Abstract
Composites comprised of Ag3PO4 and bare TiO2 (TiO2@Ag3PO4) or silver doped TiO2 (Ag@TiO2–Ag3PO4) have been synthesized by coupling sol–gel and precipitation methods. For the sake of comparison, also the bare components have been similarly prepared. All the samples have been characterized by X-ray diffraction (XRD), UV-vis diffuse reflectance spectroscopy (DRS), scanning electron microscopy (SEM), Fourier transformed infrared spectroscopy (FTIR), photoelectrochemical measurements, and specific surface area (SSA) analysis. The optoelectronic and structural features of the samples have been related to their photocatalytic activity for the degradation of 4–nitrophenol under solar and UV light irradiation. Coupling Ag3PO4 with silver doped TiO2 mitigates photocorrosion of the Ag3PO4 counterpart, and remarkably improves the photocatalytic activity under solar light irradiation with respect to the components, to the TiO2–Ag3PO4 sample, and to the benchmark TiO2 Evonik P25. These features open the route to future applications of this material in the field of environmental remediation.
Keywords: Ag@TiO2–Ag3PO4 heterojunction, solar photocatalysis, 4–nitrophenol degradation, sol–gel synthesis
1. Introduction
Each year, the surface of Earth receives 3850000 EJ of solar energy. However, only 0.014% of it is currently exploited to cover the energy demand, and to face environmental issues [1]. In fact, efficient solar light conversion is one of the hot topics of our society, which is finally aware of the need to develop and actuate sustainable processes to mitigate the dramatic climate changes and environmental disaster we are the witness of in the last years. The unique properties of visible light active semiconductors allow one to use them to convert solar energy into photogenerated charges, which, in turn, enable electricity generation or trigger useful chemical reactions. In this way, it is possible to mimic nature by storing solar energy in chemical bonds, by producing high value-added chemical compounds in a “green” way, or by removing hazardous compounds through their photoinduced mineralization. Titanium dioxide (TiO2) has been widely investigated and used to this aim, mainly due to its remarkable photoactivity, low cost, and robustness. However, TiO2 can be only activated upon UV light irradiation, which represents only ca. 5% of the solar light spectral emission. In order to overcome this limitation, TiO2 has been variously modified to extend its light absorption range toward the visible light region [2]. In many cases sensitization of TiO2 under visible light is achieved by modifying its surface with dyes [3], chromogenic species [4], or metal nanoparticles [5]. However, the resulting materials are often poorly stable, with few exceptions [6], and mainly useful for niche applications rather than for environmental remediation, where large effluent volumes need to be treated. Bulk modification, instead, provides more stable materials [7]. However, the influence of bulk defectivity on the recombination rate of the photogenerated charges, and in turn on the photocatalytic activity, is still object of scientific debate [8] due to the highly specific nature of the photoactive materials and of their interaction with light [9]. Alternatively, intrinsically visible light active semiconductors such as oxides [10], chalcogenides [11], and mixed semiconductors [12,13,14,15,16] have been recently investigated to trigger photocatalytic reactions under visible light irradiation [17]. Among them, the p-type semiconductor silver orthophosphate (Ag3PO4) has been often reported as a promising alternative to visible light active TiO2-based materials in the field of solar photocatalysis. The narrow band gap energy enables its visible light activation, and the strong oxidizing power [18,19,20] justifies its extensive investigation in scientific reports dealing with organic dye degradation, bacterial disinfection, and water splitting reaction [18,19,21]. In particular, quantum efficiency up to 90% can be achieved for O2 evolution under visible light irradiation in the presence of Ag3PO4. Worth of mention is also the great versatility of Ag3PO4, whose morphology can be easily tuned by opportunely adjusting the synthesis procedure. For instance, Zwara et al. [22] recently reported the synthesis of variously shaped Ag3PO4 samples with high photoactivity for the degradation of phenol under visible light. However, Ag3PO4 is prone to photocorrosion because photogenerated electrons easily reduce lattice silver ions to metallic silver eventually destroying the photocatalyst after few working cycles [23]. The photostability of Ag3PO4 can be enhanced in various ways. Some authors proposed the use of sacrificial reducing agents to hinder photocorrosion. However, this reduces the energy stored in the bonds of the products, which is particularly detrimental for water splitting [24] or for synthetic applications [25]. A much suitable approach consists in coupling different semiconductors [12,13,14,15,16]. To this aim, p–n heterostructures of Ag3PO4 and TiO2 are particularly promising. Yao et al. [26] reported the enhanced visible light photocatalytic activity of Ag3PO4 nanoparticles deposited onto commercial TiO2 (Evonik P25). In this case, degradation of dyes such as methylene blue and rhodamine B have been used as model visible light reactions, so that it is difficult to assess if a direct or indirect photocatalytic process is taking place [27]. However, results were confirmed by Rawal et al. [28], which used a homemade polycrystalline TiO2 coupled with Ag3PO4 for the visible light degradation of 2–propanol in gas phase. Teng et al. [29] reported the synthesis of ordered TiO2 nanotubes decorated with Ag3PO4 and Ag nanoparticles, and tested the composites for the removal of 2–chlorophenol in aqueous solution. Silver nanoparticles, deposited on the surface of Ag3PO4, acted as electron sinks thus preventing photocorrosion [30,31]. However, in the latter case, the photocatalyst is a ternary composite (Ag–Ag3PO4–TiO2) presenting exposed silver nanoparticles, which can aggregate and be easily removed during real waste water treatments. With the aim of simplifying the system for environmental applications, by providing higher robustness and simultaneously maintaining visible light absorption and high photoactivity, in the present investigation we introduced silver into the lattice of TiO2 and designed a binary heterojunction between Ag3PO4 and nanostructured Ag@TiO2. This composite provides efficient charge separation due to the unique optoelectronic features of the Ag@TiO2 counterpart, and high photocatalytic activity under solar light irradiation. In particular, the photocatalytic degradation of 4–nitrophenol (4–NP) under UV and natural solar light irradiation have been investigated, and the activity results have been related to the physico-chemical features of the samples.
2. Materials and Methods
2.1. Synthesis of the Samples
Ag3PO4 was synthesized according to a simple precipitation protocol. Silver nitrate (AgNO3, Park scientific, Northampton, UK 99.8%) and monosodium phosphate (NaH2PO4∙2H2O, Merck, Darmstadt, Germany, extra pure) salts were employed as sources of Ag+ and PO43− ions, respectively. Two aqueous solutions of Ag+ (0.1 M) and PO43− (0.3 M) were prepared separately, and 246 mL of the silver solution were added dropwise to 330 mL of the orthophosphate solution in order to provide large excess of the PO43− anions. The obtained yellow precipitate was separated by centrifuge, washed several times with distilled water, and finally dried at 80 °C overnight.
TiO2 was synthesized by dissolving 11.3 mL of titanium isopropoxide (Ti[OCH(CH3)2]4, Sigma Aldrich, St. Louis, MO, USA, 97%) in 11.4 mL isopropanol (C3H7OH, 99.8%, Sigma Aldrich, St. Louis, MO, USA). Hydrolysis started by adding 2.15 mL of acetic acid (CH3COOH, Sigma Aldrich, St. Louis, MO, USA, glacial 99.5%) and 1.35 mL distilled water, corresponding to molar ratio nH2O/nTi equal to 2. The resulting solution was stirred at 70 °C for about 20 min, until a white gel was obtained. The gel was then dried at 100 °C overnight and the resulting powder was calcined at 400 °C for 3 h in static air.
