Key Points
Question
What are the cardiovascular risk profiles and the risk of acute myocardial infarction (AMI) in patients with alopecia areata (AA)?
Findings
In this large cohort study of 4 806 606 Korean participants, those with AA tended to have better cardiovascular risk profiles than controls in all items except smoking status. There was an increased risk of AMI among patients with AA over time during the 12-year follow-up period.
Meaning
Long-term monitoring of cardiovascular health and education might be appropriate in patients with AA to prevent AMI development.
This 12-year cohort study based on data from the Korean National Health Insurance database compares the cardiovascular risk profiles of patients with alopecia areata and controls to determine the risk of acute myocardial infarction in those with alopecia areata.
Abstract
Importance
Alopecia areata (AA) is a common autoimmune disease presenting as nonscarring hair loss. Although AA can be associated with other autoimmune comorbidities or atopic diseases, little is known about the risk of cardiovascular diseases in patients with AA.
Objective
To investigate the risk of acute myocardial infarction (AMI) and cardiovascular risk profiles (CVRPs) in patients with AA via a large-scale epidemiologic study.
Design, Setting, and Participants
This was a retrospective cohort study using data from the Korean National Health Insurance claims database, including data from the National Health Screening Program. Patients aged 30 to 89 years who were newly diagnosed with AA between January 1, 2006, and December 31, 2017, and controls without AA matched by age and sex were enrolled. Data were analyzed between July 2018 and August 2019.
Exposures
Presence of AA.
Main Outcomes and Measures
The CVRPs and incidence rates of AMI were assessed in participants with and without AA. The stratified Cox regression hazard model was used to estimate the relative hazards over time.
Results
A total of 228 886 patients with AA, ranging in age from 30 to 89 years (mean [SE] age, 44.37 [0.005] years; 127 564 [55.7%] men) and 4 577 720 matched controls without AA were identified. Patients with AA tended to have slightly better CVRPs than controls in all items except smoking status before and after the diagnosis (participants with normal systolic blood pressure who were nonsmokers: 44.6% vs 42.7% and 57.8% vs 61.6% in patients with AA vs controls before and after the diagnosis, respectively). In the early phase of observation, the cumulative incidence of AMI in patients with AA was lower than that in controls (incidence rate ratio of AMI in patients with AA compared with that in controls, 0.52 [95% CI, 0.42-0.65] between 2-4 years); however, during the later phase of the 12-year follow-up period, it increased exponentially and was greater than in the control group (incidence rate ratio, 2.06 [95% CI, 1.71–2.45] between 8-10 years). Similarly, after adjusting for CVRPs, the risk of developing AMI was lower in patients with AA than in controls at the beginning of the observation period (adjusted hazard ratio (HR), 0.17 [95% CI, 0.12-0.25] between 0-2 years); however, by 8 years postdiagnosis, the risk was higher in those with AA (adjusted HR, 1.37 [95% CI, 1.11-1.70] between 8-10 years), and it increased thereafter (adjusted HR, 4.51 [95% CI, 3.65-5.58] between 10-12 years).
Conclusions and Relevance
In patients with AA, there was a significantly increased risk of AMI over time during the 12-year follow-up period independent of CVRPs. Close long-term monitoring of cardiovascular health in patients with AA might be appropriate.
Introduction
Alopecia areata (AA) is a common autoimmune hair loss disease mediated by the cytotoxic T cells attacking the hair bulb. It is well known that AA can be associated with other autoimmune comorbidities, including vitiligo and thyroiditis, or atopic diseases.1,2 To date, several studies have suggested that AA may be related to chronic immune-mediated inflammatory diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and psoriasis.3,4,5 Considering that these diseases have been proven to increase the risks of cardiovascular disease (CVD),6,7,8 AA might not be an autoimmune disease only confined to the hair follicles, but may be a systemic inflammation that increases the risk of CVDs. However, the cardiovascular effect has not been investigated much to date in patients with AA. Recently, cardiac troponin I, a heart disease biomarker, was shown to be significantly elevated in patients with AA, implying the possibility of increased heart remodeling.9 Nevertheless, to our knowledge, there has been only 1 epidemiologic study investigating the risk of ischemic heart disease, and it reported a trend toward decreased risk.10
The development of CVD is associated with cardiovascular risk profiles (CVRPs).11 Therefore, to investigate the association between CVD and AA, CVRPs must be determined in patients with AA and should be controlled in the analysis. Nevertheless, there are limited data regarding CVRPs in patients with AA. Some investigators have reported decreased prevalence of diabetes in patients with AA.12,13 Conversely, other investigators have shown that patients with AA had higher prevalence of hyperlipidemia.14,15 Another small-scale study showed a similar metabolic profile between patients with AA and controls.16 These studies have some limitations, such as small sample size and/or the definition of cardiovascular risk being heavily dependent on the diagnostic code.
In the present study, we investigated the risk of acute myocardial infarction (AMI) in patients with AA using the Korean National Health Insurance (NHI) claims database. In particular, using the National Health Screening Program (NHSP) data set included in the NHI claims database, we assessed the CVRPs of all study participants. Furthermore, we sought to determine whether there was an association between AA and the development of AMI based on age and sex after strict adjustment of CVRPs.
Methods
Study Design and Data Sources
We conducted a nationwide population-based retrospective cohort study using the Korean NHI Claims database, including the NHSP data, from January 2002 to December 2017. Data were analyzed between July 2018 and August 2019. South Korea has one of the largest NHI systems worldwide, a system that is mandated by law and covers up to 98% of the entire Korean population. The Korean NHI claims database records diagnoses based on International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. It has been used to provide reliable estimates of the prevalence of certain diseases in the country.17,18 The Korean NHI claims database also contains data from the NHSP, which is a population-based health screening program provided by the Korean NHI corporation. All insured adults are eligible for NHSP and recommended to undergo standardized medical examinations every 1 or 2 years (eMethods in the Supplement). This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital. The review board waived patient consent because of the characteristics of this study.
