Abstract
Purpose
In ICU patients with carriage of extended spectrum beta-lactamase producing Enterobacterales (ESBL-E) and suspected Gram-negative bacilli ventilator-associated pneumonia (GNB-VAP), the quantification of the rectal and throat ESBL-E carriage might predict the ESBL-E involvement in GNB-VAP. Our aim was to evaluate whether a semi-quantitative assessment of rectal/throat ESBL-E carriage can predict ESBL-E-associated VAP in medical ICU patients.
Methods
From May 2014 to May 2017, all ESBL-E carriers had a semi-quantitative assessment of ESBL-E density in swabs cultures. For those who developed GNB-VAP (diagnosed using bronchoalveolar lavage or plugged telescopic catheter with significant quantitative culture), the last positive swab collected at least 48 h before GNB-VAP onset was selected. Clinical data were extracted from a prospectively collected database.
Results
Among 365 ESBL-E carriers, 82 developed 107 episodes of GNB-VAP (ESBL-E VAP, n = 50; and non-ESBL-E GNB-VAP, n = 57) after 13 days of mechanical ventilation in median. Antimicrobials use before VAP onset was similar between groups. The last swabs were collected 5 days in median before VAP onset. ESBL-E. coli carriers developed ESBL-E VAP less frequently (n = 13, 34%) than others (n = 32, 67.3%, p < .01). Throat swab positivity (39 (78%) vs. 12 (23%), p < .01) was more frequent for ESBL-E VAP. ESBL-E VAP was associated with significantly higher ESBL-E density in rectal swabs. In multivariate models, non-E. coli ESBL-E carriage and rectal ESBL-E carriage density, or throat carriage, remained associated with ESBL-E VAP.
Conclusion
In carriers of ESBL-E other than E. coli, ESBL-E throat carriage or a high-density ESBL-E rectal carriage are risk factors of ESBL-E VAP in case of GNB-VAP.
Electronic supplementary material
The online version of this article (10.1007/s00134-020-06029-y) contains supplementary material, which is available to authorized users.
Keywords: Extended-spectrum beta-lactamase, E. coli, Ventilator-associated pneumonia, Sepsis, Outcome, Carbapenem
Take-home message
| When VAP is suspected, the risk of ESBL-E VAP in ESBL-E carriers is lower than 40%. We demonstrated that throat qualitative carriage and rectal carriage assessed semi-quantitatively, accurately predicted ESBL-E VAP in ESBL-E carriers. |
Introduction
Ventilator-associated pneumonia (VAP) is one of the most frequent causes of intensive care unit (ICU)-acquired infections [1]. The worldwide spreading of extended spectrum beta-lactamase producing Enterobacterales (ESBL-E) represents a major problem encountered at an increasing frequency in ICU [2, 3]. Among ICU patients, between 5 and 25% are ESBL-E carriers [4–6]. Whereas a previous carriage is the major risk factor associated with VAP related to ESBL-E [7, 8], only 5–20% of the ESBL-E carriers will develop a VAP related to ESBL-E [9, 10]. With regard to the occurrence of ESBL-E-associated VAP, the negative predictive value of a ESBL-E digestive carriage is high, while its positive predictive value, i.e., the probability of having an ESBL-E infection in case of ESBL-E carriage, is less than 50% [9, 11–15].
VAP due to multidrug-resistant (MDR) bacteria such as ESBL-E exposes the patient to more inadequate antimicrobial therapy and has been associated with an increased risk of death [16–21]. Given the risk of inadequate therapy, in case of previous ESBL-E carriage or colonization, clinicians start more often an empirical therapy with carbapenem, despite the potential risk of emergence of carbapenem resistance [22].
The knowledge of previous ESBL-E carriage is associated with a fourfold increase in the carbapenem use in case of VAP [9] or infection-related ventilator-associated complication (IVAC) [14]. Studies have reported a link between oropharynx colonization and lower respiratory tract infection [23, 24]. In case of community-acquired urinary tract infection, the involvement of ESBL-E might be predicted by the relative abundance (defined as the proportion of the concentration of ESBL-E vs. total concentration of Enterobacteriales in the feces) of ESBL-E carriage. Subsequently, the amount of ESBL-E in the digestive flora and the presence of an oropharyngeal colonization as compared to a rectal colonization may be associated with a higher risk of ESBL-E-associated VAP when GNB-VAP is diagnosed.
The purpose of our study was therefore to decipher the link between oropharyngeal and rectal colonization and to assess the predictive value of a semi-quantitative assessment of the ESBL-E carriage on the prediction of the involvement of ESBL-E in newly diagnosed VAP.
Materials and methods
Study characteristics
The study took place in a 20-bed medical and infectious diseases ICU between May 2014 and May 2017. We selected all patients mechanically ventilated for more than 48 h who were identified as ESBL-E carriers in throat and rectal samples at ICU admission, or weekly thereafter, before their VAP onset. Among them, we retrospectively reviewed all patients diagnosed with GNB-VAP. We then compared patients with ESBL-E VAP with patients with GNB-VAP not due to ESBL-E.
Definitions
VAP was defined as new or persistent infiltrate on chest radiography that was associated with one of the following criteria: (1) purulent tracheal secretions, (2) fever greater than or equal to 38.5 °C or hypothermia less than or equal to 36.5 °C and (3) leukocytosis greater than 109 G/L or leukopenia less than 4.108 G/L. VAP was defined as pneumonia occurring in a patient having had at least 48 h of invasive mechanical ventilation (IMV). It was confirmed by positive quantitative culture of a respiratory sample collected via bronchoalveolar lavage fluid (significant threshold, ≥ 104 cfu/ml) or plugged telescopic catheter (significant threshold, ≥ 103 cfu/ml).