Silver doped TiO2 (Ag@TiO2) was synthesized following the same procedure used for bare TiO2, but dissolving in the starting isopropanol solution an opportune amount of silver nitrate to reach an Ag/Ti molar ratio equal to 0.01. Notably, the percentage of silver used in this work is the optimum one in terms of photocatalytic activity and stability, as demonstrated in previous reports [32,33,34,35,36,37].
The composites TiO2–Ag3PO4 and Ag@TiO2–Ag3PO4 were synthesized by dispersing the as prepared TiO2 and Ag@TiO2, respectively, in the 0.1 M monosodium phosphate aqueous solution just before the addition of the 0.1 M AgNO3 solution, and by following the same procedure described for the preparation of the Ag3PO4 sample. The molar percentage of TiO2 (bare and silver doped) and Ag3PO4 was 25% and 75%, respectively, in both the composites. All the above mentioned chemicals were used as received without further purification.
2.2. Characterization
X-ray diffraction (XRD) analysis of the samples has been performed by using a Rigaku D-Max III powder diffractometer, Tokyo, Japan, in Bragg–Brentano geometry, working between 10 and 70° (2θ range) with sampling step of 0.05° and a counting time of 2 s. Monochromatic Cu Kα radiation was used, operating at a voltage of 40 kV and a current of 30 mA; a curved graphite monochromator was inserted in the diffracted beam. Both quantitative analysis and crystallite size determination were performed by means MAUD software® (Version 2.26, by Luca Lutterotti, University of Trento, 2010) [38,39], by combining the Rietveld-based method for the determination of weight percentages of phases, and line profile analysis based on the Warren–Averbach theory for the evaluation of mean crystallite sizes (D) and microstrains (ε).
UV-visible diffuse reflectance spectra were recorded by a Perkin Elmer Lambda 950 UV-vis spectrophotometer, Waltham, MS, USA, equipped with an integrating sphere, in the range of 190–800 nm and by using barium sulfate (BaSO4, Sigma Aldrich, St. Louis, MO, USA, p.a.) as the reference. Reflectance (R∞) was converted by the instrument software to F(R∞) values according to the Kubelka–Munk theory. The bandgap was obtained from a plot of [F(R∞)hν]1/2 vs. the energy of the exciting light, by assuming that all of the photocatalysts are indirect semiconductors. The band gap energy (Eg) values were determined by extrapolating the linear part of the plot to the x axis.
Scanning electron microscopy (SEM) and energy dispersive X-Ray spectroscopy (EDS) analyses were carried out using a field emission-scanning electron microscope (FE-SEM, Supra 40/40VP, Zeiss, Oberkochen, Germany), operating at a voltage of 20 kV on specimens upon which a thin layer of Pt/Pd had been deposited under Ar atmosphere. Fourier transformed infrared spectroscopy (FTIR) was carried out on a Thermo Nicolet Avatar 330 FTIR, Thermo Fisher, Waltham, MS, USA, spectrophotometer.
To determine the specific surface area (SSA) and the pore size distribution (PSD) of the photocatalysts, nitrogen adsorption measurements were performed at the liquid nitrogen temperature by using a Micromeritics, Norcross, GA, USA, ASAP 2010 porosimeter. All the samples were degassed below 1.3 Pa at 200 °C prior to the measurement. SSA’s were calculated by the Brunauer−Emmet−Teller (BET) equation in the P/P0 interval 0.05–0.33. PSD’s were calculated by using the Barrett Joyner Halenda (BJH) method applied both on the adsorption and desorption branches of the isotherms.
Determination of the flat band potential of selected samples was performed in a Pyrex round bottom flask (V = 150 mL) irradiated with a 500 W medium pressure mercury lamp. 100 mg of the photocatalyst were dispersed into 100 mL of a 0.1 M KNO3 solution. The working electrode was a platinum foil and an Ag/AgCl electrode was used as the reference. The suspension was irradiated under nitrogen bubbling for 30 min and then 20 mg of MV2+ (1,1′–dimethyl–4,4′–bipyridinium dichloride) or DP2+ (4,5–dihydro–3a,5a–diaza–pyrene dibromide) were added as the electron scavengers. A pH meter Thermo Orion (Thermo Scientific, Waltham, MS, USA 720A and a multimeter Metex 3800 (Metex Corp., Seoul, South Korea) were used to measure the values of pH and the potential (V), respectively. Potentiometric titration was carried out by adjusting the pH with HNO3 and NaOH solutions.
2.3. Photocatalytic Experiments
A Pyrex tubular photoreactor (V = 100 mL) was used for the photodegradation tests of 4–nitrophenol (4–NP, Sigma Aldrich, St. Louis, MO, USA, 98%). In a representative run the reactor contained 80 mL of an aqueous solution of 4–NP at a concentration of 10 mg∙L−1 in which 80 mg of the photocatalyst were dispersed. The catalyst amount of 1 g∙L−1 enabled the safe comparison of the results obtained in the presence of different samples. A magnetic stirrer ensured the homogeneity of the reaction mixture. The suspension was kept in the dark under agitation during 60 min prior to irradiation, in order to reach the adsorption–desorption equilibrium. Samples were taken at different time intervals and filtered using a 0.2 μm Millipore filter to separate the photocatalyst. The concentration of 4–NP during irradiation time was measured by using a Perkin Elmer 950 UV-vis spectrophotometer, Waltham, MS, USA. Tests under UV light irradiation were performed by using six Philips (Amsterdam, The Netherlands) FSL BL T8 lamps (10 W each) axially positioned around the photoreactor, with the main emission peak at 365 nm. The radiation power absorbed per unit volume of the suspension was about 0.76 mW∙mL−1 measured by a Delta Ohm photo quantum meter (model HD9021) (Selvazzano Dentro, Italy) with a LP 9021 UVA sensor probe. 4–NP photodegradation tests under solar light were carried out by exposing to natural sun light at the same time as many different reactors in the presence of the investigated samples, during a sunny day in Gabes (Tunisia, 33°53′24″N 10°06′36″E) between 11 am and 4 pm in June. This approach allows one to safely compare the photocatalytic activity of the samples under natural solar light, as all of the photoreactors worked under the same irradiation conditions. The photocatalytic results have been compared with those obtained in the presence of commercial TiO2 P25 (Evonik). The mean sun light intensity during the solar photocatalytic runs was 8.2 W∙m−2 in the range 315–400 nm, and 2800 μE∙m−2∙s−1 in the range 400–700 nm.