Identification of Study Patients and Control Group
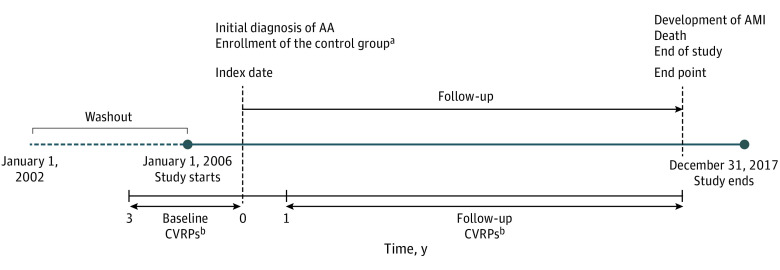
We identified patients with AA as those who had 3 or more documented physician visits per year between January 1, 2006, and December 31, 2017, with a diagnostic code for AA, alopecia totalis (AT), alopecia universalis (AU), or ophiasis (ICD-10 code L63.9, L63.0, L63.1, or L63.2, respectively). To define newly diagnosed cases, a 4-year washout period with no documented AA was established (Figure 1). The exclusion criteria were as follows: (1) patients who did not participate in a health screening 3 years before the initial diagnosis, (2) patients who had been diagnosed with AMI before the initial diagnosis, and (3) patients who were younger than 30 years or older than 80 years.
Figure 1. Overview of the Study.
AA indicates alopecia areata; AMI, acute myocardial infarction; CVRPs, cardiovascular risk profiles.
aMedian value of the date of initial diagnosis in matched patients with AA. Matching was done with age, sex, index year, and the year of health checkup.
bIf the participants engaged in multiple screening programs, the data collected closest to the index date were selected.
The control group was composed of all individuals who participated in the NHSP between 2002 and 2017 and had not been officially diagnosed with AA during the same period. The same exclusion criteria as those for study patients were applied to the control group. Next, we randomly selected controls (20 per 1 AA patient) after matching year by year with the study patients for age, sex, and the year of health checkup.
The index date for patients with AA was defined as the date of initial AA diagnosis. The index date for the controls was defined as the date of initial diagnosis in matched patients with AA. When there were multiple matched patients with AA for each matching stratum, the median value of the date of initial diagnosis of matched patients with AA was chosen (Figure 1).
Assessment of CVRPs
The CVRPs of participants, including body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP), fasting blood sugar (FBS) levels, and serum cholesterol levels, as well as smoking history, were acquired from the NHSP data. Each item of the CVRP was categorized as described in eMethods in the Supplement. We obtained the CVRPs 2 times during the study period, once before and once after the index date (Figure 1), to identify if there were any changes. First, to identify the baseline CVRPs of participants, NHSP data from 3 years before the index date were obtained. Second, to identify if there were any changes in the postdiagnosis CVRPs, we obtained the NHSP data from 1 year after the index date to the end point (follow-up CVRPs) in both patients with AA and controls. If the participants engaged in multiple screening programs, the data that were collected closest to the index date were selected in both baseline and follow-up CVRPs.
Study Outcome
Newly diagnosed AMI was the outcome of interest in this study. Patients with AMI were defined as those who had 1 or more documented hospitalization with a principal diagnosis code of AMI (ICD-10 code I21). AMI is one of the CVDs applicable to the Special Support for Serious Illness (SSI) act, which was launched by the Korean government and reduced the statutory coinsurance rate for registered patients. Because registration requires a physician’s confirmation and an additional review by another health care professional to ensure that the illness meets the diagnostic criteria, the data regarding the illnesses are considered reliable. The end point of the study was defined as death or diagnosis of AMI, or the end of the study (December 31, 2017) if neither of the former occurred (Figure 1).
Verification of Diagnosis
To verify diagnostic accuracy, we developed several algorithms based on the number of health care visits with a diagnostic code of AA (eMethods and eTable 1 in the Supplement). The algorithm selected for use in the present study (based on defining patients with AA as those having been diagnosed with AA 3 or more times) achieved a sensitivity of 90.6% and a specificity of 90.8%.
Statistical Analysis
The aim of this study is to investigate whether AA is an independent risk factor for AMI after adjusting the CVRPs. We compared baseline and follow-up CVRPs between patients with AA and controls using the McNemar test and standardized difference (eMethods in the Supplement).
We compared the AMI incidence between patients with AA and controls without AA. The person-years for each participant in both AA and control groups were calculated from the index date to their end of follow-up. The crude incidence rates (IRs) of AMI were calculated as the number of AMI cases per 1000 person-years. The IR ratio (IRR) for AMI was calculated as the IR of AMI in patients with AA compared with the IR of AMI in controls.
A multivariable stratified Cox proportional hazards regression model considering the matched study design was used to calculate the adjusted hazard ratio (HR) and corresponding 95% CIs for the association of AA and baseline CVRPs with subsequent risk of AMI. Matching variables were sex, age, index year, and the year of health checkup. The risk factors were AA and baseline CVRPs (BMI, SBP, DBP, FBS level, and serum cholesterol level). The proportional hazard assumption was tested using the Schoenfeld and scaled Schoenfeld residuals. Because AA violated the proportional hazard assumption, an extended stratified Cox regression model considering the interaction between time and AA was conducted to analyze the change in risk of AMI in patients with AA over time. The observation period was divided into 6 periods (2-year intervals) and the HRs of each period were calculated. eFigure 1 in the Supplement represents a flow chart of statistical analysis in this study. All statistical analyses were conducted using the statistical package STATA, version 15.0 (StataCorp). Statistical tests were 2-sided, and a P < .05 was considered to indicate statistical significance.
