Abstract
Purpose:
A prospective, open-label study in 20 professional swimmers evaluated the efficacy and safety of an ophthalmic solution containing crosslinked hyaluronic acid, coenzyme Q10, and vitamin E TPGS in releasing eye irritation and restoring ocular surface damages after prolonged exposure to chlorinated water.
Methods:
Individually, one eye was instilled with the ophthalmic solution and the other used as a comparator. Eye drops were self-administered three times a day for 2 months. Tear film breakup time (primary endpoint), Schirmer I test, beating of eyelashes/min, tear osmolarity, corneal and conjunctival staining with fluorescein, Ocular Surface Disease Index questionnaire, subject satisfaction, visual acuity (secondary endpoints), and Efron Grading Scale were evaluated at screening/baseline (V1), week 1 (V2), week 2 (V3), week 4 (V4), and week 8 (V5).
Results:
After 2 months, breakup time test significantly improved in the treated eyes (+1.67 s) compared to control (−3.00 s) (p = 0.0002). Corneal and conjunctival surfaces of treated eyes recovered significantly compared to control eyes when assessed by fluorescein staining (p < 0.0001), Ocular Surface Disease Index (p < 0.05), and visual analog scale (p = 0.0348) scores. Improvements were also observed with Schirmer I test, beating of eyelashes, and tear osmolarity, despite without statistical significance. Efron Grading Scale was consistent with the other tests. The ocular tolerability was excellent.
Conclusion:
The adequate combination of crosslinked hyaluronic acid, coenzyme Q10, and vitamin E TPGS, contained in the ophthalmic solution VisuXL®, has been shown to protect ocular surface from potential damages originating from prolonged exposure to chlorinated water. VisuXL may represent a compelling treatment in other situations beyond dry eye syndrome.
Keywords: Crosslinked hyaluronic acid, coenzyme Q10, vitamin E, conjunctiva, cornea, chlorinated water, ophthalmic solution, tear film
Introduction
Eye irritation induced by pool water is a well-known phenomenon. The first to speculate about a correlation between the eye and skin irritation syndromes in subjects in contact with swimming pool water treated with chlorine were Mood et al.1 Chlorine is a widespread substance in swimming pool disinfection, leading to the formation of many disinfection byproducts including trihalomethanes and chloramines, which contribute to ocular, respiratory, and skin irritation.2
Recently, the Centers for Disease Control and Prevention (CDC) published the frequency of illnesses and injuries related to pool chemicals in six national states during the period 2002–2008, participating in the Sentinel Event Notification System for Occupational Risk (SENSOR)-Pesticides surveillance program and from the National Electronic Injury Surveillance System (NEISS). Symptoms most frequently reported were respiratory symptoms, such as cough, upper respiratory irritation, and dyspnea (65% of state cases and 24% of NEISS cases), eye injuries (33% of state cases and 42% of NEISS cases), and skin injuries (18% of state cases and 19% of NEISS cases).3 The most frequent ocular symptoms are redness, itching, and irritation, with occasional signs of superficial punctate keratitis and conjunctival hyperemia. It was also hypothesized that corneal epithelial damage after swimming can be attributed to other factors, such as pH, hypotonia, or mechanical factors. However, it has not been precisely determined how these factors can induce damage to the ocular surface.
Previous studies have shown that hyaluronic acid significantly improved symptoms and signs of ocular surface damage caused by chemical (benzalkonium chloride–preserved anti-glaucoma medications) or mechanical (superficial corneal abrasion) damages4,5 and dry eye syndrome–related conditions by re-epithelialization mechanisms.6,7 On the other hand, coenzyme Q10 (CoQ10) has been demonstrated to reduce corneal damages after ultraviolet B exposure and promote corneal wound healing after corneal epithelium removal in vivo and in vitro by preserving the mitochondrial function.8 The crosslinked form of hyaluronic acid (XLHA) increases the stability, adhesion, and permanence on the ocular surface of CoQ10, allowing a longer lasting effect.
