Abstract
Sleep is fundamental for everyday functioning, yet it is often negatively impacted in critically ill patients by the intensive care setting. With a focus on the neurological intensive care unit (NeuroICU), this narrative review summarizes methods of measuring sleep and addresses common causes of sleep disturbance in the hospital including environmental, pharmacological, and patient-related factors. The effects of sleep deprivation on the cardiovascular, pulmonary, immune, endocrine, and neuropsychological systems are discussed, with a focus on short-term deprivation in critically ill populations. Where evidence is lacking in the literature, long-term sleep deprivation studies and the effects of sleep deprivation in healthy individuals are also referenced. Lastly, strategies for the promotion of sleep in the NeuroICU are presented.
Keywords: Sleep, Sleep deprivation, Intensive care unit, Brain injury, Critical illness
Introduction
Sleep is a complex physiological phenomenon necessary for restoration, growth, cognition, and survival. Sleep is particularly important among patients recovering from critical illness. Unfortunately, patients in the intensive care unit (ICU) frequently experience sleep deprivation (reduced quality and/or quantity of sleep compared to healthy subjects) [1, 2]. After reviewing methods for measuring sleep, this review highlights common causes of sleep deprivation in the ICU and its impact on the functioning of major organ systems. While there is a focus on topics pertinent to the neurological ICU (NeuroICU), we note that much of the literature comes from general critical care rather than a neurocritical patient population.
Methods
A narrative review of published literature was performed. Relevant abstracts were identified using the PubMed and Google Scholar databases. Search terms included ‘sleep deprivation,’ ‘critical care,’ ‘neurological ICU,’ and ‘neurocritical care.’ Search terms for effects on individual organ systems followed the format of ‘sleep and X’ with X being ‘heart,’ ‘cardiovascular,’ ‘blood pressure,’ ‘hypertension,’ ‘obstructive sleep apnea,’ ‘lung,’ ‘ventilator,’ ‘neuromuscular,’ ‘immune,’ ‘cytokines,’ ‘glucose,’ ‘metabolic,’ ‘temperature,’ ‘cognitive,’ ‘brain,’ ‘psychiatric,’ ‘delirium,’ and ‘pain.’ References known to the authors were also considered. Abstracts were reviewed, and articles whose abstracts addressed sleep deprivation were evaluated in full. A total of 210 articles published between 1958 and 2019 were deemed relevant for inclusion after this evaluation.
Measuring Sleep in the NeuroICU
Polysomnography (PSG) is the gold standard for measuring sleep. It involves simultaneous electroencephalogram (EEG), electromyogram, and electrooculogram and is interpreted according to American Academy of Sleep Medicine scoring guidelines [3]. Portable PSG is feasible in the ICU, but limited by cumbersome and costly equipment and the difficulties in interpretation among the critically ill [4]. Standard staging criteria in these patients have not been shown to reliably distinguish between sleep and wake as the K complexes and sleep spindles that classically characterize stage 2 sleep are often absent [5]. Thus, revised PSG scoring schemes have been proposed for the critically ill, mechanically ventilated population [6, 7].
For critically ill patients, although total sleep quantity varies, sleep is consistently highly fragmented and much less time is spent in the deeper (slow wave and REM) sleep stages as compared to healthy individuals [8–10]. ICU patients also exhibit disrupted circadian rhythms, with nearly 50% of sleep occurring during the daytime [11]. Those with sepsis have varying severity of encephalopathy and lack definable sleep–wake periods on EEG [5]. Furthermore, common ICU medications such as benzodiazepines, analgesics, and antipsychotics can lead to spindle coma patterns, burst suppression, and slowing on EEG [12], making sleep staging difficult.
Unfortunately, sleep has been especially challenging to describe in the NeuroICU population given the presence of preexisting neurological injuries that further complicate EEG interpretation. A recent study by Foreman et al. to evaluate sleep in the NeuroICU found that 65% of patient EEG recordings could not be scored, typically due to the lack of a distinguishable posterior dominant rhythm [13]. Using scorable recordings, the authors found that patients spent significantly more time in lighter (N1) than deeper sleep stages as compared to normal values. Moreover, sleep was abnormally distributed across the day–night cycle—similar to findings in critically ill patients without neurological injury. Studies of patients with subarachnoid hemorrhage (SAH) [14] and traumatic brain injury (TBI) [15] have also noted grossly abnormal or even absent sleep architecture in the majority of subjects; greater degrees of abnormality correlated with poorer prognosis.
Other tools such as actigraphy and bispectral index (BSI) have been considered as alternatives to PSG to measure sleep but present their own challenges of accuracy and reliability [16]. Actigraphy (which estimates sleep using a motion sensor worn on the wrist or ankle) is a convenient, noninvasive technique that can be used for extended periods of time; however, it likely overestimates sleep in a largely bedridden, sedated population [17]. It may be useful for assessing sleep fragmentation [18, 19]. Originally employed to monitor anesthesia in the operating room, BSI uses a single foam EEG sensor applied to the forehead. The BSI is calculated as a weighted sum of several EEG parameters and is scaled from 0 to 100, with larger values corresponding to a higher likelihood of alertness. BSI monitoring may predict depth of sedation in critically ill patients with brain injury [20], but further research is necessary to demonstrate its correlation with depth of sleep in this population [21].
Causes of Sleep Disturbance in the NeuroICU
Numerous studies have evaluated the causes of sleep disruption in the ICU setting. Environmental contributors include patient care, noise, light, and medications. Patient factors, including illness severity, mechanical ventilation requirement, and pain, can also play important roles.
Patient Care
In a recent cross-sectional, questionnaire-based study assessing sleep quantity and quality among 2005 hospitalized (ICU and other) patients in the Netherlands, 85% reported at least one nocturnal awakening, and 20% experienced sleep disturbance due to awakenings by hospital staff [22]. Our search yielded only two studies that quantify the number of care events occurring during patient sleep in the ICU. Using PSG and time-synchronized environmental monitoring, Gabor and colleagues found activities occurring approximately eight times per hour of sleep, accounting for just 7% of total arousals and awakenings (68% of sleep disruption events did not have an identifiable cause; 21% were due to sound) [8]. Tamburri et al. found an average of 42.6 interactions per night (7 p.m. to 7 a.m.), but did not directly measure sleep via PSG [23]. Nevertheless, patient care activities consistently rank as a major source of sleep disturbance in surveys of ICU survivors, even more so than noise level [5]. Although many patient care activities are unavoidable or temporally fixed, efforts have been proposed to minimize and reschedule as possible to reduce sleep disruption (see “Strategies for Sleep Promotion in the NeuroICU”). Specific to the NeuroICU, hourly neurological assessments (neurochecks) are performed on many patients and are often cited to justify increased nursing needs [24]. In conjunction with neuroimaging surveillance requiring travel off-unit, neurochecks have been demonstrated to impact surgical decision making in patients with primary intracerebral hemorrhage (ICH) [25] and severe TBI [26]. The appropriate duration and frequency of these neurochecks for most diagnoses is often at attending provider discretion [26]. Evidence from patients admitted for ICH suggests that the initial 12 h is a period of frequent and prognostically important neurological change [27], while TBI patients appear to be at highest risk of expansion of a traumatic intracerebral hematoma within 24 h from onset [28].
Noise and Light Levels
While the typical ICU is noisier than the levels recommended by the Environmental Protection Agency [29] and World Health Organization [30] (50–90 dB vs the recommended 20–45 dB) [31, 32], studies utilizing PSG have found that environmental noise causes less than 20% of all arousals and awakenings among ICU patients [5, 33]. Work by Stanchina et al. demonstrating that sleep disruption is caused by the relative noise difference from baseline to peak suggests opportunities for effective intervention with white noise if absolute noise levels cannot be reduced [34]. Environmental light exposure appears to play a smaller role in sleep disruption than patient care activities or noise, and its effect may be mediated by inadequate daytime light exposure [5, 35].