Subgroup Analysis
Subgroup analyses according to sex and age (<50 and ≥50 years) were performed; both variables are well known to be associated with development of AMI.19,20 In addition, a subgroup analysis according to subtypes of AA was carried out to identify the influence of disease extent.
Subtypes of AA were defined as AT, AU, and patch-type AA (all cases except AT and AU). Furthermore, a subgroup analysis by smoking status was also performed, where a participant was defined as a nonsmoker with pack-years of 0 and as a smoker when pack-years exceeded 0.
Sensitivity Analysis
A sensitivity analysis was conducted to identify whether the difference in health care use between patients with AA and controls affected outcomes (eMethods and eTable 2 in the Supplement). Another sensitivity analysis was conducted to identify whether the differences in timing for measuring CVRPs influenced outcomes (eMethods in the Supplement).
Results
Characteristics and CVRPs in Study Population
We identified 228 886 patients with AA (mean [SE] age, 44.37 [0.005]; 127 564 [55.7%] men) and 4 577 720 matched controls without AA (eTable 3 in the Supplement). The follow-up time was up to 12 years (mean [SD] follow up 6.2 [3.2] years in patients with AA and 6.1 [2.9] years in matched controls). Table 1 summarizes the baseline demographic data and CVRPs of the patients. In the AA group, 127 564 (55.73%) were male, and the peak age of incidence of AA was between 40 and 49 years.
Table 1. Baseline Characteristics and Cardiovascular Risk Profiles of Patients With Alopecia Areata (AA) and Matched Controls.
| Characteristic | No. (%) | Standardized differencea | ||||
|---|---|---|---|---|---|---|
| Patients with AA (n = 228 886) | Matched controls (n = 4 577 720) | |||||
| Total | AT (n = 12 131) | AU (n = 7765) | Patch-type AA (n = 208 990) | |||
| Sex | ||||||
| Male | 127 564 (55.7) | 6860 (56.6) | 4164 (53.6) | 116 540 (55.8) | 2 551 280 (55.73) | 0 |
| Female | 101 322 (44.3) | 5271 (43.4) | 3601 (46.4) | 92 450 (44.2) | 2 026 440 (44.27) | |
| Age, y | ||||||
| 30 to 39 | 55 881 (24.4) | 2750 (22.7) | 1651 (21.3) | 51 480 (24.6) | 1 117 620 (24.4) | 0 |
| 40 to 49 | 72 211 (31.6) | 3700 (30.5) | 2166 (27.9) | 66 345 (31.8) | 1 444 220 (31.6) | |
| 50 to 59 | 63 127 (27.6) | 3383 (27.9) | 2220 (28.6) | 57 524 (27.5) | 1 262 540 (27.6) | |
| 60 to 69 | 27 974 (12.2) | 1656 (13.6) | 1206 (15.5) | 25 112 (12.0) | 559 480 (12.2) | |
| 70 to 79 | 8505 (3.7) | 542 (4.5) | 446 (5.7) | 7517 (3.6) | 170 100 (3.7) | |
| 80 to 89 | 1188 (0.5) | 100 (0.8) | 76 (1.0) | 1012 (0.5) | 23 760 (0.5) | |
| BMI, kg/m2 | ||||||
| <18.5 | 8338 (3.6) | 422 (3.5) | 246 (3.2) | 7670 (3.7) | 171 390 (3.7) | 0.04 |
| 18.5 to <25 | 150 803 (65.9) | 8102 (66.8) | 5273 (67.9) | 137 428 (65.8) | 2 931 641 (64.1) | |
| ≥25 | 69 689 (30.5) | 3603 (29.7) | 2242 (28.9) | 63 844 (30.5) | 1 472 847 (32.2) | |
| Missingb | 56 (0) | 4 (0) | 4 (0) | 48 (0) | 1842 (0) | |
| Blood pressure, mm Hg | ||||||
| Systolic | ||||||
| <120 | 102 103 (44.6) | 5312 (43.8) | 3346 (43.1) | 93 445 (44.7) | 1 952 983 (42.7) | 0.06 |
| 120 to <140 | 106 679 (46.6) | 5734 (47.3) | 3666 (47.2) | 97 279 (46.6) | 2 156 799 (47.1) | |
| ≥140 | 20 069 (8.8) | 1083 (8.9) | 751 (9.7) | 18 235 (8.7) | 467 111 (10.2) | |
| Missingb | 35 (0) | 2 (0) | 2 (0) | 31 (0) | 827 (0) | |
| Diastolic | ||||||
| <80 | 132 629 (58.0) | 7010 (57.8) | 4438 (57.2) | 12 1181 (58.0) | 2 569 632 (56.1) | 0.05 |
| 80 to <90 | 75 370 (32.9) | 4015 (33.1) | 2563 (33.0) | 68 792 (33.0) | 1 534 485 (33.6) | |
| ≥90 | 20 852 (9.1) | 1104 (9.1) | 762 (9.8) | 18 986 (9.0) | 472 746 (10.3) | |
| Missingb | 35 (0) | 2 (0) | 2 (0) | 31 (0) | 857 (0) | |
| FBS, mg/dl | ||||||
| <100 | 170 254 (74.4) | 8963 (73.9) | 5745 (74.0) | 155 546 (74.4) | 3 326 978 (72.7) | 0.05 |
| 100 to <126 | 49 633 (21.7) | 2672 (22.0) | 1697 (21.9) | 45 264 (21.7) | 1 026 260 (22.4) | |
| ≥126 | 8942 (3.9) | 492 (4.1) | 322 (4.1) | 8128 (3.9) | 223 401 (4.9) | |
| Missingb | 57 (0) | 4 (0) | 1 (0) | 52 (0) | 1081 (0) | |
| Total cholesterol, mg/dl | ||||||
| <200 | 136 662 (59.7) | 7274 (60.0) | 4475 (57.6) | 124 913 (59.8) | 2 715 782 (59.4) | 0.01 |
| 200 to <240 | 68 552 (23.0) | 3650 (30.1) | 2396 (30.9) | 62 506 (29.9) | 1 372 323 (30.0) | |
| ≥240 | 23 563 (10.3) | 1201 (9.9) | 893 (11.5) | 21 469 (10.3) | 487 598 (10.6) | |
