This nonrandomized clinical trial investigates the association of combining the preoperative and postoperative Surgical Patient Safety System with the World Health Organization surgical safety checklist in perioperative care with morbidity, mortality, and length of hospital stay among patients in 3 surgical departments.
Key Points
Question
Does patient safety improve when adding the preoperative and postoperative Surgical Patient Safety System checklists to the World Health Organization’s established surgical safety checklist?
Findings
In this stepped-wedge cluster nonrandomized clinical trial with parallel controls that included 9009 surgical procedures, reductions in complications and emergency reoperations occurred when the preoperative Surgical Patient Safety System was added to the surgical safety checklist. The postoperative Surgical Patient Safety System reduced readmissions, whereas overall increased complications were found in the 9678 parallel controls.
Meaning
These findings suggest that joint use of the preoperative and postoperative Surgical Patient Safety System with the intraoperative surgical safety checklist is beneficial for patients.
Abstract
Importance
Checklists have been shown to improve patient outcomes in surgery. The intraoperatively used World Health Organization surgical safety checklist (WHO SSC) is now mandatory in many countries. The only evidenced checklist to address preoperative and postoperative care is the Surgical Patient Safety System (SURPASS), which has been found to be effective in improving patient outcomes. To date, the WHO SSC and SURPASS have not been studied jointly within the perioperative pathway.
Objective
To investigate the association of combined use of the preoperative and postoperative SURPASS and the WHO SSC in perioperative care with morbidity, mortality, and length of hospital stay.
Design, Setting, and Participants
In a stepped-wedge cluster nonrandomized clinical trial, the preoperative and postoperative SURPASS checklists were implemented in 3 surgical departments (neurosurgery, orthopedics, and gynecology) in a Norwegian tertiary hospital, serving as their own controls. Three surgical units offered additional parallel controls. Data were collected from November 1, 2012, to March 31, 2015, including surgical procedures without any restrictions to patient age. Data were analyzed from September 25, 2018, to March 29, 2019.
Interventions
Individualized preoperative and postoperative SURPASS checklists were added to the intraoperative WHO SSC.
Main Outcomes and Measures
Primary outcomes were in-hospital complications, emergency reoperations, unplanned 30-day readmissions, and 30-day mortality. The secondary outcome was length of hospital stay (LOS).
Results
In total, 9009 procedures (5601 women [62.2%]; mean [SD] patient age, 51.7 [22.2] years) were included, with 5117 intervention procedures (mean [SD] patient age, 51.8 [22.4] years; 2913 women [56.9%]) compared with 3892 controls (mean [SD] patient age, 51.5 [21.8] years; 2688 women [69.1%]). Parallel control units included 9678 procedures (mean [SD] patient age, 57.4 [22.2] years; 4124 women [42.6%]). In addition to the WHO SSC, adjusted analyses showed that adherence to the preoperative SURPASS checklists was associated with reduced complications (odds ratio [OR], 0.70; 95% CI, 0.50-0.98; P = .04) and reoperations (OR, 0.42; 95% CI, 0.23-0.76; P = .004). Adherence to the postoperative SURPASS checklists was associated with decreased readmissions (OR, 0.32; 95% CI, 0.16-0.64; P = .001). No changes were observed in mortality or LOS. In parallel control units, complications increased (OR, 1.09; 95% CI, 1.01-1.17; P = .04), whereas reoperations, readmissions, and mortality remained unchanged.
Conclusions and Relevance
In this nonrandomized clinical trial, adding preoperative and postoperative SURPASS to the WHO SSC was associated with a reduction in the rate of complications, reoperations, and readmissions.
Trial Registration
ClinicalTrials.gov Identifier: NCT01872195
Introduction
The World Health Organization surgical safety checklist (WHO SSC), now used globally, has been found to reduce complications and mortality,1,2,3,4,5,6 although negative findings have also been published.7,8 Questions have been raised regarding whether negative results are due to a lack of emphasis on the implementation and local tailoring.8,9,10 The WHO SSC is used within the operating room, aiming to improve teamwork, with shared awareness of the safety aspects of surgery.11
However, surgical complications often originate before and after operating room activities.12,13,14 The comprehensive Surgical Patient Safety System (SURPASS) developed in the Netherlands is the only surgical safety checklist to date to include specific preoperative and postoperative checklists for individual clinicians in addition to team checks in the operating room. Like the WHO SSC, SURPASS has also been found to reduce complications and mortality.15 The effect of implementing SURPASS has been replicated only in a smaller study from India,16 which found that use of SURPASS alone reduced the rate of postoperative complications in both elective and emergency operations.
To date, whether surgical safety can improve further when combining the intraoperative WHO SSC with the preoperative and postoperative SURPASS remains unknown. This study aimed to evaluate the associations of adding the preoperative and postoperative SURPASS to the intraoperative WHO SSC with surgical complications, all-cause 30-day mortality, and subsequent length of hospital stay (LOS).
Methods
Study Design, Setting, and Oversight
The trial protocol is given in Supplement 1. A prospective, stepped-wedge cluster nonrandomized clinical trial design17 was used. The study implemented the preoperative and postoperative SURPASS checklists in 3 surgical departments in a tertiary hospital in western Norway (Figure 1) in addition to the WHO SSC. The study was approved by the regional ethical research committee, the data privacy units at the Health Trust Førde, and Health Trust Fonna of Norway. After approval, the patients in the intervention departments received written information on the study and their option to refrain from data sharing. The study was exempt from written informed consent. This study followed the extension of the 2010 Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.18
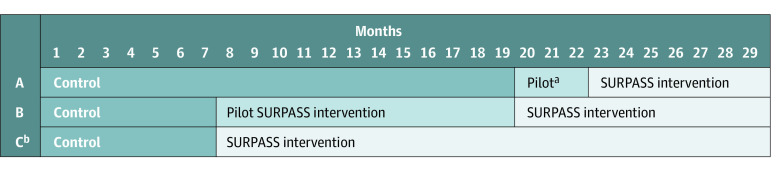
Figure 1. Stepped-Wedge Cluster Nonrandomized Clinical Trial Design.