Effective treatments of eye irritation due to prolonged exposure to swimming pool water often require anti-inflammatory drugs, but given the side effects of immune suppression and the cost of pharmaceutical preparations, subjects often suspend treatment before the benefit of therapy is obtained. Prolonged therapy with corticosteroids is potentially associated with complications (e.g. secondary glaucoma, cataract, and infections). Due to the need of identifying therapies that can alleviate dry eye symptoms without additional problems, we hypothesized that the complementary properties of the association of XLHA, CoQ10, and vitamin E TPGS (all contained in the preservative-free medical device VisuXL® ophthalmic solution) could be a beneficial treatment for ocular surface exposed to chemical challenge.
The aim of this study was to demonstrate that the clinical benefits and quality of swimmers’ life, chronically exposed to chlorinated water, were strongly linked to the long-term (2-month) application of the unique composition of VisuXL.
Methods
Study design and participants
This was a single-center, open-label study of superiority, conducted in Italy with the untreated eye considered as the comparator for the eye treated with eye drops containing a combination of XLHA, CoQ10, and vitamin E TPGS. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki revised in 2013. The protocol was first reviewed by the local Ethics Committee “Comitato Etico, Fondazione Policlinico Universitario A. Gemelli, Largo Agostino Gemelli 8, Rome—Italy” (Project identification code: 22541/18 ID:2118) on 27 June 2018 and finally approved on 31 August 2018.
Prior to enrollment, written informed consent was obtained from each participant. For athletes aged <18 years, parents’ agreement was also obtained. The study took place between 7 October 2018 (first signed informed consent form (ICF)) and 17 December 2018 (last patient visit).
Male subjects were aged 14–33 years, had a Schirmer test >10 mm at 5 min, and had discomfort of the ocular surface. The main exclusion criteria were anterior ocular segment diseases, autoimmune or metabolic diseases, entropion, trichiasis, androgen deficiency, medications interfering with the secretion of the lacrimal gland, connective tissue diseases, prior eye surgery, use of artificial tears in the 15 days before the study start, and hypersensitivity to hyaluronic acid and/or CoQ10.
Efficacy assessment
For each subject, study visits and ophthalmic tests were performed at screening/baseline (V1), week 1 (V2), week 2 (V3), week 4 (V4), and week 8 (V5). Each enrolled subject was instructed to instill 1–2 drops of VisuXL three times a day (TID) always in the left eye during the entire 2-month period of study, at morning, before training (at least 1 h before entering the pool), and after training (maximum 1 h after the end of the activity). The left eye was the one that most remained in contact with the pool water during the training sessions. Subjects continued to instill 1–2 drops TID in the same eye even in days without training (including weekends).
Primary endpoint
The primary endpoint is to evaluate the differences between the two groups in change over time in tear film breakup time (BUT) measured at each study visit.
Secondary endpoints
The secondary endpoints are to assess ocular surface health and quality of life of professional water-polo athletes by difference in change over time between the two groups of eyes determined by Schirmer I test, beating of eyelashes/min, tear osmolarity, corneal and conjunctival staining with fluorescein, Ocular Surface Disease Index (OSDI) questionnaire, subject satisfaction (10 points on the visual analog scale (VAS)), and visual acuity.
Statistical method
The calculation of the sample was not performed as this was a fact-finding pilot study in a particular category of subjects. During the study, 40 eyes (20 treated and 20 untreated controls, with one treated eye and one untreated control/subject) of 20 professional water-polo athletes (AS Roma) exposed to pool water, aged over 14 years, and diagnosed having ocular surface discomfort were evaluated. A rate of 15% dropout was considered.
Descriptive statistics reported the mean ± standard deviation for continuous parameters. Frequencies (counts and percentages) were provided for categorical variables. A mixed model for repeated measures was used for the primary and secondary endpoints. Among the latter, the staining grade with fluorescein was calculated with Wilcoxon signed-rank test. A p value ⩽ 0.05 was considered statistically significant.
