Abstract
Background
The most currently used general anaesthetics are potent potentiators of γ-aminobutyric acid A (GABAA) receptors and are invariably neurotoxic during the early stages of brain development in preclinical animal models. As causality between GABAA potentiation and anaesthetic-induced developmental neurotoxicity has not been established, the question remains whether GABAergic activity is crucial for promoting/enhancing neurotoxicity. Using the neurosteroid analogue, (3α,5α)-3-hydroxy-13,24-cyclo-18,21-dinorchol-22-en-24-ol (CDNC24), which potentiates recombinant GABAA receptors, we examined whether this potentiation is the driving force in inducing neurotoxicity during development.
Methods
The neurotoxic potential of CDNC24 was examined vis-à-vis propofol (2,6-diisopropylphenol) and alphaxalone (5α-pregnan-3α-ol-11,20-dione) at the peak of rat synaptogenesis. In addition to the morphological neurotoxicity studies of the subiculum and medial prefrontal cortex (mPFC), we assessed the extra-, pre-, and postsynaptic effects of these agents on GABAergic neurotransmission in acute subicular slices from rat pups.
Results
CDNC24, like alphaxalone and propofol, caused dose-dependent hypnosis in vivo, with a higher therapeutic index. CDNC24 and alphaxalone, unlike propofol, did not cause developmental neuroapoptosis in the subiculum and mPFC. Propofol potentiated post- and extrasynaptic GABAA currents as evidenced by increased spontaneous inhibitory postsynaptic current (sIPSC) decay time and prominent tonic currents, respectively. CDNC24 and alphaxalone had a similar postsynaptic effect, but also displayed a strong presynaptic effect as evidenced by decreased frequency of sIPSCs and induced moderate tonic currents.
Conclusions
The lack of neurotoxicity of CDNC24 and alphaxalone may be at least partly related to suppression of presynaptic GABA release in the developing brain.
Keywords: general anaesthetic, prefrontal cortex, presynaptic, subiculum, synaptic transmission, synaptogenesis
Editor's key points.
-
•
Most general anaesthetics that potentiate γ-aminobutyric acid A (GABAA) receptors are neurotoxic early in brain development, but the causal relationship is unclear.
-
•
The novel neurosteroid CDNC24, like alphaxalone, caused dose-dependent hypnosis in vivo, but did not cause developmental neuroapoptosis.
-
•
CDNC24 and alphaxalone, like propofol, potentiated postsynaptic GABAA currents, but also had a strong presynaptic effect to reduce GABA release.
-
•
The lack of neurotoxicity of CDNC24 and alphaxalone in contrast to propofol may be related to inhibition of presynaptic GABA release.
Preclinical evidence continues to mount,1, 2 and clinical studies begin to shed more light on the potential for prolonged or repeated general anaesthesia (GA) to be harmful to the immature brain and to induce long-lasting behavioural and cognitive impairment.3, 4, 5 Considering that anaesthesia is unavoidable in the clinical setting, we grapple with ways to assure the safety of children whilst providing comfort during painful procedures. Recent investigations have focused on developing novel anaesthetic agents with desirable hypnotic properties but minimal neurotoxic side-effects when administered during the critical stages of early brain development. Our investigations of novel general anaesthetics have focused on a class of agents referred to as the neuroactive steroids (neurosteroids). We recently published that the neurosteroid analogue (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) blocks T-type calcium currents (T-currents) in the subiculum,6 and is an effective hypnotic with a good safety profile when administered to rat pups.7
The most currently used general anaesthetics are potent positive modulators of postsynaptic γ-aminobutyric acid A (GABAA) receptors and are invariably neurotoxic during the early stages of brain development in preclinical animal models.3, 8, 9 As causality between GABAA potentiation and GA-induced developmental neurotoxicity has not been established, the question remains whether GABAergic activity is critical for promoting/enhancing neurotoxicity and whether T-channel blocking properties may provide ‘built-in’ protection.
Using steroid analogues that potentiate GABAA receptors either selectively ([3α,5α]-3-hydroxy-13,24-cyclo-18,21-dinorchol-22-en-24-ol [CDNC24]) or with combined T-channel blocking effect (5α-pregnan-3α-ol-11,20-dione [alphaxalone]), we examined their neurotoxic potential at the peak of rat synaptogenesis vis-à-vis the clinically used GABAergic anaesthetic propofol. In addition to the morphological neurotoxicity studies of the subiculum and medial prefrontal cortex (mPFC), we focused on careful assessment of synaptic physiology with studies of extra-, pre-, and postsynaptic effects of neuroactive steroids and propofol on GABA synaptic transmission in the developing rat subiculum.
Methods
Drugs and chemicals
The methods for the synthesis of alphaxolone and CDNC24 are provided in the Supplementary material. For in vivo experiments, alphaxalone and CDNC24 were freshly dissolved in 2-hydroxypropyl-β-cyclodextrin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (cyclodextrin 15% and 25%, respectively). Propofol (Diprivan®) was purchased from Fresenius Kabi (Lake Zurich, IL, USA). For electrophysiological studies, pure propofol solution from ICN (Aurora, OH, USA) was used. All compounds tested were prepared as 10 mM stock solutions in dimethylsulphoxide and freshly diluted to final concentrations in the external solution at the time of electrophysiological experiments.