Medication and Pharmacological Effects
Generally speaking, medications can disrupt sleep both physiologically and during their administration. In general, sedation shares characteristics with sleep (similar receptor and network effects, muscle hypotonia, altered mentation, respiratory depression) but does not produce the full spectrum of restorative benefits found in sleep [36, 37]. Benzodiazepines, while frequently prescribed for insomnia, have actually been found to suppress REM and deeper N3 sleep stages in favor of lighter N2 sleep in healthy individuals [38]. Similarly, opioids have a sedating effect, but decrease total sleep time and time spent in deeper sleep stages [39, 40]. Other sleep-disrupting medications commonly administered to ICU patients include vasopressors [41] and beta-blockers [42] via their impacts on melatonin production; antidepressants [43, 44], which can decrease time spent in REM sleep; continuous intravenous drips and diuretics, which may increase the frequency of toilet visits or require uncomfortable indwelling urinary catheters [16]; and corticosteroids, which reduce REM sleep and increase the incidence of nightmares [45].
Dexmedetomidine, a selective alpha 2-adrenoreceptor agonist, is increasingly utilized in the NeuroICU due to its analgesic, sedative, and anxiolytic effects while maintaining patient arousability and minimizing respiratory depression [46, 47]. While dexmedetomidine is more expensive, several studies have found it to be more cost-effective than traditional sedatives overall as it is associated with reduced ICU stay costs and shorter times to extubation [48–50]. Interestingly, compared to other commonly used sedative–anesthetics such as propofol; in rats, dexmedetomidine may induce a sedative response more similar to natural sleep although it seems to increase the proportion of states resembling light (stages 1 and 2) rather than deep sleep (stage 3 and REM) [51, 52].
Patient-Specific Factors
A number of patient-specific factors also contribute to sleep deprivation. Illness severity has been positively correlated with sleep disruption (e.g., in sepsis) [53]; however, this finding has not held true in studies of ICU patients across diagnoses [5, 54]. Pain, either related to illness severity or on its own, is associated with poor sleep and increased arousals due to discomfort [55, 56]. In fact, pain causing loss of sleep may trigger an unfortunate feedback loop in which lack of sleep leads to hyperalgesia, which in turn inhibits adequate rest [57]. Specific to the NeuroICU, intracranial devices such as external ventricular drains and continuous EEG may cause additional discomfort, though this remains speculation.
One area that has been thoroughly investigated is that of mechanical ventilation, and the mode most conducive to sleep. Mechanical ventilation can disrupt sleep due to mismatch between patient demand and ventilator supply (inadequate support leading to dyspnea or excessive support leading to apnea). Studies by Parthasarathy et al. [58] and Toublanc et al. [59] found that when compared to assist control ventilation (ACV), pressure support ventilation (PSV) led to episodic hyperventilation with resulting decreased partial pressure of carbon dioxide, causing apneic events. However, work by Cabello and colleagues showed no differences in sleep architecture between non-sedated ICU patients on clinically adjusted PSV, automatically adjusted PSV, or ACV. The authors therefore suggested that it is not PSV itself that causes sleep disruption but the specific ventilator settings: They used lower levels of pressure support, standard minute ventilation, and tidal volume across all modes as compared to previous authors in an effort to better mimic physiological conditions [60]. Other studies looking at proportional assist ventilation [61]—in which pressure is applied in proportion to the patient’s own inspiratory effort—and neurally adjusted ventilatory assist—in which pressure is applied in proportion to the electrical activity of the patient’s diaphragm—also find improved sleep quality [62]. Thus, evidence from multiple studies indicates that patient–ventilator mismatch, rather than any given ventilator mode, is most responsible for sleep disruption. However, it can be challenging to find the appropriate settings most reflective of patient physiology, and clinical conditions (e.g., intracranial pressure crises in the setting of hypercarbia) may demand certain ventilator modes. Additionally, when generalizing the results of critical care ventilation studies to neurological patients one must consider the underlying need for ventilation (e.g., airway protection versus hypoxemic respiratory failure). Moreover, NeuroICU patients may already experience frequent spontaneous hyperventilation and periodic breathing apneas from their primary injuries, making it difficult to apply optimal ventilator settings based on studies in non-neurological populations.
The impact of noninvasive ventilation on sleep in the critically ill population has received far less attention than invasive ventilation. It appears that poor sleep quality on PSG in the early stages of noninvasive ventilation is correlated with eventual failure (patient death, endotracheal intubation, persistent noninvasive ventilation requirement after 6 days) [63]. Of particular interest in the NeuroICU is the impact of ventilation mode on sleep in patients with neuromuscular disease. Much of the respiratory research in this population is done using noninvasive ventilation [64]. Similar to findings with invasive ventilation, Fanfulla and colleagues found that noninvasive pressure support set with physiological parameters (tailored to the patient’s respiratory effort via measurement of esophageal pressure) among neuromuscular patients with chronic respiratory failure improves nighttime gas exchange, sleep architecture, and sleep efficiency [65].
Impact on Organ Systems in Critically Ill Patients
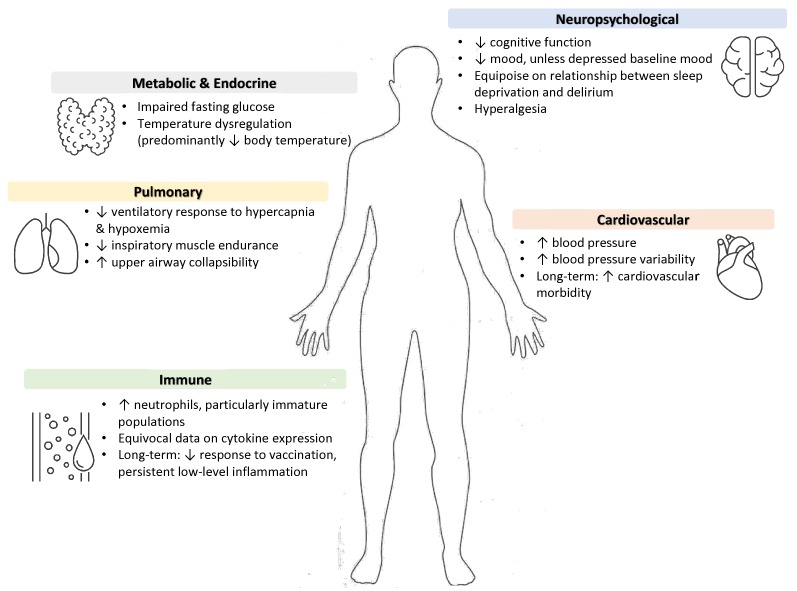
There are few data about the impact of sleep deprivation on critically ill patients as most research in this area is conducted with healthy (and typically male) subjects. What follows is an overview of the impact of sleep deprivation on specific organ systems with extrapolation to the critically ill population when possible (see Fig. 1). It emphasizes experimental studies of short-term sleep deprivation, but some long-term sequelae will be highlighted as ICU stays may be extensive or precipitate chronic problems. Notably, experimental studies of short-term sleep deprivation differ significantly in the length of sleep deprivation assessed, subject environment and behaviors, and measurement time points, which makes comparison across studies challenging. For the purposes of our discussion, short-term or acute sleep deprivation refers to sleep deprivation of less than one-week duration, while long-term or chronic deprivation is on the order of months. When possible, studies conducted in obstructive sleep apnea (OSA) patients will be discussed, as the fragmented nature of their sleep serves as an organic model system for fragmented sleep in ICU patients [66].
Fig. 1.
Suspected impact of sleep deprivation on organ systems
Cardiovascular System
Elevated blood pressure is a recognized consequence of short-term sleep deprivation. Many studies have found increases in blood pressure and heart rate after a single night of experimental deprivation [67–69], though effects can vary with subject posture during deprivation and vital sign measurement [70, 71]. Hospitalization itself is also associated with higher morning blood pressure in older adults [72]. The hypertensive effect of sleep deprivation has been attributed to an increase in sympathetic activity [73], a reduction in maximum endothelium-dependent vasodilation [52], and an upward resetting of the baroreflex set point [50, 57]. This relationship between sleep deprivation and blood pressure has particular relevance for NeuroICU patients with strict blood pressure parameters, e.g., patients with hypertensive and aneurysmal bleeds. In addition to its hypertensive effect, sleep deprivation is also correlated with increased blood pressure variability [74], which may worsen stroke outcomes [75, 76].