Bacteriological methods
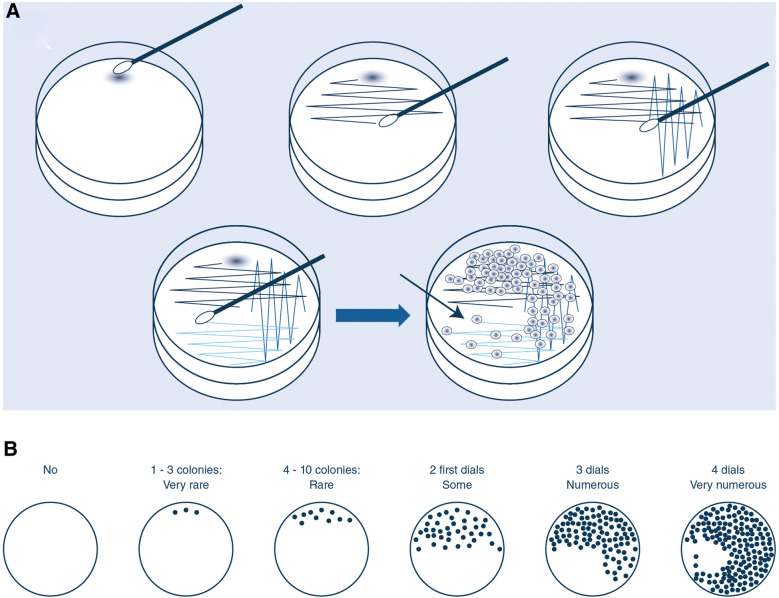
All patient admitted to the ICU were screened at ICU admission and weekly thereafter, using a throat and a rectal swab. Both swabs were plated on ChromID® ESBL agar (bioMérieux, Marcy-l’Etoile, France) and incubated for 48 h at 37 °C in aerobic conditions. Strains were identified using mass spectrometry (Maldi Biotyper, Bruker, Wissenbourg, France). Antibiotic susceptibility and ESBL-E phenotype were determined by disk diffusion and interpreted according to EUCAST (www.eucast.org). Rectal and throat samples were performed using eSwabs® (Copan Diagnostics, Brescia). At the laboratory, one drop of the Amies liquid contained in the eSwab® was put on the ChromID-ESBL agar. The liquid was streaked using the usual four-quadrant streak plate method [25] (Fig. 1). Densities of colonization were defined as follows: very low or low if, respectively, 1–3 colonies-forming unit (cfu) (“very rare” colonies) and 4–10 cfu (“rare” colonies) were present on the first half of the plate, medium when approximately 10–100 cfu (“some” colonies) were observed on the first half of the plate, high when “numerous” colonies were observed in the first three quadrants (approximately 100–1000 colonies), and very high when “very numerous” colonies were observed on the whole plate (1000 colonies or more) (Fig. 1).
Fig. 1.
A The four-quadrant streak plate method. (a) Sample on the plate. (b) With the loop, streaking quadrant 1 and 2. (c) Streaking quadrant 3. (d) Streaking quadrant 4. B Schematic representation of plates and mode of quotation used for semi-quantitative counting of bacteria (very rare colonies = very low density; rare colonies = low density; some colonies = medium density; numerous colonies = high density; very numerous colonies = high density)
Data
In order to compare ESBL-E carriers with ESBL-E VAP versus those with VAP due to other Gram-negative bacteria, we used the data prospectively captured in our database. We used data on past comorbidities, medical history, case-mix and severity on ICU admission. We also collected traditional risk factors of ESBL-E carriage and infections, including ESBL-E colonization status before admission, prevalence of ESBL-E at ICU admission, date of ESBL-E acquisition during recent hospitalization (< 3 months or < 1 year) and previous use of antimicrobials before VAP onset. We also collected procedures and treatment used in the last week before VAP. For each VAP episodes, the last ESBL-E throat and rectal sampling semi-quantitatively assessed were also collected. We do not use subglottic aspiration nor selective digestive decontamination to prevent VAP in our unit.
No specific procedure of samples was added to the standard practices. The ethical committee of hospital group Paris Nord waived the need for informed consent. Data were fully anonymized and computed in agreement to the MR-003 directive (https://www.cnil.fr/fr/declaration/mr-003-recherches-dans-le-domaine-de-la-sante-sans-recueil-du-consentement; last accessed April 20th, 2019).
Statistical analysis
Data were reported as numbers (percentages) or medians (interquartile ranges (IQR): 25th–75th percentiles). Continuous variables were compared using Mann–Whitney test and proportion using the chi-squared test.
Cochran-Armitage trend tests were used to compare the results of the semi-quantitative ESBL-E rectal and throat swabs cultures between ESBL-E VAP and non-ESBL-E VAP.
Other risk factors of ESBL-E VAP were assessed in the cohort using a logistic regression. Some patients were readmitted in the ICU and/or were reintubated and were considered as independent in the analyses (mean delay between two episodes of 24.6 days). At the last step of the selection process, ESBL-E rectal and throat carriage were successively included in the final model. The discrimination level of the final models was measured using the area under the curve (AUC), with a threshold at 0.7.
Analyses used SAS 9.4 (SAS, Inc., Cary, NC) and R (R foundation for statistical computing, Vienna, Austria) softwares.
Results
Of the 1604 patients who were admitted to the unit over the study period and experienced mechanical ventilation for more than 48 h, 365 (21.5%) were detected as ESBL-E carriers (Figure E1). One hundred and seven GNB-VAP episodes were diagnosed in 82 patients after a median duration of mechanical ventilation of 13 days. There was no case of ESBL-E VAP in patients without oropharyngeal and or rectal ESBL-E carriage.
Patients’ characteristics are presented in Table 1. Among the 50 (47%) ESBL-E VAP episodes, Klebsiella spp. was involved in 31 cases (61%), Escherichia coli in 10 cases (20%) and Enterobacter cloacae in 9 cases (18%) (Table E1).
Table 1.
