Abstract
The aim of this study was to determine whether early mobilization was associated with rehospitalization among elderly heart failure patients. We measured the time from admission to mobilization and other clinical characteristics for 190 heart failure patients (mean age, 80.7 years). The primary outcome was heart failure rehospitalization. Kaplan–Meier survival curves were plotted and the hazard ratios for rehospitalization were determined using Cox proportional hazards regression models. During a median follow-up period of 750 days, 58 patients underwent rehospitalization. The time from admission to mobilization was significantly longer for these patients than for those who were not rehospitalized. Univariate and multivariate Cox proportional hazards analyses showed that the time from admission to mobilization was an independent predictor of rehospitalization, and receiver-operating characteristic analysis determined an optimal cutoff value of 3 days for differentiating the patients more likely to experience a subsequent cardiac event (sensitivity, 76%; specificity, 69%; area under the curve, 0.667). Kaplan–Meier survival curve analysis showed a significantly lower event rate in the ≤ 3-day group (p = 0.001, log-rank test). In conclusion, the time from admission to mobilization may be one of the strongest predictors of rehospitalization in elderly heart failure patients. Early mobilization within 3 days may be an initial target for the acute phase treatment of heart failure.
Keywords: Acute heart failure, Elderly, Mobilization, Rehospitalization
Introduction
Despite a dramatic reduction in heart failure mortality rate over the last 20 years, rehospitalization due to deteriorating heart failure remains common in Japan, as well as worldwide [1, 2]. During the last 10 years, there has been a dramatic increase in heart failure among elderly patients in Japan, and it is expected that this will continue to increase over the next decade [2].
Early mobilization is a major component of acute phase rehabilitation and reported to be closely associated with better outcomes [3–5]. This was demonstrated by a randomized trial of patients on mechanical ventilation due to acute illness; those with early mobilization had higher rates of discharge home, fewer days in the intensive care unit, and fewer hospital-acquired comorbidities, such as ventilator-associated pneumonia and intensive care unit delirium [3]. A small randomized trial in patients with acute stroke showed better physical functional status among those mobilized 24–48 h after hospitalization [4]. In addition, a report based on a large-scale clinical registry found an association between early rehabilitation and the clinical outcome in heart failure patients [5]. Early ambulation within 2 days of hospitalization was associated with shorter hospital lengths of stay and fewer readmissions within 30 days of discharge.
Elderly patients in Japan often have multiple comorbidities and are physically frail. This contributes to longer hospital stays and lower rates of discharge back to the home [6]. However, little is known about the clinical impact of early mobilization on long-term clinical outcomes in elderly Japanese heart failure patients. We hypothesized that early mobilization would be associated with a lower rate of rehospitalization in elderly heart failure patients and that later mobilization may be a predictor of rehospitalization, in addition to other clinical parameters. Therefore, the aims of the present study were: (1) to investigate the relationship between early mobilization and long-term cardiac events in elderly Japanese heart failure patients and (2) to clarify the optimal cutoff time period for early mobilization associated with reduced rehospitalization.
Subjects and methods
Study population
From July 2014 to April 2018, we enrolled consecutive patients with heart failure who were admitted because of worsening heart failure to Fujita Health University Bantane Hospital, a general hospital in Nagoya City, Japan. Patients were excluded if they were less than 65 years of age, could not walk 10 m independently, had acute coronary syndrome, had severe dementia, had a history of psychiatric disorders, or if they did not wish to participate. The Research Ethics Committee of Fujita Health University approved the study (Approval No: 15-259), and all the subjects provided written informed consent.
Study design and protocol
This was a single-center registry, prospective, observational, predictive study. A baseline examination was conducted during the patients’ hospitalization. We measured physical function parameters at discharge and collected laboratory measurements from medical records.
Primary events and clinical characteristics
The primary outcomes were rehospitalization because of a deterioration of heart failure. A well-trained cardiologist at Fujita Health University determined the primary outcomes. Each patient was evaluated using all inpatient and outpatient medical record, which were generated during from discharge until November 30, 2018.