| Missingb | 109 (0) | 6 (0) | 1 (0) | 120 (0) | 2017 (0) | |
| Smoking, pack-years | ||||||
| 0 | 132 257 (57.8) | 7178 (59.2) | 4796 (61.8) | 120 283 (57.6) | 2 773 217 (61.6) | 0.06 |
| 1 to <10 | 37 271 (16.3) | 1833 (15.1) | 1169 (15.1) | 34 269 (16.4) | 697 958 (15.3) | |
| 10 to <20 | 32 619 (14.3) | 1724 (14.2) | 965 (12.4) | 29 930 (14.3) | 599 523 (13.1) | |
| 20 to <30 | 14 540 (6.3) | 693 (5.7) | 442 (5.7) | 13 405 (6.4) | 266 878 (5.8) | |
| 30 to <40 | 6057 (2.6) | 321 (2.7) | 181 (2.3) | 5555 (2.7) | 111 805 (2.4) | |
| ≥40 | 3177 (1.4) | 165 (1.4) | 100 (1.4) | 2903 (1.4) | 62 265 (1.4) | |
| Missingb | 2965 (1.3) | 217 (1.7) | 112 (1.3) | 2600 (1.2) | 66 074 (1.4) | |
Abbreviations: AT, alopecia totalis; AU, alopecia universalis; BMI, body mass index; FBS, fasting blood glucose.
SI conversion factors: to convert FBS to millimoles per liter, multiply by 0.0555; to convert total cholesterol to millimoles per liter, multiply by 0.0259. Standardized differences less than 0.1 indicates negligible differences between groups (patients with AA vs matched controls).
Missing indicates measurement error; missing data are included in totals.
In the baseline CVRP, all CVRPs except smoking status showed a tendency to be better in patients with AA (Table 1), although the differences were not big enough to be clinically significant (all standardized differences <0.1). On the other hand, the percentage of smokers was slightly higher in the AA group (25.3%) compared with the control group (23.0%) (standardized difference, 0.065; P < 0.001).
In follow-up CVRPs, similar to the baseline, all items except smoking status tended to be better in the AA group compared with the control group (eTable 4 in the Supplement). There was no significant difference in the appearance of CVRPs between the 2 groups (eTable 5 in the Supplement).
Incidence of AMI
In both AA and control groups, there were 14 028 newly diagnosed AMI cases during the 12-year follow-up period (715 in AA group, 13 313 in control group). The IR of AMI was 0.56 (95% CI, 0.52-0.60) per 1000 person-years for patients with AA and 0.52 (95% CI, 0.51-0.53) per 1000 person-years for matched controls(Table 2). The IRR of AMI between patients with AA and controls was 1.07 (95% CI, 0.99-1.15) in the total population.
Table 2. Risk of Acute Myocardial Infarction in Patients With Alopecia Areata (AA) and Matched Controls.
| Characteristic | Patients with AA | Matched controls | IRR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| No. of cases | Person-years | IR per 1000 person-years (95% CI) | No. of cases | Person-years | IR per 1000 person-years (95% CI) | ||
| Total | 715 | 1 277 270 | 0.56 (0.52-0.60) | 13 313 | 25 462 241 | 0.52 (0.51-0.53) | 1.07 (0.99-1.15) |
| AT | 41 | 76 416 | 0.54 (0.40-0.73) | NA | NA | NA | 1.03 (0.74-1.39) |
| AU | 34 | 48 915 | 0.70 (0.50-0.97) | NA | NA | NA | 1.33 (0.92-1.86) |
| Patch-type AA | 640 | 1 151 939 | 0.56 (0.51-0.60) | NA | NA | NA | 1.06 (0.98-1.15) |
| Age, y | |||||||
| <50 | 185 | 673 946 | 0.27 (0.24-0.32) | 2963 | 13 474 940 | 0.22 (0.21-0.23) | 1.25 (1.07-1.45) |
| ≥50 | 530 | 603 324 | 0.88 (0.81-0.96) | 10 350 | 11 987 301 | 0.86 (0.85-0.88) | 1.02 (0.93-1.11) |
| Sex | |||||||
| Male | 558 | 721 093 | 0.77 (0.71-0.84) | 9917 | 14 357 158 | 0.69 (0.68-0.70) | 1.12 (1.03-1.22) |
| Female | 157 | 556 177 | 0.28 (0.24-0.33) | 3369 | 11 105 083 | 0.31 (0.30-0.32) | 0.92 (0.78-1.08) |
| Smoking, pack-years | |||||||
| 0 | 281 | 760 311 | 0.38 (0.34-0.42) | 6113 | 15 843 883 | 0.39 (0.38-0.40) | 0.97 (0.86-1.09) |
| 1 to <10 | 71 | 186 095 | 0.39 (0.31-0.49) | 1204 | 3 491 540 | 0.35 (0.33-0.37) | 1.11 (0.86-1.41) |
| 10 to <20 | 141 | 184 534 | 0.77 (0.65-0.91) | 2520 | 3 376 799 | 0.75 (0.72-0.78) | 1.03 (0.86-1.22) |
| 20 to <30 | 101 | 74 795 | 1.36 (1.12-1.65) | 1560 | 1 343 731 | 1.17 (1.11-1.23) | 1.17 (0.95-1.43) |
| 30 to <40 | 57 | 30 431 | 1.88 (1.45-2.43) | 823 | 549 572 | 1.50 (1.40-1.61) | 1.26 (0.94-1.64) |
| >40 | 46 | 15 692 | 2.94 (2.20-3.92) | 743 | 295 106 | 2.52 (2.35-2.71) | 1.17 (0.85-1.57) |
| Missing | 17 | 25 412 | 0.67 (0.42-1.08) | 350 | 561 610 | 0.63 (0.57-0.70) | 0.92 (0.78-1.08) |
Abbreviations: AT, alopecia totalis; AU, alopecia universalis; IR, incidence rate; IRR, incidence rate ratio; NA, not applicable.