Implementation of the individualized preoperative and postoperative Surgical Patient Safety System (SURPASS) checklists in 3 surgical clusters in a tertiary hospital in Western Norway, November 2012 to March 2015 (29 months). A indicates gynecology; B, orthopedics; and C, neurosurgery.
aIndicates pilot SURPASS intervention.
bIndicates 3-week pilot during June and July 2012.
At the time of the study (and to date), the WHO SSC is mandatory in Norwegian operating rooms. The study design allowed introduction of the SURPASS to each department sequentially19 as opposed to a classic before-after design, wherein the switch from before/control to after/intervention is introduced for all the trial departments at the same time. The original SURPASS15 and WHO SSC20 intervention trials are classic before-after studies. Our current design allows adjustment for time trends and is advantageous in health care settings with limited resources, involving continuous advancements and change.19
Following advice from the WHO checklist implementation guideline, the trial departments were invited to participate based on their management commitment, frontline positive engagement, and high adherence to the WHO SSC.21 The SURPASS intervention followed a stepwise introduction in 1 department at a time. The departments each contributed patient data before and after the study intervention and served as their own controls, thus minimizing selection bias. Contamination between study departments and the parallel control departments—caused by information bias due to personnel working in several disciplines, sections, or departments—was avoided: The operating rooms and surgical teams were geographically separate with their own organizational units and specialized personnel (neurosurgery, orthopedic surgery, and gynecology and the parallel control departments of thoracic surgery, general surgery, vascular surgery, gastroenterology, urology, orthopedics, and ear, nose, and throat surgery). Because single surgical procedures were subjects of investigation, it was unlikely that any participant could have been in both control and intervention groups, hence within-department contamination was avoided. Three separate surgical units in different hospitals (a tertiary hospital serving a population of 1.1 million, a community hospital serving a population of 110 000, and a community hospital serving a population of 180 000) constituted additional parallel controls, with the WHO SSC as standard care but without SURPASS.
Intervention
The original SURPASS system was developed to include known risk factors described in the literature, validated against actual registered adverse events.14 The preoperative and postoperative SURPASS checklists are individualized to be performed by key clinicians in the surgical pathway. Each checklist is to be used as a last point of check before transfer to the next segment of the pathway, ensuring good planning and compliance with existing perioperative care protocols at all transfer points. Figure 2 displays the combined SURPASS and WHO SSC checklists across the surgical pathway as implemented in this study.
Figure 2. Surgical Checklist Flow.
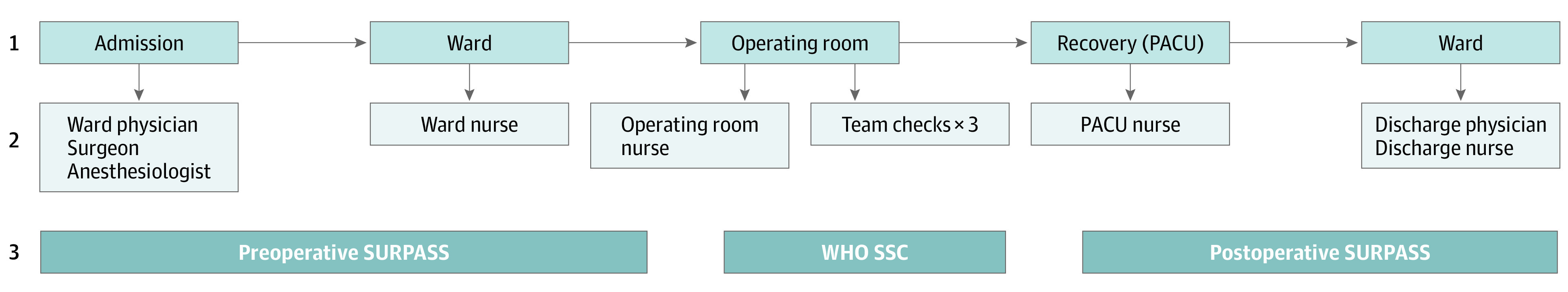
1 indicates surgical pathway; 2, checkpoints for clinicians; and 3, Surgical Patient Safety System (SURPASS) or World Health Organization surgical safety checklist (WHO SSC) applications. PACU indicates postanesthesia care unit.
Before the trial, validation of the SURPASS checklist content into a Norwegian context was performed in a neurosurgical setting.22 Before checklist implementation in a new department, tailoring for specific conditions in the different Norwegian departments was performed in accordance with advice in the WHO implementation manual.21 Implementation of SURPASS was informed by our team’s extensive experience with implementing the WHO SSC in Norway23 and also by recently compiled implementation strategies for health care compendium developed by implementation scientists.24 In brief, the implementation strategy included educational sessions with frontline clinicians emphasizing why the checklists should be used, their evidence, and the practicalities of how to apply them.25,26 Individual coaching was offered by the research team. Moreover, an information campaign in the trial departments was performed through distribution of printed posters and emails to staff. Service managers and key clinicians in the different departments were designated champions of the SURPASS intervention. Last, audit and feedback on SURPASS implementation fidelity (ie, quality of application) was provided through regular compliance reports sent directly to all service managers by the research team.
Outcome Measures
Primary outcomes were in-hospital morbidity (complications, emergency reoperations, and 30-day readmissions) and all-cause 30-day postoperative mortality. The secondary outcome measure was LOS.