The subjects’ clinical characteristics were collected from a review of medical records, recording age, sex, body mass index, the etiology of heart failure, and medications. The time from admission to mobilization was evaluated as an assessment of early mobilization. Mobilization day was defined as the day when patients started walking with stable hemodynamics. The potential prognostic variables collected from the results of the blood investigations included N-terminal pro B-type natriuretic peptide (NT-proBNP), serum albumin, serum sodium, and the estimated glomerular filtration rate (eGFR) [7]. In addition, the left ventricular ejection fraction (LVEF) and the transmitral to mitral annular early diastolic velocity ratio (E/Ea) were obtained from echocardiography findings.
Handgrip strength was measured using a Jamar dynamometer. The subject sat with the wrist in a neutral position with the elbow flexed to 90° [8]. Grip strength was measured three times for each hand, and the highest value was used in the analysis. The preparation and procedure for the 6-min walking distance test were performed in accordance with the guidelines of the American Thoracic Society [9]. In this test, the subject was asked to walk as far as possible in 6 min (using any walking aid, such as a cane or walker, the subject normally used for daily walking). Walking speed was evaluated by the 10-m usual walk test, in which the subject was asked to walk at their usual pace for 14 m, of which the middle 10 m was timed. The test was completed twice, and the faster speed was used in the analysis.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation and categorical data as percentages. The subjects were divided into two groups according to whether they experienced the primary outcome (rehospitalization) during follow-up. The baseline characteristics were compared between the rehospitalization and non-rehospitalization groups using unpaired t tests and the Mann–Whitney U test for continuous variables and the Chi-square test or Fisher’s exact test for categorical variables. Univariate and multivariate Cox regression analyses were used to evaluate predictive capability. The Cox regression analysis involved three predictive models based on preexisting potential prognostic and confounding factors: Model 1 used log NT-proBNP as an adjusting variable; Model 2 included eGFR as well as log NT-proBNP; and Model 3 included log NT-proBNP, eGFR, variables with a p value of less than 0.1 at univariate analysis. Receiver-operating characteristic (ROC) curves were constructed, and the area under the curve was analyzed to select a cutoff value for predicting rehospitalization. The difference in survival between the two groups was assessed by the Kaplan–Meier method and compared using the log-rank test. The statistical analyses were performed with SPSS 24.0 software (SPSS Japan, Tokyo, Japan), and a p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics and primary outcome events
A total of 190 patients (mean age, 80.7 ± 8.5 years; 111 women) were enrolled and successfully followed up. The mean LVEF was 53.9%, and 51 subjects (26.8%) had LVEF < 40%. According to electrocardiogram, chest X-ray, chest computed tomography, and echocardiography finding, no patients had severe respiratory disease or severe pulmonary hypertension. During the follow-up period (median, 750 days), 58 primary outcome events were observed. Table 1 shows a comparison of clinical characteristics between the rehospitalization group (n = 58) and the non-rehospitalization group (n = 132). There were no significant differences in medications and follow-up period between the groups.
Table 1.
Demographic and clinical characteristics of the rehospitalization and non-rehospitalization groups
| Total (N = 190) |
Rehospitalization (n = 58) |
Non-rehospitalization (n = 132) |
p | |
|---|---|---|---|---|
| Age | 80.7 (8.5) | 79.8 (8.3) | 81.2 (8.6) | 0.298 |