Animals
We used postnatal Day 7 (PND7) rat pups for anaesthesia exposure experiments (Sprague-Dawley; Envigo, Indianapolis, IN, USA) as this is the age when they are most vulnerable to anaesthesia-induced developmental neurotoxicity.10 For electrophysiology recordings, we used acute brain slices from PND7–9 rat pups. The animals were housed in an accredited animal facility in a 10 h light and 14 h dark cycle at a constant temperature of 21 (2)°C. All animals had ad libitum access to food and water. Experiments were approved by the Institutional Animal Care and Use Committee at the University of Colorado. All procedures were carried out in accordance with the guidelines established by the US Public Health Service Policy on Humane Care and Use of Laboratory Animals. All animal procedures were performed in an AAALAC-accredited facility in accordance with the US Public Health Service Policy and the Guide for the Care and Use of Laboratory Animals. Details of specific experimental procedures can be found in the Supplementary material.
Results
Alphaxalone and CDNC24 have comparable hypnotic properties to propofol in rat pups
We focused our studies on two neurosteroids, alphaxalone and CDNC24, and a clinically used injectable anaesthetic, propofol (2,6-diisopropylphenol) (Fig 1a).
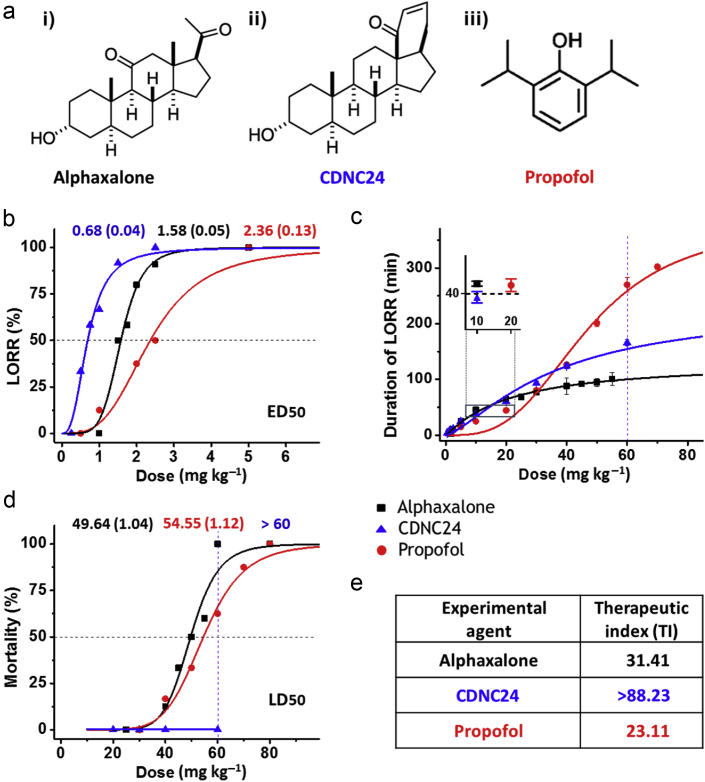
Figure 1.
Characterisation of hypnotic properties of alphaxalone, CDNC24, and propofol in postnatal Day 7 rat pups. (a) The chemical structure of tested agents: i) alphaxalone (5α-pregnan-3α-ol-11,20-dione), ii) CDNC24 ([3α,5α]-3-hydroxy-13,24-cyclo-18,21-dinorchol-22-en-24-ol) and iii) propofol (2,6-diisopropylphenol). (b) Percentage of rat pups with loss of righting reflex (LORR) with increasing doses of alphaxalone (black), CDNC24 (blue), or propofol (red) after a single i. p. injection. The dose that caused LORR in 50% of animals (marked with horizontal dashed line in the graph) was used to determine the ED50 (mg kg−1) of alphaxalone, CDNC24, or propofol (exact ED50 values for each of three agents are indicated above the graph in corresponding colours) (n=4–11 rats per each data point). (c) Duration of LORR (min) with increasing doses of alphaxalone (black), CDNC24 (blue), or propofol (red) after a single i. p. injection. The difference in the duration of LORR at the dose of 60 mg kg−1 for agents tested is presented as a vertical dashed line in the graph. Each data point is presented as mean (standard error of the mean) (n=4–16 rats per each data point). The inset highlights the assessment of the equipotent doses using the duration of LORR (min) after a single i. p. injection. Alphaxalone (black) and CDNC24 (blue), 10 mg kg−1 for both agents, are comparable with propofol (red) at 20 mg kg−1, a dose known to cause significant developmental neuroapoptosis. The dashed line in the inserted graph indicates that chosen doses of tested agents caused LORR for ∼40 min. (d) The mortality rate of rat pups with increasing doses of alphaxalone (black), CDNC24 (blue), or propofol (red) after a single i. p. injection. The dose that caused mortality in 50% of animals (outlined as a horizontal dashed line in the graph) was used to determine LD50 (mg kg−1) of alphaxalone or propofol (exact ED50 values for each of two agents are indicated above the graph in corresponding colours). Note that CDNC24 did not cause any mortality even at the highest dose tested (ED50 >60 mg kg−1). The difference in mortality rate at a dose of 60 mg kg−1 for agents tested is indicated as a vertical dashed line in the graph (n=4–10 rats per each data point). (e) Therapeutic indices of alphaxalone, CDNC24, and propofol. The therapeutic indices of tested compounds were calculated as the ratio of corresponding LD50 and ED50 values.
