | Roots | In vivo S 180 induced ascetic and solid tumor model in mice | HeLa, A-431, A-549, PC-3, HCT-116 and THP-1 | Ranging between 3 to 24 | - | - | - | [33] |
| Aristolochia longa | Roots | MTT assay | MCF-7, HT-29, H5-6 and N2A | - | - | - | - | [34] | |
| Asphodelaceae | Aloe vera | Whole Plant | MTT assay | HepG2 | 10.5 | - | - | Increased p53 and reduced Bcl-2 gene expression | [35] |
| Aspleniaceae | Asplenium nidus | Whole Plant | MTT assay | HepG2 and HeLa | - | Gliricidin-7-O- hexoside |
About 507 | - | [36] |
| Asteraceae | Artemisia annua | Aerial Parts | MTT assay | 148B, MG63, D-17, OSCA2, OSCA16 and OSCA50 | 167 and 178 for 148B and MG63, respectively | Dihydro- artemisinin |
Ranging between 8.7 to 43.6 | - | [37,38,39,40] |
| Bidens pilosa | Whole Plant | MTT and Comet assays | HeLa and KB | 88.6 and 372.4, respectively | - | - | - | [41] | |
| Centaurea antiochia | Aerial Parts | MTT assay | Vero and HeLa | - | - | - | - | [23] | |
| Centaurea nerimaniae | Aerial Parts | MTT assay | Vero and HeLa | - | - | - | - | [23,42] | |
| Chrysanthemum coronarium | Aerial Parts | In vitro MTT assay and in vivo chorioallantoic membrane (CAM) model | MCF-7, T47D, CACO-2, HRT18, A375.S2 and WM1361A | 75.7, 79.8 and 138.5 for WM1361A, T47D and MCF-7 | Campesterol | - | Inhibition of fibroblast growth factor-mediated cell proliferation | [43,44] | |
| Inula viscosa | Flowers | MTT assay | MCF-7 and Hep-2 | 15.78 for MCF-7 | - | - | - | [22] | |
| Parthenium hysterophorus | Leaves | MTT and SRB assays | DU-145, THP-1, MCF-7 and K562 | - | - | - | - | [42,45] | |
| Phagnalon rupestre | Aerial Parts | MTT assay | MCF-7 and Hep-2 | 93.3 and 97, respectively | - | - | - | [22] | |
| Scorzonera tomentosa | Aerial Parts | MTT assay | Vero and HeLa | 987 and 195, respectively | - | - | - | [23] | |
| Senecio scandens | Leaves | MTT and Clonogenic assays | DL, MCF-7 and HeLa | 27, 24 and 18, respectively | - | - | - | [46] | |
| Berberidaceae | Berberis aristata | Roots, Stem | MTT assay | MCF-7, DWD, Hop62 and A2780 | 71 and 220 for MCF-7 and DWD | - | - | - | [47,48] |
| Bignoniaceae | Tecoma stans | Leaves, Flowers | MTT assay | A549 and HepG2 | - | Rutin, luteolin, diosmetin, skytanthine, etc. | - | - | [49,50,51] |
| Boraginaceae | Cordia dichotoma | Leaves | MTT, DCFH-DA and DAPI staining assays | PC3 | 74.5 | - | - | apoptosis, nuclear condensation and ROS production | [52] |
| Bromeliaceae | Tillandsia recurvata | Whole Plant | WST-1 assay | A375, MCF-7 and PC-3 | 1, 40.5 and 6, respectively | 1,3-di-O-Cinnamoyl-glycerol and (E)-3-(cinnamoyloxy)-2-hydroxypropyl 3-(3,4-dimethoxyphenyl) acrylate | Ranging between 3 to 41 | - | [53] |
| Caesalpiniaceae | Caesalpinia sappan | Heartwood, Leaves | MTT assay | MCF-7 and A-549 | - | Brazilin A | - | Reduction of Bcl-2 apoptotic inhibitor | [54] |
| Saraca asoca | Leaves | MTT assay | AGS | 20 | - | - | - | [24] | |
| Compositae | Gnaphalium luteoalbum | Leaves | MTT assay | VERO, NIH3T3, AGS, HT-29, MCF-7 and MDA-MB-231 | 980 and 340 for AGS and MCF-7, respectively | Apigenin, luteolin, jaceosidin, gnaphalin, etc. | - | - | [24,55] |
| Dilleniaceae | Dillenia pentagyna | Stem Bark | In vivo DAL induced ascitic tumor model in mice | DL, MCF-7 and HeLa | 25.8, 41.6 and 76.8, respectively | - | - | Reduction in glutathione level | [46,56] |
| Dillenia indica | Leaves | MTT assay | MCF-7 and MDA-MB-231 | 340 and 540, respectively | - | - | - | [24] | |
| Dipsacaceae | Pterocephalus pulverulentus | Aerial Parts | MTT assay | MCF-7 and Hep-2 | - | - | - | - | [22] |
| Ebenaceae | Diospyros peregrina | Leaves, Fruits | MTT assay | MCF-7, HepG2 and MDA-MB-231 | 7, 64.4 and 33, respectively | - | - | - | [24,57] |
| Ericaceae | Arbutus andrachne | Aerial Parts | MTT assay | MCF-7, T47D, CACO-2, HRT-18, A375.S2 and WM1361A | - | - | - | - | [43] |
| Euphorbiaceae | Croton caudatus | Leaves | MTT and Clonogenic assays | DL, MCF-7 and HeLa | 29.7 for DL | - | - | - | [46] |
| Euphorbia tirucalli | Leaves, Stem | In vitro cell viability study with CCK-8 | MiaPaCa-2 | - | - | - | - | [58] | |
| Fabaceae | Adenanthera pavonina | Stem Bark | In vivo DAL induced ascitic tumor model in mice | DAL | - | - | - | - | [18] |
| Ononis hirta | Aerial Parts | MTT assay | MCF-7, Hep-2 and Vero | 28, 48.8 and 41.9, respectively | - | - | - | [22] | |
| Ononis sicula | Aerial Parts | MTT assay | MCF-7, Hep-2 and Vero | 66, 75.3 and 79.5, respectively | - | - | - | [22] | |
| Gramineae | Zea mays | Leaves | MTT and SRB assays | Hep-2, A549, SK-OV-3, SK-MEL-2, XF-489 and HCT-15 | - | Maysin | Ranging between 32 to 62 | - | [59,60] |
| Hypericaceae | Hypericum kotschyanum | Aerial Parts | MTT assay | HeLa and Vero | 507 and 367, respectively | - | - | - | [23] |
| Labiatae | Salvia pinardi | Aerial Parts | MTT assay | MCF-7 and Hep-2 | 85.5 for MCF-7 | - | - | - | [22] |
| Lamiaceae | Lavandula angustifolia | Flowers | MTT assay | MCF-7 and Hep-2 | - | - | - | - | [22] |
| Nepeta italica | Aerial Parts | MTT assay | HeLa and Vero | 980 and >1000, respectively | - | - | - | [23] | |
| Plectranthus stocksii | Leaves, Stem | MTT assay | RAW264.7, Caco-2 and MCF-7 | 9, 36.1 and 48.9, respectively | - | - | - | [61] | |
| Salvia officinalis | Leaves, Stem | “Alamar Blue” resazurin reduction assays | MCF-7, B16F10 and HeLa | Ranging between 14 – 36 | α-Humulene and trans-caryophyllene | For MCF-7, 81 and 114, respectively | - | [62] | |
| Ocimum sanctum | Leaves | In vivo S 180 induced mice model | DMBA-induced hamster and S-180 induced mice | - | - | - | Regulation of humoral immunity and stimulation of cell mediated immunity | [63,64,65,66] | |
| Origanum sipyleum | Aerial Parts | MTT assay | HeLa and Vero | >1000 | - | - | - | [23] | |
| Salvia hypargeia | Aerial Parts | MTT assay | Vero and HeLa | > 1000 | - | - | - | [23] | |
| Teucrium polium | Aerial Parts | MTT assay | MCF-7, Hep-2, T47D, CACO-2, HRT18, A375.S2 and WM1361A | - | - | - | - | [22,43] | |
| Teucrium sandrasicum | Aerial Parts | MTT assay | HeLa and Vero | 513 and 593, respectively | - | - | - | [23] | |
| Malvaceae | Hibiscus calyphyllus | Aerial Parts | MTT assay | HepG2 and MCF-7 | 14.5 and 25.1, respectively | Ursolic acid, β-sitosterol and lupeol | - | Interaction with caspases 3 and 9, topoisomerase I, Bcl-2, Bax, DNA polymerase and poly (ADP-ribose)-polymerase | [67,68,69,70] |
| Hibiscus deflersii | Aerial Parts | MTT assay | HepG2 and MCF-7 | 14.4 and 11.1, respectively | Ursolic acid, β-sitosterol and lupeol | - | Interaction with caspases 3 and 9, topoisomerase I, Bcl-2, Bax, DNA polymerase and poly (ADP-ribose)-polymerase | [67,68,69,70] | |
| Hibiscus micranthus | Aerial Parts | MTT assay | HepG2 and MCF-7 | 27.6 and 24.1, respectively | Ursolic acid, β-sitosterol and lupeol | - | Interaction with caspases 3 and 9, topoisomerase I, Bcl-2, Bax, DNA polymerase and poly (ADP-ribose)-polymerase | [67,68,69,70] | |
| Meliaceae | Azadirachta indica | Leaves, Seeds | MTT assay | HT-29, A-549, MCF-7, HepG-2, MDBK, EAC, HL60, SK-BR-3, AZ521, SW-480, and SMMC7721 | Ranging between 83.5 to 212.2 | Nimonol, 3′-(3-hydroxy-3-methyl-butyl)naringenin, 4′-O-methyl- lespedezaflavanone C, triterpenoids, limonoids etc. |
Ranging between 4.2 to 100 | - | [71,72,73,74,75,76,77] |
| Menispermaceae | Cocculus hirsutus | Aerial Parts | MTT assay | MCF-7 | 39.1 | Coclaurine, haiderine, lirioresinol etc. | - | Interactions with Aurora kinase, c-Kit, FGF, NF-kB, Bcl-xL and VEGF | [78,79] |
| Moraceae | Artocarpus heterophyllus | Seeds | SRB assay and MTT assays | HEK293, A549, HeLa, MCF-7, B16 and T47D | 35.3 for A549 | Noratocarpin, cudraflavone, artocarpin, brosimone, kuwanon, albanin, etc. | Ranging between 7 to 32 | Inhibition of melanin biosynthesis | [80,81,82,83] |
| Ficus beecheyana | Roots | MTT assay | AGS, SW-872, HL-60 and HepG2 | - | p-Coumaric acid, chlorogenic acid, caffeic acid, gallic acid, rutin etc. | - | Interaction with Fas, Fas-L, p53, Bcl-2 and caspases (3,8,9) proteins | [84] | |
| Ficus carica | Fruits, Leaves | “Alamar Blue” resazurin reduction assays | MCF-7, B16F10 and HeLa | 440, 880 and >1000, respectively | - | - | - | [62] | |
| Ficus racemosa | Fruits | SRB assay | MCF-7, DLA, HL-60, HepG2, NCI-H23 and HEK-293T | 80, 175, 276.9 and 363 for MCF-7, DLA, HL-60 and HepG2, respectively | - | - | - | [85,86,87] | |
| Morus nigra | Leaves | MTT assay | OVCAR-8, SF-295, HCT-116 and HeLa | - | - | - | - | [88,89] | |
| Myristicaceae | Myristica fragrans | Barks | MTT assay | KB, K-562, HCT-116 and MCF-7 | 75 for KB | 38 Essential oils | Ranging between 2.11 – 78.15 | Apoptosis through Bcl-2 inhibition | [90,91] |
| Myrtaceae | Syzygium cumini | Seeds | MTS cell proliferation assay | A2780, MCF-7, PC3 and H460 | 49, 110, 140 and 165, respectively | Pelargonidin-3-O- glucoside, cyanidin-3-O-malonyl glucoside, delphenidin-3-O- glucoside, ellagitannins etc. |
- | - | [92,93,94] |
| Nyctaginaceae | Mirabilis jalapa | Aerial Parts, Roots, Stem | MTT assay | HeLa, Raji, A549, HCT 116, Vero MCF-7 and Hep-2 | - | Rotenoids and mirabilis antiviral protein (MAP) | For MAP, 150, 175, 200 against HCT116, MCF-7 and A549, respectively | - | [22,95,96] |
| Oleaceae | Jasminum sambac | Flowers, Leaves | MTT assay | MCF-7 and HEP-2 | 7 for MCF-7 | - | - | - | [22,24] |
| Olea europaea | Leaves | MTT assay | MCF-7, B16F10, HeLa and Vero | 43, 170, 440 and >1000, respectively | Interaction with Bcl-2, Bax, and p53 proteins | [23,62,97] | |||
| Syringa vulgaris | Fruits | MTT assay | MCF-7 and HeLa | - | - | - | - | [22] | |
| Oxalidaceae | Averrhoa bilimbi | Fruits, Leaves | MTT assay | MCF-7 | 154.9 and 668 for fruits and leaves | Nonanal, tricosane, squalene, malonic acid, etc. | - | - | [98,99] |
| Phyllanthacae | Phyllanthus emblica | Fruits, Leaves | MTT assay | HT-29, HL-60 and SMMC-7721 | 35 for HT-29 | Trihydroxy- sitosterol |
85.56 and 165.82 for HL-60 and SMMC-7721, respectively | - | [100,101,102] |
| Plumbaginaceae | Limonium densiflorum | Shoots | Resazurin reduction assays | A-549 and DLD-1 | 29 and 85, respectively | Myricetin, isorhamnetin and trans- 3-hydroxy- cinnamic acid | - | - | [103] |
| Picrorhiza | Picrorhiza kurroa | Rhizomes | XTT and SRB assays | Hep3B, MDA-MB-43 and PC-3 | 32.6, 19 and 63.9, respectively | Curcubitacin | - | Apoptosis | [104,105] |
| Pinaceae | Cedrus deodara | Woods | SRB assay | MIA-PA-CA-2, PC3, A-2780 and Molt-4 | 74, 77, 63 and 15, respectively | - | - | Interaction with caspase 3,8 and 9 proteins | [48,106] |
| Piperaceae | Piper longum | Fruits | In vitro SRB assay, in vivo DLA and EAC induced ascites tumor model in mice | Colo-205, DLA and EAC | 18 for Colo-205 | Piperine | - | Apoptosis | [48,107] |
| Piper regnellii | Leaves | MTT assay | UACC-62, 786–0, MCF-7, NCI-H460, OVCAR-3, PC-3, HT-29 and K-562 | - | Eupomatenoid-5 | - | - | [108] | |
| Poaceae | Cenchrus ciliaris | Aerial Parts, Roots | MTT assay | A-549, CACO, HCT-116, HeLa, HepG2, MCF-7 and PC3 | Ranging between 9 to 27.2 | - | - | - | [109,110] |
| Polygonaceae | Calligonum comosum | Whole Plant | MTT assay | HepG2 | 9.6 | Catechin, kaempferol, mequilianin, etc. | - | Increased p53 and reduced Bcl-2 gene expression | [35,111] |
| Primulaceae | Aegiceras corniculatum | Fruits | MTT assay | VERO, NIH3T3, AGS HT-29, MCF-7 and MDA-MB-231 | 150, 97, 0.5, 998, 91 and 461, respectively | Aegicoroside A, sakurasosaponin, fusarine, fusamine etc. | - | - | [24,112,113] |
| Ranunculaceae | Delphinium staphisagria | Seeds | MTT assay | MCF7, HT29, N2A, H5-6 and VCREMS | 41.9, 41.1 and 14.5 for N2A, H5-6 and VCREMS, respectively | Astragalin, paeonoside, petiolaroside, etc. | - | - | [34,114] |
| Rosaceae | Crataegus microphylla | Leaves, Flowers | MTT assay | HeLa and Vero | 576 for HeLa | - | - | - | [23,115] |
| Rosa damascena | Flowers, Seeds | MTT assay | MCF-7, Hep-2, HeLa and Vero | 265 for HeLa | Nerol, geraniol, β-citronellol, linalool, nonadecane and phenylethyl alcohol | - | - | [22,23,116] | |
| Rubiaceae | Hymenodictyon excelsum | Barks, Woods | MTT assay | VERO, NIH3T3, AGS HT-29, MCF-7 and MDA-MB-231 | 230, 70, 90, 160, 80 and 440, respectively | - | - | DNA fragmentation and apoptosis | [24,117] |
| Oldenlandia corymbosa | Leaves | MTT assay | K562 | 114.4 | - | - | Apoptosis | [118] | |
| Salicaceae | Populus alba | Flowers | MTT assay | Hep-2, A549, H1299 and MCF-7 | 12.05, 10.53 and 28.16 for A549, H1299 and MCF-7 | - | - | - | [22,119] |
| Sapotaceae | Manilkara zapota | Flowers | MTT assay | MCF-7, HT-29 and HL-60 | 12.5 | Lupanes, oleananes, ursanes, manilkoraside, etc. | For manilkoraside, 64 and 24 against HT-29 and HL-60, respectively | DNA fragmentation | [28,120] |
| Saururaceae | Saururus chinensis | Roots | MTT assay | MCF-7, HT-29 and HepG2 | 91.2 | Aristolactram, dihydroguaiatric acid, sauchinone, saucerneol D, manassantin A and B, saucerneol F etc. | Ranging between 10-16 | Inhibition of DNA topoisomerase I and II | [121,122] |
| Scrophulariaceae | Verbascum sinaiticum | Aerial Parts, Flowers | MTT assay | MCF-7 and Hep-2 | Sinaiticin, hydrocarpin and flavonolignans | For hydrocarpin and sinaiticin, 1.2 and 7.7 against P-388 | - | [22,123] | |
| Solanaceae | Solanum khasianum | Fruits | MTT and Clonogenic assays | DL, MCF-7 and HeLa | 27.4, 71.2 and 62.5, respectively | - | - | - | [46] |
| Solanum nigrum | Fruits | MTT and Clonogenic assays | HeLa, Vero, HepG2 and CT26 | 265, 6.9, 56.4 and 77.6, respectively | - | - | - | [124,125] | |
| Withania coagulans | Fruits, Roots and Leaves | Presto Blue cell viability assay | HeLa, MCF-7 and RD | Ranging between 0.7 to 6.7 | Myricetin, quercetin, gallic acid, etc. | - | - | [126] | |
| Withania somnifera | Roots | SRB assay | PC3, A-549, A-2780 and K-562 | 52, 46, 79 and 41, respectively | - | - | - | [48] | |
| Sterculiaceae | Helicteres isora | Whole Plant | MTT assay | HeLa-B75, HL-60, HEP-3B and PN-15 | - | Cucurbitacin B and isocucurbitacin B | - | - | [127,128] |
| Thymelaeaceae | Aquilaria malaccensis | Leaves | In vitro TBE and MTT assay, in vivo EAC induced ascites model | HCT116, DLA and EAC | 4, 72 and 79, respectively | Benzaldehyde, pinene, octanol, germacrene, hexadecanal, etc. | - | - | [129,130,131] |
| Verbenaceae | Clerodendrum viscosum | Leaves | MTT assay | VERO, NIH3T3, AGS, HT-29, MCF-7 and MDA-MB-231 | 50 and 880 for MCF-7 and HT-29 | - | - | Apoptosis | [24] |
| Clerodendron infortunatum | Roots | DLA induced ascites tumor model in mice | DLA | - | - | - | Apoptosis through interaction with Bax, Bcl-2, caspases 8 and 10 proteins | [132] | |
| Vitaceae | Leea indica | Leaves | MTT assay | DU-145 and PC-3 | 529.4 and 677.1, respectively | - | - | - | [133] |
| Vitis vinifera | Stem | “Alamar Blue” resazurin reduction assays | MCF-7, B16F10 and HeLa | 62, 137 and 336, respectively | Quercetin 3-O-β-D-4C1 galactoside and quercetin 3-O-β-D-4C1 glucuronide | - | Apoptosis | [62,134,135] | |
| Zingiberaceae | Curcuma longa | Rhizomes | MTT assay | Hep-2 | - | Curcumin, β-sesquiphellandrene | - | Apoptosis | [136,137,138,139,140] |
| Zingiber officinale | Rhizomes | In vitro MTT assay, in vivo DLA and EAC induced ascites model | DLA, EAC, A549, SK-OV-3, SK-MEL-2, PC-3M and HCT15 | - | Gingerol | < 50 | - | [140,141] |
3.1. Acanthaceae
Barleria grandiflora, the only plant belonging to the Acanthaceae family with reported cytotoxic potential, demonstrated in vitro and in vivo anti-tumor activity against Dalton’s Lymphoma Ascites (DLA), A-549, and Vero cell lines. Efficacy as an anticancer agent was proved through the prolongation of animal lifespan. An upsurge in ascitic fluid, the major nutritional source of tumor, was witnessed in DLA bearing mice. Treatment with B. grandiflora leaves extract in these mice led to the reduction in ascetic fluid as well as cessation of tumor growth, thereby increasing animal lifespan [17,18]. While the most significant adverse effects associated with cancer chemotherapy include anemia and myelosuppression, mice treated with B. grandiflora exhibited significant improvement in the red blood cell count and hemoglobin content, and a reduction in white blood cell count, thus demonstrating its antitumor potential [17,19].