| Gender (M/F) | 79/111 | 17/41 | 62/70 | 0.095 |
| BMI (kg/m2) | 20.9 (3.4) | 20.1 (3.1) | 21.3 (3.5) | < 0.001 |
| SBP at admission (mmHg) | 140 (30.5) | 134 (32.3) | 142 (29.4) | 0.262 |
| Admission to mobilization days | 4.2 (2.8) | 5.1 (3.5) | 3.9 (2.4) | 0.006 |
| MMSE | 24.5 (4.9) | 23.9 (5.4) | 24.6 (4.7) | 0.429 |
| GNRI | 92.8 (11.9) | 89.1 (10.2) | 93.5 (11.2) | 0.010 |
| Cause of heart failure (%) | 0.353 | |||
| Ischemic cardiomyopathy | 28.4 | 36.2 | 25.0 | |
| Dilated cardiomyopathy | 30.0 | 29.3 | 30.3 | |
| Hypertensive cardiomyopathy | 16.3 | 12.0 | 18.2 | |
| Arrythmia | 16.3 | 13.7 | 17.4 | |
| Others | 9.0 | 8.8 | 9.1 | |
| Comorbidities (%) | ||||
| Hypertension | 52.1 | 49.3 | 55.3 | 0.175 |
| Diabetes | 37.8 | 31.1 | 38.6 | 0.695 |
| CKD | 59.4 | 62.0 | 58.3 | 0.437 |
| Stroke | 14.2 | 16.8 | 16.6 | 0.363 |
| Pharmacotherapy (%) | ||||
| ACEi/ARB | 50.5 | 46.5 | 52.2 | 0.443 |
| β-blocker | 80.0 | 81.0 | 79.1 | 0.331 |
| Diuretics | 82.6 | 89.6 | 79.5 | 0.386 |
| Statin | 25.7 | 32.7 | 22.7 | 0.303 |
| Oral diabetic agent | 17.8 | 22.4 | 15.9 | 0.513 |
| Serum hemoglobin (g/dl) | 11.7 (2.1) | 11.2 (1.8) | 11.9 (2.1) | 0.023 |
| Serum albumin (g/dl) | 3.5 (0.5) | 3.4 (0.5) | 3.6 (0.5) | 0.071 |
| Serum Sodium (mEq/l) | 139 (4.4) | 139 (4.5) | 139 (4.5) | 0.996 |
| eGFR | 45.3 (19.4) | 41.4 (20.7) | 47.1 (18.6) | 0.065 |
| NT-proBNP (pg/ml) | 6965 (8021) | 9820 (9018) | 5711 (7228) | 0.001 |
| LVEF (%) | 53.9 (18.2) | 49.4 (20.7) | 55.6 (16.4) | 0.030 |
| Grip (kg) | 19.2 (7.4) | 18.1 (6.1) | 19.7 (7.9) | 0.116 |
| 6-min walking distance (m) | 272 (121) | 265 (113) | 276 (125) | 0.563 |
| Walking speed (m/s) | 0.85 (0.32) | 0.87 (0.31) | 0.85 (0.33) | 0.476 |
Data are presented as mean (standard deviation)
BMI body mass index, SBP systolic blood pressure, MMSE mini-mental state examination, GNRI geriatric nutritional risk index, CKD chronic kidney disease, ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin II type I receptor blocker, eGFR estimated glomerular filtration rate, NT-proBNP N-terminal pro B-type natriuretic peptide, LVEF left ventricular ejection fraction
Univariate and multivariate analyses
Table 2 shows the results of the Cox regression analysis for rehospitalization. This showed the time from admission to mobilization to be an independent predictor of rehospitalization in our cohort, even after adjusting for the prognostic models.
Table 2.
Univariate and multivariate cox regression hazard models for rehospitalization, with admission to mobilization time as the independent factor
| B | SE | Wald | P | HR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Univariable | 0.087 | 0.039 | 5.01 | 0.025 | 1.091 | 1.011 | 1.177 |
| Multivariable | |||||||
| Model 1 | 0.084 | 0.039 | 4.585 | 0.032 | 1.088 | 1.007 | 1.175 |
| Model 2 | 0.080 | 0.039 | 4.183 | 0.041 | 1.084 | 1.003 | 1.170 |
| Model 3 | 0.083 | 0.040 | 4.355 | 0.037 | 1.086 | 1.005 | 1.174 |
Adjusted models: Model 1, included log (NT-proBNP); Model 2, Model 1 + eGFR; Model 3, Model 2 + potential confounding factor (gender, LVEF, GNRI, serum hemoglobin)
HR hazard ratio, NT-proBNP N-terminal pro B-type natriuretic peptide, eGFR estimated glomerular filtration rate
ROC and survival analyses
ROC curve analysis with the primary outcome event as the outcome identified a value of 3 days as the optimal cutoff level predictive of rehospitalization cardiac events. Using this cutoff gave a sensitivity of 76.2% and a specificity of 69.2%; the area under the curve was 0.667 (95% CI 0.577–0.743, p = 0.001) (Fig. 1). The cutoff was used to divide the subjects into two groups, and Kaplan–Meier survival curves for these groups were compared with the log-rank test; this showed a significantly lower event rate in the ≤ 3-day group compared with the ≥ 4-day group (p = 0.001) (Fig. 2). The positive and negative predictive values of cardiac events for mobilization more than 4 days were 43.7% and 82.9%, respectively.
Fig. 1.