We compared the hypnotic properties of these agents using loss of righting reflex (LORR). PND7 rats were injected i. p. with alphaxalone 1–80 mg kg−1, CDNC24 0.25–60 mg kg−1, or propofol 0.5–80 mg kg−1. Similar to propofol (red line), the neurosteroids were effective hypnotics, as both CDNC24 (blue line) and alphaxalone (black line) caused dose-dependent LORR. The neurosteroid dose–response curves were shifted to the left compared with propofol, suggesting their higher potency (Fig 1b). The calculated ED50 values were 1.58 (0.05), 0.68 (0.04), and 2.36 (0.13) mg kg−1 for alphaxalone, CDNC24, and propofol, respectively (indicated with a dotted horizontal line).
We next investigated the duration of hypnosis (Fig 1c) and the safety margins (Fig 1d). The duration of LORR for propofol was comparable with that of alphaxalone and CDNC24 at doses ≤40 mg kg−1, but substantially longer at higher doses. For example, at 60 mg kg−1, the duration of propofol hypnosis was 1.67- and 2.59-fold longer compared with CDNC24 and alphaxalone, respectively (indicated with a dotted vertical line in Fig 1c).
The calculated lethal dose LD50 was 49.6 (1.0) mg kg−1 for alphaxalone and 54.6 (1.1) mg kg−1 for propofol (indicated with a dotted horizontal line in Fig 1d). Even at the highest dose (60 mg kg−1), CDNC24 did not cause any mortality. This finding is in contrast to alphaxalone and propofol, which, at the same dose, caused 100% and 60% mortality, respectively (indicated with a dotted vertical line in Fig 1d). Although the limited solubility of CDNC24 prevented full dose–response studies, we conclude that the calculated LD50 for CDNC24 is >60 mg kg−1. Hence, the calculated therapeutic indices (TIs) were >88.2 for CDNC24, 31.4 for alphaxalone, and 23.1 for propofol (Fig 1e). This suggests that the safety margin for propofol is the lowest of the three compounds tested.
Prolonged exposure to alphaxalone or CDNC24 did not induce developmental neuroapoptosis in the subiculum and mPFC of rat pups
We have reported that propofol was neurotoxic in PND7 rat pups when injected hourly six times (20 mg kg−1, i. p.).11 Using the same experimental approach, we conducted neurotoxicity studies with alphaxalone and CDNC24 to compare with propofol (Fig 2). To assure that the neurotoxicity studies are comparable, we first determined the equipotent doses based on the recorded duration of LORR for each hypnotic, as shown in Fig 1c (inset). For the neurotoxicity comparison studies, we administered either alphaxalone (10 mg kg−1, i. p.), CDNC24 (10 mg kg−1, i. p.), or propofol (20 mg kg−1, i. p.) every hour to maintain 6 h of exposure (total of six injections of each hypnotic). The physiological parameter assessment revealed no significant change in blood glucose concentration, SpO2, or respiration rate during exposure with all three hypnotics in comparison with control animals. All three hypnotics caused a comparable decrease in heart rate (Supplementary Fig. S1).
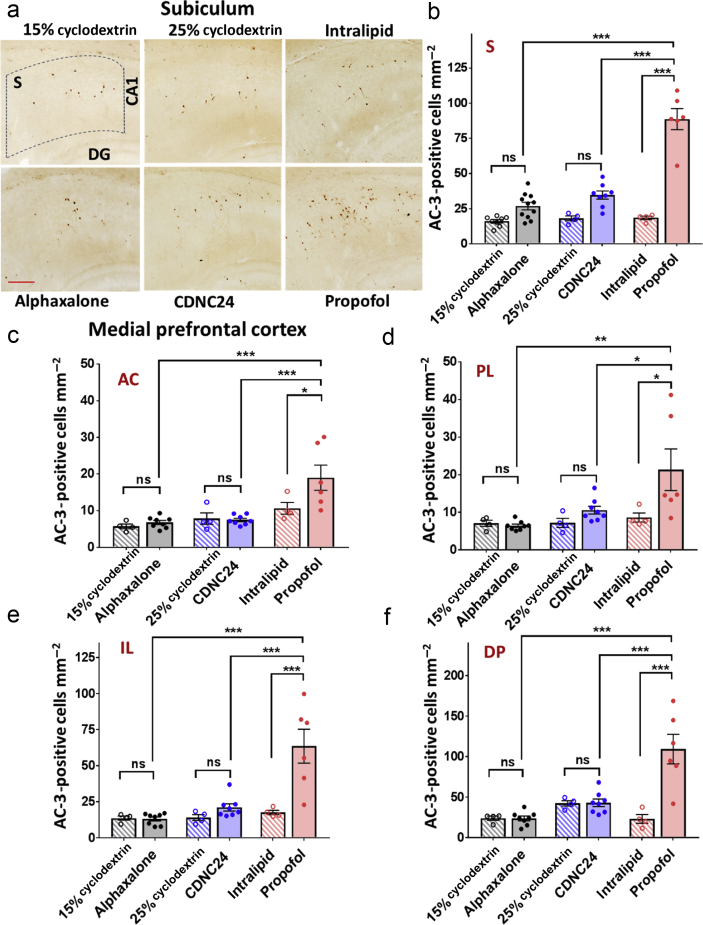
Figure 2.