Inclusion and Exclusion Criteria
The study included in-hospital patients of all ages undergoing an elective or an emergency surgical procedure. Excluded were radiological interventions, donor surgery, extracorporeal membrane oxygenation procedures, outpatients, and patients who declined to consent to the study.
Data Collection and Handling
Complications were investigated according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) as routinely recorded by physicians. The method for validating the reported in-hospital complications has been described previously.27 For the present study, all 155 ICD-10 complication codes included in the analyses were verified against each patient’s medical records by the research team.
Data on mortality, LOS, patient characteristics, and surgical procedures were collected from the hospitals’ electronic administrative systems and verified against each patient’s medical record. Checklist data were combined with outcome data after this verification procedure. Patient characteristics included age, sex, American Society of Anesthesiologists (ASA) physical health classification, urgency of surgery, type of surgery, type of anesthesia, and time (month and year) of operation. Checklist adherence (ie, fidelity of application) was recorded per SURPASS checklist item and as the proportion of individual checklists with all items checked. Thus, for the preoperative SURPASS, the proportions were 0, 0.20 for 1 checklist, 0.40 for 2, 0.60 for 3, 0.80 for 4, and 1.00 for 5 (because these have 5 parts in all). For the postoperative SURPASS and WHO SSC, the proportions were 0, 0.33 for 1 checklist, 0.66 for 2, and 1.00 for 3 (because these have 3 parts in all). All data were collected as part of daily routine patient documentation, with staff blinded to outcome measures. All data handlers were blinded to checklists used in the care of individual patients.
Statistical Analysis
Data were analyzed from September 25, 2018, to March 29, 2019. Characteristics of the preoperative and postintervention procedures and patient data were compared using the Pearson exact test with Bonferroni corrections for categorical variables and Gosset t tests for continuous variables. Intention-to-treat analyses were performed to evaluate changes in complication rates with comparison before and after the intervention regardless of SURPASS compliance. Multiple logistic regression analysis was used to evaluate associations of SURPASS with patient outcomes and including actual adherence to the checklists. Multiple Cox proportional hazards regression was used to analyze LOS. The individual checklists included in the analyses had all items checked. Preintervention and postintervention stages were analyzed while adjusting for time associations and other possible confounders in the logistic regression model,18,28 including age, sex, urgency of operation, ASA classification, anesthesia given, surgical specialty, point of time for inclusion in the study, and WHO SSC and SURPASS checklist adherence. Intention-to-treat analyses were adjusted for the same variables, except SURPASS adherence (as a proportion, as described above). With an expected mortality rate decrease from 0.015 to 0.008, a sample size of 3641 patients undergoing surgery in both preintervention and postintervention groups was required to achieve a power of 80% with an α value set to .05 (2 tailed). Intracluster correlation was not considered to affect the study power owing to heterogeneity within and between departments. The results for complications and mortality are reported as odds ratios (ORs) with 95% CIs and for LOS as hazard ratios (HRs). Two-sided P ≤ .05 was set as statistically significant. Power calculations were performed with SPPS Sample Power 2. Statistical analyses were performed using SPSS, version 24 (IBM Corporation).
Results
The study included 3892 procedures at baseline and 5117 procedures in the intervention periods during the 29 months, from November 1, 2012, to March 31, 2015 (Figure 1). A total of 7772 unique patients underwent 9009 procedures (mean [SD] patient age, 51.7 [22.2] years) in 8515 admissions within the study. Characteristics of patients and surgical procedures are reported in Table 1. The inclusion of gynecology as one of the study departments contributed to an overall higher proportion of women (5601 women [62.2%] and 3408 men [37.8%]; P < .001). A total of 5117 intervention procedures (mean [SD] patient age, 51.8 [22.4] years; 2913 women [56.9%] and 2204 men [43.1%]) and 3892 controls (mean [SD] patient age, 51.5 [21.8] years; 2688 women [69.1%] and 1204 men [30.9%]) were included.
Table 1. Characteristics of 9009 Surgical Procedures in a Stepped-Wedge Cluster Nonrandomized Clinical Trial.
| Characteristic | Study groupa | P valued | |
|---|---|---|---|
| Control (n = 3892)b | Intervention (n = 5117)c | ||
| Male sex | 1204 (30.9) | 2204 (43.1) | <.001 |
| Age, mean (SD), y | 51.5 (21.8) | 51.8 (22.4) | .49 |
| ASA risk scoree | |||
| I | 1020 (26.2) | 1385 (27.1) | .14 |
| II | 2115 (54.4) | 2630 (51.4) | |
| III | 706 (18.2) | 998 (19.5) | |
| IV | 44 (1.1) | 100 (2.0) | |
| V | 1 (0.02) | 3 (0.1) | |
| Surgery | |||
| Elective | 1878 (48.3) | 2270 (44.4) | <.001 |
| Emergency | 2014 (51.7) | 2847 (55.6) | |
| Anesthesia | |||
| Regional | 1310 (33.7) | 1794 (35.1) | .172 |
| General | 2582 (66.3) | 3323 (64.9) | |
| Surgical specialty | |||
| Neurosurgery | 636 (16.3) | 1903 (37.2) | <.001 |
| Orthopedics | 1827 (46.9) | 2612 (51.0) | |
| Gynecology | 1429 (36.7) | 602 (11.8) | |
| SURPASS preoperative checklists, No.f | |||
| 0 | NA | 216 (4.2) | NA |
| 1 | NA | 503 (9.8) | |
| 2 | NA | 1034 (20.2) | |
| 3 | NA | 1903 (37.2) | |
| 4 | NA | 1176 (23.0) | |
| 5 | NA | 285 (5.6) | |
| WHO SSC intraoperative checklists, No.f | |||
| 0 | 48 (1.2) | 39 (0.8) | <.001 |
| 1 | 192 (4.9) | 251 (4.9) | |
| 2 | 808 (20.8) | 1442 (28.2) | |
| 3 | 2844 (73.1) | 3385 (66.2) | |
| SURPASS postoperative checklists, No.f | |||
| 0 | NA | 1397 (27.3) | NA |
| 1 | NA | 2789 (54.5) | |
| 2 | NA | 595 (11.6) | |
| 3 | NA | 336 (6.6) | |
Abbreviations: ASA, American Society of Anesthesiologists’ risk score; NA, not applicable; SURPASS, Surgical Patient Safety System; WHO SSC, World Health Organization surgical safety checklist.