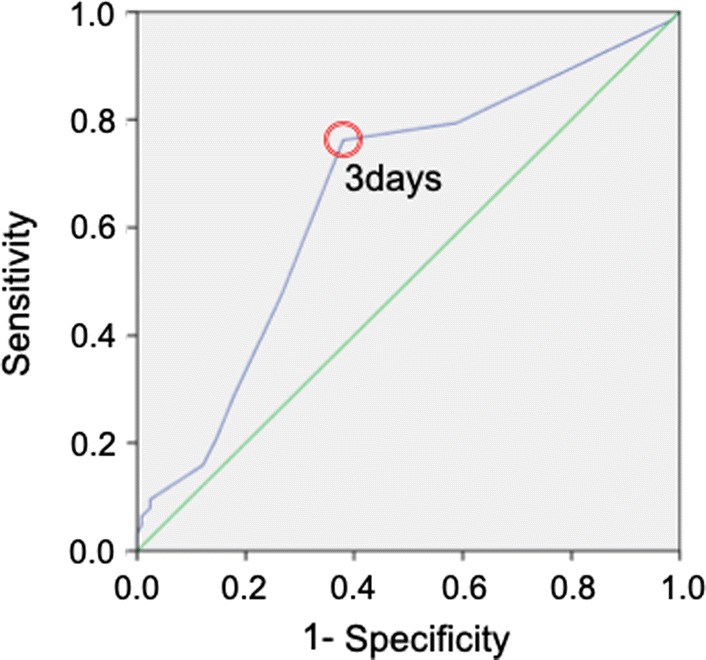
Receiver-operating characteristic (ROC) analysis of the time from admission to mobilization (in days) as a predictor of cardiac events during the follow-up period. The optimal cutoff value for distinguishing subjects who would experience a later cardiac event was 3 days. This cutoff gave a sensitivity of 76.2% and a specificity of 62.1%; the area under the curve was 0.667 (95% CI 0.577–0.743, p < 0.001)
Fig. 2.
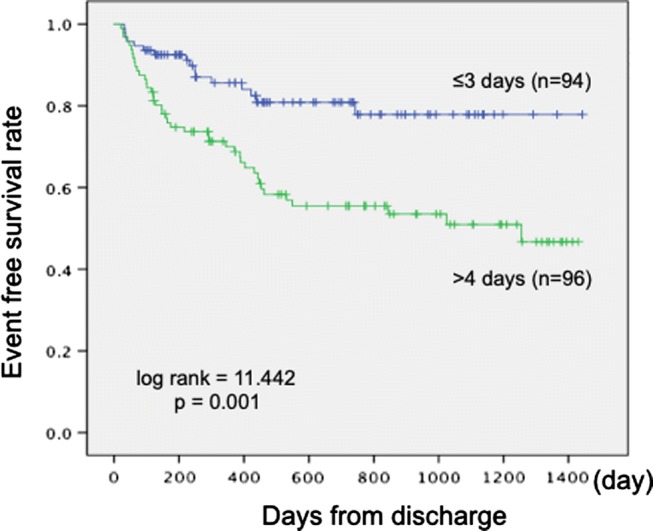
Kaplan–Meier survival curves for the period (in days) to a cardiac event. The subjects were divided into two groups according to whether the time between admission and mobilization was ≤ 3 days (blue curve) or ≥ 4 days (green curve). There was a significantly lower incidence of cardiac events in the group with earlier mobilization (log rank = 11.442, p = 0.001)
Discussion
The main finding of this study was that, for elderly patients with heart failure who were hospitalized because of deterioration in their condition, the time from admission to mobilization was a significant predictive parameter of future cardiac events. The analysis showed that 3 days was the optimal cutoff value, and the subjects who were mobilized within 3 days of admission were significantly less likely to be rehospitalized. To the best of our knowledge, this was the first study to demonstrate a relationship between early mobilization and long-term cardiac events in elderly patients with heart failure and to establish a cutoff value for distinguishing those at greater risk.