Alphaxalone and CDNC24 do not cause developmental neuroapoptosis in the subiculum and medial prefrontal cortex of postnatal Day 7 rat pups. (a) Representative images of activated caspase-3 (AC-3) immunostaining in the subiculum after six i. p. injections of alphaxalone, CDNC24, or propofol, and cyclodextrin 15%, cyclodextrin 25%, or intralipid as controls, respectively, are depicted in the panel. Scale bar is 200 μm. (b) Bar graphs showing the number of AC-3-positive cells mm−2 in subiculum after six i. p. injections of alphaxalone (filled grey bar), CDNC24 (filled blue bar), or propofol (filled red bar), and cyclodextrin 15% (patterned grey bar), cyclodextrin 25% (patterned blue bar), or intralipid (patterned red bar) as their respective vehicle controls. Propofol caused a significant increase in the number of AC-3-positive cells compared with intralipid (4.8-fold; one-way analysis of variance [anova] followed by Tukey's post hoc test: F5,35=52.27; P<0.001; ∗∗∗P<0.001), alphaxalone or CDNC24 (both ∗∗∗P<0.001). Neither neurosteroid caused an increase in the number of apoptotic cells in the immature subiculum when compared with their respective controls (P>0.05; ns, not significant). (c–f) Bar graphs showing the number of AC-3-positive cells mm−2 in (c) anterior cingulate (AC), (d) prelimbic (PL), (e) infralimbic (IL), and (f) dorsal peduncular (DP) subregions of medial prefrontal cortex (mPFC) after six i. p. injections of alphaxalone (filled grey bar), CDNC24 (filled blue bar), or propofol (filled red bar), and cyclodextrin 15% (patterned grey bar), cyclodextrin 25% (patterned blue bar), or intralipid (patterned red bar) as their vehicle controls, respectively. In the AC subregion, propofol caused a 1.8-fold increase in the number of AC-3 labelled cells in comparison with intralipid control (one-way anova followed by Tukey's post hoc test: F5,28=8.82; P<0.001; ∗P=0.033), whilst alphaxalone and CDNC24 did not show a neurotoxic effect compared with their respective controls. In PL, a 2.5-fold increase in the number of apoptotic cells was detected with propofol compared with intralipid control (one-way anova followed by Tukey's post hoc test: F5,28=5.09; P=0.002; ∗P=0.033). No difference was seen between neurosteroids and their controls (cyclodextrin 15% and cyclodextrin 25% groups). In IL, propofol increased the number of AC-3-positive cells 3.6-fold, significantly above the level detected with both neurosteroids that remained in the control level (one-way anova followed by Tukey's post hoc test: F5,28=13.60; P<0.001; ∗∗∗P<0.001). In DP, propofol caused a 4.7-fold increase in comparison with intralipid. The neurosteroids showed no neurotoxic effect when compared with controls, with the number of AC-3 labelled cells significantly lower than propofol (one-way anova followed by Tukey's post hoc test: F5,28=14.63; P<0.001; ∗∗∗P<0.001). In all mPFC subregions, the number of AC-3-positive cells in the propofol group was significantly higher than in either alphaxalone or CDNC24 group (all comparisons at least ∗P<0.05). All data are presented as mean (standard error of the mean [sem]) (n=4–11 rats per each data point). Representative images of AC-3-positive cells in subregions of mPFC can be found in Supplementary Figure S2. Exact values of mean (sem) numbers of AC-3-positive cells in each treatment and all regions presented in graphs (b–f) can be found in Supplementary Table S1.
We next stained neurones for activated caspase-3 (AC-3) in the subiculum and four distinct subregions of the mPFC: anterior cingulate (AC), prelimbic (PL), infralimbic (IL), and dorsal peduncular (DP) cortices (Fig 2). We confirmed that there is some naturally occurring neuroapoptosis in PND7 subiculum as noted by minimal AC-3 immunolabelling in all three vehicle groups (cyclodextrin 15%, cyclodextrin 25%, and intralipid) (Fig 2a). When compared with their respective experimental groups, the quantitative analysis of AC-3-positive cells (Fig 2b) showed that propofol treatment causes a 4.8-fold increase in AC-3 labelled subicular cells (P<0.001) compared with its vehicle (intralipid) controls. In contrast, when we compared the density of apoptotic cells in two experimental groups with their respective controls, neither alphaxalone nor CDNC24 exhibited significant neurotoxic effects in the subiculum. Further statistical analysis confirmed that propofol-induced developmental neuroapoptosis was significantly higher compared with either alphaxalone or CDNC24.
We observed a similar phenomenon in mPFC (Fig. 2c–f). In each of the subregions of interest (AC, Fig 2c; PL, Fig 2d; IL, Fig 2e; and DP, Fig 2f), there was significant upregulation of neuroapoptosis induced by propofol anaesthesia compared with its intralipid control. The increase in the number of AC-3-positive cells in the propofol-treated group in comparison with intralipid was 1.8-fold for AC, 2.5-fold for PL, and 3.6- and 4.7-fold for the ventral subregions of mPFC, IL and DP, respectively. Although neither alphaxalone- nor CDNC24-induced neuroapoptosis was greater than with their respective controls, propofol-induced developmental neuroapoptosis was significantly greater compared with either neurosteroid in each subregion of mPFC. The representative photomicrographs of AC-3 staining in mPFC are shown in Supplementary Figure S2a–d, whereas the mean (standard error of the mean) values detected in each of the regions examined are provided in Supplementary Table S1.