Unless otherwise indicated, data are expressed as number (percentage) of procedures. Percentages have been rounded and may not total 100. Data are from 1 hospital in Western Norway from November 2012 through March 2015.
Includes 3274 unique patients.
Includes 4498 unique patients.
Calculated from Pearson exact test with Bonferroni corrections except ASA risk score (not exact test) and age (Gosset t test).
Missing for 6 control group procedures and 1 intervention group procedure. Higher scores indicate more comorbidities.
All items of individual checklists checked.
In total, 1418 of 9009 procedures (15.7%) were associated with 1 or more complications (Table 2). In adjusted intention-to-treat analyses, the number of complications decreased (OR, 0.73; 95% CI, 0.54-0.98; P = .04).
Table 2. Characteristics of Outcomes Before and After Intervention With SURPASS Checklists Added to WHO SSC.
| Outcome | Study groupa | P valueb | |
|---|---|---|---|
| Control (n = 3892) | Intervention (n = 5117) | ||
| Respiratory | 41 (1.1) | 76 (1.5) | .08 |
| Pneumonia | 34 (0.9) | 69 (1.3) | .045 |
| Respiratory other | 10 (0.3) | 13 (0.3) | >.99 |
| Cardiac | 31 (0.8) | 27 (0.5) | .14 |
| Cardiac arrhythmia | 7 (0.2) | 14 (0.3) | .39 |
| Congestive heart failure | 14 (0.4) | 9 (0.2) | .10 |
| Cardiac other | 13 (0.3) | 7 (0.1) | .07 |
| Infections | 89 (2.3) | 161 (3.1) | .01 |
| Sepsis | 7 (0.2) | 10 (0.2) | >.99 |
| Surgical site | 13 (0.3) | 7 (0.1) | .07 |
| Urinary tract | 68 (1.7) | 138 (2.7) | .003 |
| Infections other | 4 (0.1) | 11 (0.2) | .30 |
| Surgical wound rupture | 7 (0.2) | 4 (0.1) | .23 |
| Nervous system | 11 (0.3) | 18 (0.4) | .58 |
| Delirium | 6 (0.2) | 12 (0.2) | .48 |
| Cerebral infarction | 5 (0.1) | 7 (0.1) | >.99 |
| Bleeding | 105 (2.7) | 201 (3.9) | .001 |
| Embolism | 12 (0.3) | 8 (0.2) | .17 |
| Nutrition | 21 (0.5) | 85 (1.7) | <.001 |
| Malnutrition | 7 (0.2) | 56 (1.1) | <.001 |
| Other disorders | 14 (0.4) | 44 (0.9) | .003 |
| Anesthesia | 6 (0.2) | 4 (0.1) | .35 |
| Mechanical implantation | 4 (0.1) | 3 (0.1) | .71 |
| Fall | 0 | 5 (0.1) | .07 |
| Other | 65 (1.7) | 73 (1.4) | .39 |
| Emergency reoperations | 153 (3.9) | 218 (4.3) | .45 |
| Readmissionsc | 128 (3.5) | 149 (3.1) | .32 |
| Overall complicationsd | 574 (14.7) | 844 (16.5) | .03 |
| Length of stay, dc | |||
| Mean (SD) | 5.8 (17.7) | 5.6 (5.7) | .43 |
| Median (IQR) | 4.0 (2.0-7.0) | 4.1 (2.2-6.9) | |
| Mortality within 30 d in-hospitale,f | 23 (0.7) | 28 (0.6) | .67 |
| Mortality after dischargef | 24 (0.7) | 32 (0.7) | >.99 |
Abbreviations: IQR, interquartile range; SURPASS, Surgical Patient Safety System; WHO SSC, World Health Organization surgical safety checklist.
Unless otherwise indicated, data are expressed as number (percentage) of procedures.
Calculated using the 2-sided Pearson exact test with Bonferroni corrections for binary variables and Gosset t test for length of hospital stay.
Includes 3680 admissions in the control group and 4835 in the intervention group.
Included in overall complications are 155 International Statistical Classification of Diseases and Related Health Problems, Tenth Revision complication codes verified from unique surgical procedures, and emergency reoperations and 30-day readmissions.
Indicates 30 days or less from first operation on last hospital admission.
Includes 3274 patients in the control group and 4498 in the intervention group.
To analyze associations of complications per procedure with preoperative and postoperative SURPASS added to the WHO SSC, multiple logistic regression analyses were performed accounting for the level of adherence. When adherence to the preoperative SURPASS checklists was achieved, adjusted analysis demonstrated a decrease in in-hospital complications (OR, 0.70; 95% CI, 0.50-0.98; P = .04) and emergency reoperations (OR, 0.42; 95% CI, 0.23-0.76; P = .004) (Table 3). Furthermore, adherence to the 3 postoperative SURPASS checklists was associated with a reduction of unplanned 30-day readmissions (OR, 0.32; 95% CI, 0.16-0.64; P = .001) in adjusted analyses.