This article was written using the CONSORT reporting guidelines.9
Results
A total number of 20 subjects were screened and enrolled in the study. The intention-to-treat (ITT) and safety analyses were performed on 19 subjects (38 eyes), as one was excluded from the study due to a serious adverse event (SAE) unrelated to the investigational product and occurred soon after the screening/baseline visit (V1). Visual acuity was 10/10 in both eyes for each individual at baseline and remained unchanged during the study period. The major demographic results are reported in Table 1.
Table 1.
Demographic results of professional water-polo athletes exposed to pool water (ITT population).
| Parameters | Values |
|---|---|
| Age (years) | |
| No. of subjects | 19 |
| Mean (SD) | 20.11 (6.85) |
| Median | 17.00 |
| Range | 15.00–35.00 |
| Height (cm) | |
| No. of subjects | 19 |
| Mean (SD) | 186.74 (5.76) |
| Median | 187.00 |
| Range | 178.00–198.00 |
| Weight (kg) | |
| No. of subjects | 19 |
| Mean (SD) | 82.99 (6.85) |
| Median | 84.00 |
| Range | 70.00–95.00 |
| Ethnicity (Caucasian), n | 19 |
| Medical history | |
| Ear and labyrinth disorders, n (%) | 1 (5.26) |
| Renal and urinary disorders, n (%) | 1 (5.26) |
| Subjects with concomitant medicationa, n (%) | 3 (15.78) |
| Subjects with prior medicationb, n (%) | 1 (5.26) |
ITT: intention-to-treat; SD: standard deviation.
Concomitant medications = medications started at or after the first administration of investigational product (IP), including also medications started prior to the first administration of IP but continued during the study.
Prior medications = medications started and ended prior to the first administration of investigational study medication.
BUT
The ophthalmic solution containing XLHA, CoQ10, and vitamin E TPGS (VisuXL) achieved the primary objective of superior efficacy versus no treatment in re-establishing and restoring the tear film stability in treated eyes when measured by the tear film BUT test.
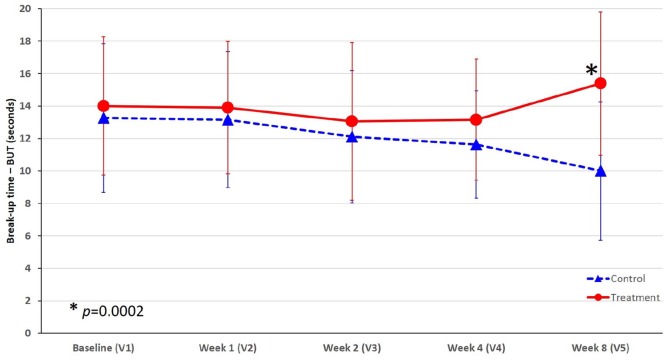
At baseline, the two groups had similar mean values (i.e. 13.26 s for the control group; 14 s for the treated group). During the first 4 weeks, in the control group, the BUT values constantly declined, with an evident mean decrease at week 4 (−1.63 s) and even more pronounced at week 8 (−3.00 s) compared to baseline. This represented a worsening of about −25% of BUT from baseline. Conversely, in the treated group of eyes, a slight decrease was observed between weeks 1 and 2, but after week 4 the mean BUT value improved significantly achieving an absolute positive change at week 8 compared to the baseline value of +1.67 s. This represented an improvement of about +10% of BUT from baseline (Figure 1).
Figure 1.
Change of tear film breakup time (seconds) over time in the treated and control eyes in professional water-polo athletes.
*p = 0.0002 indicates statistically significant difference at week 8 (V5) in favor of the group of eyes treated with VisuXL versus control.
After 8 weeks of treatment, the treated eyes registered a mean change from baseline higher of 4.67 s than the control group, and this difference was statistically significant (p = 0.0002).