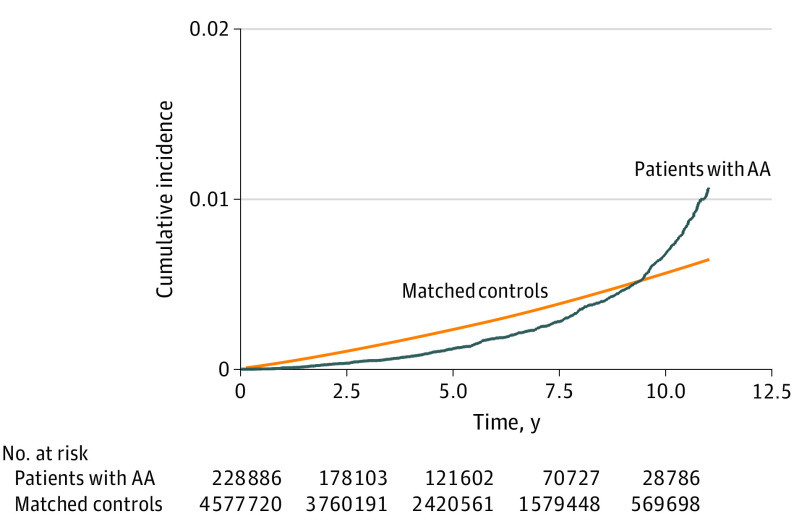
The Kaplan-Meier curves of cumulative incidence of developing AMI in patients with AA and matched controls showed a different pattern (Figure 2). Contrary to the gradual increase in incidences shown in the control group, the incidences in the AA group showed an exponential increase. The incidence of AMI in the AA group was lower than that in the control group at the beginning of the observation period; however, during the later phase, incidence of AMI in the AA group increased rapidly to be higher than that in the control group.
Figure 2. Cumulative Incidences of Acute Myocardial Infarction in Patients With Alopecia Areata (AA).
The risk of developing AMI after adjusting for CVRPs also showed a time-dependent change in patients with AA (Figure 3). In the extended Cox model considering the interaction between time and AA, the risk of patients with AA developing AMI was lower than that in the controls at the beginning of the study period (HR, 0.17 [95% CI, 0.12-0.25] between 0-2 years from the index date). However, it increased over time (HR, 0.35 [95% CI, 0.27-0.46] between 2-4 years from the index date; HR, 0.70 [95% CI, 0.57-0.87] between 4-6 years from the index date; and HR, 0.85 [95% CI, 0.69-1.06] between 6-8 years from the index date) and got significantly higher than in the controls between 8 and 10 years (HR, 1.37 [95% CI, 1.11-1.70]). Then the risk increased sharply to the end of the study period (HR, 4.51 [95% CI, 3.65-5.58] between 10-12 years from the index date).
Figure 3. Forest Plots of the Risk of Acute Myocardial Infarction in Different Groups of Patients With Alopecia Areata (AA) According to the Observation Period.