3.2. Actinidiaceae
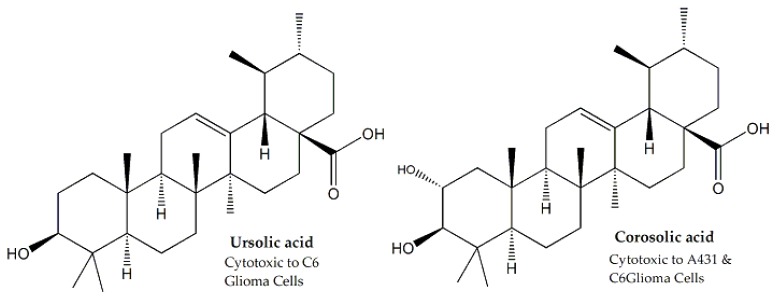
Saurauja roxburghii, belonging to the Actinidiaceae family, was assessed for cytotoxic activity. Ursolic acid and corosolic acid (Figure 1), obtained from the plant, were tested for cytotoxicity against A431 human epidermoid carcinoma and C6 rat glioma cell lines. Corosolic acid was effective against both cancer cells, while ursolic acid was cytotoxic against C6 glioma cells [20,21].
Figure 1.
Chemical structure and cytotoxic potential of ursolic and corosilic acid.
3.3. Amaryllidaceae
Narcissus tazetta was evaluated for cytotoxic potential against MCF-7 (human breast adenocarcinoma) and Hep-2 (human epithelial type 2) cells, and the flower and aerial part extracts led to reduction of cell viability by 40% and 20%, respectively [22].
3.4. Anacardiaceae
Cotinus coggygria leaf extract was reported to have moderate cytotoxic potential (IC50 = 293 µg/mL) against HeLa (Henrietta Lacks cervical cancer) and Vero (Verda Reno) cell lines (IC50 >1000 µg/mL) [23]. In addition, the leaf and bark extracts of Lannea coromandelica were reported to have cytotoxic effects against AGS (gastric adenocarcinoma), HT-29 (human colorectal adenocarcinoma), MCF-7, and MDA-MB-231 (M. D. Anderson metastatic breast cancer, Houston, TX, USA) cell lines, (IC50 values of 90, 520, 270, and 160 µg/mL, respectively) [24]. The cytotoxicity of the plant extract was attributed to the induction of apoptosis and DNA fragmentation and presence of major bioactive phytoconstituents, such as 2-palmitoylglycerol, myricadiol, pyrogallol, and isovanillin. [25,26].
3.5. Annonaceae
Annona muricata leaf extract was found to be cytotoxic against EACC, MDA, and SKBR3 cancer cell lines (IC50 values of 335, 248, and 202 µg/mL, respectively) in in vitro MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Presence of different bioactive alkaloids, flavonoids, terpenoids, and tannins were also reported [27]. The cytotoxic effects of the flower extract of A. squamosa were evaluated against MCF-7 and Vero cell lines (IC50 values of 6.9 and 75 µg/mL, respectively). The plant was reported to have several bioactive cytotoxic compounds, such as eupafolin, apigenin, and rhamnetin [28].
3.6. Apocynaceae
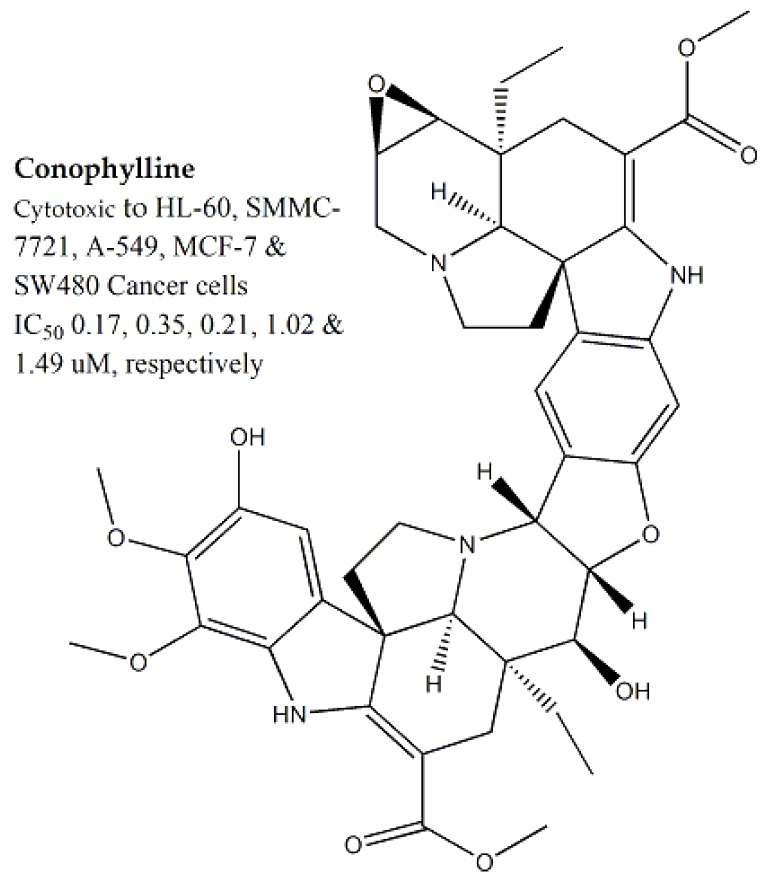
Tabernaemontana divaricate, belonging to the Apocynaceae family, was investigated for its cytotoxic potential against NIH3T3 and HeLa cell lines and the respective IC50 values were >100 μg/mL and 35.3 μg/mL, respectively. Several alkaloidal cytotoxic compounds i.e., ervachinine, cononitarine, conofoline, conophylline and cisplatin were also reported, among which conophylline (Figure 2) was found to be most potent against HL-60 (human Caucasian promyelocytic leukemia), SMMC-7721, A-549, MCF-7, and SW480 cell lines (IC50 values of 0.17, 0.35, 0.21, 1.02, and 1.49 μM, respectively) [29,30].
Figure 2.
Cytotoxic bioactive constituent from Tabernaemontana divaricata of family Apocynaceae.
3.7. Araceae
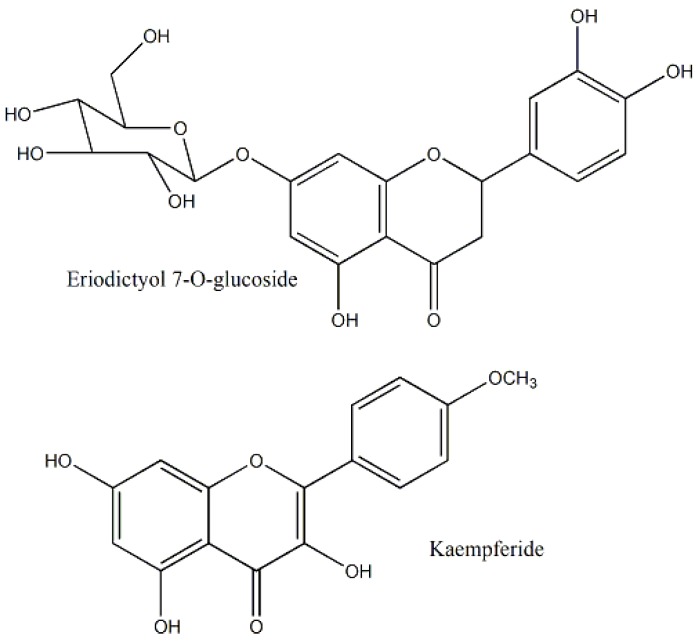
The aerial parts of Luffa cylindrica were found to be cytotoxic against MCF-7 and Hep-2 cell lines and the percentage cell viability were 83 and 120, respectively after MTT assay [22]. Another study reported the cytotoxicity of L. cylindrica against BT-474, MDA-MB-231, and HepG2 cells, with the IC50 value of 329.2 µg/mL against HepG2 [31]. The major cytotoxic compounds were kaempferide, eriodictyol 7-O-glucoside, and apigenin 7-O-glucouronide (Figure 3), which acted on caspases 3 and 8 [32].
Figure 3.
Cytotoxic constituents of Luffa cylindrica of family Araceae.
3.8. Aristolochiaceae
In the Aristolochiaceae family, Aristolochia ringens and Aristolochia longa root extracts exhibited cytotoxic effect against multiple cancer cell lines. A. ringens was reported to be effective against HeLa, A-431, A-549, PC-3 (prostate cancer), HCT-116, and THP-1 (human acute monocytic leukemia) cells, with IC50 values ranging between 3–24 µg/mL, while A. longa was found to be active against MCF-7, HT-29, H5-6, and N2A cells, with 22–83% inhibition of tumor cell growth compared to control. Moreover, while testing against S 180 ascites and S 180 solid tumor model, A. ringens demonstrated significant tumor growth inhibition in a dose-dependent manner. Furthermore, A. ringens also demonstrated a rise in mean survival time (MST) in in vivo models of L1210 lymphoid leukemia [33,34].
3.9. Asphodelaceae
Whole plant of Aloe vera of Asphodelaceae was examined against HepG2 cell lines and showed time and dose-dependent inhibition of cancer cells (IC50 value: 10.5 µg/mL). Cytotoxicity was demonstrated by apoptosis of HepG2 cells through increased expression p53 and decreased expression of Bcl-2 genes [35].
3.10. Aspleniaceae
A bioactive cytotoxic flavonoid i.e., gliricidin-7-O-hexoside was isolated from Asplenium nidus (Aspleniaceae family) and evaluated for cytotoxic effect against HepG2 and HeLa cell lines. It demonstrated moderate cytotoxic effect (IC50 value of 507 μg/mL) [36].
3.11. Asteraceae
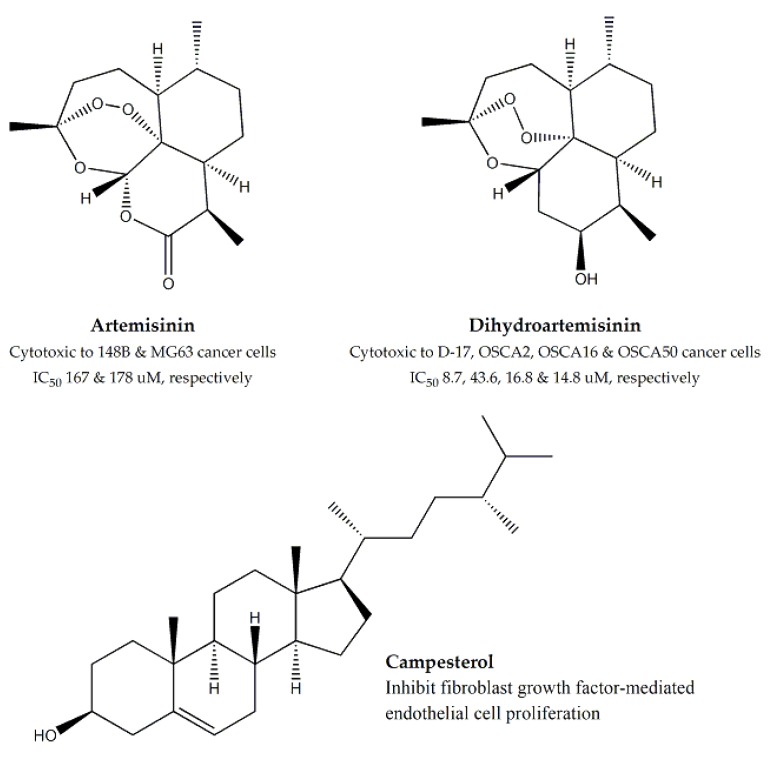
Artemisinin (Figure 4), the designated phytoconstituent of Artemisia annua, was reported to be active against two human osteosarcoma cell lines, 148B and MG63 (IC50 values were 167 μM and 178 μM, respectively) [37]. Dihydroartemisinin (Figure 4), a derivative of the compound was evaluated against canine OSA (Osteosarcoma) cell lines, D-17, OSCA2, OSCA16, and OSCA50 and the respective IC50 values were 8.7, 43.6, 16.8 and 14.8 μM [38]. Apart from this, other bioactive compounds of A. annua were also tested by evaluating the cytotoxicity of its hydro-alcoholic, dichloromethane, and methanol extracts against D-17, HeLa, and TC221 cell lines, and a dose- dependent anticancer activity was observed [39,40]. For Bidens pilosa, different fractions of whole plant were evaluated, using the MTT and Comet assays, against HeLa and KB cell lines. The chloroform fraction was found to be most effective against KB cells, while the water fraction was most active against HeLa cells, with CC50 values (concentration of the sample tolerated by 50% of the cultures exposed) of 88.6 and 372.4 µg/mL, respectively [41]. Aerial parts of both Centaurea antiochia and Centaurea nerimaniae were evaluated against Vero and HeLa cell lines and showed moderate cytotoxicity against Centaurea antiochia and significant cytotoxicity against Centaurea nerimaniae [23,42].