Schirmer I test
Both the treated and control eyes showed a constant trend over 8 weeks. The mean values in the treated group were higher than the ones of the control group at baseline (17.68 and 16.84 mm/5 s, respectively). The intergroup difference doubled at the end of the study (17.56 and 15.89 mm/5 s, respectively) but not enough to be statistically significant. Nevertheless, a global higher trend for the treated eyes indicated that the ophthalmic solution improved the lacrimal secretion over time.
Beating of eyelashes
At baseline, the two groups had the same mean number of eyelash beatings (15.26/min). During the first week, a slight increase was observed in both groups, but afterwards the number of beats decreased until day 14. At week 4, the treated and control eyes reached approximately the baseline values (15.11 and 15.37/min, respectively). At week 8, the mean number of beatings decreased again until the common value of 14.2 beats/min. The two groups of eyes showed the same trend and fluctuation over time, despite the number of beatings being slightly higher in the control group, with the exception of the baseline and at week 8. No significant difference between groups of eyes was observed.
Tear osmolarity
In the treated group of eyes, the mean baseline value of tear osmolarity was 308.21 mOsm/L. After an initial increase during the first week (314.74 mOsm/L), the mean osmolarity decreased achieving the lowest peak at week 4 (298.16 mOsm/L). The final mean value at week 8 was 306.39 mOsm/L (1.82 mOsm/L lower than the baseline value). The control group had a similar mean baseline value (305.42 mOsm/L), but a remarkable oscillation was observed during the study with increases (312.21 and 314.11 mOsm/L at weeks 2 and 8, respectively) and decreases (303.26 and 305.26 mOsm/L at weeks 1 and 4, respectively) of tear osmolarity. The end study mean value was 314.11 mOsm/L, which is 8.69 mOsm/L higher than the baseline value.
The higher the tear film osmolarity, the greater the severity of the ocular surface damage. Despite the treated eyes showing a trend toward osmolarity normalization during the study, this improvement did not achieve statistical significance compared to the worsening observed in the control eyes.
Coloration of the ocular surface (corneal and conjunctival) with fluorescein
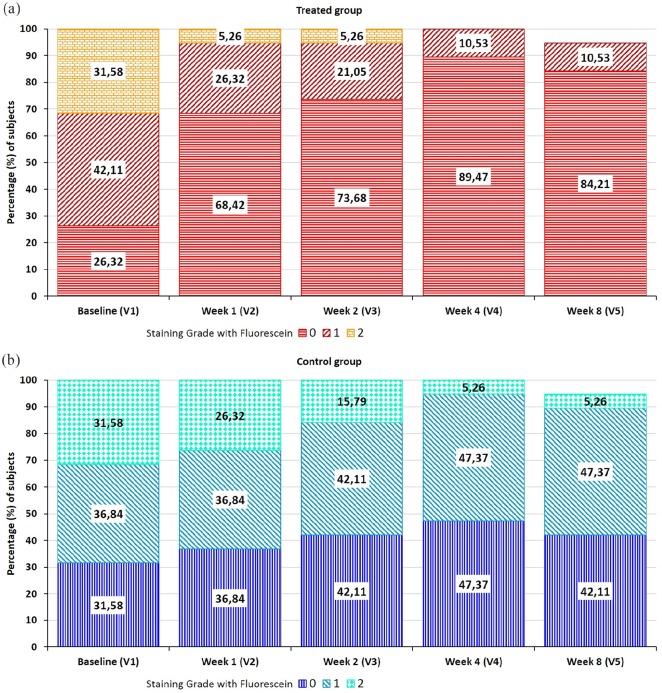
Damages of the exposed corneal and conjunctival surface are estimated by the grading staining system of 0–4 based on the extension of the area of corneal staining (Oxford schema). The total area is designated as grade 0 when there is no punctate staining, grade 1 when ⩽1/8th of the ocular surface area is stained, grade 2 when ⩽1/4th of the area is stained, grade 3 when ⩽1/2nd of the area is stained, and grade 4 when more than half or the entire area is stained.