Although an association between early mobilization and 30-day readmission has previously been reported [5], the association with long-term outcomes has not been addressed. The results of the present study showed that the early mobilization of patients after admission to hospital had a predictive impact on rehospitalization during a follow-up period lasting a median of 750 days, after adjusting for several prognostic and confounding factors, including heart failure severity and physical function. A possible explanation for this finding may be that early mobilization reduced the risk of iatrogenic sarcopenia, a major hospital-acquired comorbidity caused by excessive bed rest in an acute phase clinical setting. Previous studies of Japanese heart disease patients have shown a close association between rehospitalization and physical function, as measured by 6-min walking distance [10], gait speed [10, 11], grip strength [12], and functional capacity [13]. Elderly heart failure patients are more likely to experience sarcopenia, not only due to physical inactivity, but also because of their heart failure disease status [14]. Previous studies have reported that early mobilization had favorable effects on physical function at discharge [3–5]. However, in the present study, there was no difference in physical function between the rehospitalization and non-rehospitalization groups. A previous study of patients with heart failure reported that the prevention of functional decline during hospitalization was a positive prognostic factor [15]. Thus, the prevention of functional decline may be a factor related to our finding that early mobilization was associated with the reduced likelihood of long-term rehospitalization in elderly heart failure patients.
In the present study, the time from admission to mobilization was significantly longer for the rehospitalization group than for the non-rehospitalization group. The optimal cutoff value for differentiating between the rehospitalization and non-rehospitalization groups was 3 days, and the area under the curve was 0.667, indicating moderate ability to distinguish between the groups [16]. Furthermore, a Kaplan–Meier analysis showed a lower incidence of cardiac events for the subjects with early mobilization within 3 days of admission than for those with mobilization in 4 or more days. A previous multi-center observational study reported that elderly patients who developed sarcopenia spent an average of 5.1 days in bed compared with 3.2 days for those without sarcopenia at discharge [6]. A cutoff of 3 days could play an important role in preventing iatrogenic sarcopenia-related functional decline. Indeed, mobilization in 4 or more days had an 82% negative predictive value in the present study, suggesting that mobilization within 3 days from admission could be used to stratify patients at low risk of rehospitalization. Mobilization within 3 days and that this might be an initial target for the early mobilization for elderly patients with heart failure to improve the quality of acute phase heart failure treatment, similar to the door-to-balloon time within 90 min for coronary interventions [17].
Previous reports have identified several prognostic parameters of heart failure. Some clinical markers, such as blood laboratory test and cardiac function test results [7], have provided a basis for cardiac risk stratification; however, these tend to be complex to obtain and costly. In contrast, the time from admission to mobilization is easily evaluated and is easy to understand even for staff that are not medical specialists. Therefore, it could play an important role in disease management programs after discharge and could be used to stratify heart failure patients at high risk for rehospitalization.
This study had some potential limitations. First, it was based on a single-center registry of patients with heart failure and the results may not necessarily be generalized to groups with dissimilar demographic characteristics. Second, our patients had lower ACEi/ARB prescription about 50%. Previous large-scale registry reported that patients with older age and HFpEF were less likely to receive ACEi/ARB therapy [18, 19]. These were the reason why our patients had lower prescription of ACEi/ARB. However, rehospitalization rate in this study was almost the same rate as previous findings [19], and there was no difference between two groups in terms pharmacotherapy. Accordingly, lower ACEi/ARB prescription rate did not influence our results. Finally, this was designed as a predictive research, so the level of evidence was lower than that provided by a propensity-score matching analysis or a randomized controlled trial. Thus, we could not determine the effectiveness of early mobilization within 3 days on rehospitalization. However, there are ethical issues to performing a randomized controlled trial in an acute phase clinical setting, and the results of the present study provided novel information that early mobilization could be a key component of acute phase treatment.
In conclusion, our results showed that the time from admission to mobilization might be one of the potential predictors for rehospitalization in elderly heart failure patients. Early mobilization within 3 days from admission might be an initial target for acute phase heart failure management.
Funding
This work was supported by JSPS KAKENHI Grant no. 16K09460 (Hideo Izawa). None of the work in this article has been presented at a conference or in any other publications.