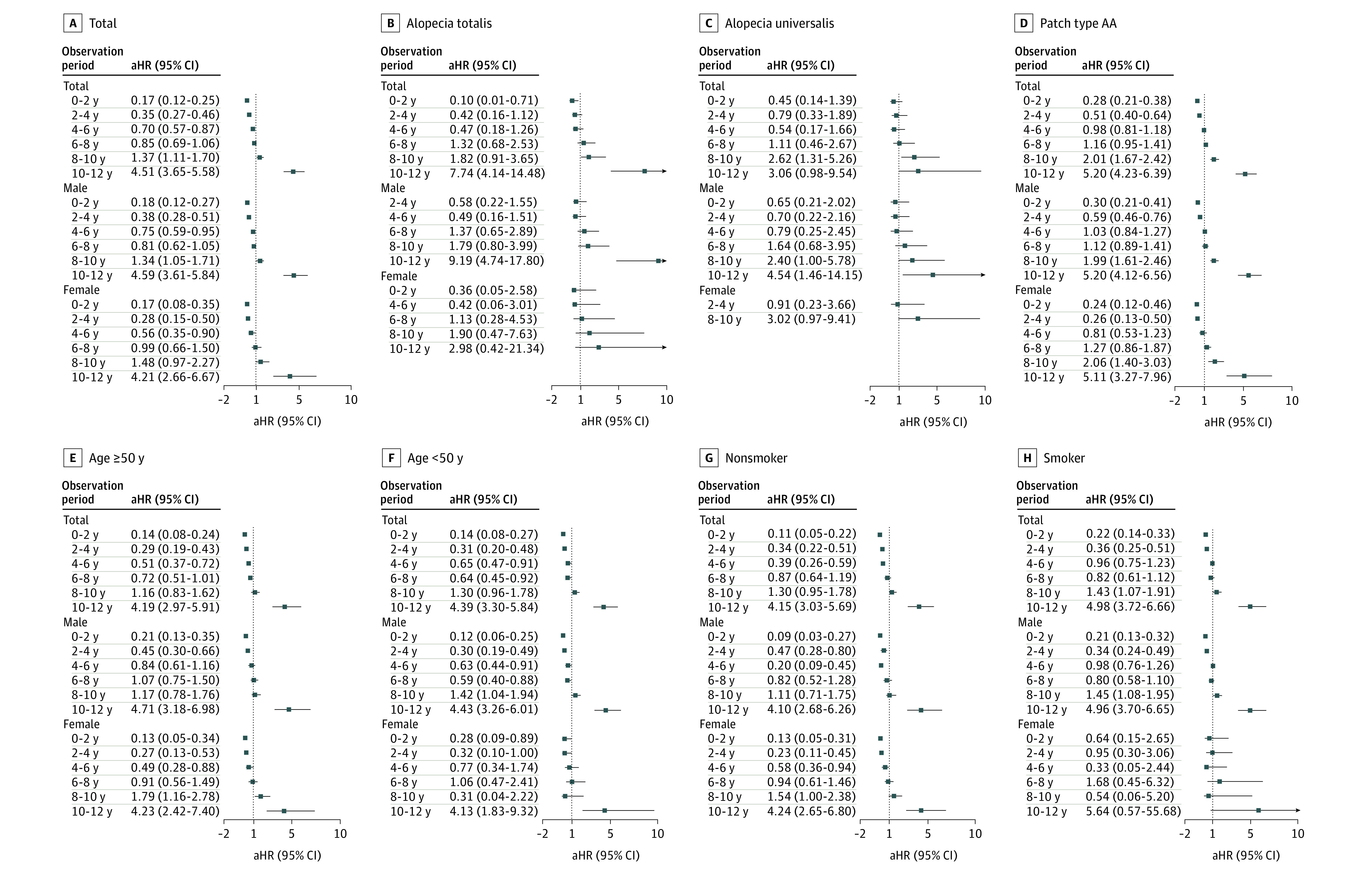
A, Total number of patients. B, Patients with alopecia totalis. C, Patients with Alopecia universalis. D, Patients with patch type AA. E, Patients 50 years or older. F, Patients younger than 50 years. G, Patients who are nonsmokers. H, Patients who are smokers. Abbreviation: aHR, adjusted hazard ratio.
Association of CVRPs With Development of AMI
As shown in eFigure 2 in the Supplement, all items defined as CVRPs in this study (BMI, SBP, DBP, FBS, cholesterol level, and smoking status) were demonstrated to be associated with AMI development in both men and women. We can confirm that an adjustment of these factors is reasonable for investigating the risk of AMI development.
Subgroup Analysis
In the subgroup analysis by sex, the IR of AMI was significantly higher in men with AA (IRR, 1.12 [95% CI, 1.03-1.22]) while there was no difference in rates in women (Table 2). After adjusting CVRPs, the time point when the risk of AMI in AA became significantly higher than in the controls was earlier in men than in women (after 8 years vs after 10 years from the index date) (Figure 3). The baseline CVRPs according to sex showed a similar pattern between men and women (eTable 6 in the Supplement).
In the subgroup analysis by age, the IR of AMI was significantly higher in the population with AA younger than 50 years (IRR, 1.25 [95% CI, 1.07-1.45]) while there was no difference in the population 50 years and older (Table 2). After adjusting CVRPs, the time point when the risk of AMI in AA became significantly higher than in the controls was 10 years from the index date in both groups (Figure 3).
In the subgroup analysis by subtypes of AA, the IR of AMI in patients with AU was nonsignificantly higher (IRR, 1.33 [95% CI, 0.92-1.86]) than in other types (Table 2). After adjusting CVRPs, all the subtypes of AA showed similar time-dependent patterns (Figure 3). Specifically, in AT, the risk of AMI was lower than in other types in the early phase of observation but increased drastically at the end of the observation period. In AU, the risk of AMI was higher than in other types in the early phase of observation and increased more slowly during the later phase.
In the subgroup analysis by smoking status (pack-years), the association between AA and development of AMI tended to be stronger in smokers than in nonsmokers (Figure 3). The time point when the risk of AMI in AA became significantly higher than in the controls was earlier in smokers than in nonsmokers (after 8 years vs after 10 years from the index date, respectively).
Sensitivity Analysis
After matching with health care use, the association of CVRPs with the risk of AMI development in patients with AA were not different from those before the matching (eTable 7 and eFigure 3 in the Supplement). In addition, the associations of CVRPs measured at 3 years after the index date were not significantly different from those measured from 1 year after the index date to the end point (eTable 4 and 5 in the Supplement).
Discussion
In this nationwide cohort study, we identified 228 886 patients with incident AA and 4 577 720 matched controls to evaluate the risk of AMI in patients with AA. To our knowledge, this is one of the first publications with a large, validated cohort of patients with AA evaluating the risk of developing AMI. Interestingly, in the present study, the risk of developing AMI was associated with unique, time-dependent changes in patients with AA. The risk was lower at the beginning of the study period; however, it increased continuously throughout the study period, with further increase at 8 years after the initial diagnosis.