Patients characteristics on admission and before VAP occurrence
| All Gram-negative VAP | ESBL-E VAP | Other VAP | p-value* | |
|---|---|---|---|---|
| n = 107 | n = 50 | n = 57 | ||
| Previous medical history | ||||
| Known ESBL-E carriage before admission | 14 (13.1) | 6 (12) | 8 (14) | 0.76 |
| Hospitalization within the previous year | 12 (11.7) | 5 (10.4) | 7 (12.7) | 0.72 |
| Antimicrobial therapy within the previous 3 months | 45 (43.7) | 24 (49) | 21 (38.9) | 0.30 |
| Diabetes mellitus | 33 (30.8) | 14 (28) | 19 (33.3) | 0.55 |
| Immunodepression | 29 (27.1) | 17 (34) | 12 (21.1) | 0.13 |
| Chronic pulmonary diseases | 22 (20.6) | 9 (18) | 13 (22.8) | 0.54 |
| Renal replacement therapy | 8 (7.5) | 4 (8) | 4 (7) | 0.85 |
| Kidney failure | 23 (21.5) | 13 (26) | 10 (17.5) | 0.29 |
| Liver insufficiency | 3 (2.8) | 0 (0) | 3 (5.3) | 0.1 |
| Solid organ transplant recipient | 25 (23.6) | 14 (28.6) | 11 (19.3) | 0.26 |
| Cancer | 2 (1.9) | 2 (4) | 0 (0) | 0.13 |
| Recent travel (within the previous 6 months) | 27 (25.2) | 10 (20) | 17 (29.8) | 0.24 |
| Recent surgery (within the previous year) | 30 (28) | 16 (32) | 14 (24.6) | 0.39 |
| Chest drain before ICU admission | 23 (21.5) | 13 (26) | 10 (17.5) | 0.29 |
| Urinary catheter for > 24 h before ICU admission | 70 (65.4) | 38 (76) | 32 (56.1) | 0.03 |
| Characteristics at ICU admission | ||||
| Hospital stay prior to ICU admission (days), median (IQR) | 1 [1; 4] | 1 [1; 5] | 1 [1; 3] | 0.42 |
| Transfer from another ICU | 43 (40.2) | 25 (50) | 18 (31.6) | 0.05 |
| Age, median (IQR) | 57 [49; 69] | 55.5 [50; 68] | 57 [48; 72] | 0.48 |
| SAPS II, median (IQR) | 51 [35; 67] | 55.5 [40; 67] | 46 [34; 67] | 0.33 |
| SOFA, median (IQR) | 8 [5; 11] | 9 [5; 11] | 8 [4; 11] | 0.19 |
| Weight, median (IQR) (kg) | 75 [66; 90] | 77 [64.5; 90] | 75 [69; 90] | 0.91 |
| BMI, median (IQR) | 25.2 [22.8; 29.4] | 26 [23.2; 30.4] | 24.9 [22.4; 28.7] | 0.62 |
| Albumin level, median (IQR) (G/L) | 25.5 [22; 32] | 24 [20; 29] | 26 [23; 32] | 0.04 |
| WBC, median (IQR) (G/L) | 12.2 [7.5; 15.6] | 12.5 [6.1; 17] | 11.2 [7.7; 15.3] | 0.64 |
| Lymphocytes, median (IQR) (G/L) | 1 [0.4; 1.5] | 0.9 [0.4; 1.4] | 1.1 [0.4; 1.8] | 0.14 |
| Time before intubation (day), median (IQR) | 1 [0; 2] | 1 [− 1; 2] | 1 [1; 1.5] | 0.46 |
| Antimicrobial use within the past 7 days before VAP | ||||
| Any antimicrobials | 84 (78.5) | 40 (80) | 44 (77.2) | 0.72 |
| Beta-lactams | 68 (63.6) | 31 (62) | 37 (64.9) | 0.75 |
| Carbapenems | 27 (25.2) | 13 (26) | 14 (24.6) | 0.86 |
| Fluoroquinolones | 12 (11.2) | 6 (12) | 6 (10.5) | 0.81 |
| 3rd and 4th gen cephalosporin | 21 (19.6) | 9 (18) | 12 (21.1) | 0.69 |
ESBL-E extended spectrum beta-lactamase producing Enterobacterales, VAP ventilator-associated pneumonia, ICU intensive care unit, IQR interquartile range, SAPS simplified acute physiology score, SOFA sequential organ failure assessment, BMI body mass index, WBC white blood cells
The duration of the hospital stay prior to the ICU admission was longer in patients who developed ESBL-E VAP. Patients from both groups were similar in term of medical history and illness severity at ICU admission, but patients with ESBL-E VAP had a higher SOFA score at VAP onset (8.5 [6.5; 11] vs. 10.5 [8; 14]; p = 0.03). ESBL-E VAP presented more frequently with ARDS (13 (22.8%) vs. 20 (40%); p = 0.05). Both groups had similar previous exposure to antimicrobials (overall and each family, including carbapenems) before the occurrence of the VAP episode (Table 2).
Table 2.
Characteristics of patients within the last week before VAP onset
| All Gram-negative VAP | EBLSE VAP | Other VAP | p-value | |
|---|---|---|---|---|
| n = 107 | n = 50 | n = 57 | ||
| ARDS | 33 (30.8) | 20 (40) | 13 (22.8) | 0.05 |
| Coma | 30 (28) | 12 (24) | 18 (31.6) | 0.38 |
| Paralytic agents | 51 (47.7) | 25 (50) | 26 (45.6) | 0.65 |
| Proton pump inhibitor | 84 (78.7) | 40 (80) | 44 (77.2) | 0.72 |
| Reintubation | 17 (15.9) | 6 (12) | 11 (19.3) | 0.3 |
| Intra-hospital transport | 65 (60.7) | 33 (66) | 32 (56.1) | 0.3 |