A link between chronic sleep deprivation and increased cardiovascular morbidity has also been demonstrated across numerous epidemiologic studies [77–79], and since ICU length of stay (LOS) can be prolonged, we will briefly discuss the relationship here. This relationship holds true in OSA, in which higher numbers of apneic events correlate with increased blood pressure variability during wakefulness, a phenomenon which may precede the development of persistent hypertension [80]. OSA patients also have increased sympathetic activity and resting heart rate. An elevation in blood pressure and inflammation—which also increases with chronic lack of sleep [81]—may underlie this relationship, increasing cumulative cardiovascular risk. For example, levels of C-reactive protein (CRP), an inflammatory marker shown to be an independent predictor of future myocardial infarction and stroke in apparently healthy individuals [82, 83], are higher after experimental sleep deprivation [84]. Newly diagnosed OSA patients have also been found to have elevated levels of CRP, with severity proportional to the CRP level [85].
Pulmonary System
The impact of sleep deprivation on pulmonary function is relevant in the NeuroICU given the proportion of patients who require ventilatory support [86, 87] and the prevalence of sleep-disordered breathing among stroke and transient ischemic attack patients [88]. As compared to ventilated non-neurological patients, those with neurological disease experience poorer outcomes with longer ICU and ventilator days, more tracheostomies, and higher mortality rates [89].
Most studies have found a reduced ventilatory response to hypercapnia and hypoxemia after 24 h of sleep deprivation [90–92], although this finding has been disputed by a newer study performed with stricter control of subject behavior and environment [93]. Short-term sleep deprivation has also been shown to lessen inspiratory muscle endurance. In humans, Chen et al. found a significant reduction in maximal voluntary ventilation after 30 h of sleeplessness, with no change in respiratory muscle strength, forced expiratory volume in one second, and forced vital capacity [94]. Studies in rats [95] and exploratory work in humans [96] have suggested that the underlying mechanism is impairment of respiratory motor plasticity (i.e., inspiratory drive in response to hypoxia) with sleep deprivation, a finding that may be especially problematic among patients hospitalized for brain injury.
Experimental sleep deprivation has also been found to increase upper airway collapsibility, which can cause or contribute to preexisting OSA and lead to challenges in extubation [97–99]. This finding may be pertinent for neurological patients with pharyngeal muscle weakness. Studies on patients with OSA have also demonstrated that cerebral blood flow (CBF) decreases during obstructive apneas [100–102], and intracranial pressure increases in linear proportion to the length of an apneic event [103]. The reduction of CBF in OSA may be attributed to damage to brain areas essential for vascular regulation (including the insular, cingulate, and medial frontal cortices; raphe and ventrolateral medulla) and an unexplained blunting of the typical vasodilator response to increased PaCO2 [104]. Such results highlight the possible complications of sleep deprivation for NeuroICU patients with ischemic brain injury, but may not be generalizable as the authors do not correlate apneas with sleep disruption.
Immune System
Conventional wisdom considers sleep essential to maintaining the body’s immunologic defenses. However, aside from response to vaccination—consistently shown to be lower in sleep-deprived individuals [105–107]—loss of sleep has not consistently demonstrated impacts on other aspects of the immune system. Short-term sleep deprivation studies largely assess the effect of varying degrees of sleep deprivation and recovery on specific cell types and cytokines. Long-term sleep deprivation has also been associated with persistent, low-level inflammation that can increase risk of conditions such as cardiovascular disease [108] and arthritis [109] and has been associated with increased risk of pneumonia [110]—at least in observational studies. The significance of these individual changes in the larger context of illness recovery or infection response is difficult to evaluate.
The impact of sleep deprivation on natural killer (NK) cells, which play a critical role in early defense against viruses and tumor cell cytotoxicity [111], has been studied extensively with great variability in results. Several authors report a decline in NK cell function with sleep deprivation, and a return to baseline after one night of recovery sleep [112–114]. One study did not find NK cell function recovery after resumed sleep [115], and one found an increase in NK cell activity after severe sleep deprivation (64 h) [116]. This variation might be explained by the difference in timing of blood draws across experiments, which may be confounded by the natural circadian rhythm of NK cell activity, and the small number of subjects in each of these studies (10 or fewer).
Neutrophils have been found to be persistently elevated after sleep deprivation, even with return to normal sleep [117, 118]. These cells are the ‘first responders’ at a site of potential injury or infection and therefore serve as a marker of acute inflammation. Christoffersson et al. demonstrated an enrichment of immature populations and a reduced capacity to produce reactive oxygen species in response to bacterial and pharmacologic stimuli, suggesting the promotion of a less effective yet pro-inflammatory state with one night of total sleep deprivation [119]. Notably, elevated neutrophil-to-lymphocyte ratios in peripheral blood have been shown to be predictive of poor outcomes among patients with TBI [120], acute ICH [121], and stroke [122].
Data on cytokine expression are equivocal. Levels of pro-inflammatory proteins ICAM-1, E-selectin, IL-1b, IL-6, and TNF-a, have been found to increase after one night of total sleep deprivation in most [123, 124] but not all studies [125]. A meta-analysis of 72 studies evaluating CRP (n > 34,000), IL-6 (n > 30,000), and TNF-a (n = 672) levels after sleep deprivation found higher levels of CRP with sleep disturbance and the extremes of short or long sleep, higher IL-6 with sleep disturbance and the extreme of long sleep, and no changes in TNF-a. Notably, neither experimental sleep deprivation nor sleep restriction was associated with statistically significant changes in any of the three inflammatory markers in this meta-analysis [60]. Regarding neurological injury specifically, acute elevation of CRP following ICH is associated with higher mortality and poor functional outcome [126], although the etiology underlying the rise in CRP in ICH is likely multifactorial.
Ultimately, increases and decreases in immune cell activity and cytokine levels are seen with inadequate sleep. Regarding acute brain injury (ABI) specifically, the pro-inflammatory milieu of biomarkers that can occur with sleep deprivation is also associated with poorer outcomes. Additional research is required to fully comprehend the physiological significance of these changes, but raises the possibility that sleep normalization and restoration of the circadian rhythm may be targets for improving outcomes in ABI. Furthermore, given the potential for inflammatory markers to predict or influence prognosis after brain injury, future studies may explore the value of sleep indices such as sleep quality or fragmentation as mediators of clinical outcomes.
Metabolic and Endocrine Systems
Acute sleep deprivation alters glucose metabolism and modulates hormone levels [127, 128]. Among hospitalized patients, shorter sleep duration and worse sleep efficiency (measured via wrist actigraphy) are associated with hyperglycemia and impaired fasting glucose [129]. This is important to consider among those with acute neurological injury due to the association between hyperglycemia and worse outcomes in patients with ischemic stroke [130], TBI [131], and SAH [132–134].
Several experimental studies have demonstrated that the loss of sleep greater than one hour per night can decrease glucose sensitivity by 20–40% among healthy subjects [44, 135]. This phenomenon may occur through the modulation of both hepatic and peripheral metabolic pathways after just a single night of sleep restriction to 4 h [136]. Changes in glucose metabolism likely contribute to the correlation between chronic sleep loss and type 2 diabetes observed in epidemiological studies [137].
Thermoregulation is also very important in the NeuroICU. Hyperthermia and fever (temperature > 38 °C) is a cause for concern among patients with stroke and TBI [138] and occurs in up to half of these patients [139–141]. Cooling techniques and pharmaceutical management can be used to target normothermia and even hypothermia. Although sleep deprivation has been associated with declines in body temperature [142–144], it is difficult to clarify the relevance of this finding in the context of acute neurological injury, which itself causes temperature dysregulation [145].