In the present study, we evaluated CVRPs in patients with AA based on the actual level of each test item using the NHSP data. Our results indicated that there was no significant difference in baseline CVRPs (SBP, DBP, BMI, FBS level, and blood cholesterol level) between patients with AA and controls, although patients with AA have generally better results compared with those without AA, except smoking status. Notably, we checked the CVRPs after the AA diagnosis, because the risk of developing AMI showed time-varying changes. The follow-up CVRP showed a similar pattern; patients with AA tended to have better CVRPs except smoking. This implies that the disease course of AA or treatment of AA might not affect CVRPs of patients. The reason for lower risk of AMI during the early phase in patients with AA might be attributed to the associated factors with better CVRPs in this study. Nonetheless, the reason for an exponential increase in the risk of developing AMI is not clear. We were able to demonstrate that this was not associated with the deterioration of CVRPs by identifying that there was no change in CVRPs after the AA diagnosis. Alopecia areata might be an independent risk factor for AMI development. Further investigations are necessary to confirm this phenomenon and elucidate the possible mechanisms.
In particular, the IRs before adjusting CVRPs demonstrated a different risk of AMI development according to sex and age. In men with AA and in patients younger than 50 years with AA, the risk of AMI was significantly increased compared with controls. However, after adjusting CVRPs, the risk of AMI in all the subgroups increased during the later phase of observation, although the point of incidence reversal was earlier in men than in women. Regarding smoking status, the association of AA with development of AMI tended to be stronger in smokers than in nonsmokers.
Huang et al10 conducted a propensity score–matched retrospective study on 1377 patients with AA and 4131 matched controls and reported a trend toward decreased risk of AMI. In this study, the sample size was relatively small, and BMI—one of the major confounders—was not considered. Moreover, other CVRPs considered as confounding factors were solely dependent on the diagnostic codes. Above all, the most prominent difference between the study by Huang et al10 and the present study is that they did not observe changes in the risk over time.
Although the cause of the association between AA and AMI remains unclear, the chronic inflammatory status of AA is considered to be a promoting and accelerating factor of AMI development. In previous studies, C-reactive protein level, which is a marker for systemic inflammation, has been reported to be elevated in patients with AA.9,21 Moreover, increased oxidative stress in patients with AA was also reported.16,22,23 Thus, it could be possible that patients with AA undergo significant pathophysiological changes in their inflammatory and oxidative statuses, which may be associated with arthrosclerosis.24 Meanwhile, Wang et al9 demonstrated that the plasma level of a major CVD marker, cardiac troponin I, was higher in patients with AA than in controls, and the plasma samples with high cardiac troponin I levels also induced significantly higher rates of cardiomyocyte apoptosis in the cell culture assay. Current evidence suggests that cardiac troponin I may be a marker not only for AMI, but also for chronic ischemic heart disease, which can also ultimately affect cardiomyocytes by itself.25 Interestingly, the elevation of plasma levels of cardiac troponin I was more prominent in younger age groups and men, indicating that younger and male individuals with AA might be affected more often by cardiac diseases, which is consistent with our results.
Limitations
This study has several limitations. First, the NHI claims database may include some misdiagnoses. To reduce such errors, we defined patients with AA as those who visited a physician at least 3 times in a given year, at which visits the patient was diagnosed with AA; we then validated the accuracy of this definition. Second, the NHI claims database did not provide detailed clinical information on individual patients, including disease onset, disease duration, or treatment histories. Third, because we only included adult patients who participated in the NHSP, only adults were included. However, considering that AMI is extremely rare in children and young adolescents, it would not have a major effect on our results. Finally, we did not match health care use between patients with AA and controls. However, according to the results from the sensitivity analysis, additional matching with health care use did not change this study’s main results. Despite these limitations, the study has certain strengths. First, to our knowledge, it is the largest study investigating comorbidities in patients with AA. With a large sample size, this study has sufficient power to study the risk of AMI in patients with AA by age and sex. Moreover, we performed an incidence-case cohort study with a sufficient follow-up duration, allowing us to observe the changes of cumulative incidence and risk of AMI in patients with AA. Finally, by using the NHSP data, we were able to accurately access the CVRPs of patients and adjust these factors.
Conclusions
In conclusion, there was a significantly increased risk of AMI over time during the 12-year follow-up period, independent of CVRPs. Whether the association between AA and AMI is causal requires further research. Acute myocardial infarction is a major cause of mortality and morbidity of CVD. These results suggest that close, long-term monitoring of cardiovascular health and education, such as antismoking measures for patients with AA, might be appropriate to prevent the development of AMI.
eMethods.
eTable 1. Validation algorithms of alopecia areata(AA).
eTable 2. Characteristics of healthcare use of AA patients and controls.
eTable 3. Number of subjects enrolled by year.
eTable 4. Follow-up cardiovascular risk profiles in patients with AA and matched controls.
eTable 5. Changes of cardiovascular risk profiles in patients with AA and matched controls.
eTable 6. The baseline cardiovascular risk profiles in patients with AA and matched controls according to sex.
eTable 7. Baseline characteristics and cardiovascular risk profiles of alopecia areata patients and healthcare use-matched controls.
eFigure 1. Flowchart of statistical analyses.
eFigure 2. Forest plots of the risk of acute myocardial infarction in cardiovascular risk profiles used in this study.
eFigure 3. Forest plots of the risk of acute myocardial infarction in alopecia areata after matching with healthcare use.