Figure 4.
Cytotoxic constituent from family Asterace.
Chrysanthemum coronarium was screened for cytotoxic activity against six cell lines i.e., MCF-7, T47D (ductal epithelial breast tumor), CACO-2 (human colon carcinoma), HRT18, A375.S2, and WM1361A (malignant melanoma), among which WM1361A and T47D were found to be most susceptible (IC50 values of 75.7 and 79.8 μg/mL, respectively), while MCF-7 breast carcinoma cell line was least susceptible (IC50 = 138.5 μg/mL) [43]. Campesterol (Figure 4), the bioactive steroid isolated from C. coronarium, was also reported to induce inhibition of fibroblast growth factor-mediated endothelial cell proliferation [44]. Inula viscosa flower extract was evaluated against MCF-7 and Hep-2 cancer cells and demonstrated cytotoxic effect against MCF-7 (IC50 value: 15.78 μg/mL) [22].
The leaf extract of Parthenium hysterophorus was evaluated for cytotoxicity against DU-145 (prostate), THP-1 (leukemia), MCF-7 (breast), and K562 cell lines, and it was observed that the tumor growth was inhibited at a concentration of about 100 μg/mL [42,45]. The aerial parts of Phagnalon rupestre were examined for cytotoxic effects against MCF-7 and Hep-2 cell lines (IC50 values: 93.3 μg/mL and 97 μg/mL, respectively) [22]. Scorzonera tomentosa extract was tested against Vero and HeLa cell lines (IC50 values were 987 and 195 μg/mL, respectively) [23]. Senecio scandens was found to be cytotoxic against DL, MCF-7, and HeLa cell lines (IC50 values were 27, 24, and 18 μg/mL, respectively) [46].
3.12. Berberidaceae
Berberis aristata stem extract was found to be cytotoxic against MCF-7 human breast cancer cell (IC50 of 220 μg/mL) [47]. The roots of the plant was found to be cytotoxic against DWD (oral), Hop62 (lungs), and A2780 (ovary) cancer cell lines, with the lowest IC50 value of 71 μg/mL observed against DWD [48].
3.13. Bignoniaceae
Tecoma stans was tested against A549 (human lung adenocarcinoma) cell line and cell viability was reduced in a dose-dependent manner, indicating a moderate cytotoxic effect [49]. Another study showed that the plant extract was cytotoxic against HepG2 cells where a reduction in viability of cancer cells was observed [50]. The major anti-proliferative phytoconstituents of the plant included rutin, luteolin, diosmetin, and skytanthine [51].
3.14. Boraginaceae
Cordia dichotoma, belonging to the Boraginaceae family, was tested against prostate carcinoma cell line (PC3). In addition, some bioactive flavonoids were isolated. The cytotoxic activity of the plant extract (IC50 value of 74.5 μg/mL) was exerted through apoptosis, nuclear condensation, and ROS (reactive oxygen species) production [52].
3.15. Bromeliaceae
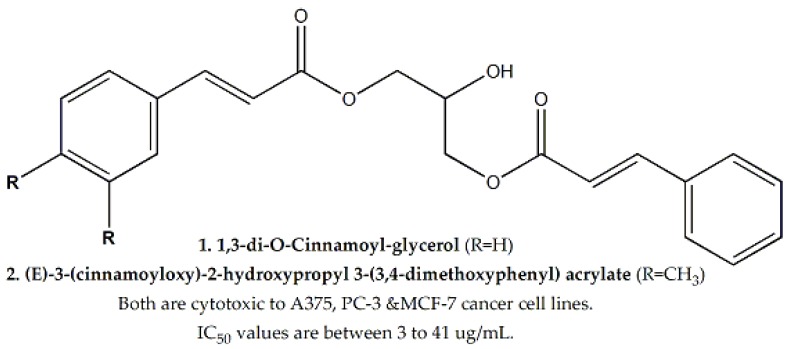
Tillandsia recurvata was evaluated against A375 (human melanoma), MCF-7, and PC-3 cell lines (IC50 values were 1, 40.5 and 6 μg/mL, respectively). Two bioactive dicinnamates i.e., 1,3-di-O-cinnamoylglycerol and (E)-3-(cinnamoyloxy)-2-hydroxypropyl 3-(3,4-dimethoxyphenyl) acrylate (Figure 5) were also isolated from the plant. Both of them exhibited activity against A375, PC-3 and MCF-7 cell lines, with IC50 values ranging between 3 to 41 μg/mL, respectively [53].
Figure 5.
Bioactive dicinnamates from Tillandsia recurvata with cytotoxic potential.
3.16. Caesalpiniaceae
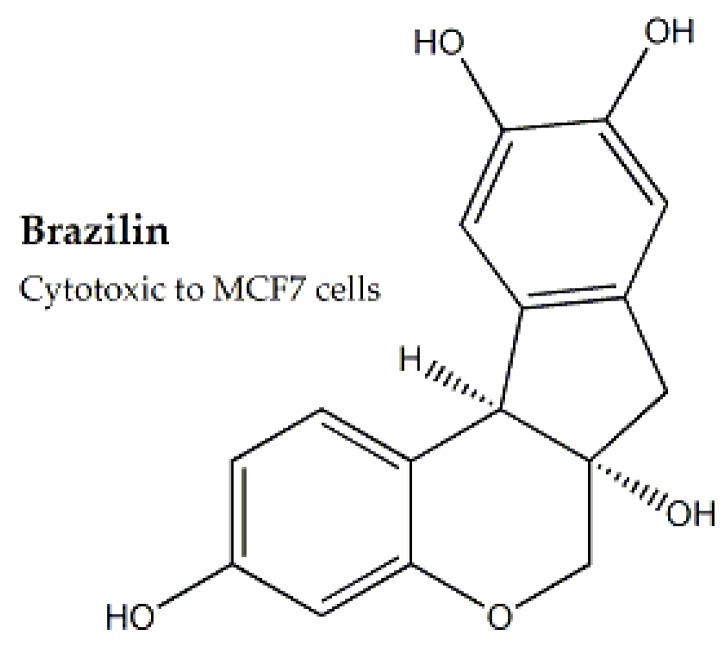
Heartwood and leaf extracts of Caesalpinia sappan were examined for cytotoxic potential against MCF-7 and A-549 cell lines. Brazilin A (Figure 6) was also isolated and evaluated against MCF-7. Significant cytotoxic property was observed for both crude extracts and bioactive compound. Further molecular docking proved the effectiveness of Brazilin A in the reduction of Bcl-2 apoptotic inhibitor [54]. Saraca asoca (Caesalpiniaceae family) was reported cytotoxic against AGS cell lines (IC50 value: 20 μg/mL). Further evaluation of the bioactive compounds was recommended [24].
Figure 6.
Cytotoxic Brazilin from Caesalpinia sappan heartwood.
3.17. Compositae
Gnaphalium luteoalbum, belonging to the Compositae family, was evaluated for cytotoxic effects against VERO, NIH3T3, AGS, HT-29, MCF-7, and MDA-MB-231 cell lines and was found to be cytotoxic against AGS and MCF-7 cells (IC50 values of 980 and 340 μg/mL). The major cytotoxic phytoconstituents included apigenin, luteolin, jaceosidin, and gnaphalin [24,55].
3.18. Dilleniaceae
The stem and bark extracts of Dillenia pentagyna (Dillenaceae family) were found to have cytotoxic activity against DL, MCF-7, and HeLa cell lines (IC50 values of 25.8, 41.6 and 76.8 µg/mL, respectively). Reduction in glutathione (GSH) level was also observed in D. pentagyna treated animal. GSH is related with onset of tumor cell proliferation through regulation of PKC (protein kinase C). Thus, D. pentagyna was reported to be beneficial in cancer treatment through depletion of GSH [46,56]. In addition, Dillenia indica leaves have also been reported to be cytotoxic against MCF-7 and MDA-MB-231 cell lines (IC50 of 340 and 540 μg/mL, respectively) [24].
3.19. Dipsacaceae
The aerial parts of Pterocephalus pulverulentus demonstrated moderate cytotoxic effects against MCF-7 and Hep-2 cell lines (percentage of remaining viable cells was 80 and 138%, respectively) [22].
3.20. Ebenaceae
Diospyros peregrina leaves were reported to have cytotoxic activity against MCF-7 and MDA-MB-231 cell lines (IC50 values were 7 and 33 μg/mL, respectively) [24]. Another study reported the cytotoxicity of different fractions of D. peregina fruits against MCF-7 and HepG2 cell lines (lowest IC50 values were 37.2 and 64.4 μg/mL, respectively) [57].
3.21. Ericaceae
Arbutus andrachne aerial part was reported to have cytotoxic activity against MCF-7, T47D, CACO-2, HRT-18, A375.S2, and WM1361A cell lines. Significant cytotoxic effect was observed against A375.S2, HRT-18, and MCF-7 cell lines, while moderate cytotoxic potential was observed for other cell lines. The percentage of remaining cell viability was within 40.56 to 121.2% [43].
3.22. Euphorbiaceae
Croton caudatus was evaluated against DL, MCF-7, and HeLa cell lines. Potent cytotoxic effect against DL cell line was observed (IC50 value: 29.7 µg/mL) [46]. The cytotoxic effect of Euphorbia tirucalli was evaluated against pancreatic cancer cell line (MiaPaCa-2) and a dose-dependent cytotoxic effect was observed with a reduction in quantity of viable cells (7% for 200 µg/mL) [58].
3.23. Fabaceae
Adenanthera pavonina of family Fabaceae was evaluated in vivo for its cytotoxic potential in DLA induced ascetic mice model. Significant reduction in volume of tumor and number of viable tumor cells were observed in mice treated with the plant extract [18]. For Ononis sicula and Ononis hirta, the antiproliferative activity of different fractions of aerial parts were evaluated against MCF-7, Hep-2 and Vero cell lines. For Ononis hirta, the lowest IC50 values were 28 (methanol fraction), 48.8 (chloroform fraction), and 41.9 μg/mL (n-hexane fraction) against MCF-7, Hep-2 and Vero cell lines, respectively. On the other hand, the chloroform fraction of Ononis sicula was most effective against MCF-7, Hep-2, and Vero cell lines (IC50 values of 66, 75.3, and 79.5 μg/mL, respectively) [22].
3.24. Gramineae
Leaves of Zea mays (Germineae family) were reported to be cytotoxic against Hep-2 cell lines and resulted in an increase in the number of apoptotic Hep-2 cells [59]. Maysin, a cytotoxic constituent isolated from the plant, demonstrated moderate cytotoxic effects against five cancer cells namely, A549, SK-OV-3 (ovarian), SK-MEL-2 (melanoma), XF-489 (CNS), and HCT-15 (colon) (IC50 of 62.24, 43.18, 16.83, 37.22, and 32.09 μg/mL, respectively) [60].
3.25. Hypericaceae
Hypericum kotschyanum aerial part was reported to have moderate cytotoxic effects against HeLa and Vero cell lines (IC50 values were 507 and 367 μg/mL, respectively) [23].
3.26. Labiatae
The aerial parts of Salvia pinardi were significantly cytotoxic against MCF-7 and Hep-2 cell lines (IC50 of 85.5 (methanol fraction), 94.8 (chloroform fraction), and 192.3 (n-hexane fraction) μg/mL) [22].
3.27. Lamiaceae
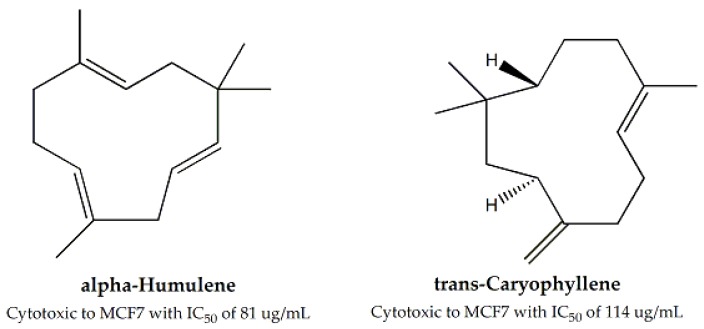
Lavandula angustifolia, belonging to the Lamiaceae family, was found to have negligible or no activity against MCF-7 and Hep-2 cell lines [22]. On the other hand, Plectranthus stocksii stem and leaf extract was significantly cytotoxic against RAW264.7, Caco-2, and MCF-7 cell lines. The least IC50 value for RAW264.7 was 9 mg/mL (stem ethyl acetate fraction), for Caco-2 it was 36.1 mg/mL (leaves ethyl acetate fraction), and for MCF-7, it was 48.9 mg/mL (leaf ethyl acetate fraction) [61]. For Salvia hypargeia, cytotoxic activity was examined against Vero and HeLa cell lines and the IC50 values were over 1000 µg/mL against both cell lines [23]. In contrast, Salvia officinalis was found to be significantly cytotoxic against MCF-7, B16F10, and HeLa cells (IC50 = 14–36 µg/mL). Two bioactive compound i.e., α-humulene and trans-caryophyllene (Figure 7) were also isolated and tested against MCF-7 cell line and the IC50 values were found to be 81 and 114 μg/mL, respectively [62]. the aerial parts of Teucrium sandrasicum were examined for cytotoxic effects against HeLa and Vero cell lines and the reported IC50 values were 513 and 593 µg/mL, respectively [23].
Figure 7.
Cytotoxic components of family Lamiaceae.
Nepeta italica, belonging to Lamiaceae family, was found to have moderate cytotoxicity against HeLa and Vero cell lines (IC50 values of 980 and >1000 μg/mL, respectively) [23]. In a DMBA (7,12-dimethylbenz[a]anthracene)-induced hamster buccal pouch carcinogenesis model, Ocimum sanctum demonstrated inhibition of tumor development and early events of carcinogenesis [63]. In S 180 induced mice model, an increase in survival rate was observed without any impact on tumor volume. These effects were attributed to its indirect or direct impact on the immune system through modulation or regulation of humoral immunity and stimulation of cell-mediated immunity, thereby resulting in inhibition of neoplasm [64,65,66]. Origanum sipyleum was found to have minimal cytotoxic effect against both HeLa and Vero cell lines, with IC50 values over 1000 μg/mL. [23]. Teucrium polium (Laminaceae family) was examined against MCF-7, Hep-2, T47D, CACO-2, HRT18, A375.S2, and WM1361A cell lines. The extract was found to be most effective against MCF-7, Hep-2, and A375.S2 cell lines (percent of remaining viable cells were 78, 58, and 61%, respectively) [22,43].
3.28. Malvaceae
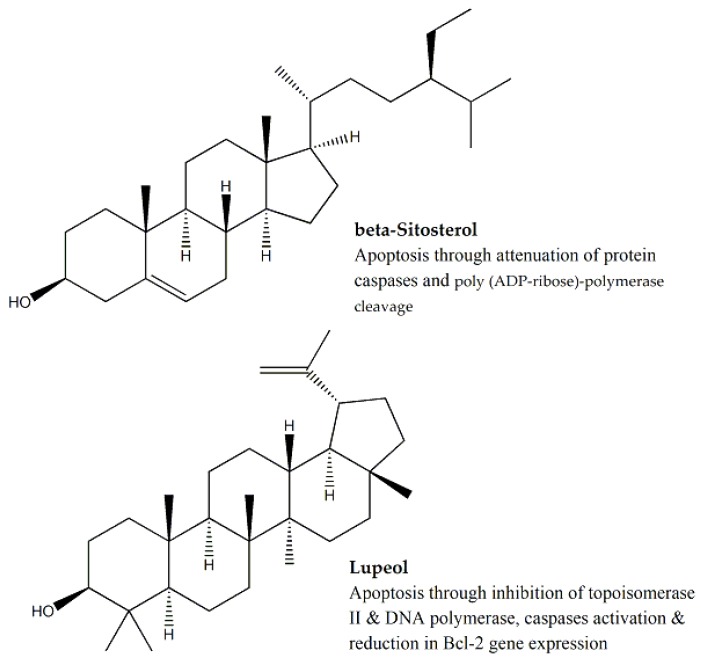
Three species of Hibiscus (Malvaceae family) i.e., Hibiscus micranthus, Hibiscus calyphyllus, and Hibiscus deflersii were evaluated for their cytotoxic effect against HepG2 and MCF-7 cell lines, using the MTT assay. The petroleum ether fraction of H. deflersii extract was found to be most potent amongst the three species against HepG2 and MCF-7 cell lines (IC50 values were 14.4 and 11.1 μg/mL, respectively). Three cytotoxic biomarkers i.e., ursolic acid, β-sitosterol, and lupeol (Figure 8) were quantified through HPLC analysis and highest concentration of these biomarkers were obtained from the petroleum ether fraction of H. deflersii extract.
Figure 8.
Biomarkers of family Malvaceae and mechanism of cytotoxicity.