At baseline, the two treatment groups were almost similar in their basal conditions (Figure 2(a) and (b)). During the study, the proportion of eyes in each staining grade changed. At week 8, the treated group showed a positive trend: the majority of treated eyes healed (i.e. 84.21% reached grade 0) and only 10.53% (i.e. two eyes) reported grade 1, while in the control group less than half (42.11%) achieved grade 0. At the end of the study visit (week 8), in the control and treatment groups, there were, respectively, 42.11% and 84.21% of eyes with grade 0, 47.37% and 10.53% with grade 1, and 5.26% and 0% with grade 2 (Figure 2(a) and (b)). The results indicate a statistically higher percentage of ocular surface healing (grade 0) or return to normalization (grade 1) in the eyes treated with the ophthalmic solution compared to control eyes in the same subjects (p < 0.0001, Wilcoxon signed-rank test).
Figure 2.
Distribution of the percentage of subjects at each visit according to the staining grade system with fluorescein: (a) treated group of eyes and (b) control group of eyes.
OSDI Questionnaire
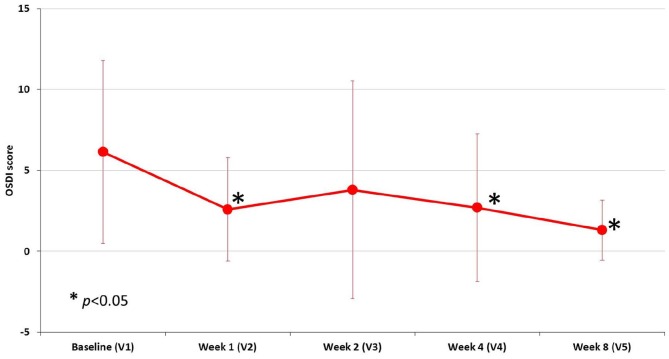
The OSDI questionnaire is a sensitive and specific index in distinguishing between normal subjects and patients with ocular discomfort (normal, mild to moderate, and severe). Thus, the OSDI score was referred to subjects and not to eyes. During the study, a decreasing trend of OSDI score was observed, with a slight increase between week 1 and week 2 (Figure 3). Two outliers (subjects #13 and #19) experienced a worsening of moderate extent during the study (OSDI score at each visit: 2.27, 2.27, 25, 17.5, 5 and 13.6, 2.27, 17.5, 10, 4.5 for each respective patient). The overall mean reduction of OSDI score at each study visit versus baseline was statistically significant (p < 0.05) except at week 2 (Figure 3).
Figure 3.
Trend of mean OSDI score during the study visits.
*p < 0.05 indicates statistically significant reduction of the mean OSDI score compared with the baseline mean value.
Subject satisfaction (VAS)
Subject satisfaction was measured with the 10-point VAS at each post-baseline visit. The mean VAS values were 6.63, 6.63, 6.95, and 7.06 at visits 2–5, respectively. Subjects experienced a robust increase in satisfaction after 8 weeks of treatment. The mean VAS achieved at visit 5 showed a statistically significant change compared to the result at visit 2 (p = 0.0348; VAS score was not collected at visit 1). The mean values at visits 3 and 4 were not statistically significant when compared to VAS at visit 2.