| Duration of sedation (days) | 5 [0; 7] | 5.5 [1; 7] | 3 [0; 7] | 0.09 |
| Norepinephrine (mg/h) | 1 [0; 6] | 1 [0; 7] | 1 [0; 5] | 0.18 |
| Chest drain | 30 (28) | 17 (34) | 13 (22.8) | 0.20 |
| SOFA at D-3 | 7 [5; 11] | 8 [6; 11.5] | 7 [5; 10] | 0.27 |
| SOFA at D0 | 9.5 [7; 12] | 10.5 [8; 14] | 8.5 [6.5; 11] | 0.03 |
| Increase in the SOFA score between D-3 and D0 | 1 [0; 3] | 1 [0; 3] | 0 [0; 4] | 0.09 |
| Duration of mechanical ventilation before VAP (days) | 13 [7; 35] | 20 [7; 41] | 12 [6; 21] | 0.09 |
| Time interval between the last screening and VAP (days) | 5 [4; 10] | 5.5 [4; 10] | 5 [4; 13] | 0.76 |
| Only ESBL E. coli previous carriage | 46 (43) | 14 (28) | 32 (56.1) | < 0.01 |
| Rectal ESBL-E colonization | 100 (93.5) | 48 (96) | 52 (91.2) | 0.32 |
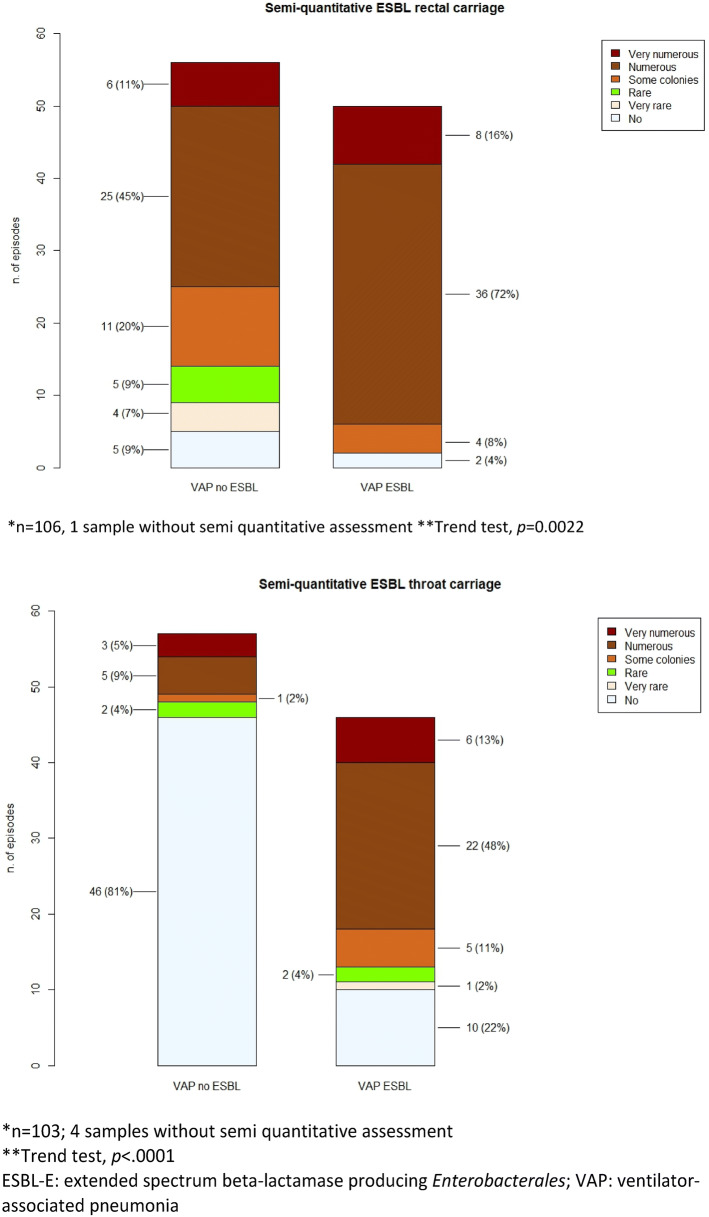
| Rectal ESBL-E colonization (semi-quantitative) | 0.0022* | |||
| None | 7 (6.6) | 2 (4) | 5 (8.9) | |
| Very rare | 4 (3.8) | 0 | 4 (7.1) | |
| Rare | 5 (4.7) | 0 | 5 (8.9) | |
| Some | 15 (14.2) | 4 (8) | 11 (19.6) | |
| Numerous | 61 (57.5) | 36 (72) | 25 (44.6) | |
| Very numerous | 14 (13.2) | 8 (16) | 6 (10.7) | |
| Rectal ESBL-E colonization only | 52 (48.6) | 9 (18) | 43 (75.4) | < 0.01 |
| Throat ESBL-E colonization | 51 (47.7) | 40 (80) | 11 (19.3) | < 0.01 |
| Throat ESBL-E colonization (semi-quantitative) | < 0.01* | |||
| None | 56 (54.4) | 10 (21.7) | 46 (80.7) | |
| Very rare | 1 (1) | 1 (2.2) | 0 | |
| Rare | 4 (3.9) | 2 (4.3) | 2 (3.5) | |
| Some colonies | 6 (5.8) | 5 (10.9) | 1 (1.8) | |
| Numerous | 27 (26.2) | 22 (47.8) | 5 (8.8) | |
| Very numerous | 9 (8.7) | 6 (13) | 3 (5.3) | |
| Carriage of other multidrug-resistant/pandrug-resistant strains | ||||
| A. baumannii (imipenem-susceptible) | 5 (4.7) | 2 (4) | 3 (5.3) | |
| Carbapenemase producer | 1 (0.9) | 1 (2) | 0 | |
| Vancomycin-resistant enterococci | 1 (0.9) | 0 (0) | 1 (1.8) | |
| P. aeruginosa | 4 (3.7) | 3 (6) | 1 (1.8) | |
| Methicillin-resistant S. aureus | 2 (1.9) | 1 (2) | 1 (1.8) | |
| S. maltophilia | 1 (0.9) | 1 (2) | 1 (1.8) | |
| CPIS, median (IQR) | 7 [6; 9] | 8 [6; 9] | 7 [6; 8] | 0.25 |
| Carbapenems included into the initial empiric therapy | 63 (61.2) | 37 (78.7) | 26 (46.4) | < 0.01 |
| Length of antimicrobial therapy | 8 [8; 9] | 8 [8; 9] | 9 [8; 11] | 0.52 |
| In-ICU mortality | 52 (48.6) | 30 (60) | 22 (38.6) | 0.03 |
Results are shown in median [interquartile range (IQR)] or n(%) for quantitative and qualitative variables, respectively
ESBL-E extended spectrum beta-lactamase producing Enterobacterales, VAP ventilator-associated pneumonia, ARDS acute respiratory distress syndrome, SOFA sequential organ failure assessment, CPIS clinical pulmonary infection score
*Trend test
The carriage of ESBL-E. coli was less frequently associated with ESBL-E VAP than the carriage of other ESBL-E (Fig. 2). A result of throat carriage positive with ESBL-E was associated with ESBL-E VAP rather than other VAP, which was not the case for positive rectal carriage. However, a semi-quantitative assessment with ESBL-E of both throat (positive predictive value (PPV): 78% 95% CI (68–86%); negative predictive value (NPV) 82%; 95% CI (72–89%)) and rectal previous colonization using none rare or very rare as reference: PPV 53% 95% CI (49–57%); NPV 88% 95% CI (64–97%) was very significantly associated with ESBL-E VAP. PPV and NPV of both tests and combination of tests are depicted in Table E2.
Fig. 2.
Semi-quantitative ESBL-E rectal (Panel 1) and throat (Panel 2) results for patients with VAP due to ESBL-E or not. ESBL-E extended spectrum beta-lactamase producing Enterobacterales, VAP ventilator-associated pneumonia
The correlation between semi-quantitative assessment of throat and rectal swabs was significant but weak (Pearson r coefficient = 0.227; p = 0.019). There was no combination of both throat and rectal samples able to rule out ESBL-E VAP diagnosis. The combination of a negative throat sample and a rectal sample less than numerous or very numerous was associated with a 4.7% (1 case out of 21) of presenting ESBLE-VAP (Table E2).