Again, the petroleum ether fraction of H. calyphyllus extract (IC50 values were 14.5 and 25.1 μg/mL against HepG2 and MCF-7, respectively) and the chloroform fraction of H. micranthus extract (IC50 values were 27.6 and 24.1 μg/mL against HepG2 and MCF-7, respectively) had the most potent cytotoxic effect [67]. Both extracts were then analyzed with HPLC for the quantification of the aforementioned biomarkers. Among the three biomarkers, the apoptotic effect of ursolic acid was reported to be mediated by cytochrome c-dependent caspase-3 activation, inhibition of DNA replication through topoisomerase I cleavage, and increase in the expression of p21WAF1 cell-cycle regulator [68]. β-Sitosterol induced apoptosis via caspase-3, caspase-9 activation and poly (ADP-ribose)-polymerase cleavage. In addition, reduction in anti-apoptotic Bcl-2 protein expression and increase in pro-apoptotic Bax protein expression were reported [69]. The cytotoxic effects of lupeol were attributed to the inhibition of topoisomerase II, DNA polymerase, angiogenesis, and induction of apoptosis through caspases activation, poly (ADP-ribose)-polymerase cleavage, and decreased Bcl-2 expression [70].
3.29. Meliaceae
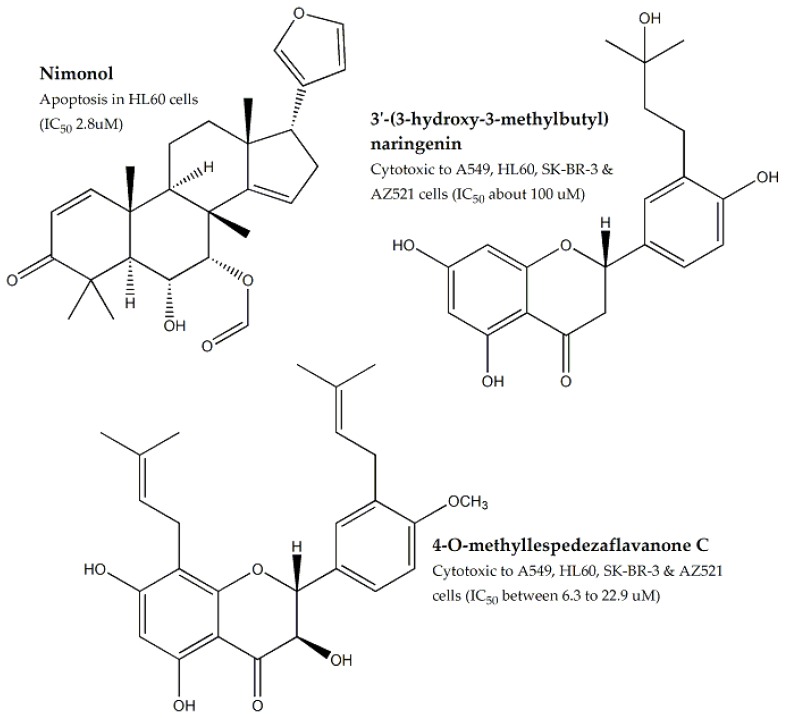
Azadirachta indica, an important medicinal plant found on the Indian subcontinent, was evaluated for its cytotoxic effect against HT-29, A-549, MCF-7, HepG-2, MDBK, and EAC cell lines (IC50 values: 83.5–212.2 μg/mL) [71,72]. Multiple cytotoxic triterpenoids, limonoids (tetranortriterpenoids), and flavonoids were isolated from A. indica, and their cytotoxic effects have been reported in numerous studies. One study reported the isolation of 17 limonoids (including three new compounds) and assessed them for cytotoxic potential against lung (A549), leukemia (HL60), breast (SK-BR-3), and stomach (AZ521) cancer cell lines. Seven compounds exhibited significant cytotoxic potential (IC50 values: 0.1–9.9 μM). Among them, nimonol (Figure 9) induced apoptosis in HL60 cells (IC50 value: 2.8 μM) [73]. In another study, 36 limonoids were isolated (including six new compounds) and the anti-tumor potential was evaluated against the aforementioned cell lines (IC50 0.1–9.3 μM). Sixteen compounds were reported to induce apoptosis in AZ521 cells [74,75]. In addition, two new flavonoids, 3′-(3-hydroxy-3-methylbutyl) naringenin and 4′-O-methyllespedezaflavanone C (Figure 9), and seven known flavonoids were isolated from the plant and were found to be potentially cytotoxic against A549, HL60, SK-BR-3, and AZ521 cell lines (IC50 4.2–100 μM) [76]. Furthermore, two new terpeniods, isolated from A. indica, were found to be cytotoxic against A549, HL60, SW-480, SMMC7721, and MCF-7 cells (IC50 values: 0.8 to 4.5 μM) [77].
Figure 9.
Cytotoxic components of Azadirachta indica.
3.30. Menispermaceae
The aerial parts of Cocculus hirsutus were evaluated for cytotoxic effects against MCF-7 cell line using in vitro MTT assay. The methanol extract of the plant demonstrated cytotoxic potential (IC50 value: 39.1 μg/mL). Multiple bioactive anticancer compounds were isolated from this plant, including coclaurine, haiderine, and lirioresinol, which exerted anti-tumor effects by interacting with cell-cycle regulatory proteins, such as Aurora kinase, c-Kit, FGF, Nuclear Factor-Kappa B (NF-kB), Bcl-xL, and VEGF [78,79].
3.31. Moraceae
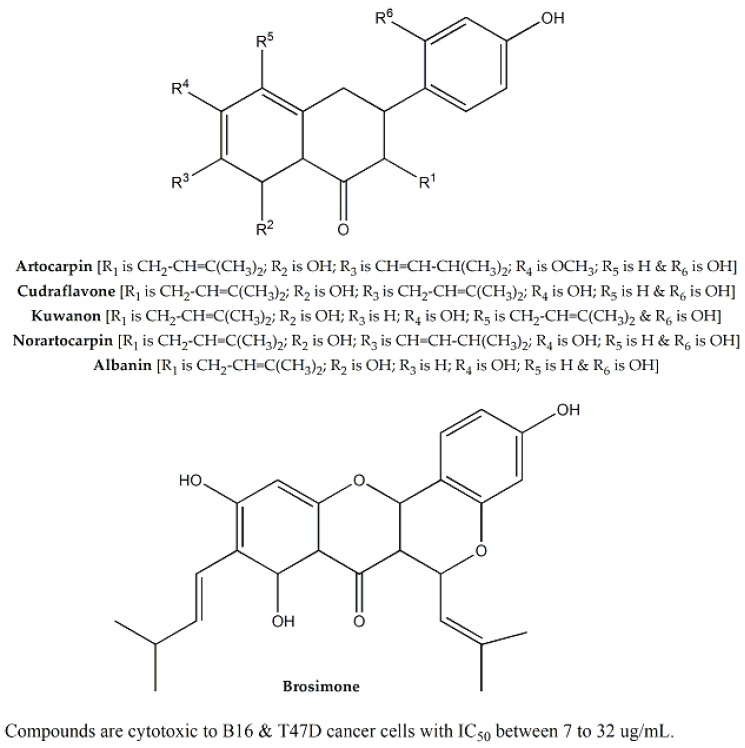
Artocarpus heterophyllus, belonging to the Moraceae family, was examined for cytotoxic effect against HEK293, A549, HeLa, and MCF-7 cell lines using the MTT and sulforhodamine B (SRB) assays. The plant extract was found to have cytotoxic effect against A549 cell line (IC50 value of 35.3 μg/mL) [80]. In other studies, nine bioactive flavonoids were isolated and evaluated against B16 melanoma cells and T47D cells. The compounds with significant cytotoxic effects were noratocarpin, cudraflavone, artocarpin, brosimone, kuwanon, and albanin. (Figure 10), with IC50 values ranging between 7 to 32 μg/mL [81,82]. The effect was reported to be due to inhibition of melanin biosynthesis [83]. Among the three Ficus species, i.e., F. beecheyana, F. carica, and F. racemosa, F. beecheyana led to a dose-dependent reduction in cell viability of AGS, SW-872, HL-60, and HepG2 cells due to cellular apoptosis. Apoptosis was thought to be induced by polyphenolic compounds, such as p-coumaric acid, chlorogenic acid, caffeic acid, gallic acid, and rutin through interaction with Fas, Fas-L, p53, Bcl-2, and caspases (3,8,9) proteins [84]. In addition, F. carica was found to have cytotoxic activity against MCF-7, B16F10, and HeLa cell lines (IC50 values were 440, 880, and >1000 μg/mL, respectively) [62].
Figure 10.
Cytotoxic phytoconstituents of family Moraceae.
Different parts of F. racemosa extracts were evaluated for cytotoxic effects against MCF-7, DLA, HL-60, HepG2, NCI-H23, and HEK-293T cell lines. Cytotoxicity was observed against MCF-7, DLA, HL-60, and HepG2 cells (IC50 values were 80, 175, 276.9, and 363 μg/mL, respectively) [85,86,87]. The extract of Morus nigra was evaluated for cytotoxic effects against OVCAR-8 (ovarian), SF-295 (brain), HCT-116 (colon), and HeLa (cervix) cancer cells, and demonstrated significant cytotoxicity against HeLa (% inhibition 89.5–32) [88,89].
3.32. Myristicaceae
Myristica fragrans was found to have cytotoxic activity against KB cell lines (IC50 value: 75 μg/mL). Cytotoxicity was induced by apoptosis of cancer cells through interaction with Bcl-2 protein [10]. Other studies demonstrated 2 new phenolic and 38 essential oils from M. fragrans which were tested against K-562, HCT-116, and MCF-7 cells, and the IC50 values were found to be within the range 2.11–78.15 μg/mL [90,91].
3.33. Myrtaceae
Syzygium cumini, belonging to the Myrtaceae family, was reported to have cytotoxic effects against A2780, MCF-7, PC3, and H460 cell lines (IC50 values were 49, 110, 140, and 165 μg/mL, respectively) [92]. In addition, the cytotoxic effects of S. cumini against U251, HepG2, and HeLa cell lines were also reported [93,94]. The major bioactive components in S. cumini were anthocyanins (pelargonidin-3-O-glucoside, pelargonidin-3,5-O-diglucoside, cyanidin-3-O-malonyl glucoside, and delphenidin-3-O-glucoside), tannins (ellagic acid, ellagitannins etc.), and polyphenols [92,93].
3.34. Nyctaginaceae
The aerial parts of Mirabilis jalapa were tested for cytotoxic activity against MCF-7 and Hep-2 cell lines and the percentage of remaining viable cells after treatment were 60 and 78, respectively [22]. Studies have reported the presence of bioactive rotenoids and Mirabilis antiviral protein (MAP) in M. jalapa, which were found to be cytotoxic against HeLa, Raji, A549, HCT 116, and Vero cell lines. The IC50 values of MAP against HCT116, MCF-7, and A549 cell lines were 150, 175, and 200 µg/mL, respectively [95,96].
3.35. Oleaceae
The flowers of Jasminum sambac were observed to have moderate cytotoxicity against MCF-7 and HEP-2 cell lines [22]. Other studies reported the cytotoxicity of the leaves of J. sambac against MCF-7 cell lines (IC50: 7 µg/mL) [24]. Olea europaea leaves have also been reported to have cytotoxic activity against MCF-7, B16F10, HeLa, and Vero cell lines (IC50 values were 43, 170, 440, and >1000 µg/mL, respectively) [23,62]. Cytotoxicity of O. europaea was due to the induction of apoptosis through interaction with Bcl-2, Bax, and p53 proteins [97]. The fruits of Syringa vulgaris were found to be cytotoxic against MCF-7 and HeLa cell lines (viability of cells were 71 and 122%, respectively) [22].
3.36. Oxalidaceae
The leaves and fruits of Averrhoa bilimbi were found to be cytotoxic against MCF-7 breast cancer cell lines (IC50 was 154.9 and 668 µg/mL for fruits and leaves, respectively) [98]. The major bioactive phytoconstituents included nonanal, tricosane, squalene, and malonic acid [99].
3.37. Phyllanthacae
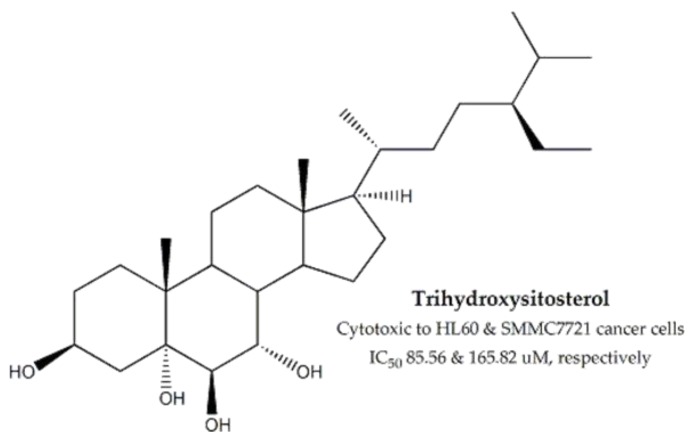
Phyllanthus emblica, belonging to the Phyllanthacae family, was found to be cytotoxic against HT-29 colon cancer cell lines (IC50 value: ~35 µg/mL) [100]. Amongst the bioactive compounds, a new apigenin glucoside and 14 sterols have been isolated (including two new compounds) and screened for cytotoxic activity against HL-60 and SMMC-7721. Among these compounds, trihydroxysitosterol (Figure 11) exhibited cytotoxicity against both HL-60 and SMMC-7721 (IC50 values of 85.56 and 165.82 µg/mL, respectively) [101,102].
Figure 11.
Bioactive cytotoxic component of Phyllanthus emblica.
3.38. Plumbaginaceae
Limonium densiflorum halophyte was reported to have cytotoxicity against A-549 and DLD-1 cancer cell lines (IC50 values were 29 and 85 μg/mL, respectively). The major bioactive phytoconstituents included myricetin, isorhamnetin, and trans 3-hydroxycinnamic acid [103].
3.39. Picrorhiza
Picrorhiza kurroa rhizomes, belonging to the Picrorhiza family, were found to have cytotoxic effects against Hep3B (hepatocellular carcinoma), MDA-MB-435S (breast carcinoma), and PC-3 (prostate) cancer cell lines (lowest IC50 values were 32.6, 19, and 63.9 μg/mL, respectively). Cytotoxicity was induced in the target cells through apoptosis [104]. Among the phytoconstituents isolated from the plant, curcubitacin was a potent cytotoxic and antitumor agent [105].
3.40. Pinaceae
The cytotoxic activity of Cedrus deodara, belonging to the Pinaceae family, was tested against multiple cancer cell lines. It was found to be cytotoxic against Mia-Pa-Ca-2, PC3, and A-2780 (ovary) cancer cells (IC50 of 74, 77, and 63 μg/mL, respectively) [48]. In addition, the plant extract was found to have cytotoxic potential against Molt-4 cancer cells (IC50 value: 15 μg/mL). Cytotoxicity was induced by apoptosis of cancer cells through interaction with caspase 3, 8, and 9 proteins [106].
3.41. Piperaceae
The cytotoxicity of Piper longum, belonging to the Piperaceae family, was evaluated against multiple cancer cell lines. Significant cytotoxic effects were observed against Colo-205 colon cancer cell lines (IC50 was 18 μg/mL) [48]. Other studies reported cytotoxicity of P. longum fruits against DLA and EAC cancer cells. The major cytotoxic phytoconstituent was piperine and its antitumor effect was due to the induction of apoptosis [107]. The cytotoxicity of P. regnelli was tested against melanoma (UACC-62), kidney (786-0), breast (MCF-7), lung (NCI-H460), ovary (OVCAR-3), prostate (PC-3), colon (HT-29), and leukemia (K-562) cancer cells. The major bioactive agent of P. regnelli was eupomatenoid-5 and it demonstrated cytotoxicity against PC3, OVCAR-3, 786-0, and MCF-7 cells [108].
3.42. Poaceae
The aerial parts and roots of Cenchrus ciliaris were evaluated for cytotoxic activity against A-549, CACO, HCT-116, HeLa, HepG2, MCF-7, and PC3 cell lines. Significant cytotoxicity was observed against HepG2 (IC50 values were 12 and 9 µg/mL for aerial parts and roots, respectively), CACO (IC50 values were 27.2 and 20.5 µg/mL for aerial parts and roots, respectively), and A-549 (IC50 values were 14.5 and 11.1 µg/mL for aerial parts and roots, respectively) [109,110].
3.43. Polygonaceae
Calligonum comosum, belonging to the Polygonaceae family, was found to be cytotoxic against HepG2 cancer cell lines (IC50 value: 9.60 µg/mL). The major cytotoxic phytoconstituents of the plant were catechin and its derivatives as well as kaempferol and its derivatives, including mequilianin. Cytotoxicity was induced by apoptosis through increased expression of p53 and reduced expression of Bcl-2 gene [35,111].
3.44. Primulaceae
Aegiceras corniculatum, a well-known medicinal plant belonging to the Primulaceae family, was found to have significant cytotoxic effect against VERO, NIH3T3, AGS HT-29, MCF-7, and MDA-MB-231 cell lines. (IC50 values of 150, 97, 0.5, 998, 91, and 461 μg/mL, respectively) [24]. The isolated bioactive constituents were aegicoroside A, sakurasosaponin, fusarine, and fusamine. [112,113].