Conjunctival hyperemia (Efron Grading Scale)
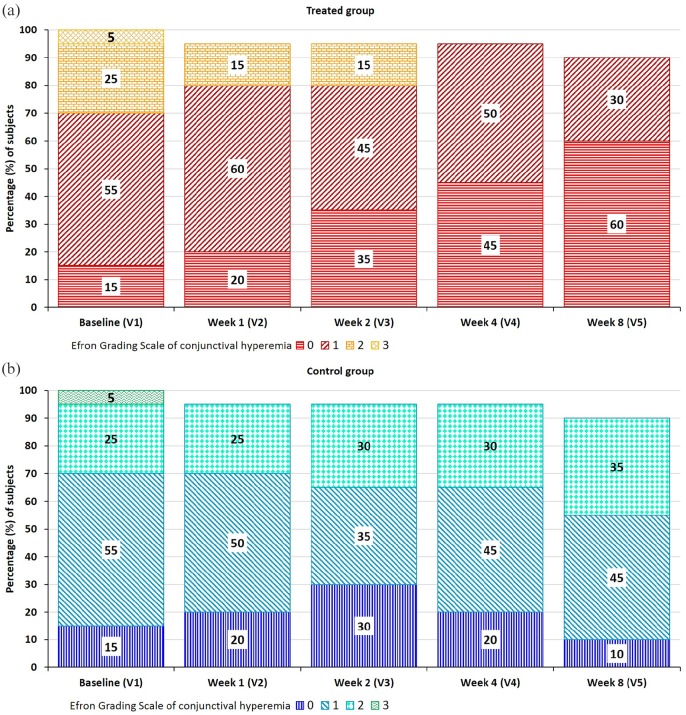
Efron Grading Scale is reported as a descriptive measure of conjunctival hyperemia, not being included among secondary endpoints. At baseline, the treated and control groups of eyes presented the same basal conditions. During the study, in the group of treated eyes, the percentage of eyes in class 0 increased over time (to 20%, 35%, 45%, and 60% after 1, 2, 4, and 8 weeks, respectively), and the proportion of eyes in class 1 decreased consequently (Figure 4(a)). After 4 weeks, there were no more eyes in class 2. Conversely, in the control group, the proportion of eyes in classes 0 and 1 remained rather unvaried over time, and the proportion of eyes in class 2 slightly increased (Figure 4(b)). At the end of the study (week 8), the majority of treated eyes healed (i.e. reached grade 0) and only the 30% (i.e. six eyes) were graded 1, while in the control group only the 10% of the eyes achieved grade 0.
Figure 4.
Distribution of the percentage of subjects at each visit according to Efron Grading Scale of conjunctival hyperemia: (a) treated group of eyes and (b) control group of eyes.
Safety analysis
Among the 19 professional water-polo athletes analyzed for safety, 7 subjects (36.8%) reported eight adverse events (AEs) but none was related to the long-term application of the ophthalmic solution. Five AEs were of mild intensity, two moderate, and one was an SAE (life threatening or disabling) due to a car accident occurred soon after the sign of the ICF (Table 2). The SAE led to exclusion of the injured subject from the study.
Table 2.
Adverse events reported by professional swimmers during the study (safety population).
| Patient No. | Age | Event | Outcome | Severity | Relationship with study device |
|---|---|---|---|---|---|
| 01 | 35 | Fever | Recovered/resolved | Mild | None |
| 02 | 33 | Fever | Recovered/resolved | Mild | None |
| 02 | 33 | Abdominal pain | Recovered/resolved | Mild | None |
| 13 | 16 | Ocular burning and itch | Recovered/resolved | Moderate | None |
| 14 | 17 | Flu-like symptoms | Recovered/resolved | Mild | None |
| 17 | 15 | Headache | Recovered/resolved | Mild | None |
| 18 | 15 | Gastritis | Recovered/resolved | Moderate | None |
| 20 | 19 | Car accident | Recovered/resolved with residual effects | Life threatening or disabling | None |
Discussion
Hyaluronic acid is a natural constituent found in the lacrimal gland, corneal epithelium, vitreous, conjunctiva,10–12 and tear fluid.13,14 The crosslinked form (XLHA) is more stable and efficacious compared to non-stabilized polymeric structure, provided with increased rigidity of the polymer network, extended permanence on the site of application, and decreased susceptibility to enzymatic degradation, thus reducing the daily number applications of a formulation. Its viscoelastic, mucomimetic, and moisturizing properties are thus enhanced. CoQ10 is an organic constituent of biological membranes, particularly of mitochondria where it works as an electron transporter.15 The local administration of CoQ10 after corneal epithelium removal promotes corneal wound healing and reduces corneal damages by preserving mitochondrial function.8 Vitamin E TPGS is an esterified form of vitamin E succinate with polyethylene glycol 1000 with well-established properties in wetting, emulsification, solubilization, spreading, and detergency.16
Our results sustain the efficacy of the long-term local application of the combination of XLHA, CoQ10, and vitamin E TPGS in restoring the corneal and conjunctival health of professional water-polo athletes after prolonged exposure to water added with chlorine. The local instillation of the ophthalmic solution clearly improved the tear film health (BUT) in the treated eyes compared to the untreated ones, and this difference was statistically significant. It may be supposed that the continuous exposure of professional swimmers to chlorinated water could have prolonged the time of the healing process. The evident deterioration