Neuropsychological Effects
It is known that sleep deprivation impacts neuropsychological function in healthy individuals. The impact of sleep deprivation on various aspects of neuropsychological functioning among ICU patients is not as well studied, and what we do know is addressed as follows:
Cognitive Function
Cognitive performance declines with sleep deprivation and fragmentation in healthy individuals [146]. A wide range of cognitive tasks—attention, working memory, short-term memory, learning, and situational awareness—are negatively impacted by lack of sleep and worsen as sleep-deprived subjects spend more time on task [147]. In a landmark meta-analysis of 19 studies evaluating the neurocognitive consequences of sleep deprivation in 1932 non-critically ill patients, Pilcher et al. found that a person at the 50th percentile (composite of motor, cognitive, and mood domains) in the sleep-deprived group performs at approximately the same level as a person at the 9th percentile in the non-sleep-deprived group [148]. In healthy individuals, improvement in cognitive impairment can be seen with adequate rest [149, 150].
Critical illness itself—independent of sleep deprivation—appears to have an effect on cognitive performance. ICU survivors exhibit cognitive impairments across a similarly wide range of domains so-called post-intensive care syndrome. Between 25 and 78% of ICU survivors experience neurocognitive impairments, with acute respiratory distress syndrome patients particularly afflicted [151–154]. While cognitive function has been found to improve in the first year after discharge, long-term studies assessing outcomes several years post-ICU stay have found persistent impairment after 6 years [155]. The extent of this cognitive impairment is notable: Jackson et al. found impairments in visual domains, psychomotor speed, and verbal fluency in ICU survivors at 6-month follow-up that were clinically equivalent to mild dementia [156]. The mechanisms by which this impairment occurs in the ICU include hypotension, hypoxemia, hyperglycemia, and delirium during admission [73]. The relationship between sleep deprivation and cognitive impairment in this setting has not been investigated directly.
Psychiatric Effects
Sleep deprivation for one night has been observed to alleviate depressive symptoms in 40–60% of patients with preexisting depression [157], though this effect is limited by relapse with recovery sleep [158]. However, sleep deprivation of healthy subjects impairs mood as assessed using the Profile of Mood States rating scale [64, 159]. Underlying confinement to a laboratory setting can have a negative impact on mood [49, 160], which may confound findings in sleep studies lacking controls [69]. There is evidence to suggest that sleep deprivation may reset circadian clock gene abnormalities that contribute to depression [161]. Perhaps sleep deprivation can only have a beneficial effect in depressed patients with preexisting circadian rhythm disturbances.
In addition to new cognitive symptoms, ICU survivors may also display post-traumatic stress disorder (PTSD), depression, and anxiety. Depression and anxiety have a documented prevalence of 17–43% [62, 162] and 23–48% [163], respectively. Estimates of the prevalence of ICU-PTSD vary from 19% in systematic review [164] to 64% (although these authors ultimately felt the variability among studies to be too significant to draw strong conclusions) [165, 166]. These new psychiatric symptoms can have long-lasting effects on sleep; for instance, PTSD often causes persistent distress and nightmares [167].
While sleep deprivation outside of the ICU has a negative effect on mood and subjective stress [168], its effects in the ICU are more speculative. Studies of daily sedation interruption have found an improvement in PTSD symptoms, theoretically by facilitating the formation of factual rather than delusional memories which are characteristic of the condition [169]. It remains unclear whether sleep deprivation, which has different physiological consequences from sedation interruption, would alter psychiatric symptoms in the same way.
Delirium
Delirium is a clinical syndrome of fluctuations in consciousness, attention, and cognition. It is an independent predictor of mortality [170–172], increased hospital LOS [50, 173, 174], and persistently worsened physical and mental status after discharge [175, 176]. There is significant overlap in the risk factors, pathophysiology, and presentation of sleep deprivation and delirium, particularly the hypoactive subtype which is characterized by lethargy and sedation [177].
Three neuroanatomical pathways of attention have been proposed [178, 179], and those same regions evidence abnormalities (utilizing functional magnetic resonance imaging [180], positron emission tomography [181], and EEG [182]) in both sleep deprivation and delirium [183]. Both conditions also appear to share the neurochemical alterations of cholinergic deficiency and dopaminergic excess, although the former has only been seen in rat models [57].
Despite these similarities, no studies have definitively shown that sleep deprivation is itself a risk factor for delirium, although there is equipoise on the topic [184]. Notably, sleep-promoting interventions have been shown to reduce the number and duration of delirium episodes among general medical ward [185], medical ICU [186], and surgical ICU [187, 188] patients.
Pain Perception
There appears to be a bidirectional relationship between pain perception and sleep deprivation. The conventional perspective is that pain impairs sleep, but sleep deprivation may also interfere with pain processing. Correlational studies in fibromyalgia patients have found more arousal episodes during sleep as compared to healthy controls [189], as well as an inverse relationship between subjective sleep quality and sensitivity to pressure pain [190]. In the acute setting, poor sleep has also been associated with a significantly greater pain experience among hospitalized burn patients [191]. Experimental work in healthy subjects has found that 24 to 40 h of total sleep deprivation causes either no [192, 193] or a generalized hyperalgesic effect [194], with a return to baseline following a night of recovery sleep [195]. The disruption of slow wave sleep seems particularly important in lowering the pain threshold [196, 197].
Strategies for Sleep Promotion in the NeuroICU
The critical care environment and circumstances surrounding admission to the ICU will disrupt sleep for many patients. Without data demonstrating a direct and significant impact on patient outcomes, sleep cannot take precedence over patient care activities. Nevertheless, there are several opportunities to address some of the causes of sleep deprivation discussed above.
Patient care may be consolidated and discretionary care activities (e.g., baths, phlebotomy, dressing changes) performed during the daytime. Hourly neurochecks may be limited to the most crucial initial 12 h after presentation or surgery, or for an extended period in patients more likely to require intervention, such as those with vasospasm or malignant cerebral edema. Basic noise reduction interventions such as earplugs and white noise machines have been shown to promote longer and better quality sleep [198, 199]. New ICUs can consider noise reduction in their designs [200]. Quiet time to allow naps in the afternoon and evening hours is a simple policy that may increase patient satisfaction with sleep [201], especially as patient satisfaction is becoming an important benchmark of care.
Inappropriate light deprivation or exposure may be corrected through the adjustment of window blinds, use of personal eyeshades, and appropriate timing of artificial light sources. The timing of indoor lighting to reflect or augment the physiological 24-h circadian sleep–wake cycle has been demonstrated to improve mood in seasonal affective disorder and non-seasonal depression [202]. A small pilot study with hematopoietic stem cell transplant survivors found that full-spectrum white light administered for 30 min every morning for 4 weeks improved cancer-related fatigue as compared to non-stimulating dim red light [203]. For Alzheimer’s disease and related dementia patients residing in healthcare facilities, lighting schemes that favor stimulating light with blue undertones in the morning appear to improve sleep time and efficiency [204]. Artificial light exposure during the daytime has been shown to decrease the incidence and duration of delirium [184]. Although more research is necessary to determine the benefits of circadian lighting schemes in the ICU setting, past work has shown subjective patient satisfaction with dynamic lighting environments without clear sleep benefits [205].
Patients receiving continuous sedation should receive daily sedative interruptions—when not limited by clinical contraindication—given its association with decreased duration of mechanical ventilation and LOS [206]. For mechanically ventilated patients, the ventilation mode should be tailored to pulmonary requirements. Given the association between sleep-disordered breathing and stroke/transient ischemic attack, simple interventions including avoidance of the supine position if possible, administering low concentrations of oxygen, and continuous positive airway pressure for hemorrhagic stroke patients may also be implemented [207]. Complementary therapies including massage [208–210] and music [211] have been found to promote sleep in the ICU setting, and should be further investigated.