3.45. Ranunculaceae
The seeds of Delphinium staphisagria (Ranunculaceae family) were found to be cytotoxic against MCF7, HT29, N2A, H5-6, and VCREMS cell lines. In addition, cytotoxicity was observed against N2A, H5-6, and VCREMS (IC50 values were 41.9, 41.1, and 14.5 μg/mL, respectively) [34]. The major constituents of the plant were astragalin, paeonoside, and petiolaroside. [114].
3.46. Rosaceae
The leaves of Crataegus microphylla, belonging to the Rosaceae family, were found to have cytotoxic activity against HeLa and Vero cell lines (IC50 of 576 and >1000 μg/mL, respectively) [23]. Another study reported cytotoxicity of the flowers of C. microphylla against HeLa cells (IC50 871 μg/mL) [115]. The cytotoxic effects of Rosa damascene were evaluated against MCF-7, Hep-2, HeLa, and Vero cell lines, and significant cytotoxic activity was observed against HeLa cells (IC50 value of 265 μg/mL) [22,23]. The cytotoxic constituents of R. damascena included nerol, geraniol, β-citronellol, linalool, nonadecane, and phenylethyl alcohol [116].
3.47. Rubiaceae
The bark and wood of Hymenodictyon excelsum, belonging to the Rubiaceae family, were screened for cytotoxicity against Vero, NIH3T3, AGS HT-29, MCF-7, and MDA-MB-231 cell lines, and significant cytotoxic effects of the bark were observed against all cell types (IC50 values were 230, 70, 90, 160, 80, and 440 μg/mL, respectively) [24]. The cytotoxicity was induced by DNA fragmentation and apoptosis of cancer cells [117]. The leaves of Oldenlandia corymbosa were reported to be cytotoxic against K562 cells (IC50 of 114.4 μg/mL) and the toxicity was induced by apoptosis [118].
3.48. Salicaceae
The flowers of Populus alba were reported to be moderately cytotoxic against MCF-7 and Hep-2 cells. The percentage of residual cell viability was 100% for both cell lines at 100 μg/mL dose [22]. Another study revealed the cytotoxicity of the essential oils from the plant against A549, H1299 (human non-small cell lung cancer), and MCF-7 cancer cells (IC50 values were 12.05, 10.53, and 28.16 μg/mL, respectively) [119].
3.49. Sapotaceae
Flowers of Manilkara zapota, belonging to the Sapotaceae family, were found to be cytotoxic against MCF-7 cell line (IC50 was 12.5 μg/mL) via DNA fragmentation [28]. Eleven triterpenoid saponins, including lupanes, oleananes, ursanes, and manilkoraside were isolated from the plant, among which manilkoraside exhibited the highest cytotoxic effects against HT-29 and HL-60 cell lines (EC50 of 64 and 24 μg/mL, respectively) [120].
3.50. Saururaceae
Saururus chinensis roots were found to be cytotoxic against MCF-7 breast cancer cell lines in the MTT assay. The lowest IC50 value calculated was 91.2 μg/mL (water fraction). The major bioactive constituents included aristolactram, dihydroguaiatric acid, and sauchinone [121]. Saucerneol D, manassantin A and B, and saucerneol F were some of the lignans isolated from S. chinensis, which had cytotoxic effects against HT-29 and HepG2 cells (IC50: 10–16 μg/mL). The cytotoxicity was due to the inhibition of DNA topoisomerase I and II [122].
3.51. Scrophulariaceae
The flowers and aerial parts of Verbascum sinaiticum were reported to have moderate cytotoxicity against MCF-7 and Hep-2 cancer cells (cell viability of 60 and 80%, respectively [22]. Another study reported cytotoxic effects of two flavonolignans, novel sinaiticin and hydrocarpin, obtained from V. sinaticum. They were tested against P-388 cells and found cytotoxic with ED50 of 1.2 and 7.7 μg/mL for hydrocarpin and sinaiticin, respectively [123].
3.52. Solanaceae
Solanum khasianum, belonging to the Solanaceae family, was screened for cytotoxicity against DL, MCF-7, and HeLa cancer cells (IC50 values were 27.4, 71.2, and 62.5 μg/mL, respectively) [46]. The cytotoxic effects of the fruits of S. nigrum were evaluated against HeLa, Vero, HepG2, and CT26 cancer cell lines (IC50 values were 265, 6.9, 56.4, and 77.6 μg/mL, respectively) [124,125].
Two species of Withania i.e., W. coagulans and W. somnifera were also evaluated for their cytotoxic potential. The fruits, roots, and leaves of W. coagulans were screened for cytotoxic effects against HeLa, MCF-7, and RD cancer cell lines. The IC50 values for leaves and roots were 0.7–4.7 μg/mL and 0.7–6.7 μg/mL, respectively. The major constituents were myricetin, quercetin, and gallic acid [126]. W. somnifera demonstrated cytotoxicity against PC3, A-549, A-2780, and K-562 cell lines (IC50 of 52, 46, 79, 41 μg/mL, respectively) [48].
3.53. Sterculiaceae
Helicteres isora whole plant was screened for cytotoxicity against HeLa-B75, HL-60, HEP-3B, and PN-15 cells. Moderate effectiveness was observed against all cell types. The major cytotoxic components of this plant were cucurbitacin B and isocucurbitacin B [127,128].
3.54. Thymelaeaceae
Aquilaria malaccensis, belonging to the Thymelaeaceae family, was found to be cytotoxic against DLA and EAC cancer cells (IC50 values were 72 and 79 μg/mL, respectively). However, the cytotoxicity against normal cells was negligible [129]. Another study evaluated the cytotoxicity of the oil fraction of the plant extract against HCT116 colon cancer cells and reported an IC50 value 4 μg/mL [130]. The major phytoconstituents of the plant were benzaldehyde, pinene, octanol, germacrene, and hexadecanal [131].
3.55. Verbenaceae
Clerodendrum viscosum leaf extract was screened against VERO, NIH3T3, AGS, HT-29, MCF-7, and MDA-MB-231 cancer cell lines and was found to have cytotoxic effects against MCF-7 and HT-29 cells (IC50 of 50 and 880 μg/mL, respectively) [24]. The roots of C. infortunatum, belonging to the same family, exhibited in vivo cytotoxicity in DLA-induced ascetic mice model and the induction of apoptosis was observed through interaction with Bax, Bcl-2, caspases 8, and 10 proteins [132].
3.56. Vitaceae
Leea indica, belonging to the Vitaceae family, was found to be cytotoxic against DU-145 and PC-3 prostate cancer cell lines (IC50 values were 529.4 and 677.1 μg/mL, respectively) [133]. In case of Vitis vinifera, the stem was screened against MCF-7, B16F10, and HeLa cancer cell lines and the lowest IC50 values were 62, 137, and 336 μg/mL, respectively for the acetone fraction of the extract [62]. Two major cytotoxic polyphenols in V. vinifera i.e. quercetin 3-O-β-d-4C1 galactoside and quercetin 3-O-β-d-4C1 glucuronide, were isolated. The cytotoxic effects were induced through apoptosis [134,135].
3.57. Zingiberaceae
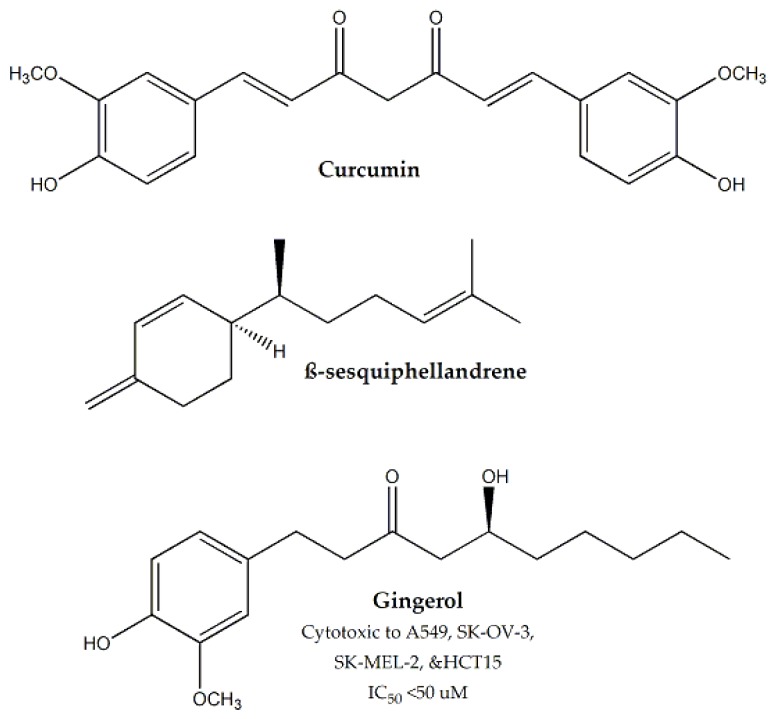
Rhizomes of Curcuma longa, belonging to the Zingiberaceae family, were found to have 97% cytotoxicity against Hep-2 cell line, at a dose of 1000 μg/mL [136]. The major phytoconstituent of this plant is curcumin (Figure 12), which has been reported to have anticancer activity in previous studies [137,138]. Amongst other phytoconstituent in C. longa, β-sesquiphellandrene (Figure 13) has also been reported to possess anticancer potential [139]. The cytotoxic effect of curcumin was through the induction of apoptosis [140].
Figure 12.
Cytotoxic compounds isolated from plants of family Zingiberaceae.
Figure 13.
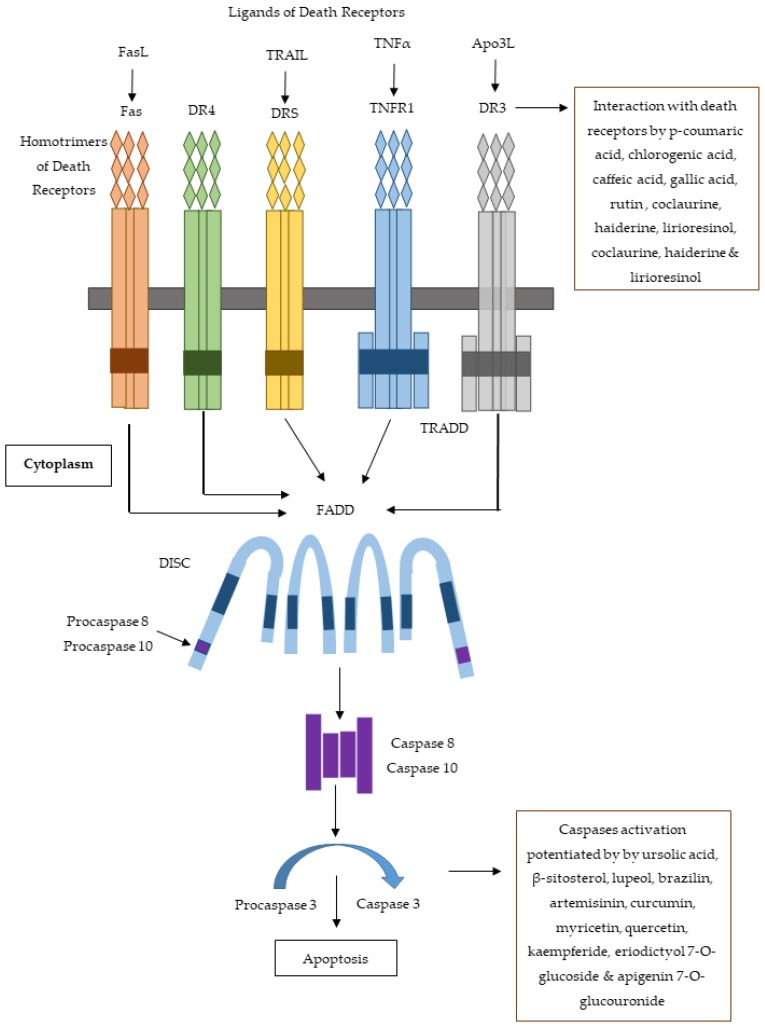
Extrinsic apoptosis pathway showing the sites of action of cytotoxic constituents obtained from medicinal plants of Indian subcontinent.
The major constituents of Zingiber officinale, i.e., gingerol (Figure 13) and its derivatives were screened against A549, SK-OV-3, SK-MEL-2, and HCT15 and were found to be cytotoxic (IC50 < 50 μM). Many essential oils of Z. officinale have also been reported to have cytotoxic effects [141,142]. The combination effect of C. longa and Z. officinale were observed to have synergistic cytotoxic effect against PC-3M cancer cells [143].
4. Reported Cytotoxic Constituents: Therapeutic Perspective and Future Directions
The aforementioned cytotoxic phytoconstituents, isolated from different plants of Indian subcontinent, were further examined for their significance in cancer chemotherapy. The DTP database of the National Cancer Institute (NCI), USA, was utilized to assess the therapeutic effects of these bioactive constituents [144].
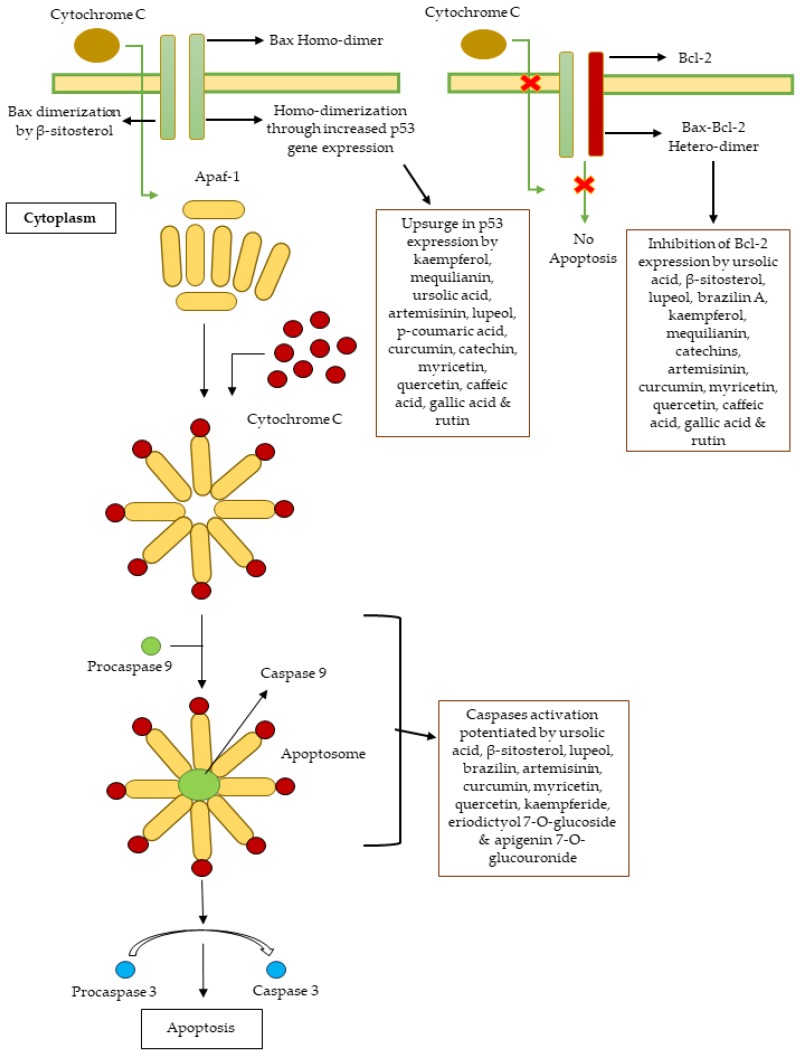
Ursolic acid, a pentacyclic triterpenoid, was found to have significant anticancer effect against MCF-7 and MDA-MB-231 breast cancer cell lines. The reported mechanisms of cytotoxicity included reduction in cyclin D1, STAT3 (signal transducer and activator of transcription), CDK4 (cyclin dependent kinase), Bcl-2, AKT, and MMP-2 (matrix metallopeptidases) proteins as well as activation of Bax, caspase 3, caspase 8, caspase 9, PARP (poly(ADP-ribose)polymerase), p53, and p21 proteins (Figure 13 and Figure 14) [145]. DTP database revealed several in vivo animal model studies against L1210, P388, and B16 cancer cells. Although the compound showed low toxicity in in vivo studies, the major impediment in the therapeutic development of ursolic acid was its poor bioavailability and short plasma-half life. Currently, attempts are being made to improve its pharmacokinetic parameters by utilizing nano-particle-based drug delivery techniques [146].
Figure 14.
Intrinsic apoptosis pathway with sites of action of cytotoxic constituents obtained from medicinal plants of Indian subcontinent.
Artemisinin, a well-known anti-malarial drug and its derivative dihydroartemisinin, were found to have anticancer activity in numerous in vitro and in vivo studies. The cytotoxic effects were attributed to DNA damage by base excision or homologous recombination, cell death by apoptosis, autophagy, and necrosis; inhibition of angiogenesis via AMPK (AMP activated protein kinase) pathway; reduction in CyclinD1, CyclinE, and Bcl-2; and activation of Bax, Caspase 3, Caspase 8, Caspase 9, PARP, p53, and p21 (Figure 13 and Figure 14). Currently, clinical trials are being conducted to establish artemisinin as a potential anticancer agent [147].