3. Results
3.1. Structure and Morphology of the Samples
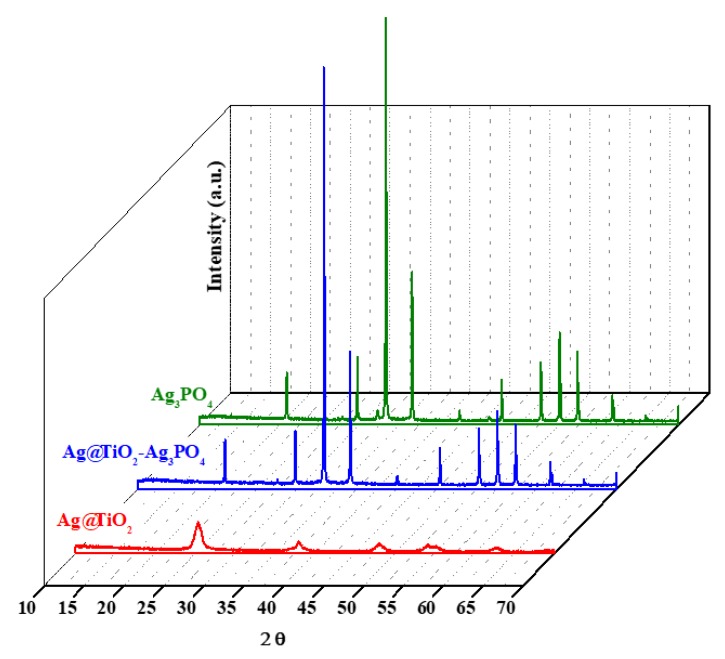
Figure 1 reports the XRD patterns of Ag3PO4, Ag@TiO2, and Ag@TiO2–Ag3PO4 samples. The XRD patterns of TiO2 and of TiO2–Ag3PO4 are reported in the Supporting Information (Figures S1–S4), being similar to those of Ag@TiO2 and Ag@TiO2–Ag3PO4, respectively.
Figure 1.
XRD patterns of Ag3PO4, Ag@TiO2, and Ag@TiO2–Ag3PO4 composites.
Ag3PO4 presents body centered cubic structure (JCPDS N°06-0505) while bare TiO2 shows the typical patterns of the anatase structure (JCPDS N°21-1272). Ag3PO4 signals were sharper with respect to those of TiO2 indicating the lower crystallinity of the latter, typical of powders obtained from the sol–gel method. The Ag@TiO2 sample presents qualitatively the same patterns of the TiO2 sample indicating that the anatase structure is not affected upon silver doping (Ag/Ti molar ratio = 0.01). Moreover, no traces of impurities ascribable to silver containing compounds such as oxides can be observed, suggesting the successful incorporation of silver into the TiO2 lattice. On the basis of XRD data, the lattice parameters (a, b, and c) and the mean crystallites dimension (D) were calculated for the pure components as summarized in Table 1, which also reports the microstrain values (ε), the specific surface area (SSA), and the pores volume (Vp).
Table 1.
Lattice parameters (a, b, and c), mean crystallites dimension (D), microstrain values (ε), specific surface area (SSA and BET), and pores volume (Vp) of the samples indicated. Digits under brackets represent relative error influencing the last significant digit of the calculated value.
| Samples | a (Å) | b (Å) | c (Å) | D (nm) | ε | SSA (m2∙g−1) | Vp (cm3∙g−1) |
|---|---|---|---|---|---|---|---|
| Ag3PO4 | 6.0209(1) | 6.0209(1) | 6.0209(1) | 332(8) | - | 0.5 | 0.003 |
| TiO2 | 3.7911(3) | 3.791 | 9.523(1) | 26.1(4) | 0.00288(3) | 80 | 0.205 |
| Ag@TiO2 | 3.794(1) | 3.794 | 9.499(3) | 23.6(8) | 0.0058(2) | 54 | 0.109 |
| TiO2–Ag3PO4 | - | - | - | - | - | 1.9 | 0.005 |
| Ag@TiO2–Ag3PO4 | - | - | - | - | - | 1.7 | 0.004 |
By comparing the lattice parameters and the crystallite size of TiO2 and Ag@TiO2 in Table 1, it is evident that in the presence of silver the unit cell shrinked along c and became larger along a and b directions. These changes, due to an isotropic size dependent effect [40], can be attributed to the insertion of substitutional silver ions into the TiO2 lattice. In fact, the bigger size of Ag+ (1.26 Å) with respect to that of Ti4+ ions (0.60 Å) modifies the lattice parameter and influences the crystal growth and the particle size. Literature reports suggest that silver doping generally hinders the growth of crystallites [41] due to grain boundary restraining caused by symmetry-breaking effects of the dopant [36]. At the same time, a decrease in crystallite dimensions is associated to an increase of microstrain values, reported in Table 1, due to the presence of surface tensions that provoke an increase of stresses [42]. This is confirmed also in this work, being the size of particles slightly smaller in the silver doped TiO2 with respect to the bare sample. Similar effects have been observed in literature when doping metal oxides with other metal ions [43].
The specific surface area of Ag3PO4 sample is much lower than that of the TiO2-based ones, in agreement with its higher crystallinity observed by XRD analysis. The mixed samples present intermediate specific surface area, closer to that of Ag3PO4, which is present in a higher amount with respect to TiO2.
The morphology and the composition of the samples were investigated by using scanning electron microscopy (SEM). Figure 2 shows the SEM images of all the samples. EDS analyses, reported in the Supporting Information (Figure S5a–c), confirm the presence of the expected atoms in percentage relatively close to the estimated one. No impurities containing foreign atoms could be detected. Images of TiO2 and Ag@TiO2 samples do not present significant differences, and are composed of micrometric agglomerates comprised of small nanoparticles of few tens of nanometers. This is in agreement with the crystallites dimension retrieved by XRD analysis. On the other hand, the Ag3PO4 particles are in the range of 1 to 5 micrometers and appear quite homogeneous in morphology and size. The composites TiO2–Ag3PO4 and Ag@TiO2–Ag3PO4 are comprised, respectively, of large TiO2 or Ag@TiO2 agglomerates upon which Ag3PO4 particles can be observed.
Figure 2.
SEM images of TiO2, Ag@TiO2, Ag3PO4, TiO2–Ag3PO4, and Ag@TiO2–Ag3PO4 samples.
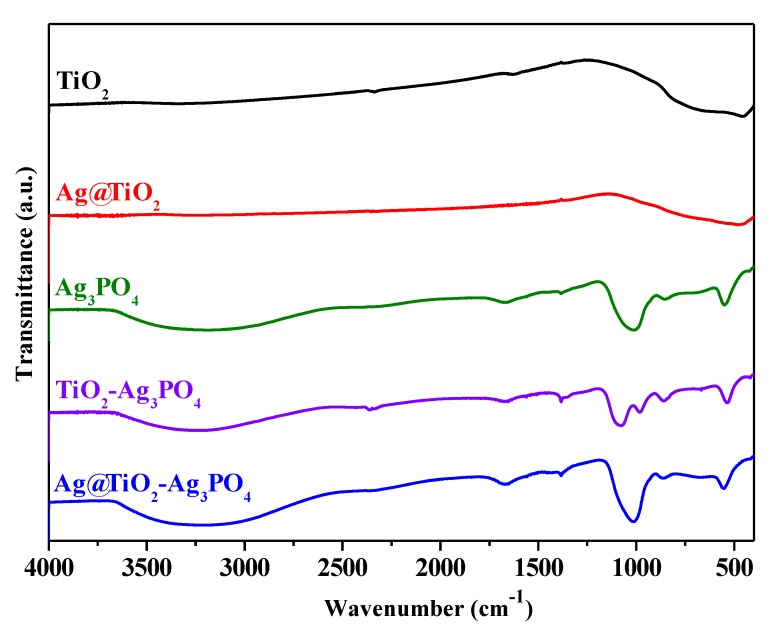
Infrared spectra of the samples are shown in Figure 3.