Based on our neurotoxicity studies in the subiculum and mPFC (Fig 2), we conclude that, unlike propofol, alphaxalone and CDNC24, at equipotent doses, do not cause developmental neuroapoptosis.
Propofol increased the spontaneous inhibitory postsynaptic current decay time without affecting event frequency in the immature subiculum
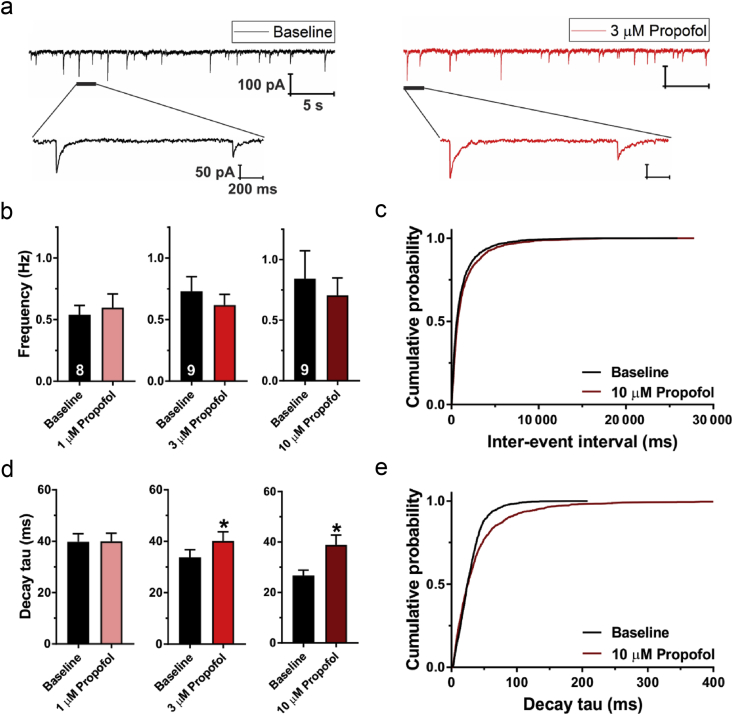
We examined the effects of propofol at clinically relevant brain concentrations of 1 and 3 μM12 on spontaneous inhibitory postsynaptic currents (sIPSCs) in PND7–9 rat pups. Representative traces of sIPSCs before and after the perfusion with propofol 3 μM are shown in Fig 3a. Either 1 or 3 μM propofol did not affect baseline sIPSC frequency (Fig 3b). However, diffusion of propofol into rat brain slices can be very slow.13 Therefore, we also tested propofol 10 μM, which did not significantly change the event frequency, as shown in the cumulative probability plots (Fig 3c). Conversely, both propofol 3 and 10 μM prolonged the sIPSC decay time by about 1.20- and 1.46-fold, respectively (Fig 3d). This is shown by the rightward shift of the propofol cumulative probability plot (Fig 3e). We conclude that the GABA-enhancing properties of propofol in the developing rat brain are mostly mediated through postsynaptic targets.
Figure 3.
Effects of propofol on spontaneous inhibitory postsynaptic currents (sIPSCs) in the subiculum of postnatal Days 7–9 rat pups. (a) Original traces from a representative subicular neurone in baseline conditions (black) and after addition of propofol 3 μM (red trace). Expanded traces show changes in sIPSC event amplitude and kinetics after propofol application. (b) Propofol (1, 3, and 10 μM) did not significantly affect sIPSC frequency. The number of neurones is shown at the bottom of each bar graph. (c) Cumulative probability plots of sIPSC inter-event intervals were similar for baseline (2329 events) and propofol 10 μM (1969 events). (d) Propofol significantly prolonged sIPSC decay time (paired t-test [3 μM]: t8=2.94; ∗P=0.019; n=9 neurones, four rats; 10 μM: t8=3.21; ∗P=0.012; n=9 neurons, seven rats). (e) Cumulative probability plots revealed a rightward shift (longer sIPSC decay times) after application of propofol 10 μM (1543 events) compared with baseline (1566 events). Results are expressed as mean (standard error of the mean).