Brazilin was reported to have cytotoxic effects against TCA8113, MG-63, and T24 cancer cells. The reported mechanisms of cytotoxicity included interaction with c-Fos, inhibition of Bcl-2, p62, and p-mTOR as well as enhancement of Bax, caspase-3, LC3B, and p-AMPK (Figure 13 and Figure 14) [148,149]. Future studies should focus on the pharmacokinetic evaluation of this compound.
β-Sitosterol was found to be have anti-cancer effects against breast, prostate, lung, colon, stomach, and ovarian cancers as well as leukemia. The established mechanisms of cytotoxicity were through interaction with cell signaling pathway, apoptosis, invasion, angiogenesis, metastasis, and proliferation. Although the compound was reported to be nontoxic, it was found to be less potent. The use of cell-specific or liposome-based drug delivery is proposed for the successful application of this compound as an anticancer agent [150].
Lupeol was reported to be cytotoxic against HeLa, CWR22Rt1, A549, 451Lu, SMMC7721, and WM35 cancer cells by downregulation of cyclin D1, CDK2, and Bcl-2 and upregulation of Bax, caspase-3, and p38 (Figure 13 and Figure 14). The compound is currently under investigation in various clinical trials, and if successful, it might be used as a novel adjuvant therapeutic agent for treating multiple cancers in human [151].
Curcumin is a prominent natural phytoconstituent used in the treatment of several types of cancers, including prostate, pancreatic, colorectal, breast, and lung cancers as well as multiple myeloma and leukemia. Reported molecular targets for curcumin include Bcl-2, Bax, p53, caspases, p38, NF-kB, PARP, and cyclin proteins (Figure 13 and Figure 14). The limited bioavailability of curcumin, owing to poor absorption and rapid metabolism, has been improved by synthesizing structural analogues and development of liposomal, nanoparticle, and phospholipid complex-based drug delivery systems. Several clinical trials have been conducted to evaluate the efficacy of curcumin. Further studies are required to enhance the bioavailability of this compound [152].
A large number of studies have reported the cytotoxicity of catechins, such as epicatechin, epigallecatechin-3-gallate etc. Possible mechanisms of cytotoxicity include interaction with Bcl-2, STAT, FYN, p53, CDKs, and vascular endothelial growth factor (VEGF). Several in vivo studies have also demonstrated the effectiveness of catechins in animal models. Additional pharmacokinetic and pharmacodynamic studies in humans are required [153].
Myricetin is a natural flavonoid that has extensively been reported for its antitumor and cytotoxic potential against gastric, esophageal, ovarian, colon, and cervical cancers and multiple leukemia, melanoma, and sarcoma. The compound was tested against many cell lines, including HGC-27, MCF-7, HCT-15, OVCAR-3, HepG2, A549, HeLa, and PC3. Myricetin was found to interact with multiple proteins, such as Bcl-2, Bax, Caspases, Bcl-xl, p53, p21, NF-kB, and cyclin, IL (Figure 13 and Figure 14) [154]. Despite being a potential cytotoxic agent, poor aqueous solubility and poor absorption of this compound has led to its reduced bioavailability. Formulation into nano-suspensions or micro-emulsions was proposed to enhance its absorption. More studies are required in this regard [155].
Quercetin, another naturally occurring polyphenolic flavonoid, has already been reported to be effective against breast, colon, pancreatic, lung, liver, prostate, bladder, gastric, bone, blood, brain, cervical, skin, eye, ovarian, thyroid, and kidney cancers. The cytotoxicity of the compound has been attributed to its interaction with p51, p21, caspases, TNF, IL, Bcl-2, Bax, p53, myeloid cell leukemia (MCL), and cyclin proteins (Figure 13 and Figure 14) [156]. Despite of its low toxicity, multiple clinical trials have been conducted with quercetin. However, extensive studies are required before the development of an established anticancer quercetin formulation [157].
Among the other reported phytoconstituents, caryophyllene, campesterol, rutin, gallic acid, and caffeic acid have been assessed in vivo by DTP of NCI, USA in mice models of P388, L1210, Friend virus leukemia, and Lewis lung carcinoma. However, data regarding the remaining bioactive cytotoxic phytoconstituents reported in this review article, is very limited. Hence, in future, further in vivo as well as mechanistic studies are warranted to assess the activity of these cytotoxic compounds.
Moreover, out of almost 50,000 plant species found in the Indian subcontinent, only a handful of them have been properly examined for their cytotoxic potentials [158]. The diverse environmental conditions such as dry forests, mangrove forests, deserts, aquatic reservoirs, and hill tracts have made the Indian subcontinent a natural treasure. However, the studies conducted so far have just explored the tip of the iceberg. Therefore, the Indian subcontinent still remains a vast unexplored region with great number of exclusive medicinal plants. Further studies should investigate these endemic plants with respect to their cytotoxic potential, mechanism of action, and isolation of active constituents.
Acknowledgments
The authors wish to acknowledge the support of Osaka University, Osaka, Japan and Jashore University of Science and Technology, Jashore, Bangladesh for providing software access and other technological assistances.
Abbreviations
| A549 | Human Lung Adenocarcinoma |
| AGS | Gastric Adenocarcinoma |
| AMPK | AMP Activated Protein Kinase |
| CACO | Human Colon Carcinoma |
| CCK | Cell Counting Kit |
| CDK | Cyclin Dependent Kinase |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DCFH-DA | Dichlorodihydrofluorescein diacetate |
| DLA/DAL | Dalton’s Lymphoma Ascites/Dalton’s Ascites Lymphoma |
| DTP | Developmental Therapeutic Program |
| GSH | Glutathione |
| H1299 | Human Non-Small Cell Lung Cancer |
| HCT/HT | Human Colorectal Adenocarcinoma |
| HeLa | Henrietta Lacks Cervical Cancer |
| Hep | Human Epithelial |
| HL | Human Caucasian Promyelocytic Leukemia |
| IC50/EC50 | Concentration Required to Inhibit/Destroy 50% Cells |
| NF-kB | Nuclear Factor-Kappa B |
| MCF | Human Breast Adenocarcinoma |
| MCL | Myeloid Cell Leukemia |
| MDA-MB | M. D. Anderson Metastasis Breast Cancer |
| MMP | Matrix Metallopeptidase |
| MST | Mean Survival Time |
| MTT | 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| OSA | Osteosarcoma |
| PARP | PolyADP-ribosepolymerase |
| PC | Prostate Cancer |
| PKC | Protein Kinase C |
| SRB | Sulforhodamine B |
| STAT | Signal Transducer and Activator of Transcription |
| T47D | Ductal Epithelial Breast Tumor |
| TBE | Trypan Blue Exclusion |
| THP | Human Acute Monocytic Leukemia |
| VEGF | Vascular Endothelial Growth Factor |
| Vero | Verda Reno |
| WM | Malignant Melanoma |
| WST | Water Soluble Tetrazolium |
| XTT | Cell Proliferation Kit II |
Author Contributions
Conceptualization, K.M. and K.F.; methodology, K.M., B.B. and I.M.R.; software, B.B. and I.M.R.; investigation, K.M., B.B. and I.M.R.; resources, K.M. and K.F.; data curation, K.M. and K.F.; writing—original draft preparation, B.B. and I.M.R.; writing—review and editing, K.M. and K.F.; supervision, K.M. and K.F.; funding acquisition, K.M. and K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and the APC was funded by Prof. Koichi Fukase, Graduate School of Science Osaka University 1-1 Machikaneyama, Toyonaka, Osaka, 560-0043, Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Iqbal J., Abbasi B.H., Mahmood T., Kanwal S., Ali B., Shah S.A., Khalil A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7:1129–1150. doi: 10.1016/j.apjtb.2017.10.016. [DOI] [Google Scholar]
- 2.Krishnamurthi K. 17-screening of natural products for anticancer and antidiabetic properties. Cancer. 2007;3:69–75. [Google Scholar]
- 3.Thirumal M., Kishore G., Prithika R., Das S., Nithya G. In vitro anticancer activity of Tecoma stans (L) ethanolic leaf extract on human breast cancer cell line (MCF-7) Intl. J. Pharma Bio Sci. 2012;2:488–493. [Google Scholar]
- 4.Zyad A., Leouifoudi I., Tilaoui M., Mouse H.A., Khouchani M., Jaafari A. Natural products as cytotoxic agents in chemotherapy against cancer. Cytotoxicity. 2018:65–88. doi: 10.5772/intechopen.72744. [DOI] [Google Scholar]
- 5.Merina N., Chandra K.J., Jibon K. Medicinal plants with potential anticancer activities: A review. Int. Res. J. Pharm. 2012;3:26–30. [Google Scholar]
- 6.Tilaoui M., Mouse H.A., Jaafari A., Zyad A. Comparative phytochemical analysis of essential oils from different biological parts of Artemisia herba alba and their cytotoxic effect on cancer cells. PLoS ONE. 2015;10:e0131799. doi: 10.1371/journal.pone.0131799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla Y., Pal S.K. Dietary cancer chemoprevention: An overview. Int. J. Hum. Genet. 2004;4:265–276. doi: 10.1080/09723757.2004.11885905. [DOI] [Google Scholar]
- 8.Latosińska J.N., Latosińska M. Anticancer drug discovery—From serendipity to rational design. Drug Discov. 2013:35–74. doi: 10.5772/52507. [DOI] [Google Scholar]
- 9.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Rengasamy G., Venkataraman A., Veeraraghavan V.P., Jainu M. Cytotoxic and apoptotic potential of Myristica fragrans Houtt. (mace) extract on human oral epidermal carcinoma KB cell lines. Braz. J. Pharm. Sci. 2018;54:54. doi: 10.1590/s2175-97902018000318028. [DOI] [Google Scholar]
- 11.Verdine G.L. The combinatorial chemistry of nature. Nature. 1996;384:11–13. doi: 10.1038/384011a0. [DOI] [PubMed] [Google Scholar]
- 12.Demain A.L., Vaishnav P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011;4:687–699. doi: 10.1111/j.1751-7915.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hücre B.T.B.F.K., Aktiviteleri H.S. Cytotoxic activities of certain medicinal plants on different cancer cell lines. Turk. J. Pharm. Sci. 2017;14:222–230. doi: 10.4274/tjps.80299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg A., Darokar M.P., Sundaresan V., Faridi U., Luqman S., Khanuja S. Anticancer activity of some medicinal plants from high altitude evergreen elements of Indian Western Ghats. J. Res. Educ. Indian Med. 2007;13:1–6. [Google Scholar]
- 16.Umadevi M., Kumar K.S., Bhowmik D., Duraivel S. Traditionally used anticancer herbs in India. J. Med. Plants Stud. 2013;1:56–74. [Google Scholar]
- 17.Manglani N., Vaishnava S., Dhamodaran P., Sawarkar H. In vitro and in vivo anticancer activity of leaf extract of Barleria grandiflora. Int. J. Pharm. Pharm. Res. 2014;6:70–72. [Google Scholar]
- 18.Kumar A.S.G., Javvadi R.K., Kumar V.K., Reddy M.E., Reddy V.Y., Harshavardhan G., Akbar M. Effect of methanolic extract of Adenanthera pavonina Linn on Dalton’s ascitic lymphoma. Indian J. Res. Pharm. Biotech. 2013;1:138. [Google Scholar]
- 19.Trease G., Evans W. Text book of Pharmacognosy. Bailliare Tindall; London, UK: 1983. pp. 193–336. [Google Scholar]
- 20.Mazumder K., Siwu E.R., Nozaki S., Watanabe Y., Tanaka K., Fukase K. Ursolic acid derivatives from Bangladeshi medicinal plant, Saurauja roxburghii: Isolation and cytotoxic activity against A431 and C6 glioma cell lines. Phytochem. Lett. 2011;4:287–291. doi: 10.1016/j.phytol.2011.04.019. [DOI] [Google Scholar]
- 21.Mazumder K., Tanaka K., Fukase K. Cytotoxic activity of ursolic acid derivatives obtained by isolation and oxidative derivatization. Molecules. 2013;18:8929–8944. doi: 10.3390/molecules18088929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talib W.H., Mahasneh A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 2010;78:33–45. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artun F.T., Karagoz A., Ozcan G., Melikoglu G., Anil S., Kultur S., Sutlupinar N. In vitro anticancer and cytotoxic activities of some plant extracts on HeLa and Vero cell lines. JBUON: Off. J. Balk. Union Oncol. 2016;21:720–725. doi: 10.3390/proceedings1101019. [DOI] [PubMed] [Google Scholar]
- 24.Akter R., Uddin S.N., Grice I.D., Tiralongo E. Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J. Nat. Med. 2013;68:246–252. doi: 10.1007/s11418-013-0789-5. [DOI] [PubMed] [Google Scholar]
- 25.Weerapreeyakul N., Junhom C., Barusrux S., Thitimetharoch T. Induction of apoptosis in human hepatocellular carcinoma cells by extracts of Lannea coromandelica (Houtt.) Merr. and Diospyros castanea (Craib) Fletcher. Chin. Med. 2016;11:19. doi: 10.1186/s13020-016-0091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun X.-J., Shu H.-M., Chen G.-Y., Ji M.-H., Ding J.-Y. Chemical constituents from barks of Lannea coromandelica. Chin. Herb. Med. 2014;6:65–69. doi: 10.1016/S1674-6384(14)60009-5. [DOI] [Google Scholar]
- 27.Gavamukulya Y., Abou-Elella F., Wamunyokoli F., El-Shemy H.A. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola) Asian Pac. J. Trop. Med. 2014;7:S355–S363. doi: 10.1016/S1995-7645(14)60258-3. [DOI] [PubMed] [Google Scholar]
- 28.Sumithra P., Gricilda Shoba F., Vimala G., Sathya J., Sankar V., Saraswathi R. Anticancer activity of Annona squamosa and Manilkara zapota flower extract against MCF-7 cell line. DerPharm. Sin. 2014;5:98–100. [Google Scholar]
- 29.Dantu A.S., Shankarguru P., Ramya D.D., Vedha H.B. Evaluation of in vitro anticancer activity of hydroalcoholic extract of Tabernaemontana divaricata. Asian J. Pharm. Clin. Res. 2012;5:59–61. [Google Scholar]
- 30.Bao M.-F., Yan J.-M., Cheng G.-G., Li X.-Y., Liu Y.-P., Li Y., Cai X.-H., Luo X.-D. Cytotoxic indole alkaloids from Tabernaemontana divaricata. J. Nat. Prod. 2013;76:1406–1412. doi: 10.1021/np400130y. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Salam I.M., Awadein N.E.-S., Ashour M. Cytotoxicity of Luffa cylindrica (L.) M. Roem. extract against circulating cancer stem cells in hepatocellular carcinoma. J. Ethnopharmacol. 2019;229:89–96. doi: 10.1016/j.jep.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Salam I.M., Ashmawy A.M., Hilal A.M., Eldahshan O.A., Ashour M. Chemical composition of aqueous ethanol extract of Luffa cylindrica leaves and its effect on representation of caspase-8, caspase-3, and the proliferation marker Ki67 in intrinsic molecular subtypes of breast cancer in vitro. Chem. Biodivers. 2018;15:e1800045. doi: 10.1002/cbdv.201800045. [DOI] [PubMed] [Google Scholar]
- 33.Akindele A., Wani Z., Mahajan G., Sharma S., Aigbe F.R., Satti N., Adeyemi O.O., Mondhe D.M. Anticancer activity of Aristolochia ringens Vahl. (Aristolochiaceae) J. Tradit. Complement. Med. 2014;5:35–41. doi: 10.1016/j.jtcme.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daoudi A., El Yoube A., Bagrel D., Aarab L. In vitro anticancer activity of some plants used in Moroccan traditional medicine. J. Med. Plants Res. 2013;7:1182–1189. [Google Scholar]
- 35.Shalabi M., Khilo K., Zakaria M.M., Elsebaei M.G., Abdo W., Awadin W. Anticancer activity of Aloe vera and Calligonum comosum extracts separetely on hepatocellular carcinoma cells. Asian Pac. J. Trop. Biomed. 2015;5:375–381. doi: 10.1016/S2221-1691(15)30372-5. [DOI] [Google Scholar]