Figure 3.
FTIR spectra of TiO2 (black spectrum), Ag@TiO2 (red spectrum), Ag3PO4 (green spectrum), TiO2–Ag3PO4 (purple spectrum), and Ag@TiO2–Ag3PO4 (blue spectrum) samples.
The spectra of the Ag3PO4 containing samples present a signal at 1649 cm−1 and a broad band between 3000 and 3600 cm−1 that can be attributed to the bending and to the stretching of O–H groups of adsorbed water, respectively [44,45]. The characteristic peaks of the PO43− groups are present at 1010 cm−1 and 558 cm−1 corresponding to P–O stretching vibrations [44,45]. The IR spectra of TiO2 and Ag@TiO2 present a broad band between 500 and 900 cm−1, ascribable to Ti–O vibrations [46].
3.2. Optical Properties
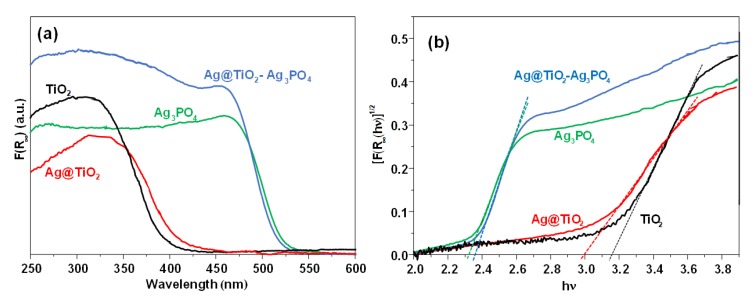
The absorption spectra of the samples are presented in Figure 4a. The values of F(R∞), calculated according to the Kubelka–Munk theory from the values of diffuse reflectance, were proportional to the absorbance. By considering that all of the samples are indirect crystalline semiconductors [47], the band gap energy can be obtained by extrapolating the linear part of a plot of [F(R∞)hν]1/2 vs. the energy of the exciting light (Figure 4b).
Figure 4.
(a) UV-vis absorption spectra and (b) Tauc plot of the TiO2 (black line), Ag@TiO2 (red line), Ag3PO4 (green line), and Ag@TiO2–Ag3PO4 (blue spectrum) samples.
The adsorption edge of the Ag3PO4 containing samples is at about 540 nm due to the narrow band gap of this semiconductor. The conduction band of Ag3PO4 presents Ag 5s and 5p character, while the valence band is composed of Ag 4d and O 2p orbitals [48,49]. As expected, Ag@TiO2–Ag3PO4 sample presented lower absorption in the visible region with respect to bare Ag3PO4, but higher absorption in the UV region due to the typical transition of TiO2. By comparing the spectra of bare TiO2 and of Ag@TiO2, it was evident the red shift of the doped sample moved towards the visible region due to the presence of silver ions into the lattice. Notably, the Ag@TiO2 spectrum does not show the typical plasmon resonance of silver nanoparticles. This is a further evidence of the successful incorporation of silver ions into the lattice of TiO2. The introduction of silver ions, in fact, is reported to introduce intermediate energy states deriving from the strong electronic coupling between the orbitals of TiO2 and of silver. In particular, spin polarized density of states analysis revealed strong hybridization between the silver orbitals and the Ti 3d and O 2p bands near the Fermi level [50]. The delocalized electron cloud results in a strong bonding between Ag atoms and the oxygen of TiO2. This strongly interacting electronic structure results in a bathochromic shift of the absorption edge, similarly to what reported for strongly interacting components of a solid solution, as in the case of vanadium, chromium, iron, or nickel doped TiO2 prepared by ion implantation [51] or for Fe2O3 heterojunctions with ZnO [16] and TiO2 [52]. This behavior significantly differs from the more common weak interaction case, as in the case of silver islands on the surface of TiO2, usually generating novel absorption shoulders. According to the Kubelka–Munk theory the band gap energy of bare Ag3PO4, TiO2–Ag3PO4, and Ag@TiO2–Ag3PO4 was ca. 2.3 eV, while the band gap of TiO2 and Ag@TiO2 were 3.2 and 3.0 eV, respectively. The narrow band gap of the Ag@TiO2 with respect to TiO2 makes it a better candidate to be part of a visible light active heterojunction with Ag3PO4. However, the knowledge of the potential edge of the photogenerated electrons is required to establish the nature of the interfacial electron transfer between the components. To this aim we experimentally determined the quasi Fermi level of both Ag@TiO2 and TiO2 samples by means of the method proposed by Roy et al. [53]. This method allows one to estimate the quasi Fermi level of a powdered semiconductor in water suspension and under irradiation, i.e., under experimental conditions similar to those used during the photocatalytic experiments. In fact, increasing the pH of the suspension under irradiation shifts cathodically the band edges of the semiconductor, until the one-electron reduction of the electron scavenger is thermodynamically allowed. This occurs at the inflection point of the potentiometric curve, where the quasi Fermi level of electrons equals the pH independent reduction potential of the electron acceptor.
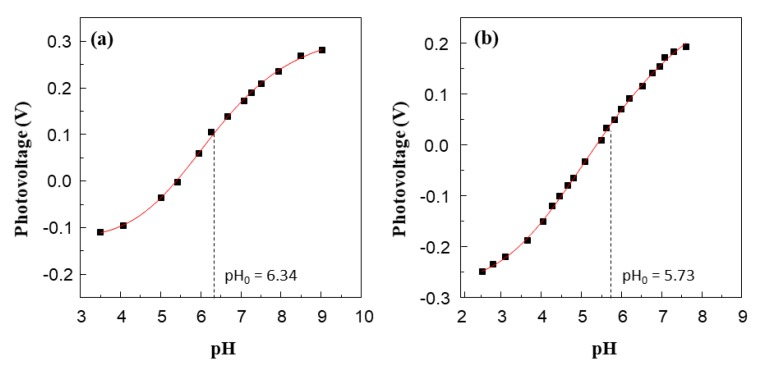
Trials to determine the quasi Fermi level of Ag3PO4 with this method failed, so that we used the conduction band edge reported in literature (0.45 V vs. NHE at pH 7) [54,55]. Figure 5a,b show the photovoltage vs. pH curves obtained by irradiating TiO2 and Ag@TiO2 suspensions in the presence of MV2+ and DP2+, respectively, as the electron acceptors. The use of DP2+ instead of MV2+, whose structures are shown in the Supporting Information (Figure S6), was necessary because in the presence of MV2+ the flex point would have been evident at too high pH values (ca. 9). Notably, the determination of the quasi-Fermi level of electrons of TiO2 does not depend on the nature of the electron scavenger used, as demonstrated by Kisch et al. [56].
Figure 5.
Effect of pH on the photovoltage developed by irradiation of aqueous suspension of TiO2 (a) and Ag@TiO2 (b) in the presence of MV2+ and DP2+, respectively, as electron scavengers. Each experiment has been repeated 3 times and reproducibility was better than ± 0.02 V.