The AUC of semi-quantitative rectal colonization (0.774 vs. 0.651, p = 0.27) and throat (0.797 vs. 0.817, p = 0.88) colonization was not significantly different whether the time interval of the last available densities was less or greater or equal to 5 days.
In the multivariate analysis (Table 3), using successively rectal and throat carriage, when using only the rectal samples, the final model selected both a positive semi-quantitative assessment (p = 0.0076) and a colonization solely with ESBL-E. coli only (OR = 0.29 [0.1223; 0.70]; p = 0.0055) to predict ESBL-E VAP. The AUC of the model was 0.77. When using only the throat samples, the best prediction was obtained using the qualitative assessment and the presence of an urinary bladder at admission. The AUC of this model was 0.84, i.e., significantly discriminant. In both models, none of the clinical variables or antimicrobial pre-exposure remained associated with ESBL-E VAP.
Table 3.
Summary of the results of the multivariate models of ESBL-E VAP (all variables significant in the univariate analysis (see Tables 1, 2) were included in the selection process at the first step of the stepwise selection, i.e., urinary bladder before ICU admission, albumin level, ARDS in the 7 days before VAP; ESBL E. coli only, SOFA score at the time of VAP)
| Parameter | Odds ratio | CI 95% min | CI 95% max | Pr > chi-sq |
|---|---|---|---|---|
| Model 1 including rectal semi-quantitative result (AUC 0.769) | ||||
| Rectal colonization (semi-quantitative) density | 0.0076* | |||
| None/very rare/rare | 1 | |||
| Some | 3.103 | 0.457 | 21.072 | 0.2467 |
| Numerous | 11.594 | 2.343 | 57.379 | 0.0027 |
| Very numerous | 11.166 | 1.715 | 72.698 | 0.0116 |
| ESBL E. coli only | 0.291 | 0.122 | 0.695 | 0.0055 |
| Model 2 including throat qualitative result (AUC 0.838) | ||||
| Throat colonization with ESBL-E (qualitative) | 17.321 | 6.452 | 46.503 | < 0.0001 |
| Urinary bladder at admission | 2.727 | 0.954 | 7.799 | 0.0613 |
| Model 3 including both throat and rectal semi-quantitative results (AUC: 0.872) | ||||
| Throat colonization with ESBL-E (qualitative) | 21.161 | 6.91 | 64.802 | < 0.0001 |
| Rectal colonization (semi-quantitative) density | 0.0117* | |||
| None/very rare/rare | 1 | |||
| Some | 2.61 | 0.3 | 22.705 | 0.3846 |
| Numerous | 15.732 | 2.469 | 100.225 | 0.0035 |
| Very numerous | 7.701 | 0.908 | 65.308 | 0.0613 |
ESBL-E extended spectrum beta-lactamase producing Enterobacterales, VAP ventilator-associated pneumonia
*The p value referred to the global impact of the semi-quantitative value. OR: italicized referred to comparison of one particular density to the reference
Finally, rectal and throat samples were both introduced into the same model. The qualitative assessment of the throat samples (p < 0.0001) and the semi-quantitative assessment of the rectal samples (p = 0.012) were both independently associated with ESBL-E VAP. Interestingly, in this model, colonization with ESBL-E. coli was no longer significant. The AUC of the model was 0.872, i.e., significantly discriminant.
The empirical therapy was adequate in 39/50 (78%) cases of ESBL-E VAP as compared to 42/57 (74%) cases of other VAP. The empiric use of carbapenems was more frequent in ESBL-E VAP than in other VAP (37 (78.7%) vs. 26 (46.4%); p < .01), while the Gram-negative pathogen was eventually resistant to carbapenem in 6/26 cases (Pseudomonas aeruginosa: 4; Acinetobacter baumannii: 1, Carbapenemase-producer Enterobacter cloacae: 1). In-ICU death occurred for 52 patients, more frequently after ESBL-E VAP than other VAP [30 (60%) vs. 22 (38.6%); p = 0.03].
Discussion
This retrospective analysis of a prospective cohort of 107 VAP due to Gram-negative bacilli in ESBL-E colonized patients confirmed that factors such as the previous carriage assessed weekly but only qualitatively, clinical data or previous antimicrobial exposure in the days before VAP onset were insufficiently discriminant for predicting the involvement of ESBL-E in the VAP. The ESBL-E coli carriage was confirmed as less frequently associated with ESBL-E VAP than the carriage of non-E. coli ESBL-E [11]. Importantly, this study showed that both rectal and throat samples became strong predictors of ESBL-E VAP when assessed using a semi-quantitative method. Our study pointed out that both the quantification and the site of the digestive tract-yielding ESBL-E carriage were the only important factors that should be considered to evaluate the risk of ESBL-E VAP when a GNB-VAP is suspected.
The prevalence of colonization with ESBL-E is rising steadily in critically ill patients, owing to a continuous influx from both the community and healthcare system, and ICU acquisition, leading to frequent reports of carriage rates above 20% [2, 5, 9, 11]. Recent studies showed that ESBL-E colonization often precedes the ESBL-E VAP, with a positive predictive value of more than 97% [7]. However, in the ESBL-E-colonized patients who are suspected of GNB-VAP, the risk of ESBL-E VAP reached only 20–48% [7–11, 14]. Hence, the management of suspected VAP in ESBL-E carriers is a daily challenge for the intensivists, who need to select the most likely active antibacterial without overusing those active against highly resistant pathogens.
Carbapenems are along this strategy, considered as the drug of choice in case of suspected ESBL-E severe infections [26], including VAP [27], although using them for all ESBL-E carriers exposes to an overuse of carbapenems as empirical therapy. This overuse has two potential adverse effects: (1) the emergence of carbapenem-resistant bacteria [22, 28–31]; and (2) an inadequate initial antimicrobial therapy if the VAP is due to carbapenem-resistant bacteria, thus a risk of increased mortality and length of stay [28, 30, 32, 33].
Therefore, predicting ESBL-E VAP in known ESBL-E carriers is instrumental for the appropriate choice of the initial therapy at VAP onset. Previous studies suggested that the illness severity at admission and at VAP onset was higher in case of ESBL-E VAP [11]. In this study, ESBL-E VAP was indeed associated with a higher SOFA score at VAP onset and a higher mortality. However, this study showed that illness severity was no longer a risk factor of ESBL-E VAP when semi-quantitative assessment of the rectal and throat swabs and the distinction between carriage of ESBL-E. coli versus other ELBL-E were included in the logistic regression model.