- 36.Jarial R., Thakur S., Sakinah M., Zularisam A., Sharad A., Kanwar S., Singh L. Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus. J. King Saud Univ.-Sci. 2018;30:185–192. doi: 10.1016/j.jksus.2016.11.006. [DOI] [Google Scholar]
- 37.Jirangkul P., Srisawat P., Punyaratabandhu T., Songpattanaslip T., Mungthin M. Cytotoxic effect of artemisinin and its derivatives on human osteosarcoma cell lines. J. Med. Assoc. Thail. = Chotmaihet thangphaet. 2014;97:215–221. [PubMed] [Google Scholar]
- 38.Hosoya K., Murahari S., Laio A., London C.A., Couto C.G., Kisseberth W.C. Biological activity of dihydroartemisinin in canine osteosarcoma cell lines. Am. J. Veter- Res. 2008;69:519–526. doi: 10.2460/ajvr.69.4.519. [DOI] [PubMed] [Google Scholar]
- 39.Efferth T., Herrmann F., Tahrani A., Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959–969. doi: 10.1016/j.phymed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Isani G., Bertocchi M., Andreani G., Farruggia G., Cappadone C., Salaroli R., Forni M., Bernardini C. Cytotoxic effects of Artemisia annua L. and pure artemisinin on the D-17 canine osteosarcoma cell line. Oxidative Med. Cell. Longev. 2019;2019:1615758–1615759. doi: 10.1155/2019/1615758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundararajan P., Dey A., Smith A., Doss A.G., Rajappan M., Natarajan S. Studies of anticancer and antipyretic activity of Bidens pilosa whole plant. Afr. Health Sci. 2006;6:27–30. doi: 10.5555/afhs.2006.6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saranya K., Manivasagan V., Kanakadurga R., Mohan Babu V.P., Ramesh Babu N.G. A survey on anticancer properties of Indian medicinal plants – A broad spectrum analysis. Int. J. Pharm. Sci. Res. 2019;10:3635–3640. [Google Scholar]
- 43.Abu-Rish E.Y., Kasabri V., Hudaib M., Mashalla S.H., Alalawi L.H., Tawaha K., Mohammad M.K., Mohamed Y.S., Bustanji Y. Evaluation of antiproliferative activity of some traditional anticancer herbal remedies from Jordan. Trop. J. Pharm. Res. 2016;15:469. doi: 10.4314/tjpr.v15i3.6. [DOI] [Google Scholar]
- 44.Choi J.-M., Lee E.-O., Lee H.-J., Kim K.-H., Ahn K.-S., Shim B.-S., Kim N.-I., Song M.-C., Baek N.-I., Kim S.-H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007;21:954–959. doi: 10.1002/ptr.2189. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S., Chashoo G., Saxena A.K., Pandey A.K. Parthenium hysterophorus: A probable source of anticancer, antioxidant and anti-HIV agents. BioMed Res. Int. 2013;2013:1–11. doi: 10.1155/2013/810734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosangkima G., Jagetia G. In vitro anticancer screening of medicinal plants of Mizoram State, India, against Dalton’s lymphoma, MCF-7 and HELA cells. Int. J. Recent Sci. Res. 2015;6:5648–5653. [Google Scholar]
- 47.Serasanambati M., Chilakapati S.R., Manikonda P.K., Kanala J.R. Anticancer activity of methanolic extract of Berberis aristata in MCF-7 human breast cancer cell lines. Int. J. Life Sci. Biotech. Pharma Res. 2015;4:31–35. [Google Scholar]
- 48.Gaidhani S., Singh A., Kumari S., Lavekar G., Juvekar A., Sen S., Padhi M. Evaluation of some plant extracts for standardization and anticancer activity. Indian. J. Tradit. Know. 2013;12:682–687. [Google Scholar]
- 49.Jayachandran P.R., Suriya K., Subbaiya R., Ponmurugan P. Antioxidant and cytotoxic activity of Tecoma stans against lung cancer cell line (A549) Braz. J. Pharm. Sci. 2017;53:53. doi: 10.1590/s2175-97902017000300204. [DOI] [Google Scholar]
- 50.Zhu J., Viñas R., Smith E.E. In vitro evaluation of human liver cancer cells and the potential cytotoxicity of Tecoma stans (Bignoniaceae) and Brickellia cavanillesi (Asteraceae) both single and in combination. Toxicol. Environ. Chem. 2008;90:801–808. doi: 10.1080/02772240701740387. [DOI] [Google Scholar]
- 51.Marzouk M.S., Gamal-Eldeen A., Mohamed M., El-Sayed M. Anti-proliferative and antioxidant constituents from Tecoma stans. Zeitschrift für Naturforschung C. 2007;61:783–791. [PubMed] [Google Scholar]
- 52.Rahman A., Sahabjada, Akhtar J. Evaluation of anticancer activity of Cordia dichotoma leaves against a human prostate carcinoma cell line, PC3. J. Tradit. Complement. Med. 2016;7:315–321. doi: 10.1016/j.jtcme.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowe H.I., Toyang N.J., Watson C., Badal S., Bahado-Singh P., Bryant J. In vitro anticancer activity of the crude extract and two dicinnamate isolates from the Jamaican ball moss (Tillandsia Recurvata L.) Am. Int. J. Contemp. Res. 2013;3:93–96. [PMC free article] [PubMed] [Google Scholar]
- 54.Bukke A.N., Hadi F.N., Babu K.S., Shankar P.C. In vitro studies data on anticancer activity of Caesalpinia sappan L. heartwood and leaf extracts on MCF7 and A549 cell lines. Data Brief. 2018;19:868–877. doi: 10.1016/j.dib.2018.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Snafi A.E. The medical benefit of Gnaphalium luteoalbum —A review. IOSR J. Pharm. 2019;9:40–44. [Google Scholar]
- 56.Prasad S., Rosangkima G., Rongpi T. Role of glutathione and glutathione-related enzymes in the antitumor activity of Dillenia pentagyna in Dalton’s lymphoma-bearing mice. Int. J. Cancer Res. 2008;4:92–102. doi: 10.3923/ijcr.2008.92.102. [DOI] [Google Scholar]
- 57.Alex A.T., Nawagamuwa N.H., Joseph A., Rao J.V., Mathew J.A., Udupa N. In vitro anticancer and antioxidant activity of different fractions of Diospyros peregrina unripe fruit extract. Free. Radicals Antioxidants. 2012;2:45–49. doi: 10.5530/ax.2012.4.8. [DOI] [Google Scholar]
- 58.Munro B.R., Vuong Q., Chalmers A.C., Goldsmith C., Bowyer M.C., Scarlett C.J. Phytochemical, antioxidant and anti-cancer properties of Euphorbia tirucalli methanolic and aqueous extracts. Antioxidants. 2015;4:647–661. doi: 10.3390/antiox4040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balasubramanian K., Padma P.R. Anticancer activity of Zea mays leaf extracts on oxidative stress-induced Hep2 Cells. J. Acupunct. Meridian Stud. 2013;6:149–158. doi: 10.1016/j.jams.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.L., Snook M.E., Lee J.O. Radical scavenging activity and cytotoxicity of maysin (C-glycosylflavone) isolated from silks of Zea mays L. Korean J. Crop. Sci. 2003;48:392–396. [Google Scholar]
- 61.Muniyandi K., George E., Mudili V., Kalagatur N.K., Anthuvan A.J., Krishna K., Thangaraj P., Natarajan G. Antioxidant and anticancer activities of Plectranthus stocksii Hook. f. leaf and stem extracts. Agric. Nat. Resour. 2017;51:63–73. doi: 10.1016/j.anres.2016.07.007. [DOI] [Google Scholar]
- 62.Jawad A., Balayeshwanth R.V., Rami A., Waleed R., Hatem S., Nathan W.L., Alzeer J., Vummidi B.R., Arafeh R., Rimawi W., et al. The influence of extraction solvents on the anticancer activities of Palestinian medicinal plants. J. Med. Plants Res. 2014;8:408–415. doi: 10.5897/JMPR2013.5044. [DOI] [Google Scholar]
- 63.Karthikeyan K., Ravichandran P., Govindasamy S. Chemopreventive effect of Ocimum sanctum on DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 1999;35:112–119. doi: 10.1016/S1368-8375(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 64.Godhwani S., Godhwani J., Was D. Ocimum sanctum — A preliminary study evaluating its immunoregulatory profile in albino rats. J. Ethnopharmacol. 1988;24:193–198. doi: 10.1016/0378-8741(88)90151-1. [DOI] [PubMed] [Google Scholar]
- 65.Mediratta P.K., Dewan V., Bhattacharya S.K., Gupta V.S., Maiti P.C., Sen P. Effect of Ocimum sanctum Linn. on humoral immune responses. Indian J. Med. Res. 1988;87:384–386. [PubMed] [Google Scholar]
- 66.Mandal S., Chatterjee A. Seminar on research in Ayurveda and Siddha. CCRAS; New Delhi, India: 1994. pp. 58–59. [Google Scholar]
- 67.Alam P., Al-Yousef H.M., Siddiqui N.A., Alhowiriny T.A., Alqasoumi S.I., Amina M., Hassan W.H.B., Abdelaziz S., Abdalla R.H. Anticancer activity and concurrent analysis of ursolic acid, β-sitosterol and lupeol in three different Hibiscus species (aerial parts) by validated HPTLC method. Saudi Pharm. J. 2018;26:1060–1067. doi: 10.1016/j.jsps.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim D.K., Baek J.H., Kang C.M., Yoo M.A., Sung J.W., Kim D.K., Chung H.Y., Kim N.D., Choi Y.H., Lee S.H. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int. J. Cancer. 2000;87:629–636. doi: 10.1002/1097-0215(20000901)87:5<629::AID-IJC2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 69.Choi Y., Kong K., Kim Y.-A., Jung K.-O., Kil J.-H., Rhee S.-H., Park K.-Y. Induction of Bax and activation of caspases during β-sitosterol-mediated apoptosis in human colon cancer cells. Int. J. Oncol. 2003;23:1657–1662. doi: 10.3892/ijo.23.6.1657. [DOI] [PubMed] [Google Scholar]
- 70.Gallo M.B., Sarachine M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009;3:46–66. [Google Scholar]
- 71.Amer H., Helmy W.A., Taie H.A. In vitro antitumor and antiviral activities of seeds and leaves Neem (Azadirachta indica) extracts. Int. J. Acad. Res. 2010;2:47–51. [Google Scholar]
- 72.Jafari S., Saeidnia S., Hajimehdipoor H., Reza S.A.M., Faramarzi M.A., Hadjiakhoondi A., Khanavi M. Cytotoxic evaluation of Melia azedarach in comparison with, Azadirachta indica and its phytochemical investigation. DARU J. Pharm. Sci. 2013;21:37. doi: 10.1186/2008-2231-21-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takagi M., Tachi Y., Zhang J., Shinozaki T., Ishii K., Kikuchi T., Ukiya M., Banno N., Tokuda H., Akihisa T. Cytotoxic and melanogenesis-inhibitory activities of limonoids from the leaves of Azadirachta indica (Neem) Chem. Biodivers. 2014;11:451–468. doi: 10.1002/cbdv.201300348. [DOI] [PubMed] [Google Scholar]
- 74.Akihisa T. ChemInform abstract: Limonoids from Azadirachta indica var. siamensis extracts and their cytotoxic and melanogenesis-inhibitory activities. ChemInform. 2014;45:505–531. doi: 10.1002/chin.201438216. [DOI] [PubMed] [Google Scholar]
- 75.Kikuchi T., Ishii K., Noto T., Takahashi A., Tabata K., Suzuki T., Akihisa T. Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (Neem) J. Nat. Prod. 2011;74:866–870. doi: 10.1021/np100783k. [DOI] [PubMed] [Google Scholar]
- 76.Kitdamrongtham W., Ishii K., Ebina K., Zhang J., Ukiya M., Koike K., Akazawa H., Manosroi A., Manosroi J., Akihisa T. ChemInform abstract: Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. ChemInform. 2014;45:73–84. doi: 10.1002/chin.201420214. [DOI] [PubMed] [Google Scholar]
- 77.Chen J., Chen J., Sun Y., Yan Y., Kong L.-M., Li Y., Qiu M. Cytotoxic triterpenoids from Azadirachta indica. Planta Medica. 2011;77:1844–1847. doi: 10.1055/s-0030-1271197. [DOI] [PubMed] [Google Scholar]
- 78.Thakkar K., Prasad A., Nayak J., Iyer S., Kumar S. Antioxidant and in vitro cytotoxic activity of extracts of aerial parts of Cocculus hirsutus (L) using cell line cultures (breast cell line) J. Phytopharmacol. 2014;3:395–399. [Google Scholar]
- 79.Thavamani B.S., Mathew M., Dhanabal S.P. Cocculus hirsutus: Molecular docking to identify suitable targets for hepatocellular carcinoma by in silico technique. Pharmacogn. Mag. 2016;12:S350–S352. doi: 10.4103/0973-1296.185769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel R.M., Patel S.K. Cytotoxic activity of methanolic extract of Artocarpus heterophyllus against A549, Hela and MCF-7 cell lines. J. Appl. Pharm. Sci. 2011;1:167–171. [Google Scholar]
- 81.Arung E.T., Yoshikawa K., Nakagawa T., Kondo R. Isoprenoid-substituted flavonoids from wood of Artocarpus heterophyllus on B16 melanoma cells: Cytotoxicity and structural criteria. Fitoterapia. 2010;81:120–123. doi: 10.1016/j.fitote.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Arung E.T., Wicaksono B.D., Handoko Y.A., Kusuma I.W., Nakagawa T., Yulia D., Sandra F. Cytotoxic effect of artocarpin on T47D cells. J. Nat. Med. 2010;64:423–429. doi: 10.1007/s11418-010-0425-6. [DOI] [PubMed] [Google Scholar]
- 83.Arung E.T., Nakagawa T., Kondo R. Structure–activity relationship of prenyl-substituted polyphenols from Artocarpus heterophyllus as Inhibitors of melanin biosynthesis in cultured melanoma cells. Chem. Biodivers. 2007;4:2166–2171. doi: 10.1002/cbdv.200790173. [DOI] [PubMed] [Google Scholar]
- 84.Yen G., Chen C.-S., Chang W.-T., Wu M.-F., Cheng F.-T., Shiau D.-K., Hsu C.-L. Antioxidant activity and anticancer effect of ethanolic and aqueous extracts of the roots of Ficus beecheyana and their phenolic components. J. Food Drug Anal. 2018;26:182–192. doi: 10.1016/j.jfda.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khan A., Anand V., Badrinarayanan V., Thirunethiran K., Natarajan P. In vitro antioxidant and cytotoxicity analysis of leaves of Ficus racemosa. Free. Radic. Antioxid. 2016;7:8–12. doi: 10.5530/fra.2017.1.2. [DOI] [Google Scholar]
- 86.Sukhramani P.S., Vidyasagar G., Patel P.M. In vitro screening of Ficus racemosa for anticancer activity. Res. J. Pharmacog. Phytochem. 2013;5:119–122. [Google Scholar]
- 87.Gavhane D.S., Moregaonkar S.D., Mhase A.K. Cytotoxic and anticancer activity of Ficus racemosa fruit extract on MCF7 human breast cancer cell line by SRB method. J. Anim. Res. 2016;6:43–47. doi: 10.5958/2277-940X.2016.00008.5. [DOI] [Google Scholar]
- 88.Qadir M.I., Ali M., Ibrahim Z. Anticancer activity of Morus nigra leaves extract. Bangladesh J. Pharmacol. 2014;9:496–497. doi: 10.3329/bjp.v9i4.19783. [DOI] [Google Scholar]
- 89.Souza G.R., Oliveira-Júnior R.G., Diniz T.C., Branco A., Lima-Saraiva S.R.G., Guimarães A.L., Oliveira A.P., Pacheco A.G.M., Silva M.G., Moraes-Filho M.O., et al. Assessment of the antibacterial, cytotoxic and antioxidant activities of Morus nigra L. (Moraceae) Braz. J. Boil. 2017;78:248–254. doi: 10.1590/1519-6984.05316. [DOI] [PubMed] [Google Scholar]
- 90.Piaru S.P., Mahmud R., Majid A.M.S.A., Ismail S., Nin Man C. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012;92:593–597. doi: 10.1002/jsfa.4613. [DOI] [PubMed] [Google Scholar]
- 91.Duan L., Tao H.-W., Hao X.-J., Gu Q.-Q., Zhu W. Cytotoxic and antioxidative phenolic compounds from the traditional chinese medicinal plant, Myristica fragrans. Planta Medica. 2009;75:1241–1245. doi: 10.1055/s-0029-1185506. [DOI] [PubMed] [Google Scholar]
- 92.Yadav S.S., Meshram G., Shinde D., Patil R., Manohar S.M., Upadhye M.V. Antibacterial and anticancer activity of bioactive fraction of Syzygium cumini L. seeds. HAYATI J. Biosci. 2011;18:118–122. doi: 10.4308/hjb.18.3.118. [DOI] [Google Scholar]
- 93.Nazif N.M. The anthocyanin components and cytotoxic activity of Syzygium cumini (L.) fruits growing in Egypt. Nat. Prod. Sci. 2007;13:135–139. [Google Scholar]
- 94.Banerjee J., Narendhirakannan R. Phytochemical analyses, antibacterial, in vitro antioxidant and cytotoxic activities of ethanolic extract of Syzygium cumini (L.) seed extract. Int. J. Pharm. Sci. Res. 2011;2:1799–1806. [Google Scholar]
- 95.Kale D.K.C., Mukundan U. Cytotoxicity against tumor cell lines of a purified mirabilis antiviral protein isolated from root of Mirabilis jalapa. World J. Pharm. Res. 2015;4:1696–1710. [Google Scholar]