Table 3. Results From Logistic Regression of the Effects of Preoperative and Postoperative SURPASS Checklists Plus WHO SSC on 1 or More Complications in 9002 Surgical Proceduresa.
| Variables | Unadjusted model | Fully adjusted model | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| SURPASS preoperative | 0.97 (0.82-1.15) | .74 | 0.70 (0.50-0.98) | .04 |
| SURPASS postoperative | 1.02 (0.99-1.05) | .20 | 1.01 (0.97-1.05) | .65 |
| WHO SSC | 0.72 (0.55-0.94) | .02 | 0.90 (0.68-1.19) | .46 |
| Male sex | 0.92 (0.82-1.04) | .18 | 0.97 (0.85-1.11) | .67 |
| Age, per 10 y | 1.24 (1.20-1.27) | <.001 | 1.11 (1.08-1.15) | <.001 |
| Month of operationb | 1.14 (1.06-1.22) | <.001 | 1.23 (1.08-1.40) | .002 |
| ASA risk scorec | 2.21 (2.04-2.39) | <.001 | 1.80 (1.65-1.97) | <.001 |
| Urgency of surgery | ||||
| Elective | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Emergency | 2.32 (2.05-2.62) | 2.34 (2.02-2.71) | ||
| Anesthesia | ||||
| General | 1 [Reference] | <.001 | 1 [Reference] | .99 |
| Regional | 1.58 (1.40-1.77) | 1.00 (0.87-1.16) | ||
| Surgical specialty | ||||
| Neurosurgery | 1 [Reference] | .006 | 1 [Reference] | .02 |
| Orthopedics | 1.12 (0.98-1.28) | 0.82 (0.69-0.96) | ||
| Gynecology | 0.89 (0.75-1.05) | 1.03 (0.84-1.28) | ||
Abbreviations: ASA, American Society of Anesthesiologists; OR, odds ratio, effect size; SURPASS, Surgical Patient Safety System; WHO SSC, World Health Organization surgical safety checklist.
Calculated as proportions of checklists used. SURPASS included 5 preoperative checklists and 1 postoperative postanesthesia care unit nurse checklist; WHO SCC, 3 checklists. Preoperative SURPASS includes 0 for no checklist and 1 to 5 checklists (proportions, 0.20, 0.40, 0.60, 0.80, and 1.00); postoperative SURPASS and WHO SSC, 0 for no checklist and 1 to 3 checklists (proportions, 0.33, 0.66, and 1.00).
Time for inclusion in the study, per year.
Scores range from I to V, with higher scores indicating greater comorbidities.
Analyzing time trends in adjusted Cox proportional hazards regression showed an overall shorter LOS from early to later in the study period (ie, an increasing chance of earlier discharge; HR, 1.07 per year; 95% CI, 1.02-1.13; P = .003). Added use of the SURPASS checklists showed no significant associations with LOS.
The 30-day in-hospital mortality associated with using the preoperative SURPASS was nonsignificant (OR, 0.28; 95% CI, 0.04-1.78; P = .17). For postoperative SURPASS, the association was likewise nonsignificant (OR, 0.86; 95% CI, 0.68-1.08; P = .18). Similarly, there was no change in 30-day mortality after discharge associated with use of either the preoperative SURPASS (OR, 1.67; 95% CI, 0.38-7.44; P = .50) or the postoperative SURPASS (OR, 0.64; 95% CI, 0.17-2.45; P = .51) in all adjusted analyses.
The 3 parallel control surgical units included 9678 procedures during the study period (mean [SD] patient age, 57.4 [22.2] years; 4124 women [42.6%] and 5554 men [57.4%]). A CONSORT flow diagram describing eligible procedures is represented in the eFigure in Supplement 2. Characteristics of the procedures and outcome measures are reported in eTables 1 and 2 in Supplement 2, respectively. There was an overall decrease in LOS in the control units during the study period, with an increased chance of being more rapidly discharged (HR, 1.07 per year; 95% CI, 1.04-1.11; P < .001). We also found an increase in complications over time (OR, 1.09; 95% CI, 1.01-1.17; P = .04) (eTable 3 in Supplement 2). In adjusted analyses, no changes were observed in emergency reoperations (OR, 0.96; 95% CI, 0.82-1.12; P = .57), 30-day readmissions (OR, 1.17; 95% CI, 0.96-1.42; P = .11), 30-day in-hospital mortality (OR, 0.95; 95% CI, 0.70-1.29; P = .73), or 30-day mortality after discharge (OR, 1.14; 95% CI, 0.81-1.59; P = .46) in these departments.
Discussion
Findings from this study demonstrate that adding the preoperative and postoperative SURPASS checklists to the intraoperative WHO SSC may be clinically advantageous. We found that the joint application of the 2 surgical checklist systems was associated with reduced in-hospital complications, emergency reoperations, and hospital readmissions.