Ultimately, ICU staff must be educated regarding the benefits of minimizing sleep deprivation for their patients. The exact form and quantity of interventions undertaken may vary by ICU, but increased awareness of this issue, real-time identification of areas for improvement, and active intentions to address them are necessary.
Conclusion
During their hospital admission, critically ill patients suffer from sleep disturbance due to environmental and personal factors, with detriment to various organ systems. Although the relevance of specific sleep-deprivation-related impacts to hospital and long-term outcomes requires further characterization, it is clear that low-quality sleep harms patients’ subjective well-being.
Critically ill patients with brain injuries face several unique challenges related to sleep deprivation. In the NeuroICU, patients are subject to hourly neurochecks and uncomfortable devices, in addition to the issues with the hospital environment, mechanical ventilation, and medications that other ICU patients experience. Impacts on the endocrine system such as glucose regulation and thermoregulation, in addition to potentially compounding the effect of sleep deprivation on preexisting neurological injury in neuropsychological domains, are particularly relevant in the NeuroICU population. Several straightforward interventions are possible to improve patient sleep quality and quantity. It should be noted that the major limitation of this review is the paucity of data derived from the NeuroICU patient population. Therefore, the continued optimization of care will require further investigation in this specific population regarding the impact of sleep deprivation on outcome measures and the utility of various interventions on sleep specifically in the neurocritically ill.
Authors’ Contribution
VA Chang was responsible for primary review of the literature and drafting of the manuscript, as well as providing final approval for submission. RL Owens provided revisions and feedback and was involved in final approval for submission. JN LaBuzetta was responsible for conception of the manuscript, extensive revisions and feedback, and providing final approval for submission.
Source of Support
No financial support was utilized in the preparation of this review article.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval/Informed Consent
This article does not contain any studies with human performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Victoria A. Chang, Email: vachang@uw.edu
Robert L. Owens, Email: rowens@ucsd.edu
Jamie N. LaBuzetta, Email: jlabuzetta@ucsd.edu
References
- 1.Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571–572. doi: 10.1016/S0140-6736(99)02728-2. [DOI] [PubMed] [Google Scholar]
- 2.Sarah R. An exploratory study of patients’ perceptions, memories and experiences of an intensive care unit. J Adv Nurs. 2001;29(4):783–791. doi: 10.1046/j.1365-2648.1999.00953.x. [DOI] [PubMed] [Google Scholar]
- 3.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. The AASM manual for the scoring of sleep and associated events. Am Acad Sleep Med. 2013;53(9):1689–1699. [Google Scholar]
- 4.Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43(5):445–452. doi: 10.1016/j.hrtlng.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 6.Drouot X, Roche-Campo F, Thille AW, et al. A new classification for sleep analysis in critically ill patients. Sleep Med. 2012;13(1):7–14. doi: 10.1016/j.sleep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41(8):1958–1967. doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically Ill patients requiring mechanical ventilation. Chest. 2000;117(3):809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 9.Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma Acute Care Surg. 2007;63(6):1210. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- 10.Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: anobservational study. Crit Care. 2013;17(2):R46. doi: 10.1186/cc12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 12.Blume WT. Drug effects on EEG. J Clin Neurophysiol. 2006;23(4):306. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- 13.Foreman B, Westwood AJ, Claassen J, Bazil CW. Sleep in the neurological intensive care unit: feasibility of quantifying sleep after melatonin supplementation with environmental light and noise reduction. J Clin Neurophysiol. 2015;32(1):66. doi: 10.1097/WNP.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 14.Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4(2):103–112. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- 15.Bergamasco B, Bergamini L, Doriguzzi T. Clinical value of the sleep electroencephalographic patterns in post-traumatic coma. Acta Neurol Scand. 1968;44(4):495–511. doi: 10.1111/j.1600-0404.1968.tb05588.x. [DOI] [PubMed] [Google Scholar]
- 16.Nasraway SA, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM. How reliable is the bispectral index in critically ill patients? A prospective, comparative, single-blinded observer study. Crit Care Med. 2002;30(7):1483. doi: 10.1097/00003246-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Schwab KE, Ronish B, Needham DM, To AQ, Martin JL, Kamdar BB. Actigraphy to evaluate sleep in the intensive care unit. A systematic review. Ann Am Thorac Soc. 2018;15(9):1075–1082. doi: 10.1513/AnnalsATS.201801-004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34(11):2076–2083. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- 19.van der Kooi AW, Tulen JHM, van Eijk MMJ, et al. Sleep monitoring by actigraphy in short-stay ICU patients. Crit Care Nurs Q. 2013;36(2):169. doi: 10.1097/CNQ.0b013e318283cff3. [DOI] [PubMed] [Google Scholar]
- 20.Deogaonkar A, Gupta R, DeGeorgia M, et al. Bispectral Index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32(12):2403. doi: 10.1097/01.CCM.0000147442.14921.A5. [DOI] [PubMed] [Google Scholar]
- 21.Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88(3):659. doi: 10.1213/00000539-199903000-00035. [DOI] [PubMed] [Google Scholar]
- 22.Wesselius HM, van den Ende ES, Alsma J, et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018;178(9):1201–1208. doi: 10.1001/jamainternmed.2018.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13(2):102–113. doi: 10.4037/ajcc2004.13.2.102. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin DC, Hartjes TM, Freeman WD. Sleep deprivation in neurointensive care unit patients from serial neurological checks: how much is too much? J Neurosci Nurs. 2018;50(4):205–210. doi: 10.1097/JNN.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 25.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81(2):107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone JJ, Childs S, Smith LE, Battin M, Papadakos PJ, Huang JH. Hourly neurologic assessments for traumatic brain injury in the ICU. Neurol Res. 2014;36(2):164–169. doi: 10.1179/1743132813Y.0000000285. [DOI] [PubMed] [Google Scholar]
- 27.Maas MB, Berman MD, Guth JC, Liotta EM, Prabhakaran S, Naidech AM. Neurochecks as a biomarker of the temporal profile and clinical impact of neurologic changes after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24(9):2026–2031. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaki T, Hirakawa K, Ueguchi T, Tenjin H, Kuboyama T, Nakagawa Y. Chronological evaluation of acute traumatic intracerebral haematoma. Acta Neurochir (Wien) 1990;103(3):112–115. doi: 10.1007/BF01407516. [DOI] [PubMed] [Google Scholar]
- 29.EPA . Information on levels of environmental noise requisite to protect public health and welfare with an adequate margin of safety. Washington, D.C: EPA; 1974. pp. 1–242. [Google Scholar]
- 30.Berglund B, Lindvall T, Schwela DH. Guideline Values. Guidelines for community noise (1999). (http://www.who.int/docstore/peh/noise/Commnoise4.htm).