References
- 1.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part 1. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62(2):177-188. doi: 10.1016/j.jaad.2009.10.032 [DOI] [PubMed] [Google Scholar]
- 2.Barahmani N, Schabath MB, Duvic M; National Alopecia Areata Registry . History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61(4):581-591. doi: 10.1016/j.jaad.2009.04.031 [DOI] [PubMed] [Google Scholar]
- 3.Hordinsky M, Ericson M. Autoimmunity: alopecia areata. J Investig Dermatol Symp Proc. 2004;9(1):73-78. doi: 10.1111/j.1087-0024.2004.00835.x [DOI] [PubMed] [Google Scholar]
- 4.Ishak RS, Piliang MP. Association between alopecia areata, psoriasis vulgaris, thyroid disease, and metabolic syndrome. J Investig Dermatol Symp Proc. 2013;16(1):S56-S57. doi: 10.1038/jidsymp.2013.22 [DOI] [PubMed] [Google Scholar]
- 5.Petukhova L, Christiano AM. Functional interpretation of genome-wide association study evidence in alopecia areata. J Invest Dermatol. 2016;136(1):314-317. doi: 10.1038/JID.2015.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Tong Q, Guo L, et al. Risk of coronary artery disease in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Am J Med Sci. 2018;356(5):451-463. doi: 10.1016/j.amjms.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303-1307. doi: 10.1161/01.CIR.0000054612.26458.B2 [DOI] [PubMed] [Google Scholar]
- 8.Vena GA, Vestita M, Cassano N. Psoriasis and cardiovascular disease. Dermatol Ther. 2010;23(2):144-151. doi: 10.1111/j.1529-8019.2010.01308.x [DOI] [PubMed] [Google Scholar]
- 9.Wang EH, Santos L, Li XY, et al. Alopecia areata is associated with increased expression of heart disease biomarker cardiac troponin 1. Acta Derm Venereol. 2018;98(8):776-782. doi: 10.2340/00015555-2964 [DOI] [PubMed] [Google Scholar]
- 10.Huang KP, Joyce CJ, Topaz M, Guo Y, Mostaghimi A. Cardiovascular risk in patients with alopecia areata (AA): a propensity-matched retrospective analysis. J Am Acad Dermatol. 2016;75(1):151-154. doi: 10.1016/j.jaad.2016.02.1234 [DOI] [PubMed] [Google Scholar]
- 11.Riccioni G, Sblendorio V. Atherosclerosis: from biology to pharmacological treatment. J Geriatr Cardiol. 2012;9(3):305-317. doi: 10.3724/SP.J.1263.2012.02132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conic RZ, Miller R, Piliang M, Bergfeld W, Atanaskova Mesinkovska N. Comorbidities in patients with alopecia areata. J Am Acad Dermatol. 2017;76(4):755-757. doi: 10.1016/j.jaad.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 13.Chu SY, Chen YJ, Tseng WC, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011;65(5):949-956. doi: 10.1016/j.jaad.2010.08.032 [DOI] [PubMed] [Google Scholar]
- 14.Kang JH, Lin HC, Kao S, Tsai MC, Chung SD. Alopecia areata increases the risk of stroke: a 3-year follow-up study. Sci Rep. 2015;5:11718. doi: 10.1038/srep11718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149(7):789-794. doi: 10.1001/jamadermatol.2013.3049 [DOI] [PubMed] [Google Scholar]
- 16.Incel-Uysal P, Akdogan N, Alli N, et al. Assessment of metabolic profile and ischemia-modified albumin level in patients with alopecia areata: a case-control study. Indian J Dermatol. 2019;64(1):12-18. doi: 10.4103/ijd.IJD_238_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. doi: 10.1093/heapol/czn037 [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Choi KH, Hwang SW, Lee YB, Park HJ, Bae JM. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76(1):40-48. doi: 10.1016/j.jaad.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Lee CH, Cheng CL, Yang YH, et al. Trends in the incidence and management of acute myocardial infarction from 1999 to 2008: Get With the Guidelines performance measures in Taiwan. J Am Heart Assoc. 2014;3(4):e001066 . doi: 10.1161/JAHA.114.001066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champney KP, Frederick PD, Bueno H, et al. ; NRMI Investigators . The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart. 2009;95(11):895-899. doi: 10.1136/hrt.2008.155804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gade VKV, Mony A, Munisamy M, Chandrashekar L, Rajappa M. An investigation of vitamin D status in alopecia areata. Clin Exp Med. 2018;18(4):577-584. doi: 10.1007/s10238-018-0511-8 [DOI] [PubMed] [Google Scholar]
- 22.Cwynar A, Olszewska-Słonina D, Czajkowski R, et al. Investigation of oxidative stress in patients with alopecia areata by measuring the levels of malondialdehyde and ceruloplasmin in the blood. Postepy Dermatol Alergol. 2018;35(6):572-576. doi: 10.5114/pdia.2017.68047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yenin JZ, Serarslan G, Yönden Z, Ulutaş KT. Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin Exp Dermatol. 2015;40(6):617-621. doi: 10.1111/ced.12556 [DOI] [PubMed] [Google Scholar]
- 24.Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845. doi: 10.1155/2019/8563845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everett BM. Cardiac troponin as a novel tool for cardiovascular risk prediction in ambulatory populations. Trends Cardiovasc Med. 2017;27(1):41-47. doi: 10.1016/j.tcm.2016.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Validation algorithms of alopecia areata(AA).
eTable 2. Characteristics of healthcare use of AA patients and controls.
eTable 3. Number of subjects enrolled by year.
eTable 4. Follow-up cardiovascular risk profiles in patients with AA and matched controls.
eTable 5. Changes of cardiovascular risk profiles in patients with AA and matched controls.
eTable 6. The baseline cardiovascular risk profiles in patients with AA and matched controls according to sex.
eTable 7. Baseline characteristics and cardiovascular risk profiles of alopecia areata patients and healthcare use-matched controls.
eFigure 1. Flowchart of statistical analyses.
eFigure 2. Forest plots of the risk of acute myocardial infarction in cardiovascular risk profiles used in this study.
eFigure 3. Forest plots of the risk of acute myocardial infarction in alopecia areata after matching with healthcare use.