A decade ago, the WHO SSC was initially implemented in 2 Norwegian hospitals (one being the present trial hospital), resulting in a 42% relative risk reduction of complications from 19.9% to 12.4%.23 Although the WHO SSC has become national clinical policy for surgery, evidence shows that surgical complications often originate outside the operating room.12,13,14 Logically, this outcome suggests that a checklist to improve flow of information and completeness of requisite clinical care protocols before a patient reaches the operating room can reduce unwanted variation in preparation and planning and improve care.29 Our findings suggest that effective application of the preoperative SURPASS may achieve this. The reduction in emergency reoperations when preoperative SURPASS had been used replicates studies showing a decrease in reoperations after implementing intraoperative surgical checklists.20,30,31
Furthermore, better use of the 3 postoperative SURPASS checklists was associated with decreased readmissions to hospital within 30 days. Improved communications optimize care delivery in transfer of patients to other units.12,32,33,34,35 The clinical associations we observed could be owing to the SURPASS discharge checklists supporting better preparation of patients when leaving the hospital (ie, plans for their medications and expectations regarding their ongoing recovery). Other studies have found that patient discharge is strengthened by use of checklists,36 and decreased readmissions have been linked to use of the WHO SSC.37
The parallel control units had increased complication rates over time, whereas rates of emergency reoperations, 30-day readmissions, and mortality remained unchanged. Over time, we observed an overall increased complication rate in both trial and control units. We do not have data directly addressing this finding. We hypothesize, however, that the national context of the study may account for this pattern. National economic incentive systems reimbursing ICD-10 codes for complicated admissions have increased hospitals’ focus on coding practices.38 A possible explanation may be increased hospital focus on more accurate coding practice for reimbursement purposes by individual physicians throughout the study period. The increase in complications is unlikely to reflect a lack of effect from the checklist intervention, because when adjusted regression analyses were performed, the intervention was associated with lower risk of complications. Also, stricter adherence to the SURPASS checklists had a lower risk of complications than looser adherence, indicating a dose-response effect. Furthermore, use of the stepped-wedge design allowed us to adjust for time trends in complication rates.17 Both the trial departments and the control units had an overall decrease in LOS over time, and LOS was not associated with use of the SURPASS in the intervention departments. This finding contrasts with those of previous studies, which showed reduction in LOS with checklist use.23,39 We consider it possible that maximum reduction of LOS had been reached in our study owing to the national Norwegian context. Specifically, a national coordination reform took effect in January 2012.40 One of the main goals of the reform was to reduce LOS in hospitals by a build-up and enhancement of publicly funded nursing homes. This national policy program likely affected discharge decision-making throughout the study period and thus affected our findings. Our findings cannot directly support this explanation, which can be evaluated further through longitudinal outcome studies.
Strengths and Limitations
This study has several strengths, including the prestudy SURPASS validation process, study design, long-term collection of data, and strong engagement from hospital leaders, managers, and influential clinicians when implementing the SURPASS intervention, thus achieving good fidelity. In addition, the validation procedures with exact and extensive verification of in-hospital ICD-10 codes for complications, emergency reoperations, readmissions, mortality, and LOS from patient records linked to actual checklist adherence allowed reliable outcome measurement.27 Furthermore, the study allows distinguishing which checklists are associated with improvements on which outcomes. For example, combined use of the preoperative and postanesthesia care unit nurses’ SURPASS with the WHO SSC may improve in-hospital complications, in-hospital mortality, and LOS. Use of preoperative SURPASS checklists and the WHO SSC may influence emergency reoperations. A combined use of preoperative and postoperative SURPASS and WHO SSC may influence unplanned hospital readmissions and mortality after discharge.
The study also has limitations. The nonsignificant change in mortality could be owing to an underpowered sample size. The calculation was performed in 2012 based on the published literature.15,20 However, the number of patients dying in our sample was lower than anticipated. Furthermore, an important consideration is whether there could be any residual confounders explaining the observed higher rate of complications after the intervention. For example, were more complex procedures performed in sicker patients after the intervention? In Table 1, we showed that there is no difference in comorbidity (ASA classification) between control and intervention departments. In the regression analyses, we have adjusted for case mixes, including age, sex, emergency procedures, ASA classification, anesthesia given, surgical specialty, point of time for inclusion in the study, and checklist use. Additional comorbidity measures such as the Charlson comorbidity index were not part of the original study protocol. However, with these rigorous adjusted analyses, we believe that very little residual confounding has remained unexplained.
The parallel control units contributed different surgical procedures and specialties to the trial compared with the intervention departments. Comparing outcome data on similar procedures and specialties would have been ideal. However, morbidity and mortality trends in the parallel controls were similar to those of the intervention departments. The actual complexity of the SURPASS intervention, involving different professional groups across different departments, added an inherent limitation, because randomizing the start-up of the intervention with the time and resources available became unfeasible.
In addition, overall high-fidelity application of all checklists across all professional groups for all surgical procedures was not obtained. Known implementation barriers affect checklist use globally (eg, information technology systems, checklist and personnel flow, checklist resistance, and/or checklist fatigue) and could have resulted in underestimations of the sizes of associations of intervention and clinical outcomes in our analyses. Other investigators41 have also raised these issues. Further studies of how to improve fidelity in delivering clinically effective checklists in surgical pathways are warranted.
Conclusions
Our findings suggest that combinations of the WHO and SURPASS checklists throughout the perioperative pathway may be clinically advantageous in improving processes of care and patient safety further with reductions in complications, reoperations, and readmissions beyond what sole use of the WHO checklist in the operating room achieves. The WHO checklist has been adopted globally for use in operating rooms. The next step to increase surgical patient safety is to use safety checklists throughout the perioperative pathway, as when combining the WHO checklist with SURPASS checklists. Rigorous large-scale multicenter randomized clinical trials are recommended to investigate this further.