- 31.Balogh D, Kittinger E, Benzer A, Hackl JM. Noise in the ICU. Intensive Care Med. 1993;19(6):343–346. doi: 10.1007/BF01694709. [DOI] [PubMed] [Google Scholar]
- 32.Martin C. Noise levels in a general intensive care unit: a descriptive study. Nurs Crit Care. 2007;12(4):188–197. doi: 10.1111/j.1478-5153.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 33.Jaiswal SJ, Garcia S, Owens RL. Sound and Light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017;12(10):798–804. doi: 10.12788/jhm.2826. [DOI] [PubMed] [Google Scholar]
- 34.Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423–428. doi: 10.1016/j.sleep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal SJ, Garcia S, Owens RL. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017;12(10):798–804. doi: 10.12788/jhm.2826. [DOI] [PubMed] [Google Scholar]
- 36.Alkire MT, Haier RJ, Barker SJ, et al. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82(2):393–403. doi: 10.1097/00000542-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Sebel PS, Bovill JG, Wauquier A, et al. Effects of high dose fentanyl anesthesia on the electroencephalogram. Anesthesiology. 1981;55(3):203–211. doi: 10.1097/00000542-198109000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Gevins AS, Stone RK, Ragsdale SD. Differentiating the effects of three benzodiazepines on non-REM sleep EEG spectra. Neuropsychobiology. 1988;19(2):108–115. doi: 10.1159/000118444. [DOI] [PubMed] [Google Scholar]
- 39.Kay DC, Pickworth WB, Neidert GL, Falcone D, Fishman PM, Othmer E. Opioid effects on computer-derived sleep and EEG parameters in nondependent human addicts. Sleep. 1979;2(2):175–191. doi: 10.1093/sleep/2.2.175. [DOI] [PubMed] [Google Scholar]
- 40.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3(01):33–36. [PubMed] [Google Scholar]
- 41.Andrzejowski J, Sleigh JW, Johnson IAT, Sikiotis L. The effect of intravenous epinephrine on the bispectral index and sedation. Anaesthesia. 2000;55(8):761–763. doi: 10.1046/j.1365-2044.2000.01532.x. [DOI] [PubMed] [Google Scholar]
- 42.Stoschitzky K, Sakotnik A, Lercher P, et al. Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol. 1999;55(2):111–115. doi: 10.1007/s002280050604. [DOI] [PubMed] [Google Scholar]
- 43.Kupfer DJ, Ehlers CL, Pollock BG, Swami Nathan R, Perel JM. Clomipramine and EEG sleep in depression. Psychiatry Res. 1989;30(2):165–180. doi: 10.1016/0165-1781(89)90158-3. [DOI] [PubMed] [Google Scholar]
- 44.Feuillade P, Pringuey D, Belugou JL, Robert P, Darcourt G. Trimipramine: acute and lasting effects on sleep in healthy and major depressive subjects. J Affect Disord. 1992;24(3):135–145. doi: 10.1016/0165-0327(92)90061-A. [DOI] [PubMed] [Google Scholar]
- 45.Turner R, Elson E. Sleep disorders. Steroids cause sleep disturbance. BMJ Br Med J. 1993;306(6890):1477. doi: 10.1136/bmj.306.6890.1477-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 47.Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med. 2004;30(12):2188–2196. doi: 10.1007/s00134-004-2417-z. [DOI] [PubMed] [Google Scholar]
- 48.Turunen H, Jakob SM, Ruokonen E, et al. Dexmedetomidine versus standard care sedation with propofol or midazolam in intensive care: an economic evaluation. Crit Care. 2015;19(1):67. doi: 10.1186/s13054-015-0787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bioc JJ, Magee C, Cucchi J, et al. Cost effectiveness of a benzodiazepine vs a nonbenzodiazepine-based sedation regimen for mechanically ventilated, critically ill adults. J Crit Care. 2014;29(5):753–757. doi: 10.1016/j.jcrc.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Dasta JF, Kane-Gill SL, Pencina M, et al. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38(2):497–503. doi: 10.1097/CCM.0b013e3181bc81c9. [DOI] [PubMed] [Google Scholar]
- 51.Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M. Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med. 2012;38(12):1982–1989. doi: 10.1007/s00134-012-2685-y. [DOI] [PubMed] [Google Scholar]
- 52.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52(2):289–294. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 53.Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30(3):536. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Bihari S, Doug McEvoy R, Matheson E, Kim S, Woodman RJ, Bersten AD. Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8(3):301–307. doi: 10.5664/jcsm.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atkinson JH, Ancoli-Israel S, Slater MA, Garfin SR, Gillin C. Subjective sleep disturbance in chronic back pain. Clin J Pain. 1988;4(4):225. doi: 10.1097/00002508-198812000-00007. [DOI] [Google Scholar]
- 56.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23(1):1–13. doi: 10.1023/A:1005444719169. [DOI] [PubMed] [Google Scholar]
- 57.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1–3):56. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166(11):1423–1429. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 59.Toublanc B, Rose D, Glérant J-C, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33(7):1148–1154. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 60.Cabello B, Thille AW, Drouot X, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36(6):1749. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 61.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35(4):1048. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 62.Delisle S, Ouellet P, Bellemare P, Tétrault J-P, Arsenault P. Sleep quality in mechanically ventilated patients: comparison between NAVA and PSV modes. Ann Intensive Care. 2011;1:42. doi: 10.1186/2110-5820-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campo FR, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):477. doi: 10.1097/CCM.0b013e3181bc8243. [DOI] [PubMed] [Google Scholar]
- 64.Luo F, Annane D, Orlikowski D, et al. Invasive versus non-invasive ventilation for acute respiratory failure in neuromuscular disease and chest wall disorders. Cochrane Database Syst Rev. 2017;12:CD008380. doi: 10.1002/14651858.CD008380.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fanfulla F, Delmastro M, Berardinelli A, Lupo ND, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med. 2005;172(5):619–624. doi: 10.1164/rccm.200406-694OC. [DOI] [PubMed] [Google Scholar]
- 66.Mills PJ, Dimsdale JE. Sleep apnea: a model for studying cytokines, sleep, and sleep disruption. Brain Behav Immun. 2004;18(4):298–303. doi: 10.1016/j.bbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12(11):63–68. doi: 10.1016/S0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 68.Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26(8):986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 69.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27(6):1318–1324. doi: 10.1161/01.HYP.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 70.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35(5):1173–1175. doi: 10.1161/01.HYP.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 71.Holmes AL, Burgess HJ, Dawson D. Effects of sleep pressure on endogenous cardiac autonomic activity and body temperature. J Appl Physiol. 2002;92(6):2578–2584. doi: 10.1152/japplphysiol.01106.2001. [DOI] [PubMed] [Google Scholar]
- 72.Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186. doi: 10.1111/j.1532-5415.2011.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhong X. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98(6):2024–2032. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 74.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, DeMeersman RE, Basner RC. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98(6):2024–2032. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 75.Stead LG, Gilmore RM, Vedula KC, Weaver AL, Decker WW, Brown RD. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. 2006;66(12):1878–1881. doi: 10.1212/01.wnl.0000219628.78513.b5. [DOI] [PubMed] [Google Scholar]
- 76.Chang JY, Jeon SB, Lee JH, Kwon OK, Han MK. The relationship between blood pressure variability, recanalization degree, and clinical outcome in large vessel occlusive stroke after an intra-arterial thrombectomy. Cerebrovasc Dis. 2018;46(5–6):279–286. doi: 10.1159/000495300. [DOI] [PubMed] [Google Scholar]
- 77.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007;49(4):777–783. doi: 10.1161/01.HYP.0000258215.26755.20. [DOI] [PubMed] [Google Scholar]
- 78.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Jama Internal Medicine. 2003;163(2):3–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 79.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. J Am Med Assoc. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Narkiewicz K, Montano N, Cogliati C, Van De Borne PJH, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(11):1071–1077. doi: 10.1161/01.CIR.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 81.Palagini L, Maria Bruno R, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19(13):2409–2419. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- 82.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 83.Koenig W, Sund M, Fröhlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–242. doi: 10.1161/01.CIR.99.2.237. [DOI] [PubMed] [Google Scholar]
- 84.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiat. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shamsuzzaman ASM, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. doi: 10.1161/01.CIR.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 86.Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33(3):654. doi: 10.1097/01.CCM.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]
- 87.Solenski NJ, Haley ECJ, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Crit Care Med. 1995;23(6):1007. doi: 10.1097/00003246-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 88.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137. doi: 10.5664/jcsm.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pelosi P, Ferguson ND, Frutos-Vivar F, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39(6):1482. doi: 10.1097/CCM.0b013e31821209a8. [DOI] [PubMed] [Google Scholar]
- 90.Cooper KR, Phillips BA. Effect of short-term sleep loss on breathing. J Appl Physiol. 1982;53(4):855–858. doi: 10.1152/jappl.1982.53.4.855. [DOI] [PubMed] [Google Scholar]
- 91.Schiffman PL, Trontell MC, Mazar MF, Edelman NH. Sleep deprivation decreases ventilatory response to CO2 But not load compensation. Chest. 1983;84(6):695–698. doi: 10.1378/chest.84.6.695. [DOI] [PubMed] [Google Scholar]
- 92.White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984–986. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- 93.Spengler CM, Shea SA. Sleep deprivation per Se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med. 2000;161(4):1124–1128. doi: 10.1164/ajrccm.161.4.9906026. [DOI] [PubMed] [Google Scholar]
- 94.Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. Am Rev Respir Dis. 1989;140(4):907–909. doi: 10.1164/ajrccm/140.4.907. [DOI] [PubMed] [Google Scholar]
- 95.Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. In: New frontiers in respiratory control; 2010. p. 289–292. [DOI] [PubMed]
- 96.Rault C, Diaz V, Frat JP et al. Sleep deprivation reduces inspiratory endurance by altering central command. In: Healthy subjects. A29 updates in control of breathing; 2017. p. A1237.