Similarly, the previous antibiotics use was not an independent risk factor for ESBL-E VAP in our cohort. Indeed, the previous use of beta-lactams/beta-lactamases inhibitors was an independent risk factor of ESBL-E ICU-acquired pneumonia in one study only [11]. In another multicenter analysis of the Outcomerea study group, the use of carbapenems in the past few days was associated with a lower risk of ESBL-E VAP in case of infection-related ventilator-associated complication [14].
The relationship between rectal and oropharyngeal GNB colonization and VAP has not been fully addressed in the literature. In a monocenter study performed in patients receiving selective digestive decontamination [34], Frencken et al. showed that a rectal colonization predicted the respiratory tract colonization for both Enterobacterales and non-fermentative GNB. They also showed that both rectal and respiratory tract colonization with Enterobacterales predicted the involvement of these bacteria in the investigated ICU-acquired infections, including bacteremia. Another monocenter study found that the monitoring of both respiratory and rectal colonization increased the adequacy of antibiotic therapy of VAP and BSI due to Gram-negative bacteria (mainly P. aeruginosa and Acinetobacter spp.) [35]. More recently, in acutely ill patients who developed VAP caused by Enterobacterales species, a prospective observational case–control study suggested that the causative pathogens gained access to the oropharynx early after the start of the mechanical ventilation and outgrew the commensal members of the microbiome [36]. The results called for assessing whether a specific pattern of the oropharyngeal microbiome could predict VAP. Carbonne et al. assessed the presence of ESBL-E in respiratory samples collected in ESBL-E-positive rectal carriers, irrespective of any clinical symptoms. Respiratory samples were significantly more frequently positive when the sample was collected after an ICU stay longer than 5 days [13]. A meta-analysis [37] showed that respiratory tract colonization with multi-drug-resistant bacteria including Enterobacterales predicted the risk of subsequent VAP. The prediction was better when surveillance tracheal cultures were systematically performed at least twice a week.
Few studies suggested that the predictive value for VAP of oropharyngeal samples positive with Enterobacterales was higher than that of gastric samples [24, 38]. Indeed, the modulation of the oropharyngeal flora by a selective oral decontamination reduced the oropharyngeal colonization and the rate of VAP, without influencing the gastric colonization with GNB [38].
In our study, we confirmed that a throat ESBL-E colonization is important to differentiate ESBL-E carriers with subsequent ESBL-E VAP or other VAP due to other GNB. We identified a significant but weak relationship between semi-quantitative rectal colonization and the presence of ESBL-E in the throat that may partly explain this result.
We used a very simple and inexpensive semi-quantitative assessment of the abundance of ESBL-E bacteria in the culture in selected culture media of the colonization swabs to predict the impact of the colonization by ESBL-E bacteria. Although we did not check for its reproducibility, the technique is routinely performed in all microbiological laboratories and has been previously validated [25]. Of note, in this study, we did not try to demonstrate that this semi-quantitative assessment was linked to ESBL-E-relative abundance. Some studies performed in critically ill patients identified a relationship between the relative abundance in the stool of enterococci [39], ESBL-E [40] and carbapenemase-producing Enterobacterales [41], and subsequent infections due to these organisms. Indeed, the amount and the proportion of multi-drug-resistant GNB in the digestive microbiota are linked to the bacterial translocation in immunocompromised patients [42]. It would have been interesting to compare ESBL-E bacterial density with the ESBL-E-relative abundance. Unfortunately, the density of susceptible Enterobacterales was not assessed.
As stools cannot be collected at scheduled frequency in very severe ICU patients with abnormal digestive transit, we postulated that rectal swabs may be used as a surrogate of stool samples to quantify ESBL-E bacteria in the microbiota. Indeed, rectal swabs have been similarly preferred to stool samples to assess the relative abundance of carbapenemase-producing K. pneumoniae [43].
In our study, the digestive samples were performed on admission and weekly. We do not consider that the results will change dramatically if samples are collected in a more frequent way. Indeed, a recent study with daily rectal swabs found that, contrarily to the carriage of cephalosporinase hyperproducing-Enterobacterales, ESBLE-E intermittent rectal carriage was not frequent [44]. Considering that the median time interval between colonization and infection was 5.9 days on a metanalysis of available data, it is therefore possible that few cases of colonization acquisition could have been missed [8].
Other limitations should be acknowledged. First, the study was performed in one unit without confirmatory data on other independent samples and its results may not be extrapolated to other ICUs. Indeed, our patients were most often ventilated for more than 7 days. Our results need to be confirmed in patients with early onset VAP. Considering the poor link between rectal colonization and early-onset ICU-acquired infections [12] or positive respiratory samples [13] in previous studies, we consider that our results should not be generalized to early onset infections without further validation. Finally, the link between ESBL-E VAP and ESBL-E digestive carriage might be different from ESBL-E. coli and other Enterobacterales producers of ESBL. However, our result suggested that the quantification of the digestive carriage is a more important factor than the nature of the Enterobacterales species to predict ESBL-E VAP (Table 3, Model 3).
Further studies are needed to confirm the value of semi-quantitative culture of oropharyngeal swabs with highly resistant Gram-negative bacteria (i.e., ESBL-E, AmpC hyperproducer Enterobacterales, non-fermentative Gram-negative bacteria) to predict the pathogens involved in VAP.
In conclusion, we showed that the presence of ESBL-E in the throat and/or high densities of ESBL-E carriage in the rectum might be a powerful tool to estimate the risk of subsequent ESBL-E VAP, thus to initiate an empiric antibiotic therapy in ICU patients which would more likely be appropriate. These results should be confirmed in a multicentric study with a more diversified case mix of patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Celine Feger, MD (EMIBiotech) for her editorial support.
Funding
The research leading to these results has received partial support from the Innovative Medicines Initiative Joint Undertaking under Grant Agreement No. 115523 COMBACTE, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies, in-kind contribution.