Trial Protocol
eFigure. CONSORT Flow Diagram
eTable 1. Characteristics of 9678 Surgical Procedures With Care as Usual Over 29 Months in 3 Control Hospitals in Western Norway From November 1, 2012, Through March 31, 2015
eTable 2. Characteristics of Outcomes in 9678 Surgical Procedures Over 29 Months in 3 Control Hospitals in Western Norway From November 1, 2012, Through March 31, 2015
eTable 3. Results From Logistic Regression of Change in 1 or More Verified Complications in 9669 Surgical Procedures With Care as Usual in 3 Hospitals in Western Norway Over 29 Months, From November 1, 2012, Through March 31, 2015
References
- 1.Abbott TEF, Ahmad T, Phull MK, et al. ; International Surgical Outcomes Study (ISOS) group . The surgical safety checklist and patient outcomes after surgery: a prospective observational cohort study, systematic review and meta-analysis. Br J Anaesth. 2018;120(1):146-155. doi: 10.1016/j.bja.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 2.Bergs J, Hellings J, Cleemput I, et al. Systematic review and meta-analysis of the effect of the World Health Organization surgical safety checklist on postoperative complications. Br J Surg. 2014;101(3):150-158. doi: 10.1002/bjs.9381 [DOI] [PubMed] [Google Scholar]
- 3.Borchard A, Schwappach DL, Barbir A, Bezzola P. A systematic review of the effectiveness, compliance, and critical factors for implementation of safety checklists in surgery. Ann Surg. 2012;256(6):925-933. doi: 10.1097/SLA.0b013e3182682f27 [DOI] [PubMed] [Google Scholar]
- 4.Boyd J, Wu G, Stelfox H. The impact of checklists on inpatient safety outcomes: a systematic review of randomized controlled trials. J Hosp Med. 2017;12(8):675-682. doi: 10.12788/jhm.2788 [DOI] [PubMed] [Google Scholar]
- 5.Lau CSM, Chamberlain RS. The World Health Organization surgical safety checklist improves post-operative outcomes: a meta-analysis and systematic review. Surg Sci. 2016;7(4):206-217. doi: 10.4236/ss.2016.74029 [DOI] [Google Scholar]
- 6.Thomassen Ø, Storesund A, Søfteland E, Brattebø G. The effects of safety checklists in medicine: a systematic review. Acta Anaesthesiol Scand. 2014;58(1):5-18. doi: 10.1111/aas.12207 [DOI] [PubMed] [Google Scholar]
- 7.Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med. 2014;370(11):1029-1038. doi: 10.1056/NEJMsa1308261 [DOI] [PubMed] [Google Scholar]
- 8.Reames BN, Krell RW, Campbell DA Jr, Dimick JB. A checklist-based intervention to improve surgical outcomes in Michigan: evaluation of the Keystone Surgery program. JAMA Surg. 2015;150(3):208-215. doi: 10.1001/jamasurg.2014.2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbach DR. The challenge of quality improvement in surgical care. JAMA Surg. 2015;150(3):215. doi: 10.1001/jamasurg.2014.2908 [DOI] [PubMed] [Google Scholar]
- 10.Leape LL. The checklist conundrum. N Engl J Med. 2014;370(11):1063-1064. doi: 10.1056/NEJMe1315851 [DOI] [PubMed] [Google Scholar]
- 11.Russ S, Rout S, Sevdalis N, Moorthy K, Darzi A, Vincent C. Do safety checklists improve teamwork and communication in the operating room? a systematic review. Ann Surg. 2013;258(6):856-871. doi: 10.1097/SLA.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 12.Greenberg CC, Regenbogen SE, Studdert DM, et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533-540. doi: 10.1016/j.jamcollsurg.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 13.Griffen FD, Stephens LS, Alexander JB, et al. The American College of Surgeons’ closed claims study: new insights for improving care. J Am Coll Surg. 2007;204(4):561-569. doi: 10.1016/j.jamcollsurg.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 14.de Vries EN, Hollmann MW, Smorenburg SM, Gouma DJ, Boermeester MA. Development and validation of the Surgical Patient Safety System (SURPASS) checklist. Qual Saf Health Care. 2009;18(2):121-126. doi: 10.1136/qshc.2008.027524 [DOI] [PubMed] [Google Scholar]
- 15.de Vries EN, Prins HA, Crolla RM, et al. ; SURPASS Collaborative Group . Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363(20):1928-1937. doi: 10.1056/NEJMsa0911535 [DOI] [PubMed] [Google Scholar]
- 16.Mehta N, Amaranathan A, Jayapal L, Kundra P, Nelamangala Ramakrishnaiah VP. Effect of comprehensive surgical safety system on patients’ outcome: a prospective clinical study. Cureus. 2018;10(5):e2601. doi: 10.7759/cureus.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown C, Hofer T, Johal A, et al. An epistemology of patient safety research: a framework for study design and interpretation—part 2, study design. Qual Saf Health Care. 2008;17(3):163-169. doi: 10.1136/qshc.2007.023648 [DOI] [PubMed] [Google Scholar]
- 18.Hemming K, Taljaard M, McKenzie JE, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. 2018;363:k1614. doi: 10.1136/bmj.k1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mdege ND, Man MS, Taylor Nee Brown CA, Torgerson DJ. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. J Clin Epidemiol. 2011;64(9):936-948. doi: 10.1016/j.jclinepi.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Haynes AB, Weiser TG, Berry WR, et al. ; Safe Surgery Saves Lives Study Group . A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491-499. doi: 10.1056/NEJMsa0810119 [DOI] [PubMed] [Google Scholar]
- 21.World Alliance for Patient Safely; World Health Organization Implementation Manual: Surgical Safety Checklist. Published May 2008. Accessed February 16, 2020. https://www.who.int/patientsafety/safesurgery/tools_resources/SSSL_Manual_finalJun08.pdf