The pH values of the inflection point (pH0) of the obtained sigmoidal titration curves, i.e., pH0 equal to 6.34 and 5.73 for TiO2 and Ag@TiO2 samples, allow one to calculate the flat band potential at pH 7 using Equation (1):
| EFB (pH = 7) = E EA2+/EA+∙ + 0.059 (pH0 − 7) | (1) |
where E EA2+/EA+∙ is the standard potential of the electron acceptor used, i.e. MV2+/MV+˙ equal to −0.45 V vs. NHE and DP2+/DP+˙ equal to −0.27 V vs. NHE [54]. The resulting potentials of the photogenerated electrons were −0.5 and −0.34 V for TiO2 and Ag@TiO2, respectively.
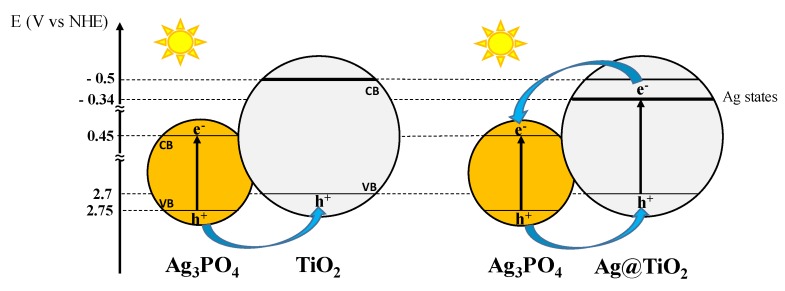
By taking into account these values, the one reported in literature for Ag3PO4 [54,55], and the measured band gap energies, the simplified electronic map of the two heterojunctions Ag@TiO2–Ag3PO4 and TiO2–Ag3PO4, shown in Figure 6, can be proposed.
Figure 6.
Mechanism of charge separation in the systems TiO2–Ag3PO4 and Ag@TiO2–Ag3PO4. Notably, under solar light irradiation also excitation of bare TiO2 take place, even if at lower extent due to the low fraction of UV photons (ca. 5%) in the solar light. For this reason, and for the sake of clarity, Figure 6 does not show this effect.
The visible part of the solar spectrum is not able to excite the TiO2 counterpart of the TiO2–Ag3PO4 sample, while the presence of substitutional silver within the bulk of Ag@TiO2 enables visible light excitation of the Ag@TiO2 nanoparticles in the Ag@TiO2–Ag3PO4 sample. In both the composites the photogenerated charges are efficiently separated; however, the excitation of the Ag@TiO2 counterpart enables generation of electrons at the silver intermediate energy states from which they can be more efficiently injected into the conduction band of Ag3PO4.
3.3. Photocatalytic Activity under UV and Natural Solar Light Irradiations
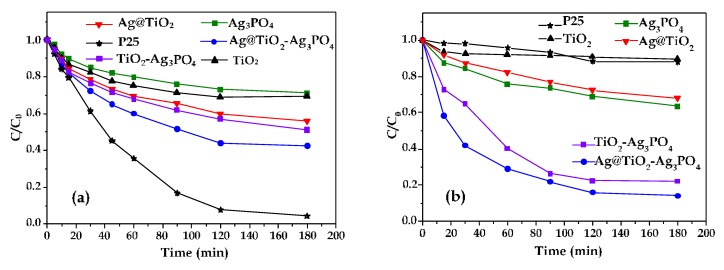
Figure 7 shows the photodegradation of the 4–NP under UV light (Figure 7a) and under natural solar light (Figure 7b) in the presence of the investigated photocatalysts. For the sake of comparison, results obtained in the presence of the commercial TiO2 P25 Evonik are also reported.
Figure 7.
Normalized concentration of 4–NP during the irradiation time (a) under UV light and (b) under natural solar light of the (■) Ag3PO4, (▼) Ag@TiO2, (▲) TiO2, (*) P25, (●) Ag@TiO2–Ag3PO4, and (■) TiO2–Ag3PO4 samples.
The observed initial rate constants (kobs) for each run in Figure 7 have been calculated by differentiating the experimental data at the initial time. The obtained values are reported in Table 2.
Table 2.
Observed initial rate constants (kobs (mg∙L−1∙min−1)) of 4–NP degradation under UV and natural solar light irradiation in the presence of the considered photocatalytic powders.
| kobs∙102 | Ag3PO4 | TiO2 | P25 | Ag@TiO2 | TiO2–Ag3PO4 | Ag@TiO2–Ag3PO4 |
|---|---|---|---|---|---|---|
| UV light | 6.8 | 8.8 | 15.8 | 10.4 | 11.1 | 12.6 |
| Solar light | 6.8 | 3.8 | 0.6 | 5.5 | 13.5 | 26.9 |
Under UV light (Figure 7a), the commercial P25 was the most active sample. The composites, and in particular the Ag@TiO2–Ag3PO4 one, were more active than TiO2, Ag3PO4, and Ag@TiO2 samples. Ag3PO4 showed the lowest activity despite its narrow band gap. The situation dramatically changes under solar light irradiation (Figure 7b), which mainly contains visible light photons, being the UV component only ca. 5%. TiO2 and P25 samples were poorly active under solar light, according to their optical properties, while the presence of substitutional silver into the lattice of Ag@TiO2 endows it with higher photocatalytic activity, comparable with that of Ag3PO4. Moreover, the composites, and in particular the Ag@TiO2–Ag3PO4 one, were the most active samples. Notably, Ag doping almost doubles the initial degradation rate in the Ag@TiO2–Ag3PO4 sample with respect to the TiO2–Ag3PO4 one.
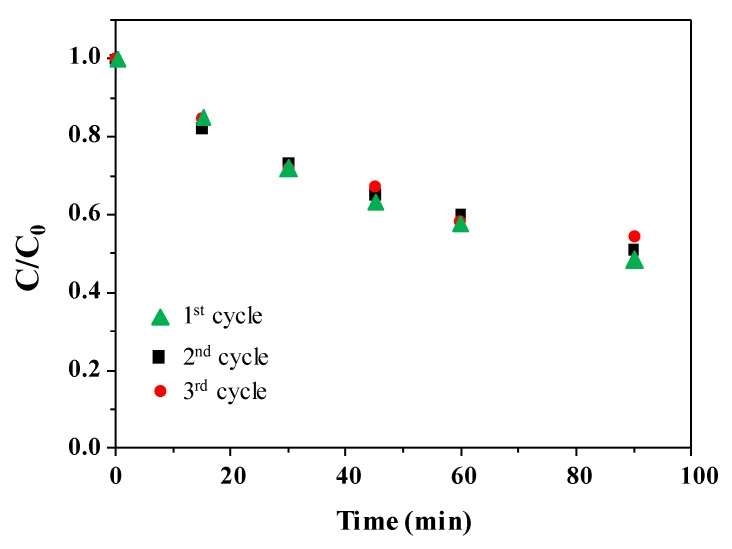
It is known that coupling Ag3PO4 with TiO2 mitigates the photocorrosion of the Ag3PO4 counterpart [29]. In order to check if the same holds also for the Ag@TiO2–Ag3PO4 under UV light, three photocatalytic runs were consecutively performed by using the same powder recovered after each cycle. Results are shown in Figure 8.