Compliance with ethical standards
Conflicts of interest
All authors reported no conflict of interests for the submitted work. Outside of the submitted work, JFT reports research grants to my university from Merck, Pfizer, 3M. Lecture fees from Merck Biomerieux, Pfizer, Nabriva; fees for scientific board participation from Paratek, Merck, Bayer Pharma, Gilead.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT) Eur Respir J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 2.Timsit JF, Bassetti M, Cremer O, Daikos G, de Waele J, Kallil A, Kipnis E, Kollef M, Laupland K, Paiva JA, Rodriguez-Bano J, Ruppe E, Salluh J, Taccone FS, Weiss E, Barbier F. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019;45:172–189. doi: 10.1007/s00134-019-05520-5. [DOI] [PubMed] [Google Scholar]
- 3.de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect. 2013;19:860–868. doi: 10.1111/1469-0691.12028. [DOI] [PubMed] [Google Scholar]
- 4.Zahar JR, Blot S, Nordmann P, Martischang R, Timsit JF, Harbarth S, Barbier F. Screening for intestinal carriage of ESBL-producing Enterobacteriaceae in critically ill patients: expected benefits and evidence-based controversies. Clin Infect Dis. 2018;68:2125–2130. doi: 10.1093/cid/ciy864. [DOI] [PubMed] [Google Scholar]
- 5.Pilmis B, Cattoir V, Lecointe D, Limelette A, Grall I, Mizrahi A, Marcade G, Poilane I, Guillard T, Bourgeois Nicolaos N, Zahar JR, Le Monnier A. Carriage of ESBL-producing Enterobacteriaceae in French hospitals: the PORTABLSE study. J Hosp Infect. 2018;98:247–252. doi: 10.1016/j.jhin.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Derde LPG, Cooper BS, Goossens H, Malhotra-Kumar S, Willems RJL, Gniadkowski M, Hryniewicz W, Empel J, Dautzenberg MJD, Annane D, Aragao I, Chalfine A, Dumpis U, Esteves F, Giamarellou H, Muzlovic I, Nardi G, Petrikkos GL, Tomic V, Marti AT, Stammet P, Brun-Buisson C, Bonten MJM. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houard M, Rouze A, Ledoux G, Six S, Jaillette E, Poissy J, Preau S, Wallet F, Labreuche J, Nseir S, Voisin B. Relationship between digestive tract colonization and subsequent ventilator-associated pneumonia related to ESBL-producing Enterobacteriaceae. PLoS ONE. 2018;13:e0201688. doi: 10.1371/journal.pone.0201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detsis M, Karanika S, Mylonakis E. ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med. 2017;45:705–714. doi: 10.1097/CCM.0000000000002253. [DOI] [PubMed] [Google Scholar]
- 9.Barbier F, Pommier C, Essaied W, Garrouste-Orgeas M, Schwebel C, Ruckly S, Dumenil AS, Lemiale V, Mourvillier B, Clec’h C, Darmon M, Laurent V, Marcotte G, Lucet JC, Souweine B, Zahar JR, Timsit JF. Colonization and infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother. 2016;71:1088–1097. doi: 10.1093/jac/dkv423. [DOI] [PubMed] [Google Scholar]
- 10.Bruyere R, Vigneron C, Bador J, Aho S, Toitot A, Quenot JP, Prin S, Charles PE. Significance of prior digestive colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae in patients with ventilator-associated pneumonia. Crit Care Med. 2016;44:699–706. doi: 10.1097/CCM.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 11.Razazi K, Mekontso Dessap A, Carteaux G, Jansen C, Decousser JW, de Prost N, Brun-Buisson C. Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Ann Intensive Care. 2017;7:61. doi: 10.1186/s13613-017-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razazi K, Derde LP, Verachten M, Legrand P, Lesprit P, Brun-Buisson C. Clinical impact and risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria in the intensive care unit. Intensive Care Med. 2012;38:1769–1778. doi: 10.1007/s00134-012-2675-0. [DOI] [PubMed] [Google Scholar]
- 13.Carbonne H, Le Dorze M, Bourrel AS, Poupet H, Poyart C, Cambau E, Mira JP, Charpentier J, Amarsy R. Relation between presence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in systematic rectal swabs and respiratory tract specimens in ICU patients. Ann Intensive Care. 2017;7:13. doi: 10.1186/s13613-017-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbier F, Bailly S, Schwebel C, Papazian L, Azoulay E, Kallel H, Siami S, Argaud L, Marcotte G, Misset B, Reignier J, Darmon M, Zahar JR, Goldgran-Toledano D, de Montmollin E, Souweine B, Mourvillier B, Timsit JF. Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Intensive Care Med. 2018;44:616–626. doi: 10.1007/s00134-018-5154-4. [DOI] [PubMed] [Google Scholar]
- 15.Emmanuel Martinez A, Widmer A, Frei R, Pargger H, Tuchscherer D, Marsch S, Egli A, Tschudin-Sutter S. ESBL-colonization at ICU admission: impact on subsequent infection, carbapenem-consumption, and outcome. Infect Control Hosp Epidemiol. 2019;40:1–6. doi: 10.1017/ice.2019.5. [DOI] [PubMed] [Google Scholar]
- 16.Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, Paiva JA, Cakar N, Ma X, Eggimann P, Antonelli M, Bonten MJ, Csomos A, Krueger WA, Mikstacki A, Lipman J, Depuydt P, Vesin A, Garrouste-Orgeas M, Zahar JR, Blot S, Carlet J, Brun-Buisson C, Martin C, Rello J, Dimopoulos G, Timsit JF. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38:1930–1945. doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 18.Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, Agodi A, Frank U, Mertens K, Schumacher M, Wolkewitz M. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2012;11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 19.Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B, Ruckly S, Dumenil AS, Kallel H, Argaud L, Marcotte G, Barbier F, Laurent V, Goldgran-Toledano D, Clec’h C, Azoulay E, Souweine B, Timsit JF. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74:131–141. doi: 10.1016/j.jinf.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Barbier F, Lisboa T, Nseir S. Understanding why resistant bacteria are associated with higher mortality in ICU patients. Intensive Care Med. 2016;42:2066–2069. doi: 10.1007/s00134-015-4138-x. [DOI] [PubMed] [Google Scholar]
- 21.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596. doi: 10.1186/s13054-014-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armand-Lefevre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppe E, Bronchard R, Lepeule R, Lucet JC, El Mniai A, Wolff M, Montravers P, Plesiat P, Andremont A. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother. 2013;57:1488–1495. doi: 10.1128/AAC.01823-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahrani-Mougeot FK, Paster BJ, Coleman S, Barbuto S, Brennan MT, Noll J, Kennedy T, Fox PC, Lockhart PB. Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 2007;45:1588–1593. doi: 10.1128/JCM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrouste-Orgeas M, Chevret S, Arlet G, Marie O, Rouveau M, Popoff N, Schlemmer B. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am J Respir Crit Care Med. 1997;156:1647–1655. doi: 10.1164/ajrccm.156.5.96-04076. [DOI] [PubMed] [Google Scholar]