- 97.Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481–485. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 98.Berry RB, Kouchi KG, Bower JL, Light RW. Effect of upper airway anesthesia on obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151(6):1857–1861. doi: 10.1164/ajrccm.151.6.7767531. [DOI] [PubMed] [Google Scholar]
- 99.Eikermann M, Vogt FM, Herbstreit F, et al. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med. 2007;175(1):9–15. doi: 10.1164/rccm.200512-1862OC. [DOI] [PubMed] [Google Scholar]
- 100.Netzer N, Werner P, Jochums I, Lehmann M, Strohl KP. Blood flow of the middle cerebral artery with sleep-disordered breathing: correlation with obstructive hypopneas. Stroke. 1998;29(1):87–93. doi: 10.1161/01.STR.29.1.87. [DOI] [PubMed] [Google Scholar]
- 101.Hsiu-Ling C, Hsin-Ching L, Cheng-Hsien L, et al. Systemic inflammation and alterations to cerebral blood flow in obstructive sleep apnea. J Sleep Res. 2017;26(6):789–798. doi: 10.1111/jsr.12553. [DOI] [PubMed] [Google Scholar]
- 102.Joo EY, Tae WS, Han SJ, Cho J-W, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30(11):1515–1520. doi: 10.1093/sleep/30.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jennum P, Børgesen SE. Intracranial pressure and obstructive sleep apnea. Chest. 1989;95(2):279–283. doi: 10.1378/chest.95.2.279. [DOI] [PubMed] [Google Scholar]
- 104.Yadav SK, Kumar R, Macey PM, et al. Regional cerebral blood flow alterations in obstructive sleep apnea. Neurosci Lett. 2013;555:159–164. doi: 10.1016/j.neulet.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunizaton. JAMA. 2002;288(12):1471–1472. doi: 10.1001/jama.288.12.1469. [DOI] [PubMed] [Google Scholar]
- 106.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis a vaccination. Psychosom Med. 2003;65(5):831–835. doi: 10.1097/01.PSY.0000091382.61178.F1. [DOI] [PubMed] [Google Scholar]
- 107.Benedict C, Brytting M, Markstrom A, Broman JE, Schioth HB. Acute sleep deprivation has no lasting effects on the human antibody titer response following a novel influenza A H1N1 virus vaccination. BMC Immunol. 2012;13(1):1–5. doi: 10.1186/1471-2172-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-Reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 109.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56(2):51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 110.Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35(1):97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 112.Oztürk L, Pelin Z, Karadeniz D, Kaynak H, Çakar L, Gözükirmizi E. Effects of 48 hours sleep deprivation on human immune profile. Sleep Res Online. 1999;2(4):107–111. [PubMed] [Google Scholar]
- 113.Irwin M, Mascovich A, Gilun JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–498. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 114.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10(5):643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 115.Moldofsky H, Lue F, Davidson JR, Gorcyznski R. Effects of sleep deprivation on human immune functions. FASEB J. 1989;3(8):1972–1977. doi: 10.1096/fasebj.3.8.2785942. [DOI] [PubMed] [Google Scholar]
- 116.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural-killer-cell function parallel neurobehavioral fatigue-induced by 64 hours of sleep-deprivation. J Clin Investig. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boudjeltia KZ, Faraut B, Stenuit P, et al. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: a pilot study. Vasc Health Risk Manag. 2008;4(6):1467–1470. doi: 10.2147/VHRM.S3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lasselin J, Rehman JU, Akerstedt T, Leandker M, Axelsson J. Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav Immun. 2015;47:93–99. doi: 10.1016/j.bbi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Christoffersson G, Vågesjö E, Petterson US, et al. Acute sleep deprivation in healthy young men: impact on population diversity and function of circulating neutrophils. Brain Behav Immun. 2014;41(1):162–172. doi: 10.1016/j.bbi.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Zhao JL, Du ZY, Yuan Q, Yu J, Sun YR, Wu X, Li ZQ, Wu XH, Hu J. Prognostic value of neutrophil-to-lymphocyte ratio in predicting the 6-month outcome of patients with traumatic brain injury: a retrospective study. World Neurosurg. 2019;124:e411. doi: 10.1016/j.wneu.2018.12.107. [DOI] [PubMed] [Google Scholar]
- 121.Simona L, Claudia C, Leandro P, Mauro S. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47(6):1654–1657. doi: 10.1161/STROKEAHA.116.013627. [DOI] [PubMed] [Google Scholar]
- 122.Tokgoz S, Kayrak M, Akpinar Z, Seyithanoğlu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. 2013;22(7):1169–1174. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 123.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 124.Irwin MR. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 125.Redwine L. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85(10):3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 126.Di Napoli M, Slevin M, Popa-Wagner A, Singh P, Lattanzi S, Divani AA. Monomeric C-reactive protein and cerebral hemorrhage: from bench to bedside. Front Immunol. 2018;9:1921. doi: 10.3389/fimmu.2018.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20(10):865–870. [PubMed] [Google Scholar]
- 128.Spiegel K, Leproult R, Van Cauer E. Impact of sleep debt on metabolic and endocrine function. The Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 129.DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188–193. doi: 10.2337/dc16-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52(1):20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 131.Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 2009;11(2):151. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- 132.Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34(3):617. doi: 10.1097/01.CCM.0000201903.46435.35. [DOI] [PubMed] [Google Scholar]
- 133.Badjatia N, Topcuoglu MA, Buonanno FS, et al. Relationship between hyperglycemia and symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med. 2005;33(7):1603. doi: 10.1097/01.CCM.0000168054.60538.2B. [DOI] [PubMed] [Google Scholar]
- 134.Kimura K, Iguchi Y, Inoue T, et al. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci. 2007;255(1):90–94. doi: 10.1016/j.jns.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 135.Zielinski MR, Kline CE, Kripke DF, Bogan RK, Youngstedt SD. No effect of 8-week time in bed restriction on glucose tolerance in older long sleepers. J Sleep Res. 2008;17(4):412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95(6):2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 137.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury. A comprehensive meta-analysis. Stroke. 2008;39(11):3029–3035. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 139.Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47(4):850–856. doi: 10.1097/00006123-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 140.Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the neurologic intensive care unit. Neurology. 2003;60(5):837–841. doi: 10.1212/01.WNL.0000047344.28843.EB. [DOI] [PubMed] [Google Scholar]
- 141.Rabinstein AA, Sandhu K. Non-infectious fever in the neurological intensive care unit: incidence, causes and predictors. J Neurol Neurosurg Psychiatry. 2007;78(11):1278–1280. doi: 10.1136/jnnp.2006.112730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vaara J, Kyröläinen H, Koivu M, Tulppo M, Finni T. The effect of 60-h sleep deprivation on cardiovascular regulation and body temperature. Eur J Appl Physiol. 2009;105(3):439–444. doi: 10.1007/s00421-008-0921-5. [DOI] [PubMed] [Google Scholar]
- 143.Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol (Amst) 1985;58(2):123–139. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- 144.Landis CA, Savage MV, Lentz MJ, Brengelmann GL. Sleep deprivation alters body temperature dynamics to mild cooling and heating not sweating threshold in women. Sleep. 1998;21(1):101–108. doi: 10.1093/sleep/21.1.101. [DOI] [PubMed] [Google Scholar]
- 145.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24(1):45–61. doi: 10.1080/07420520601142569. [DOI] [PubMed] [Google Scholar]
- 146.Sahakian B, LaBuzetta JN. Bad Moves: How decision making goes wrong, and the ethics of smart drugs. Oxford: OUP; 2013. pp. 99–100. [Google Scholar]
- 147.Durmer JS, Dinges D. Neurocognitive consequences of sleep deprivation neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 148.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 149.Ikegami K, Ogyu S, Arakomo Y, et al. Recovery of cognitive performance and fatigue after one night of sleep deprivation. J Occup Health. 2009;51(5):412–422. doi: 10.1539/joh.L8127. [DOI] [PubMed] [Google Scholar]