Figure 8.
Normalized concentration of 4–NP during the irradiation time for three consecutive photocatalytic runs in the presence of the same Ag@TiO2–Ag3PO4 powder recovered after each cycle.
The superior activity of the Ag@TiO2–Ag3PO4 photocatalyst reflects the above discussed charge transfer mechanism (Figure 6). The existence of heterojunctions between TiO2 and Ag3PO4 has been already reported to enhance photocatalytic activity of the composites with respect to the components. In fact, it is known that the close contact between two semiconductors of suitable electronic structure triggers interfacial electron transfer, effective spatial separation and longer life time of the photogenerated charges, which in turn account for the higher activity. This mechanism can be also invoked in the present case for both the Ag@TiO2–Ag3PO4 and TiO2–Ag3PO4 systems. In fact, the suitable relative position of the valence and conduction bands in both cases allows migration of the holes generated under visible light irradiation in the valence band of Ag3PO4 to the valence band of TiO2. However, in this paper we highlight that silver modification of the TiO2 counterpart results in an even higher activity under solar light irradiation. In fact, according to the DRS results, silver doping enables the visible absorption of the Ag@TiO2 material, so that under solar light irradiation it is possible to generate higher amount of excitons localized on both Ag@TiO2 and Ag3PO4. Moreover, in this case electrons generated into the conduction band (or into intermediate energy states) of Ag@TiO2 can also migrate toward the conduction band of Ag3PO4 thus enhancing the spatial charge separation. Finally, these effects not only account for the improved photocatalytic activity of the heterojunction, but also for its photo stability. In fact, the improved interfacial electron transfer delocalizes the photogenerated holes also into the valence band of Ag@TiO2, thus reducing the reported photocorrosion of Ag3PO4.
4. Conclusions
Heterojunction between silver doped TiO2 (Ag@TiO2) and Ag3PO4 has been proved to be a suitable candidate for photocatalytic remediation under solar light irradiation due to its outstanding photocatalytic activity. Physico-chemical characterization of the composites suggests the reasons of the superior performances with respect to the bare components and to the commercial benchmark TiO2 P25 (Evonik). The optoelectronic features of the Ag@TiO2–Ag3PO4 sample played a key role and determine its superior performances. In fact, fast charge transfer between the components resulted in efficient charge separation and higher photocatalytic efficiency. In particular, the photocatalytic activity of Ag@TiO2–Ag3PO4 was higher than that of TiO2–Ag3PO4. In fact, silver doping enables visible absorption also of the TiO2 counterpart and resulted in the generation of higher amount of excitons localized on both Ag@TiO2 and Ag3PO4 under solar light irradiation. This also improved spatial charge separation with respect to the TiO2–Ag3PO4 sample, because electrons photogenerated in the conduction band of Ag@TiO2 could now migrate toward the conduction band of Ag3PO4. For the outstanding photocatalytic activity highlighted in this work, Ag@TiO2–Ag3PO4 could be proposed for solar light photocatalytic applications.
Acknowledgments
L. Moschini and A. Elaziouti are kindly acknowledged for the SEM and EDS analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/10/4/795/s1, Figure S1: XRD patterns of the TiO2 sample. Figure S2: XRD patterns of the Ag@TiO2 sample. Figure S3: XRD patterns of the Ag3PO4 sample. Figure S4: XRD patterns of the TiO2–Ag3PO4 sample. Figure S5: EDS analysis of (a) Ag3PO4, (b,) Ag@TiO2 and (c) Ag@TiO2–Ag3PO4 samples. Figure S6: Structures of the electron acceptors DP2+ and MV2+.
Author Contributions
Conceptualization, A.H. and F.P.; methodology, A.R.; data analysis, R.C.; investigation, H.A.; data curation, R.C.; writing—original draft preparation, A.H.; writing—review and editing, F.P.; supervision, L.P.; funding acquisition, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: Encouragement of Young Researchers Project (PEJC) awarded to Dr. A. Hamrouni from the Tunisian Ministry of the Higher Education and Scientific Research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Ahmad T., Farooq U., Phul R. Fabrication and photocatalytic applications of perovskite materials with special emphasis on alkali-metal-based niobates and tantalates. Ind. Eng. Chem. Res. 2017;57:18–41. doi: 10.1021/acs.iecr.7b04641. [DOI] [Google Scholar]
- 2.Guarisco C., Palmisano G., Calogero G., Ciriminna R., Di Marco G., Loddo V., Pagliaro M., Parrino F. Visible-light driven oxidation of gaseous aliphatic alcohols to the corresponding carbonyls via TiO2 sensitized by a perylene derivative. Environ. Sci. Pollut. Res. 2014;21:11135–11141. doi: 10.1007/s11356-014-2546-z. [DOI] [PubMed] [Google Scholar]
- 3.Abd-Elaal A., Parrino F., Ciriminna R., Loddo V., Palmisano L., Pagliaro M. Alcohol-selective oxidation in water under mild conditions via a novel approach to hybrid composite photocatalysts. ChemistryOpen. 2015;4:779–785. doi: 10.1002/open.201500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y., He T., Guo L., Yang Y., Guo L., Tang Y., Cao Y. Efficient visible-light photocatalytic degradation system assisted by conventional Pd catalysis. Sci. Rep. 2015;5:9561. doi: 10.1038/srep09561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellardita M., El Nazer H., Loddo V., Parrino F., Venezia A., Palmisano L., El Nazer H.A. Photoactivity under visible light of metal loaded TiO2 catalysts prepared by low frequency ultrasound treatment. Catal. Today. 2017;284:92–99. doi: 10.1016/j.cattod.2016.11.026. [DOI] [Google Scholar]
- 6.Liu J., de la Garza L., Zhang L., Dimitrijevic N.M., Zuo X., Tiede D.M., Rajh T. Photocatalytic probing of DNA sequence by using TiO2/dopamine-DNA triads. Chem. Phys. 2007;339:154–163. doi: 10.1016/j.chemphys.2007.07.040. [DOI] [Google Scholar]
- 7.Carini G., Parrino F., Palmisano G., Scandura G., Citro I., Calogero G., Bartolotta A., Di Marco G. Nanostructured anatase TiO2 densified at high pressure as advanced visible light photocatalysts. Photochem. Photobiol. Sci. 2015;14:1685–1693. doi: 10.1039/C5PP00149H. [DOI] [PubMed] [Google Scholar]