- 25.Sanders ER. Aseptic laboratory techniques: plating methods. J Vis Exp JoVE. 2012;63:e3064. doi: 10.3791/3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timsit JF, Pilmis B, Zahar JR. How should we treat hospital-acquired and ventilator-associated pneumonia caused by extended-spectrum beta-lactamase-producing enterobacteriaceae? Sem Respir Crit Care Med. 2017;38:287–300. doi: 10.1055/s-0037-1603112. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Zhang Y, Yao X, Xian H, Liu Y, Li H, Chen H, Wang X, Wang R, Zhao C, Cao B, Wang H. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis. 2016;35:1679–1689. doi: 10.1007/s10096-016-2710-0. [DOI] [PubMed] [Google Scholar]
- 29.Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, Zotou A, Spyropoulou A, Koutsileou K, Vamvakopoulou S, Sioulas N, Karamouzos V, Anastassiou ED, Spiliopoulou I, Christofidou M, Marangos M. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis. 2017;36:1125–1131. doi: 10.1007/s10096-017-2899-6. [DOI] [PubMed] [Google Scholar]
- 30.Mariappan S, Sekar U, Kamalanathan A. Carbapenemase-producing Enterobacteriaceae: risk factors for infection and impact of resistance on outcomes. Int J Appl Basic Med Res. 2017;7:32–39. doi: 10.4103/2229-516X.198520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planquette B, Timsit JF, Misset BY, Schwebel C, Azoulay E, Adrie C, Vesin A, Jamali S, Zahar JR, Allaouchiche B, Souweine B, Darmon M, Dumenil AS, Goldgran-Toledano D, Mourvillier BH, Bedos JP. Pseudomonas aeruginosa ventilator-associated pneumonia. Predictive factors of treatment failure. Am J Respir Crit Care Med. 2013;188:69–76. doi: 10.1164/rccm.201210-1897OC. [DOI] [PubMed] [Google Scholar]
- 32.Ibn Saied W, Mourvillier B, Cohen Y, Ruckly S, Reignier J, Marcotte G, Siami S, Bouadma L, Darmon M, de Montmollin E, Argaud L, Kallel H, Garrouste-Orgeas M, Soufir L, Schwebel C, Souweine B, Glodgran-Toledano D, Papazian L, Timsit JF. A comparison of the mortality risk associated with ventilator-acquired bacterial pneumonia and nonventilator ICU-acquired bacterial pneumonia. Crit Care Med. 2019;47:345–352. doi: 10.1097/CCM.0000000000003553. [DOI] [PubMed] [Google Scholar]
- 33.Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2018;7:79. doi: 10.1186/s13756-018-0370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frencken JF, Wittekamp BHJ, Plantinga NL, Spitoni C, van de Groep K, Cremer OL, Bonten MJM. Associations between enteral colonization with Gram-negative bacteria and intensive care unit-acquired infections and colonization of the respiratory tract. Clin Infect Dis. 2018;66:497–503. doi: 10.1093/cid/cix824. [DOI] [PubMed] [Google Scholar]
- 35.Papadomichelakis E, Kontopidou F, Antoniadou A, Poulakou G, Koratzanis E, Kopterides P, Mavrou I, Armaganidis A, Giamarellou H. Screening for resistant gram-negative microorganisms to guide empiric therapy of subsequent infection. Intensive Care Med. 2008;34:2169–2175. doi: 10.1007/s00134-008-1247-9. [DOI] [PubMed] [Google Scholar]
- 36.Sommerstein R, Merz TM, Berger S, Kraemer JG, Marschall J, Hilty M. Patterns in the longitudinal oropharyngeal microbiome evolution related to ventilator-associated pneumonia. Antimicrob Resist Infect Control. 2019;8:81. doi: 10.1186/s13756-019-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brusselaers N, Labeau S, Vogelaers D, Blot S. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med. 2013;39:365–375. doi: 10.1007/s00134-012-2759-x. [DOI] [PubMed] [Google Scholar]
- 38.Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, Beysens AJ, de Leeuw PW, Stobberingh EE. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164:382–388. doi: 10.1164/ajrccm.164.3.2005003. [DOI] [PubMed] [Google Scholar]
- 39.Freedberg DE, Zhou MJ, Cohen ME, Annavajhala MK, Khan S, Moscoso DI, Brooks C, Whittier S, Chong DH, Uhlemann AC, Abrams JA. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018;44:1203–1211. doi: 10.1007/s00134-018-5268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruppe E, Lixandru B, Cojocaru R, Buke C, Paramythiotou E, Angebault C, Visseaux C, Djuikoue I, Erdem E, Burduniuc O, El Mniai A, Marcel C, Perrier M, Kesteman T, Clermont O, Denamur E, Armand-Lefevre L, Andremont A. Relative fecal abundance of extended-spectrum-beta-lactamase-producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrob Agents Chemother. 2013;57:4512–4517. doi: 10.1128/AAC.00238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimasaki T, Seekatz A, Bassis C, Rhee Y, Yelin RD, Fogg L, Dangana T, Cisneros EC, Weinstein RA, Okamoto K, Lolans K, Schoeny M, Lin MY, Moore NM, Young VB, Hayden MK. Increased relative abundance of klebsiella pneumoniae carbapenemase-producing klebsiella pneumoniae within the gut microbiota is associated with risk of bloodstream infection in long-term acute care hospital patients. Clin Infect Dis. 2019;68:2053–2059. doi: 10.1093/cid/ciy796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woerther PL, Micol JB, Angebault C, Pasquier F, Pilorge S, Bourhis JH, de Botton S, Gachot B, Chachaty E. Monitoring antibiotic-resistant enterobacteria faecal levels is helpful in predicting antibiotic susceptibility of bacteraemia isolates in patients with haematological malignancies. J Med Microbiol. 2015;64:676–681. doi: 10.1099/jmm.0.000078. [DOI] [PubMed] [Google Scholar]
- 43.Lerner A, Romano J, Chmelnitsky I, Navon-Venezia S, Edgar R, Carmeli Y. Rectal swabs are suitable for quantifying the carriage load of KPC-producing carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2013;57:1474–1479. doi: 10.1128/AAC.01275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grohs P, Podglajen I, Guerot E, Bellenfant F, Caumont-Prim A, Kac G, Tillecovidin B, Carbonnelle E, Chatellier G, Meyer G, Fagon JY, Gutmann L. Assessment of five screening strategies for optimal detection of carriers of third-generation cephalosporin-resistant Enterobacteriaceae in intensive care units using daily sampling. Clin Microbiol Infect. 2014;20:O879–O886. doi: 10.1111/1469-0691.12663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.