- 150.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatric Dis Treat. 2007;3(5):553–567. [PMC free article] [PubMed] [Google Scholar]
- 151.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 152.Sukantarat KT, Burgess PW, Williamson RCN, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005;60(9):847–853. doi: 10.1111/j.1365-2044.2005.04148.x. [DOI] [PubMed] [Google Scholar]
- 153.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 154.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rothenhäusler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23(2):90–96. doi: 10.1016/S0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 156.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 157.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 158.Pflug B, Tolle R. Disturbance of the 24-hour rhythm in endogenous depression and the treatment of endogenous depression by sleep deprivation. Int Pharmacopsychiatry. 1971;6:187–196. doi: 10.1159/000468269. [DOI] [PubMed] [Google Scholar]
- 159.Dinges DF, PacK F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 160.Paterson JL, Dorrian J, Ferguson SA, Jay SM, Dawson D. What happens to mood, performance and sleep in a laboratory study with no sleep deprivation? Sleep Biol Rhythms. 2013;11(3):200–209. doi: 10.1111/sbr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiat. 2013;73(12):1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 162.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35(5):796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506–1518. doi: 10.1007/s00134-007-0730-z. [DOI] [PubMed] [Google Scholar]
- 166.Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11(1):R27. doi: 10.1186/cc5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29(6):310–316. doi: 10.1016/j.iccn.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 168.Scott JPR, McNaughton LR, Polman RCJ. Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav. 2006;87(2):396–408. doi: 10.1016/j.physbeh.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 169.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 170.Ely E, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 171.Ouimet S, Kavanaugh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 172.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KLB, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ely E, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thomason JWW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9(4):R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Med. 2010;38(7):1513. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. Can Med Assoc J. 2001;165(5):575–583. [PMC free article] [PubMed] [Google Scholar]
- 177.Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, Ely EW. Bench-to-bedside review: delirium in ICU patients—importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13(1):25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 180.Habeck C, Rakitin BC, Moeller J, et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cogn Brain Res. 2004;18(3):306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 181.Maria T, Helen S, Gregory B, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2008;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 182.Smith ME, McEvoy LK, Gevins A. The impact of moderate sleep loss on neurophysiologic signals during working-memory task performance. Sleep. 2002;25(7):56–66. doi: 10.1093/sleep/25.7.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Koponen H, Hurri L, Stenbäck U, Mattila E, Soininen H, Riekkinen PJ. Computed tomography findings in delirium. J Nerv Ment Dis. 1989;177(4):226–231. doi: 10.1097/00005053-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 184.Flannery AH, Oyler DR, Weinhouse GL. The impact of interventions to improve sleep on delirium in the ICU: a systematic review and research framework. Crit Care Med. 2016;44(12):2231. doi: 10.1097/CCM.0000000000001952. [DOI] [PubMed] [Google Scholar]
- 185.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 186.Kamdar BB, King LM, Collop NA, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41(3):800–809. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540–549. doi: 10.1111/anae.12638. [DOI] [PubMed] [Google Scholar]
- 188.Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit improved the outcomes of older adults. J Surg Res. 2014;190(1):280–288. doi: 10.1016/j.jss.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 189.Jennum P, Drewes AM, Andreasen A, Nielsen KD. Sleep and other symptoms in primary fibromyalgia and in healthy controls. J Rheumatol. 1993;20(10):1756–1759. [PubMed] [Google Scholar]
- 190.Aǧargün MY, Tekeoǧlu I, Güneş A, Adak B, Kara H, Ercan M. Sleep quality and pain threshold in patients with fibromyalgia. Compr Psychiatry. 1999;40(3):226–228. doi: 10.1016/S0010-440X(99)90008-1. [DOI] [PubMed] [Google Scholar]
- 191.Raymond I, Nielsen TA, Lavigne G, Manzini C, Choinière M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92(3):381. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 192.Older SA, Battafarano DF, Danning CL, Ward JA, Grady EP, Derman S, et al. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–1186. [PubMed] [Google Scholar]
- 193.Arima T, Svensson P, Rasmussen C, Nielsen KD, Drewes AM, Arendt-Nielsen L. The relationship between selective sleep deprivation, nocturnal jaw-muscle activity and pain in healthy men. J Oral Rehabil. 2009;28(2):140–148. doi: 10.1046/j.1365-2842.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- 194.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2008;10(1):35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 195.Schuh-Hofer S, Wodarski R, Pfau DB, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154(9):1613. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 196.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37(4):341. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 197.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38(1):35. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 198.Litton E, Carnegie V, Elliott R, Webb SAR. The efficacy of earplugs as a sleep hygiene strategy for reducing delirium in the ICU: a systematic review and meta-analysis. Crit Care Med. 2016;44(5):992. doi: 10.1097/CCM.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 199.Kahn DM, Cook TE, Carlisle CC, Nelson DL, Kramer NR, Millman RP. Identification and modification of environmental noise in an ICU setting. Chest. 1998;114(2):535–540. doi: 10.1378/chest.114.2.535. [DOI] [PubMed] [Google Scholar]
- 200.Soubra M, Harb YA, Hatoum S, et al. Effect of a quality improvement project to reduce noise in a pediatric unit. MCN Am J Matern Nurs. 2018;43(2):83–88. doi: 10.1097/NMC.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 201.Maidl CA, Leske JS, Garcia AE. The influence of “Quiet Time” for patients in critical care. Clin Nurs Res. 2014;23(5):544–559. doi: 10.1177/1054773813493000. [DOI] [PubMed] [Google Scholar]
- 202.Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162(4):656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 203.Redd WH, Valdimarsdottir H, Wu LM, et al. Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology. 2014;23(12):1431–1434. doi: 10.1002/pon.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Engwall M, Fridh I, Johansson L, Bergbom I, Lindahl B. Lighting, sleep and circadian rhythm: an intervention study in the intensive care unit. Intensive Crit Care Nurs. 2015;31(6):325–335. doi: 10.1016/j.iccn.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 206.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 207.Watson NF. Stroke and sleep specialists: an opportunity to intervene? J Clin Sleep Med. 2010;6(2):138–139. doi: 10.5664/jcsm.27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Christine D, Jennifer S, David C. Sensing an improvement: an experimental study to evaluate the use of aromatherapy, massage and periods of rest in an intensive care unit. J Adv Nurs. 2018;21(1):34–40. doi: 10.1046/j.1365-2648.1995.21010034.x. [DOI] [PubMed] [Google Scholar]
- 209.Shinde MB, Anjum S. Effectiveness of slow back massage on quality of sleep among ICU patent’s. IJSR. 2014;3(3):292–298. [Google Scholar]
- 210.Nerbass FB, Feltrim MIZ, de Souza SA, Ykeda DS, Lorenzi-Filho G. Effects of massage therapy on sleep quality after coronary artery bypass graft surgery. SciELO. 2010;65:1105–1110. doi: 10.1590/S1807-59322010001100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hu R-F, Jiang X-Y, Hegadoren KM, Zhang Y-H. Effects of earplugs and eye masks combined with relaxing music on sleep, melatonin and cortisol levels in ICU patients: a randomized controlled trial. Crit Care. 2015;19(1):115. doi: 10.1186/s13054-015-0855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]