Alphaxalone and CDNC24 increase sIPSC decay time but decrease event frequency in the immature subiculum
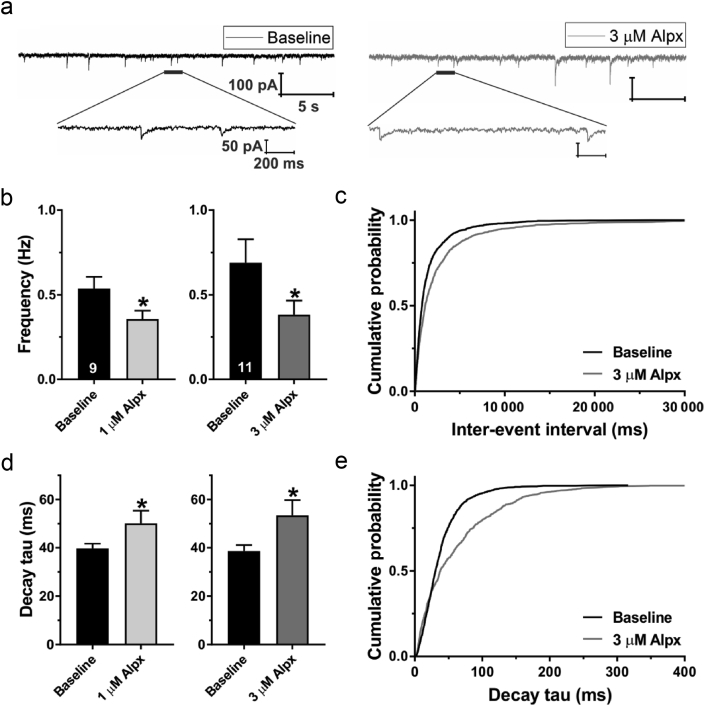
We examined the effects of neurosteroids on GABAA-mediated transmission. Representative traces of sIPSCs before and after the bath perfusion with alphaxalone 3 μM on the same neurone are shown in Fig 4a. Unlike propofol, alphaxalone decreased the baseline sIPSC frequency in subicular neurones after perfusion of either 1 or 3 μM by about 0.33- and 0.44-fold, respectively (Fig 4b). Cumulative probability plots presented in Fig 4c confirmed this finding. The decay time of sIPSCs was increased after alphaxalone 1 μM (Fig 4d, left graph) and 3 μM (Fig 4d, right graph) by about 1.26- and 1.38-fold, respectively. This effect was also evident when we presented all events using the cumulative probability plots (Fig 4e).
Figure 4.
Effects of alphaxalone on spontaneous inhibitory postsynaptic currents (sIPSCs) in the subiculum of postnatal Days 7–9 rat pups. (a) Original traces from a representative subicular neurone in baseline conditions (black) and after addition of alphaxalone 3 μM (Alpx; grey trace). The lower traces show single sIPSC events at an expanded scale. Note the change in decay time after Alpx application. (b) The frequency of sIPSC events was significantly decreased after the application of Alpx 1 μM (paired t-test: t8=2.37; ∗P=0.045; n=9 neurones, four rats) or 3 μM (t10=2.91; ∗P=0.016; n=11 neurones, six rats). The number of neurones is shown at the bottom of each bar graph. (c) Cumulative probability plots revealed longer sIPSC inter-event intervals in neurones treated with Alpx 3 μM (1329 events) compared with baseline (1812 events). (d) The decay time of sIPSCs was significantly prolonged after application of Alpx 1 μM (t8=2.90; ∗P=0.020) or 3 μM (t10=2.27; ∗P=0.046). (e) Cumulative probability plots show prolonged sIPSC decay times after application of Alpx 3 μM (929 events) compared with baseline (1493 events). Results are expressed as mean (standard error of the mean).
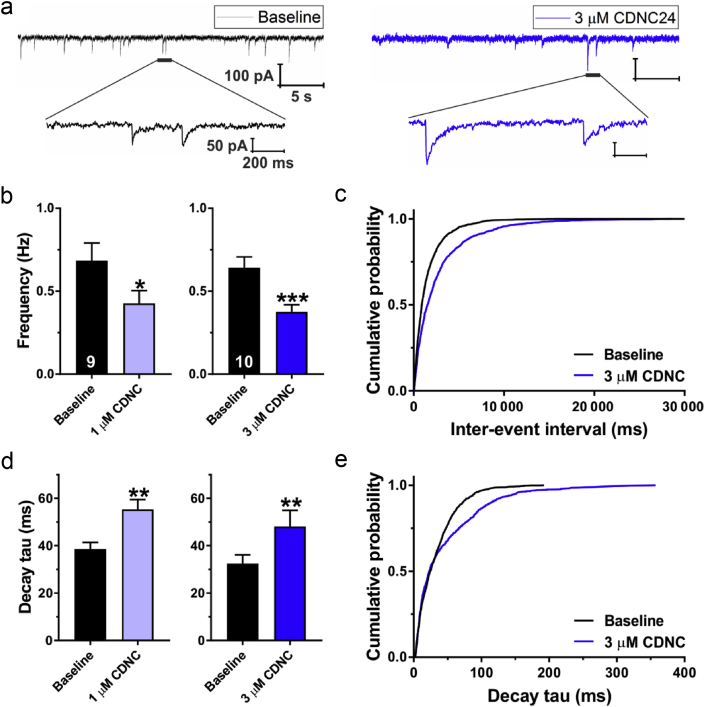
Representative traces of sIPSCs before and after perfusion with CDNC24 3 μM are shown in Fig 5a. Both CDNC24 1 μM (Fig 5b, left graph) and 3 μM (Fig 5b, right graph) decreased the sIPSC frequency of subicular neurones by about 0.37- and 0.40-fold, respectively. This was also evidenced in the probability plots shown in Fig 5c. In addition, both CDNC24 1 μM (Fig 5d, left graph) and 3 μM (Fig 5d, right graph) increased the sIPSC decay time by about 1.43- and 1.48-fold, respectively. The cumulative probability plots confirmed that CDNC24 prolongs decay times (Fig 5e).