- 8.Guo S.Q., Hu Z., Zhen M., Gu B., Shen B., Dong F. Insights for optimum cation defects in photocatalysis: A case study of hematite nanostructures. Appl. Catal. B: Environ. 2020;264:118506. doi: 10.1016/j.apcatb.2019.118506. [DOI] [Google Scholar]
- 9.Parrino F., Loddo V., Augugliaro V., Camera-Roda G., Palmisano G., Palmisano L., Yurdakal S. Heterogeneous photocatalysis: Guidelines on experimental setup, catalyst characterization, interpretation, and assessment of reactivity. Catal. Rev. 2018;61:163–213. doi: 10.1080/01614940.2018.1546445. [DOI] [Google Scholar]
- 10.Parrino F., García-López E., Marcì G., Palmisano L., Felice V., Sora I.N., Armelao L. Cu-substituted lanthanum ferrite perovskites: Preparation, characterization and photocatalytic activity in gas-solid regime under simulated solar light irradiation. J. Alloy. Compd. 2016;682:686–694. doi: 10.1016/j.jallcom.2016.05.017. [DOI] [Google Scholar]
- 11.Ye X., Chen Y., Ling C., Zhang J., Meng S., Fu X., Wang X., Chen S. Chalcogenide photocatalysts for selective oxidation of aromatic alcohols to aldehydes using O2 and visible light: A case study of CdIn2S4, CdS and In2S3. Chem. Eng. J. 2018;348:966–977. doi: 10.1016/j.cej.2018.05.035. [DOI] [Google Scholar]
- 12.Hamrouni A., Moussa N., Di Paola A., Parrino F., Houas A., Palmisano L. Characterization and photoactivity of coupled ZnO–ZnWO4 catalysts prepared by a sol–gel method. Appl. Catal. B: Environ. 2014;154:379–385. doi: 10.1016/j.apcatb.2014.02.042. [DOI] [Google Scholar]
- 13.Hamrouni A., Moussa N., Di Paola A., Palmisano L., Houas A., Parrino F. Photocatalytic activity of binary and ternary SnO2–ZnO–ZnWO4 composites. J. Photochem. Photobiol. A: Chem. 2015;309:47–54. doi: 10.1016/j.jphotochem.2015.05.001. [DOI] [Google Scholar]
- 14.Hamrouni A., Moussa N., Parrino F., Di Paola A., Houas A., Palmisano L. Sol–gel synthesis and photocatalytic activity of ZnO–SnO2 composites. J. Mol. Catal. A: Chem. 2014;390:133–141. doi: 10.1016/j.molcata.2014.03.018. [DOI] [Google Scholar]
- 15.Hamrouni A., Lachheb H., Houas A. Synthesis, characterization and photocatalytic activity of ZnO–SnO2 composites. Mater. Sci. Eng. B. 2013;178:1371–1379. doi: 10.1016/j.mseb.2013.08.008. [DOI] [Google Scholar]
- 16.Lachheb H., Ajala F., Hamrouni A., Houas A., Parrino F., Palmisano L. Electron transfer in ZnO–Fe2O3 aqueous slurry systems and its effects on visible light photocatalytic activity. Catal. Sci. Technol. 2017;7:4041–4047. doi: 10.1039/C7CY01085K. [DOI] [Google Scholar]
- 17.Parrino F., Bellardita M., García-López E.I., Marci G., Loddo V., Palmisano L. Heterogeneous photocatalysis for selective formation of high-value-added molecules: Some chemical and engineering aspects. ACS Catal. 2018;8:11191–11225. doi: 10.1021/acscatal.8b03093. [DOI] [Google Scholar]
- 18.Gou J., Ma Q., Cui Y., Deng X., Zhang H., Cheng X., Li X., Xie M., Cheng Q., Liu H. Visible light photocatalytic removal performance and mechanism of diclofenac degradation by Ag3PO4 sub-microcrystals through response surface methodology. J. Ind. Eng. Chem. 2017;49:112–121. doi: 10.1016/j.jiec.2017.01.015. [DOI] [Google Scholar]
- 19.Seo Y., Yeo B.-E., Cho Y.-S., Park H., Kwon C., Huh Y.-D. Photo-enhanced antibacterial activity of Ag3PO4. Mater. Lett. 2017;197:146–149. doi: 10.1016/j.matlet.2017.03.105. [DOI] [Google Scholar]
- 20.Baďurová K., Monfort O., Satrapinskyy L., Dworniczek E., Gościniak G., Plesch G. Photocatalytic activity of Ag3PO4 and some of its composites under non-filtered and UV-filtered solar-like radiation. Ceram. Int. 2017;43:3706–3712. doi: 10.1016/j.ceramint.2016.11.217. [DOI] [Google Scholar]
- 21.Miyasato R., Fujiwara M., Sato H., Yano T., Hashimoto H. Particle size effects of tetrahedron-shaped Ag3PO4 photocatalyst on water-oxidation activity and carrier recombination dynamics. Chem. Phys. Lett. X. 2019;2:100023. doi: 10.1016/j.cpletx.2019.100023. [DOI] [Google Scholar]
- 22.Zwara J., Grabowska E., Klimczuk T., Lisowski W., Zaleska-Medynska A. Shape-dependent enhanced photocatalytic effect under visible light of Ag3PO4 particles. J. Photochem. Photobiol. A: Chem. 2018;367:240–252. doi: 10.1016/j.jphotochem.2018.08.006. [DOI] [Google Scholar]
- 23.Luo L., Li Y., Hou J., Yang Y. Visible photocatalysis and photostability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2014;319:332–338. doi: 10.1016/j.apsusc.2014.04.154. [DOI] [Google Scholar]
- 24.Kubacka A., Fernández-García M., Colón G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2011;112:1555–1614. doi: 10.1021/cr100454n. [DOI] [PubMed] [Google Scholar]
- 25.Palmisano F.P.A.L. Reactions in the presence of irradiated semiconductors: Are they simply photocatalytic? Mini-Rev. Org. Chem. 2018;15:157–164. [Google Scholar]
- 26.Yao W., Zhang B., Huang C., Ma C., Song X., Xu Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012;22:405–4055. doi: 10.1039/c2jm14410g. [DOI] [Google Scholar]
- 27.Parrino F., De Pasquale C., Palmisano L. Influence of surface-related phenomena on mechanism, selectivity, and conversion of TiO2-induced photocatalytic reactions. ChemSusChem. 2018;12:589–602. doi: 10.1002/cssc.201801898. [DOI] [PubMed] [Google Scholar]
- 28.Rawal S.B., Do Sung S., Lee W.I. Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2–propanol under visible-light irradiation. Catal. Commun. 2012;17:131–135. doi: 10.1016/j.catcom.2011.10.034. [DOI] [Google Scholar]
- 29.Teng W., Li X., Zhao Q., Chen G. Fabrication of Ag/Ag3PO4/TiO2 heterostructure photoelectrodes for efficient decomposition of 2–chlorophenol under visible light irradiation. J. Mater. Chem A. 2013;1:9060–9068. doi: 10.1039/c3ta11254c. [DOI] [Google Scholar]
- 30.Teng W., Li X., Zhao Q., Zhao J., Zhang D. In situ capture of active species and oxidation mechanism of RhB and MB dyes over sunlight-driven Ag/ Ag3PO4 plasmonic nanocatalyst. Appl. Catal. B: Environ. 2012;125:538–545. doi: 10.1016/j.apcatb.2012.05.043. [DOI] [Google Scholar]