Compliance with ethical standards
Conflicts of interest
Hideo Izawa has received grant support through his institution from Takeda, Shionogi, Dainippon-Sumitomo, Otsuka, Pfizer, and Daiichi-Sankyo and honoraria for lectures from Otsuka and Daiichi-Sankyo.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shah RU, Tsai V, Klein L, Heidenreich PA. Characteristics and outcomes of very elderly patients after first hospitalization for heart failure. Circ Heart Fail. 2011;4:301–307. doi: 10.1161/CIRCHEARTFAILURE.110.959114. [DOI] [PubMed] [Google Scholar]
- 2.Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, Suzuki K, Tanabe N, Kodama M, Aizawa Y. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72:489–491. doi: 10.1253/circj.72.489. [DOI] [PubMed] [Google Scholar]
- 3.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1474–1482. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, Bernhardt J. Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke. 2011;42:153–158. doi: 10.1161/STROKEAHA.110.594598. [DOI] [PubMed] [Google Scholar]
- 5.Fleming LM, Zhao X, DeVore AD, Heidenreich PA, Yancy CW, Fonarow GC, Hernandez AF, Kociol RD. Early ambulation among heart failure patients is associated with reduced length of stay and 30-day readmissions. Circ Heart Fail. 2018;11:e004634. doi: 10.1161/CIRCHEARTFAILURE.117.004634. [DOI] [PubMed] [Google Scholar]
- 6.Martone AM, Bianchi L, Abete P, Bellelli G, Bo M, Cherubini A, Corica F, Di Bari M, Maggio M, Manca GM, Marzetti E, Rizzo MR, Rossi A, Volpato S, Landi F. The incidence of sarcopenia among hospitalized older patients: results from the Glisten study. J Cachexia Sarcopenia Muscle. 2017;8:907–914. doi: 10.1002/jcsm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 8.Fess EE, Moran CA. Clinical assessment recommendations. Indianapolis: American Society of Hand Therapists; 1981. pp. 6–8. [Google Scholar]
- 9.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya K, Hamazaki N, Matsue Y, Mezzani A, Corrà U, Matsuzawa R, Nozaki K, Tanaka S, Maekawa E, Noda C, Yamaoka-Tojo M, Matsunaga A, Masuda T, Ako J. Gait speed has comparable prognostic capability to six-minute walk distance in older patients with cardiovascular disease. Eur J Prev Cardiol. 2018;25:212–219. doi: 10.1177/2047487317735715. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Nakayama N, Kiyokuni M, Sumita S, Ebina T, Kosuge M, Hibi K, Tsukahara K, Iwahashi N, Endo M, Maejima N, Saka K, Hashiba K, Okada K, Taguri M, Morita S, Sugiyama S, Ogawa H, Sashika H, Umemura S, Kimura K. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Izawa KP, Watanabe S, Osada N, Kasahara Y, Yokoyama H, Hiraki K, Morio Y, Yoshioka S, Oka K, Omiya K. Handgrip strength as a predictor of prognosis in Japanese patients with congestive heart failure. Eur J Cardiovasc Prev Rehabil. 2009;16:21–27. doi: 10.1097/HJR.0b013e32831269a3. [DOI] [PubMed] [Google Scholar]
- 13.Yamada S, Shimizu Y, Suzuki M, Izumi T, PTMaTCH collaborators Functional limitations predict the risk of rehospitalization among patients with chronic heart failure. Circ J. 2012;76:1654–1661. doi: 10.1253/circj.CJ-11-1178. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passantino A, Lagioia R, Mastropasqua F, Scrutinio D. Short-term change in distance walked in 6 min is an indicator of outcome in patients with chronic heart failure in clinical practice. J Am Coll Cardiol. 2006;48:99–105. doi: 10.1016/j.jacc.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 16.Portney LG, Watkins MP (2000) Validity of measurements, Chapter 6. Foundations of clinical research applications to practice, 2nd edn. Prentice-Hall Inc., Upper Saddle River NJ, pp 92–101
- 17.Sutton NR, Gurm HS. Door to balloon time: is there a point that is too short? Prog Cardiovasc Dis. 2015;58:230–240. doi: 10.1016/j.pcad.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, et al. Influence of patient age and sex on delivery of guideline-recommended heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Am Heart J. 2009;157:754–762. doi: 10.1016/j.ahj.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchihashi-Makaya Miyuki, Hamaguchi Sanae, Kinugawa Shintaro, Yokota Takashi, Goto Daisuke, Yokoshiki Hisashi, Kato Norihiro, Takeshita Akira, Tsutsui Hiroyuki, for the JCARE-CARD Investigators Characteristics and Outcomes of Hospitalized Patients With Heart Failure and Reduced vs Preserved Ejection Fraction. Circulation Journal. 2009;73(10):1893–1900. doi: 10.1253/circj.CJ-09-0254. [DOI] [PubMed] [Google Scholar]