- 96.Xu J.-J., Qing C., Lv Y.-P., Liu Y.-M., Liu Y., Chen Y.-G. Cytotoxic rotenoids from Mirabilis jalapa. Chem. Nat. Compd. 2010;46:792–794. doi: 10.1007/s10600-010-9744-9. [DOI] [Google Scholar]
- 97.Fares R., Bazzi S., Baydoun S.E., Abdel-Massih R.M. The antioxidant and anti-proliferative activity of the Lebanese Olea europaea extract. Plant. Foods Hum. Nutr. 2011;66:58–63. doi: 10.1007/s11130-011-0213-9. [DOI] [PubMed] [Google Scholar]
- 98.Nair M.S., Soren K., Singh V., Boro B. Anticancer activity of fruit and leaf extracts of Averrhoa bilimbi on MCF-7 human breast cancer cell lines: A preliminary study. Austin J. Pharmacol. Ther. 2016;4:1082. [Google Scholar]
- 99.Ahmed Q.U., Alhassan A. Averrhoa bilimbi Linn.: A review of its ethnomedicinal uses, phytochemistry, and pharmacology. J. Pharm. Bioallied Sci. 2016;8:265–271. doi: 10.4103/0975-7406.199342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sumalatha D. Antioxidant and antitumor activity of Phyllanthus emblica in colon cancer cell lines. Int. J. Curr. Microbiol. Appl. Sci. 2013;2:189–195. [Google Scholar]
- 101.Qi W., Li Y., Hua L., Wang K., Gao K. Cytotoxicity and structure activity relationships of phytosterol from Phyllanthus emblica. Fitoterapia. 2013;84:252–256. doi: 10.1016/j.fitote.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Desouky S., Ryu S.Y., Kim Y.-K. A new cytotoxic acylated apigenin glucoside from Phyllanthus emblica L. Nat. Prod. Res. 2008;22:91–95. doi: 10.1080/14786410701590236. [DOI] [PubMed] [Google Scholar]
- 103.Medini F., Bourgou S., Lalancette K., Snoussi M., Mkadmini K., Coté I., Abdelly C., Legault J., Ksouri R. Phytochemical analysis, antioxidant, anti-inflammatory, and anticancer activities of the halophyte Limonium densiflorum extracts on human cell lines and murine macrophages. S. Afr. J. Bot. 2015;99:158–164. doi: 10.1016/j.sajb.2015.04.007. [DOI] [Google Scholar]
- 104.Rajkumar V., Guha G., Kumar R.A. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem. Toxicol. 2011;49:363–369. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 105.Masood M. Picrorhiza kurroa: An ethnopharmacologically important plant species of Himalayan region. Pure Appl. Boil. 2015;4:407–417. doi: 10.19045/bspab.2015.43017. [DOI] [Google Scholar]
- 106.Shashi B., Jaswant S., Madhusudana R.J., Kumar S.A., Nabi Q.G. A novel lignan composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide. 2006;14:72–88. doi: 10.1016/j.niox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 107.Sunila E., Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J. Ethnopharmacol. 2004;90:339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 108.Longato G., Rizzo L., Sousa I.D.O., Tinti S., Possenti A., Figueira G., Ruiz A.L.T.G., Foglio M., De Carvalho J. In vitro and in vivo anticancer activity of extracts, fractions, and eupomatenoid-5 obtained from Piper regnellii leaves. Planta Med. 2011;77:1482–1488. doi: 10.1055/s-0030-1270889. [DOI] [PubMed] [Google Scholar]
- 109.Alothman E.A., Awaad A.S., Al-Qurayn N.A., Al-Kanhal H.F., El-Meligy R.M., Zain Y.M., Alasmary F.A., Alqasoumi S.I. Anticancer effect of Cenchrus ciliaris L. Saudi Pharm. J. 2018;26:952–955. doi: 10.1016/j.jsps.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Awaad A.S., Al Qurain N.A., Alkanhal H.F., El-Meligy R.M., Al-Asamary F.A. Cenchrus ciliaris L. as an Anticancer Agent. application no. 15/882,929. U.S. Patent. 2019
- 111.Badria F.A., Ameen M., Akl M.R. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Zeitschrift für Naturforschung C. 2007;62:656–660. doi: 10.1515/znc-2007-9-1005. [DOI] [PubMed] [Google Scholar]
- 112.Vinh L.B., Nguyet N.T.M., Yang S.Y., Kim J.H., Van Thanh N., Cuong N.X., Nam N.H., Van Minh C., Hwang I., Kim Y.H. Cytotoxic triterpene saponins from the mangrove Aegiceras corniculatum. Nat. Prod. Res. 2017;33:628–634. doi: 10.1080/14786419.2017.1402320. [DOI] [PubMed] [Google Scholar]
- 113.Ding L., Dahse H.-M., Hertweck C. Cytotoxic alkaloids from Fusarium incarnatum associated with the mangrove tree Aegiceras corniculatum. J. Nat. Prod. 2012;75:617–621. doi: 10.1021/np2008544. [DOI] [PubMed] [Google Scholar]
- 114.Ramírez-Macías I., Marín C., Díaz J.G., Rosales M.J., Gutierrez-Sanchez R., Sánchez-Moreno M. Leishmanicidal activity of nine novel flavonoids from Delphinium staphisagria. Sci. World J. 2012;2012:203646. doi: 10.1100/2012/203646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bura F.T., Firuzja R.A., Nemati F. Cytotoxic effect of the flower and leaf bud extract of Crataegus microphylla C. Koch on HeLa cell line. IIOAB J. 2016;7:214–218. [Google Scholar]
- 116.Abdel-Hameed E.-S.S., Bazaid S.A., Hagag H.A. Chemical characterization of Rosa damascena Miller var. trigintipetala Dieck essential oil and its in vitro genotoxic and cytotoxic properties. J. Essent. Oil Res. 2016;28:121–129. doi: 10.1080/10412905.2015.1099120. [DOI] [Google Scholar]
- 117.Khairunnisa K., Karthik D. Evaluation of in vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Dalton’s lymphoma ascites (DLA) and Lung fibroblast-Mouse L929 cell lines. J. Appl. Pharm. Sci. 2014;4:11–17. [Google Scholar]
- 118.Pandey K., Sharma P., Dudhe R. Anticancer activity of Parthenium hysterophorus Linn. and Oldenlandia corymbosa Lam. by SRB Method. Open Acces Sci. Rep. 2012;1 [Google Scholar]
- 119.Gezici S., Sekeroglu N., Kijjoa A. In vitro anticancer activity and antioxidant properties of essential oils from Populus alba L. and Rosmarinus officinalis L. from South Eastern Anatolia of Turkey. Indian J. Pharm. Educ. Res. 2017;51:498–503. doi: 10.5530/ijper.51.3s.74. [DOI] [Google Scholar]
- 120.Awasare S., Bhujbal S., Nanda R. In vitro cytotoxic activity of novel oleanane type of triterpenoid saponin from stem bark of Manilkara zapota Linn. Asian J. Pharm. Clin. Res. 2012;5:183–188. [Google Scholar]
- 121.Alaklabi A., Arif I.A., Ahamed A., Kumar R.S., Idhayadhulla A. Evaluation of antioxidant and anticancer activities of chemical constituents of the Saururus chinensis root extracts. Saudi J. Boil. Sci. 2017;25:1387–1392. doi: 10.1016/j.sjbs.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee Y.-K., Seo C.-S., Lee C.-S., Lee K.-S., Kang S.-J., Jahng Y., Chang H.W., Son J.K. Inhibition of DNA topoisomerases I and II and cytotoxicity by lignans from Saururus chinensis. Arch. Pharmacal Res. 2009;32:1409–1415. doi: 10.1007/s12272-009-2010-7. [DOI] [PubMed] [Google Scholar]
- 123.Afifi M.S., Ahmed M.M., Pezzuto J.M., Kinghornt A.D. Cytotoxic flavonolignans and flavones from Verbascum sinaiticum leaves. Phytochemistry. 1993;34:839–841. doi: 10.1016/0031-9422(93)85369-3. [DOI] [Google Scholar]
- 124.Shokrzadeh M., Azadbakht M., Ahangar N., Hashemi A., Saravi S.S.S. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn. Mag. 2010;6:176–179. doi: 10.4103/0973-1296.66931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel S., Gheewala N., Suthar A., Shah A. In vitro cytotoxicity activity of Solanum nigrum extract against HeLa cell line and Vero cell line. Int. J. Pharm. Pharm. Sci. 2009;1:38–46. [Google Scholar]
- 126.Maqsood M., Qureshi R., Ikram M., Ahmad M.S., Jabeen B., Asi M.R., Khan J.A., Ali S., Lilge L. In vitro anticancer activities of Withania coagulans against HeLa, MCF-7, RD, RG2, and INS-1 cancer cells and phytochemical analysis. Integr. Med. Res. 2018;7:184–191. doi: 10.1016/j.imr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shaikh R., Pund M., Dawane A., Iliyas S. Evaluation of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J. Tradit. Complement. Med. 2014;4:253–257. doi: 10.4103/2225-4110.128904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bean M.F., Antoun M., Abramson D., Chang C.-J., McLaughlin J.L., Cassady J.M. Cucurbitacin B and Isocucurbitacin B: Cytotoxic components of Helicteres isora. J. Nat. Prod. 1985;48:500. doi: 10.1021/np50039a033. [DOI] [PubMed] [Google Scholar]
- 129.Hegde K., Fathima Jazeela M., Vijetha Poojary K., Satish S. Anticancer potentials of the plant Aquilaria malaccensis leaves. Indian J. Pharm. Pharmacol. 2018;5:135–140. doi: 10.18231/2393-9087.2018.0029. [DOI] [Google Scholar]
- 130.Ibrahim A., Al-Rawi S., Majid A.A., Rahman N.A., Salah K.A.-, Ab Kadir M. Separation and fractionation of Aquilaria Malaccensis oil using supercritical fluid extraction and tthe cytotoxic properties of the extracted oil. Procedia Food Sci. 2011;1:1953–1959. doi: 10.1016/j.profoo.2011.09.287. [DOI] [Google Scholar]
- 131.Adam A., Tajuddin S., Sudmoon R., Chaveerach A., Abdullah U., Mahat M., Mohamed R. Chemical constituents and toxicity effects of leaves from several agarwood tree species (Aquilaria) J. Trop. For. Sci. 2018;30:342–353. doi: 10.26525/jtfs2018.30.3.342353. [DOI] [Google Scholar]
- 132.Chacko T., Menon A., Nair S.V., AlSuhaibani E., Nair C.K.K. Cytotoxic and antitumor activity of the extract of Clerodendron infortunatum: A mechanistic study. Am. J. Phytomed. Clin. Therapeut. 2015;2:145–158. [Google Scholar]
- 133.Ghagane S.C., Puranik S.I., Kumbar V.M., Nerli R.B., Jalalpure S.S., Hiremath M.B., Neelagund S., Aladakatti R. In vitro antioxidant and anticancer activity of Leea indica leaf extracts on human prostate cancer cell lines. Integr. Med. Res. 2017;6:79–87. doi: 10.1016/j.imr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Handoussa H., Hanafi R., Eddiasty I., El-Gendy M., El Khatib A., Linscheid M.W., Mahran L., Ayoub N. Anti-inflammatory and cytotoxic activities of dietary phenolics isolated from Corchorus olitorius and Vitis vinifera. J. Funct. Foods. 2013;5:1204–1216. doi: 10.1016/j.jff.2013.04.003. [DOI] [Google Scholar]
- 135.Nirmala J.G., Akila S., Narendhirakannan R., Chatterjee S. Vitis vinifera peel polyphenols stabilized gold nanoparticles induce cytotoxicity and apoptotic cell death in A431 skin cancer cell lines. Adv. Powder Technol. 2017;28:1170–1184. doi: 10.1016/j.apt.2017.02.003. [DOI] [Google Scholar]
- 136.Srivastava P., Srivastava A. In vitro anticancer activity of ethanolic extract of Curcumin longa (Turmeric) in HEp-2 cell lines. Int. J. Eng. Res. General Sci. 2015;3:495–508. [Google Scholar]
- 137.Ramsewak R., DeWitt D., Nair M. Cytotoxicity, antioxidant and anti-inflammatory activities of Curcumins I–III from Curcuma longa. Phytomedicine. 2000;7:303–308. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 138.Kuttan R., Bhanumathy P., Nirmala K., George M. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 139.Tyagi A.K., Prasad S., Yuan W., Li S., Aggarwal B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs. 2015;33:1175–1186. doi: 10.1007/s10637-015-0296-5. [DOI] [PubMed] [Google Scholar]
- 140.Atsumi T., Murakami Y., Shibuya K., Tonosaki K., Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, α-diisoeugenol. Anticancer Res. 2005;25:4029–4036. [PubMed] [Google Scholar]
- 141.Kim J.S., Lee S.I., Park H.W., Yang J.H., Shin T.-Y., Kim Y.-C., Baek N.-I., Kim S.-H., Choi S.U., Kwon B.-M., et al. Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch. Pharmacal Res. 2008;31:415–418. doi: 10.1007/s12272-001-1172-y. [DOI] [PubMed] [Google Scholar]
- 142.Jeena K., Liju V.B., Kuttan R. Antitumor and cytotoxic activity of ginger essential oil (Zingiber officinale Roscoe) Int. J. Pharm. Pharm. Sci. 2015;7:341–344. [Google Scholar]
- 143.Kurapati K.R.V., Samikkannu T., Kadiyala D.B., Zainulabedin S.M., Gandhi N., Sathaye S.S., Indap M.A., Boukli N., Rodriguez J.W., Nair M. Combinatorial cytotoxic effects of Curcuma longa and Zingiber officinale on the PC-3M prostate cancer cell line. J. Basic Clin. Physiol. Pharmacol. 2012;23:139–146. doi: 10.1515/jbcpp-2012-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Monga M., Sausville E. Developmental Therapeutics Program at the NCI: Molecular target and drug discovery process. Leukeumia. 2002;16:520–526. doi: 10.1038/sj.leu.2402464. [DOI] [PubMed] [Google Scholar]
- 145.Jaman S., Sayeed A. Ellagic acid, sulforaphane, and ursolic acid in the prevention and therapy of breast cancer: Current evidence and future perspectives. Breast Cancer. 2018;25:517–528. doi: 10.1007/s12282-018-0866-4. [DOI] [PubMed] [Google Scholar]
- 146.Manayi A., Nikan M., Nobakht-Haghighi N., Abdollahi M., Haghighid N.N. Advances in the anticancer value of the ursolic acid through nanodelivery. Curr. Med. Chem. 2019;25:4866–4875. doi: 10.2174/0929867324666170713102918. [DOI] [PubMed] [Google Scholar]
- 147.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Boil. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 148.Jia Y., Tong X., Fan J. Effect of brazilin on apoptosis and autophagy of tongue cancer Tca8113 cells and its molecular mechanism. J. South. Med. Univ. 2019;39:351–356. doi: 10.12122/j.issn.1673-4254.2019.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang T., He J., Zhang S., Wang G., Ma E., Wang J., Yang X., Zheng Y.-W., Zhang J. Brazilin induces T24 cell death through c-Fos and GADD45β independently regulated genes and pathways. IUBMB Life. 2018;70:1101–1110. doi: 10.1002/iub.1921. [DOI] [PubMed] [Google Scholar]
- 150.Bin Sayeed M.S., Ameen S.S. Beta-Sitosterol: A promising but orphan nutraceutical to fight against cancer. Nutr. Cancer. 2015;67:1216–1222. doi: 10.1080/01635581.2015.1087042. [DOI] [PubMed] [Google Scholar]
- 151.Tsai F.-S., Lin L.-W., Wu C.-R. Drug Discovery from Mother Nature. Volume 929. Springer Science and Business Media; Basel, Switzerland: 2016. Lupeol and its role in chronic diseases; pp. 145–175. [DOI] [PubMed] [Google Scholar]
- 152.Devassy J.G., Nwachukwu I., Jones P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015;73:155–165. doi: 10.1093/nutrit/nuu064. [DOI] [PubMed] [Google Scholar]
- 153.Yang C.S., Wang H. Cancer preventive activities of tea catechins. Molecules. 2016;21:1679. doi: 10.3390/molecules21121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jiang M., Zhu M., Wang L., Yu S. Antitumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019;120:109506. doi: 10.1016/j.biopha.2019.109506. [DOI] [PubMed] [Google Scholar]
- 155.Devi K.P., Rajavel T., Habtemariam S., Nabavi S.F., Nabavi S.M. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015;142:19–25. doi: 10.1016/j.lfs.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 156.Rauf A., Imran M., Khan I.A., Ur-Rehman M.-, Gilani S.A., Mehmood Z., Mubarak M.S. Anticancer potential of quercetin: A comprehensive review. Phytotherapy Res. 2018;32:2109–2130. doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 157.Reyes-Farias M., Carrasco-Pozo C. The Anticancer effect of Quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019;20:3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rupani R., Chavez A. Medicinal plants with traditional use: Ethnobotany in the Indian subcontinent. Clin. Dermatol. 2018;36:306–309. doi: 10.1016/j.clindermatol.2018.03.005. [DOI] [PubMed] [Google Scholar]