of the tear film in the untreated eyes and the fact that the treated and untreated eyes belonged to the same subjects (this feature of the study design allowed to exclude variability of the results in case different groups of subjects would have been compared) are conditions that allow us to attribute the benefit obtained to the ophthalmic solution used. Apparently, the 2-month treatment period may seem too long before obtaining an evident beneficial tear film effect, but other experiences confirm that the tear BUT in patients with evaporative dry eye improved at 1–3 months after treatment with hyaluronic acid combined with other compounds (i.e. lipid-targeting agents or aqueous supplements).17
Consistent with the BUT results, the outcomes of corneal and conjunctival staining with fluorescein improved significantly in the group of eyes treated with the ophthalmic solution, thus suggesting that damages of the exposed corneas and conjunctivas regressed significantly in the group of treated eyes compared to the control group. This difference became evident just after 1 week of treatment. At the end of the first week, swimmers experienced a significant improvement in their initial ocular disability (OSDI score), probably due to the fast restoration of the tear film rheological properties in the treated eyes, as gathered also by results of the ocular surface staining. Nevertheless, it is difficult to explain the trend reversal recorded at the end of the second week. We can only speculate in a temporary decrease of compliance in the local instillation of the ophthalmic solution TID and/or an increase of ocular discomfort/very slow progression to improvement of conjunctival hyperemia as registered by the Efron Grading Scale. Finally, we can hypothesize that, in assessing the overall condition of the ocular health status subjectively reported by each subject, the improvement recorded after 1 month and further increased after 2 months in the treated eyes had greater weight compared to the sensation of discomfort experienced in the untreated eyes. Our results on the stable improvement of the ocular surface health after 1 month of treatment are consistent with those of Postorino et al.18 They showed that when the same combination of XLHA, CoQ10, and vitamin E TPGS was instilled in patients affected by mild to moderate dry eye disease, the OSDI score decreased significantly after 1 month and the improvement remained stable at the 90-day visit.
Other tests as Schirmer I test, beating of eyelashes, and tear osmolarity did not report a significant difference between treated and untreated eyes, but they all showed a better trend over time of eyes instilled with the ophthalmic solution. In addition, personal satisfaction improved significantly at the end of the study, reflecting the overall improvements achieved in the treated eyes and measured with the variety of tests used in this study.
The ocular tolerability of the medical device was excellent, as none of the AEs observed were related to the investigational product.
Although this was a pilot study performed on a small sample size, the peculiar nature of the study population (professional water-polo athletes), the stressful environment for ocular surface (prolonged exposure to chlorinated water), and the careful feature of the study design (treated and untreated eyes belonged to the same subject to avoid interindividual variability) allow us to believe that the adequate combination of XLHA, CoQ10, and vitamin E TPGS contained in a preservative-free solution (VisuXL) can effectively treat corneal and conjunctival injuries after environmental stress.
Acknowledgments
This study was managed by CRO 1MED Via Campagna 13, 6982 Agno—CH.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by VISUFARMA S.p.A., Via Canino 21, 00191 Rome, Italy.
Trial registration: This trial was registered at ClinicalTrials.gov (Evaluation of the VisuXL performance on ocular surface discomfort, https://clinicaltrials.gov/ct2/show/NCT03844737, ClinicalTrials.gov identifier: NCT03844737).