Figure 5.
Effects of CDNC24 on spontaneous inhibitory postsynaptic currents (sIPSCs) in the subiculum of postnatal Days 7–9 rat pups. (a) Original traces from a representative subicular neurone in baseline conditions (black) and after the addition of CDNC24 3 μM (blue trace). Extended traces show changes in sIPSC event amplitude and kinetics after CDNC24 application. (b) The frequency of sIPSC events was significantly decreased after the application of CDNC24 1 μM (paired t-test: t8=2.76; ∗P=0.025; n=9 neurones, six rats) or 3 μM (t9=6.04; ∗∗∗P<0.001; n=10 neurones, seven rats). The number of neurones is shown at the bottom of each bar graph. (c) Cumulative probability plots revealed longer sIPSC inter-event intervals in neurones treated with CDNC24 3 μM (1224 events) compared with baseline (1811 events). (d) The decay time of sIPSCs was significantly prolonged after the application of CDNC24 1 μM (t8=4.60; ∗∗P=0.002) or 3 μM (t9=4.29; ∗∗P=0.002). (e) Cumulative probability plots show prolonged sIPSC decay times after application of CDNC24 3 μM (896 events) compared with baseline (1398 events). Results are expressed as mean (standard error of the mean).
Different effects of propofol from alphaxalone or CDNC24 on phasic and tonic GABA currents
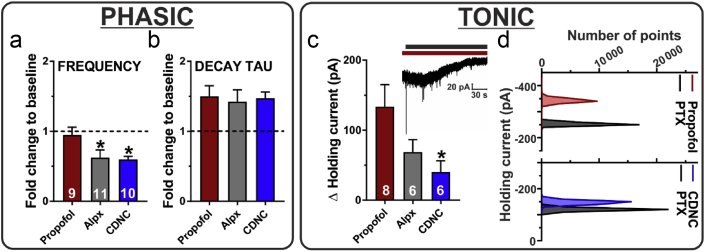
We investigated the differences in anaesthetic actions on pre-vs postsynaptic mechanisms by comparing the effects of alphaxalone and CDNC24 (3 μM) on sIPSC frequency and kinetics to those of propofol 10 μM. This concentration was selected to ensure a robust and relatively quick penetration of propofol into the brain slice. Both neurosteroids significantly decreased sIPSC frequency compared with propofol (Fig 6a). However, all three compounds had almost identical effects on sIPSC decay time (Fig 6b). We conclude that both neurosteroids potentiate the phasic GABAA currents via postsynaptic mechanisms similar to propofol, but differ from propofol in their significant presynaptic effects, as documented by the decreased frequency of sIPSCs.
Figure 6.
Comparison of effects of propofol, alphaxalone, and CDNC24 on phasic and tonic GABAergic inhibition in the subiculum of postnatal Days 7–9 rat pups. (a) The decrease in spontaneous inhibitory postsynaptic current (sIPSC) frequency observed after alphaxalone (Alpx) 3 μM or CDNC24 3 μM was significantly greater compared with propofol 10 μM (one-way analysis of variance followed by Dunnett's post hoc: F2,27=4.05; P=0.029; ∗P=0.042 [Alpx] and ∗P=0.030 [CDNC], both vs propofol). (b) All three compounds similarly potentiated sIPSC decay time. (c) The change in holding current after the application of picrotoxin (PTX) was significantly smaller in the CDNC24 group (F2,17=3.81; P=0.043; ∗P=0.031; n=6 neurones, four rats), but not in the Alpx group (P=0.145; n=6 neurones, three rats) compared with the propofol group (n=8 neurones, seven rats). The inset shows an original trace from a representative subicular neurone after propofol (dark red bar) and PTX (black bar) applications. Note the outward shift in the holding current after the application of PTX, which indicates inhibition of tonic γ-aminobutyric acid current. (d) All points count histograms of the same experiment shown in the inset of (c) (top) and a representative neurone in the CDNC24 group (bottom). Results are expressed as mean (standard error of the mean).
All three compounds induced tonic currents acting through extrasynaptic GABAA receptors (Fig 6c), as evidenced by the outward shift in the holding current after the application of picrotoxin (see inset in the figure). However, propofol was the most effective and had a greater than three-fold larger effect in comparison with CDNC24. This difference in the tonic current sensitivity was evident in all point count histograms of the representative subicular neurones (Fig 6d). These patch-clamp studies indicate that neurosteroids have distinct effects on both phasic and tonic GABAergic transmission compared with propofol.
Discussion
Most general anaesthetics targeting postsynaptic GABAA receptors are neurotoxic for the developing rodent11, 14, 15, 16, 17 and primate brains.8, 9, 18 We show here that two neurosteroids with prominent GABAergic properties, alphaxalone and CDNC24, are potent hypnotic agents with safety profiles more favourable than that of the clinically used anaesthetic propofol. Their promising safety profile is apparent not only in terms of improved pharmacological profile (i.e. higher TI and potency), but also in their lack of developmental neurotoxicity. We hypothesise that, although alphaxalone and CDNC24, like propofol, directly modulate GABAA receptors, both neurosteroids, unlike propofol, suppress GABA release, most likely via presynaptic mechanisms, and therefore, curtailing excessive activation of post- and extrasynaptic GABAA receptors.