- 22.Storesund A, Haugen AS, Wæhle HV, et al. Validation of a Norwegian version of Surgical Patient Safety System (SURPASS) in combination with the World Health Organizations’ surgical safety checklist (WHO SSC). BMJ Open Qual. 2019;8(1):e000488. doi: 10.1136/bmjoq-2018-000488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haugen AS, Søfteland E, Almeland SK, et al. Effect of the World Health Organization checklist on patient outcomes: a stepped wedge cluster randomized controlled trial. Ann Surg. 2015;261(5):821-828. doi: 10.1097/SLA.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 24.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(21):21. doi: 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conley DM, Singer SJ, Edmondson L, Berry WR, Gawande AA. Effective surgical safety checklist implementation. J Am Coll Surg. 2011;212(5):873-879. doi: 10.1016/j.jamcollsurg.2011.01.052 [DOI] [PubMed] [Google Scholar]
- 26.Haynes AB, Berry WR, Gawande AA. What do we know about the safe surgery checklist now? Ann Surg. 2015;261(5):829-830. doi: 10.1097/SLA.0000000000001144 [DOI] [PubMed] [Google Scholar]
- 27.Storesund A, Haugen AS, Hjortås M, et al. Accuracy of surgical complication rate estimation using ICD-10 codes. Br J Surg. 2019;106(3):236-244. doi: 10.1002/bjs.10985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. doi: 10.1136/bmj.h391 [DOI] [PubMed] [Google Scholar]
- 29.de Vries EN, Eikens-Jansen MP, Hamersma AM, Smorenburg SM, Gouma DJ, Boermeester MA. Prevention of surgical malpractice claims by use of a surgical safety checklist. Ann Surg. 2011;253(3):624-628. doi: 10.1097/SLA.0b013e3182068880 [DOI] [PubMed] [Google Scholar]
- 30.van Boxtel AGM, van Veghel D, Soliman Hamad MA, Schulz DN, Stepaniak PS, van Straten AHM. Use of an intraoperative checklist to decrease the incidence of re-exploration for postoperative bleeding after cardiac surgery. Interact Cardiovasc Thorac Surg. 2017;25(4):555-558. doi: 10.1093/icvts/ivx130 [DOI] [PubMed] [Google Scholar]
- 31.Lepänluoma M, Rahi M, Takala R, Löyttyniemi E, Ikonen TS. Analysis of neurosurgical reoperations: use of a surgical checklist and reduction of infection-related and preventable complication-related reoperations. J Neurosurg. 2015;123(1):145-152. doi: 10.3171/2014.12.JNS141077 [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Maturo S, Dwyer D, et al. Interdisciplinary development and implementation of communication checklist for postoperative management of pediatric airway patients. Otolaryngol Head Neck Surg. 2012;146(1):129-134. doi: 10.1177/0194599811421745 [DOI] [PubMed] [Google Scholar]
- 33.Graan SM, Botti M, Wood B, Redley B. Nursing handover from ICU to cardiac ward: standardised tools to reduce safety risks. Aust Crit Care. 2016;29(3):165-171. doi: 10.1016/j.aucc.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 34.Johnston MJ, Arora S, King D, et al. A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery. 2015;157(4):752-763. doi: 10.1016/j.surg.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 35.Nagpal K, Vats A, Lamb B, et al. Information transfer and communication in surgery: a systematic review. Ann Surg. 2010;252(2):225-239. doi: 10.1097/SLA.0b013e3181e495c2 [DOI] [PubMed] [Google Scholar]
- 36.Drake K, McBride M, Bergin J, Vandeweerd H, Higgins A. Ensuring safe discharge with a standardized checklist and discharge pause. Nursing. 2017;47(8):65-68. doi: 10.1097/01.NURSE.0000521042.81195.86 [DOI] [PubMed] [Google Scholar]
- 37.Rodella S, Mall S, Marino M, et al. Effects on clinical outcomes of a 5-year surgical safety checklist implementation experience: a large-scale population-based difference-in-differences study. Health Serv Insights. 2018;11:1178632918785127. doi: 10.1177/1178632918785127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Office of the Auditor General of Norway. The Office of the Auditor General’s investigation of medical coding practice within the health enterprises. Document 3:5 (2016-2017). Accessed February 16, 2020. https://www.riksrevisjonen.no/globalassets/reports/en-2016-2017/codingpracticehealthenterprises.pdf
- 39.de Jager E, Gunnarsson R, Ho YH. Implementation of the World Health Organization surgical safety checklist correlates with reduced surgical mortality and length of hospital admission in a high-income country. World J Surg. 2019;43(1):117-124. doi: 10.1007/s00268-018-4703-x [DOI] [PubMed] [Google Scholar]
- 40.Ministry of Health and Care Services Report No. 47 (2008–2009) to the Storting. The Coordination Reform—proper treatment at the right place and right time. Full version in Norwegian. Summary in English: Oslo, Norway. Published 2008. Accessed September 30, 2019. https://www.regjeringen.no/en/dokumenter/report.no.-47-to-the-storting-2008-2009/id567201/?ch=1&q=#?ch=1&q=&_t_dtq=true&_suid=146037692843204369084028942121
- 41.Russ SJ, Sevdalis N, Moorthy K, et al. A qualitative evaluation of the barriers and facilitators toward implementation of the WHO surgical safety checklist across hospitals in England: lessons from the “Surgical Checklist Implementation Project”. Ann Surg. 2015;261(1):81-91. doi: 10.1097/SLA.0000000000000793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. CONSORT Flow Diagram
eTable 1. Characteristics of 9678 Surgical Procedures With Care as Usual Over 29 Months in 3 Control Hospitals in Western Norway From November 1, 2012, Through March 31, 2015
eTable 2. Characteristics of Outcomes in 9678 Surgical Procedures Over 29 Months in 3 Control Hospitals in Western Norway From November 1, 2012, Through March 31, 2015
eTable 3. Results From Logistic Regression of Change in 1 or More Verified Complications in 9669 Surgical Procedures With Care as Usual in 3 Hospitals in Western Norway Over 29 Months, From November 1, 2012, Through March 31, 2015