- 31.Wu M.-C., Chan S.-H., Jao M.-H., Su W.-F. Enhanced short-circuit current density of perovskite solar cells using Zn-doped TiO2 as electron transport layer. Sol. Energy Mater. Sol. Cells. 2016;157:447–453. doi: 10.1016/j.solmat.2016.07.003. [DOI] [Google Scholar]
- 32.Rtimi S., Baghriche O., Sanjines R., Pulgarin C., Bensimon M., Kiwi J. TiON and TiON–Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A: Chem. 2013;256:52–63. doi: 10.1016/j.jphotochem.2013.02.005. [DOI] [Google Scholar]
- 33.Singh S., Singh P.K., Mahalingam H. Novel floating Ag+ doped TiO2/polystyrene photocatalysts for the treatment of dye wastewater. Ind. Eng. Chem. Res. 2014;53:16332–16340. doi: 10.1021/ie502911a. [DOI] [Google Scholar]
- 34.Goei R., Lim T.-T. Ag-decorated TiO2 photocatalytic membrane with hierarchical architecture: Photocatalytic and anti-bacterial activities. Water Res. 2014;59:207–218. doi: 10.1016/j.watres.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Lei X., Xue X., Yang H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr(VI) under visible light. Appl. Surf. Sci. 2014;321:396–403. doi: 10.1016/j.apsusc.2014.10.045. [DOI] [Google Scholar]
- 36.Mogal S.I., Gandhi V.G., Mishra M.K., Tripathi S., Shripathi T., Joshi P.A., Shah D.O. Single-step synthesis of silver-doped titanium dioxide: Influence of silver on structural, textural, and photocatalytic properties. Ind. Eng. Chem. Res. 2014;53:5749–5758. doi: 10.1021/ie404230q. [DOI] [Google Scholar]
- 37.Chang L.-H., Cho C.-P. Enhanced photocatalytic characteristics by Ag-sensitized TiO2 photocatalysts with mixed phases. Mater. Chem. Phys. 2019;223:683–693. doi: 10.1016/j.matchemphys.2018.11.063. [DOI] [Google Scholar]
- 38.Lutterotti L., Ceccato R., Maschio R.D., Pagani E. Quantitative analysis of silicate glass in ceramic materials by the rietveld method. Mater. Sci. Forum. 1998;278:87–92. doi: 10.4028/www.scientific.net/MSF.278-281.87. [DOI] [Google Scholar]
- 39.Lutterotti L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. B: Beam Interact. Mater. At. 2010;268:334–340. doi: 10.1016/j.nimb.2009.09.053. [DOI] [Google Scholar]
- 40.Tobaldi D.M., Pullar R.C., Gualtieri A.F., Seabra M.P., Labrincha J.A. Phase composition, crystal structure, and microstructure of silver and tungsten doped TiO2 nanopowders with tunable photochromic behaviour. Acta Mater. 2013;61:5571–5585. doi: 10.1016/j.actamat.2013.05.041. [DOI] [Google Scholar]
- 41.Depero L.E., Sangaletti L., Allieri B., Blasenbauer D., Marino A., Zocchi M. Correlation between crystallite sizes and microstrains in TiO2 nanopowders. J. Cryst. Growth. 1999;198:516–520. doi: 10.1016/S0022-0248(98)01086-0. [DOI] [Google Scholar]
- 42.Ahmad A., Thiel J., Shah S.I. Structural effects of niobium and silver doping on titanium dioxide nanoparticles. J. Physics: Conf. Ser. 2007;61:11–15. doi: 10.1088/1742-6596/61/1/003. [DOI] [Google Scholar]
- 43.Ajala F., Hamrouni A., Houas A., Lachheb H., Megna B., Palmisano L., Parrino F. The influence of Al doping on the photocatalytic activity of nanostructured ZnO: The role of adsorbed water. Appl. Surf. Sci. 2018;445:376–382. doi: 10.1016/j.apsusc.2018.03.141. [DOI] [Google Scholar]
- 44.Dhanabal R., Chithambararaj A., Velmathi S., Bose A.C. Visible light driven degradation of methylene blue dye using Ag3PO4. J. Environ. Chem. Eng. 2015;3:1872–1881. doi: 10.1016/j.jece.2015.06.001. [DOI] [Google Scholar]
- 45.Liang Q., Ma W., Shi Y., Li Z., Yang X. Hierarchical Ag3PO4 porous microcubes with enhanced photocatalytic properties synthesized with the assistance of trisodium citrate. CrystEngComm. 2012;14:2966. doi: 10.1039/c2ce06425a. [DOI] [Google Scholar]
- 46.Tom R.T., Nair A.S., Singh N., Aslam M., Nagendra C.L., Philip R., Vijayamohanan K., Pradeep T. Freely dispersible Au@TiO2, Au@ZrO2, Ag@TiO2, and Ag@ZrO2, core-shell nanoparticles: One-step synthesis, characterization, spectroscopy, and optical limiting properties. Langmuir. 2003;19:3439–3445. doi: 10.1021/la0266435. [DOI] [Google Scholar]
- 47.Jia L., Wu C., Li Y., Han S., Li Z., Chi B., Pu J., Jian L. Enhanced visible-light photocatalytic activity of anatase TiO2 through N and S coupling. Appl. Phys. Lett. 2011;98:211903. doi: 10.1063/1.3593147. [DOI] [Google Scholar]
- 48.Yi Z., Ye J., Kikugawa N., Kako T., Ouyang S., Stuart-Williams H., Yang H., Cao J., Luo W., Li Z., et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010;9:559–564. doi: 10.1038/nmat2780. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Fu X., Chen S.F., Zhu Y.F. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 2011;99:191903. doi: 10.1063/1.3660319. [DOI] [Google Scholar]
- 50.Dorothy A.A., Subramaniam N.G., Panigrahi P., Subramaniam G. Tuning electronic and optical properties of TiO2 with Pt/Ag doping to a prospective photocatalyst: A first principles DFT study. Mater. Res. Express. 2019;6:045913. doi: 10.1088/2053-1591/aafc56. [DOI] [Google Scholar]
- 51.Ji P., Takeuchi M., Cuong T.-M., Zhang J., Matsuoka M., Anpo M. Recent advances in visible light-responsive titanium oxide-based photocatalysts. Res. Chem. Intermed. 2010;36:327–347. doi: 10.1007/s11164-010-0142-5. [DOI] [Google Scholar]
- 52.Tada H., Jin Q., Nishijima H., Yamamoto H., Fujishima M., Okuoka S.-I., Hattori T., Sumida Y., Kobayashi H. Titanium(IV) dioxide surface-modified with iron oxide as a visible light photocatalyst. Angew. Chem. Int. Ed. 2011;50:3501–3505. doi: 10.1002/anie.201007869. [DOI] [PubMed] [Google Scholar]
- 53.Roy A. Determination of the flatband potential of semiconductor particles in suspension by photovoltage measurement. Int. J. Hydrogen Energy. 1995;20:627–630. doi: 10.1016/0360-3199(94)00105-9. [DOI] [Google Scholar]
- 54.Li Y., Wang P., Huang C., Yao W., Wu Q., Xu Q.-J. Synthesis and photocatalytic activity of ultrafine Ag3PO4 nanoparticles on oxygen vacated TiO2. Appl. Catal. B: Environ. 2017;205:489–497. doi: 10.1016/j.apcatb.2016.12.059. [DOI] [Google Scholar]
- 55.Chen X., Dai Y., Wang X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloy. Compd. 2015;649:910–932. doi: 10.1016/j.jallcom.2015.07.174. [DOI] [Google Scholar]
- 56.Kisch H., Burgeth G., Macyk W. Visible light photocatalysis by a titania transition metal complex. Adv. Inorg. Chem. 2004;56:241–259. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.