ORCID iD: Costanza Tredici  https://orcid.org/0000-0003-0419-6605
https://orcid.org/0000-0003-0419-6605
References
- 1. Mood EW, Clarke CC, Gelperin A. The effect of available residual chlorine and hydrogen ion concentration upon the eyes of swimmers. Am J Hyg 1951; 54(1): 144–149. [DOI] [PubMed] [Google Scholar]
- 2. Florentin A, Hautemaniere A, Hartemann P. Health effects of disinfection by-products in chlorinated swimming pools. Int J Hyg Environ Health 2011; 214(6): 461–469. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Acute illness and injury from swimming pool disinfectants and other chemicals: United States, 2002–2008. MMWR Morb Mortal Wkly Rep 2011; 60(39): 1343–1347. [PubMed] [Google Scholar]
- 4. Liu X, Yu FF, Zhong YM, et al. Therapeutic effects of sodium hyaluronate on ocular surface damage induced by benzalkonium chloride preserved anti-glaucoma medications. Chin Med J 2015; 128(18): 2444–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin T, Gong L. Sodium hyaluronate eye drops treatment for superficial corneal abrasion caused by mechanical damage: a randomized clinical trial in the People’s Republic of China. Drug Des Devel Ther 2015; 9: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ang BCH, Sng JJ, Wang PXH, et al. Sodium hyaluronate in the treatment of dry eye syndrome: a systematic review and meta-analysis. Sci Rep 2017; 7(1): 9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fallacara A, Vertuani S, Panozzo G, et al. Novel artificial tears containing cross-linked hyaluronic acid: an in vitro re-epithelialization study. Molecules 2017; 22(12): E2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mencucci R, Favuzza E, Boccalini C, et al. CoQ10-containing eye drops prevent UVB-induced cornea cell damage and increase cornea wound healing by preserving mitochondrial function. Invest Ophthalmol Vis Sci 2014; 55(11): 7266–7271. [DOI] [PubMed] [Google Scholar]
- 9. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stuart JC, Linn JG. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann Ophthalmol 1985; 17(3): 190–192. [PubMed] [Google Scholar]
- 11. Yoshida K, Nitatori Y, Uchiyama Y. Localization of glycosaminoglycans and CD44 in the human lacrimal gland. Arch Histol Cytol 1996; 59(5): 505–513. [DOI] [PubMed] [Google Scholar]
- 12. Lapcik L, Jr, Lapcik L, De Smedt S, et al. Hyaluronan: preparation, structure, properties, and applications. Chem Rev 1998; 98(8): 2663–2684. [DOI] [PubMed] [Google Scholar]
- 13. Frescura M, Berry M, Corfield A, et al. Evidence of hyaluronan in human tears and secretions of conjunctival cultures. Biochem Soc Trans 1994; 22(2): 228S. [DOI] [PubMed] [Google Scholar]
- 14. Fukuda M, Miyamoto Y, Miyara Y, et al. Hyaluronic acid concentrations in human tear fluids. Invest Ophthalmol Vis Sci 1996; 37: S848. [Google Scholar]
- 15. Lenaz G, Fato R, Castelluccio C, et al. The function of coenzyme Q in mitochondria. Clin Invest 1993; 71: S66–S70. [DOI] [PubMed] [Google Scholar]
- 16. Guo Y, Luo J, Tan S, et al. The applications of vitamin E TPGS in drug delivery. Eur J Pharm Sci 2013; 49(2): 175–186. [DOI] [PubMed] [Google Scholar]
- 17. Kim YH, Kang YS, Lee HS, et al. Effectiveness of combined tear film therapy in patients with evaporative dry eye with short tear film breakup time. J Ocul Pharmacol Ther 2017; 33(8): 635–643. [DOI] [PubMed] [Google Scholar]
- 18. Postorino EI, Rania L, Aragona E, et al. Efficacy of eyedrops containing cross-linked hyaluronic acid and coenzyme Q10 in treating patients with mild to moderate dry eye. Eur J Ophthalmol 2018; 28(1): 25–31. [DOI] [PubMed] [Google Scholar]