Potentiation of GABAergic neurotransmission was suggested as the most likely mechanism responsible for neurodevelopmental anaesthetic toxicity.19 Propofol, an anaesthetic often used in the paediatric population,20 enhances phasic currents mediated by GABAA receptors.21 However, little is known about its effects on the GABAergic system during early brain development. Here, we show that propofol increases the sIPSC decay time without changing the event frequency, which would strongly suggest that propofol acts mainly postsynaptically. As sIPSCs represent the response of postsynaptic GABAA receptors not only to quantal (action potential independent) release of GABA, but also to release caused by spontaneous action potentials,22 they provide a realistic approximation of physiological conditions. Propofol was the most effective agent in activating tonic GABAA currents in the immature subiculum. Upregulation of tonic GABAA conductance has been linked to epilepsy and neurodevelopmental disorders,23 and may also contribute to memory impairment after GA.24
Modulation of ion channel function and neuronal excitability by neurosteroids is an area of increasing interest. Much of this interest is focused on modulation of postsynaptic GABAA receptor function by anaesthetic neurosteroids, including alphaxalone.25, 26 However, little is known about their presynaptic effects in the CNS. We have reported that 3β-OH, at a hypnotic brain concentration (3 μM), presynaptically inhibits AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)-mediated evoked synaptic currents in the subiculum of rat pups without affecting GABA-mediated and N-methyl-D-aspartate-mediated responses.7 We also found that spontaneous GABA release was decreased with 3β-OH 10 μM. Here, we show that alphaxalone- and CDNC24-induced postsynaptic potentiation of GABAA receptors is accompanied by significant suppression of spontaneous GABA release, similar to allopregnanolone, an endogenous analogue of alphaxalone.7, 27 Although it remains to be confirmed, it is plausible that the favourable safety profile of neurosteroids could be attributed to this suppression of GABA release. This may in turn dampen the activation of post- and extrasynaptic GABAA receptors, and minimise excessive depolarisation, given that GABA may have excitatory properties in the immature mammalian brain.28
Although the precise mechanism of CDNC24-induced decrease in GABA release remains to be examined, the inhibitory effects of alphaxalone on voltage-gated Ca2+ channels (VGCCs)29, 30 provide a plausible explanation for its presynaptic actions. Presynaptic VGCCs control both activity-dependent (evoked) and random (spontaneous) presynaptic GABA release,31 suggesting that these channels control two distinct synaptic vesicle pools.32, 33 It is reasonable to propose that alphaxalone, by the virtue of blocking presynaptic VGCCs, suppresses both evoked and spontaneous presynaptic GABA release, thus taming the activation of postsynaptic GABAA receptors.
CDNC24 does not inhibit T-currents in acutely dissociated rat sensory neurones.34 However, it is possible that the strong presynaptic effects of CDNC24 are at least partly mediated by effects on other members of the VGCC family, or alternatively that other effects on the presynaptic release machinery may be involved. Some non-steroidal anaesthetics, including propofol, may increase the frequency of IPSCs in rat hippocampal slices.35 Alternatively, some neurosteroids may induce signalling via nuclear hormone receptors (e.g. progesterone receptors) that regulate RNA expression.36 It is possible that this traditional neuroprotective mechanism may work in concert with a new one that we propose here, such as presynaptic suppression of GABA release. This remains an important area of future investigation.
We focused on the subiculum and mPFC in this study for two main reasons: first, both regions are exquisitely sensitive to anaesthesia-induced developmental neurotoxicity,1, 37, 38, 39 and second, both regions are important in cognitive development. It is believed that the mPFC controls higher-order decision-making, establishment of short-term memory, and retrieval and consolidation of long-term memory.40 Although it is promising that alphaxalone and CDNC24 do not induce developmental neuroapoptosis in mPFC and subiculum, the vulnerability of other brain regions remains to be examined.
After almost 20 yr of intense preclinical research, there is very little doubt that early exposure to clinically used general anaesthetics can cause substantial developmental neurotoxicity in young mammalian brain. We propose a need to design improved general anaesthetics that would be safe and effective for use in paediatric anaesthesia especially when prolonged or repeated exposures are necessary. Synthetic neuroactive steroids that target presynaptic mechanisms of GABA release may prove to be promising novel anaesthetic agents.
Authors' contributions
Study design: VJ-T, DFC, NQ, SMT
Experimentation: VT, SMJ, KK
Data analysis: VT, SMJ, KK
Overall project supervision: VJ-T, DFC, NQ, SMT
Final manuscript preparation: VJ-T, DFC, NQ, SMT
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Department of Anesthesiology, University of Colorado Anschutz Medical Campus; National Institutes of Health (R01 GM123746) to SMT and VJ-T; (R01 GM118197, R01 HD144517, R21 HD080281, and GM118197-S) to VJ-T; March of Dimes National Award to SMT; CU Medicine Endowment to VJ-T; Taylor Family Institute for Innovative Psychiatric Research to DFC.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.01.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jevtovic-Todorovic V., Hartman R.E., Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V. Exposure of developing brain to general anesthesia. Anesthesiology. 2018;128:832–839. doi: 10.1097/ALN.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin E.P., Lee J.-R., Lee C.S., Deng M., Loepke A.W. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol Teratol. 2017;60:117–128. doi: 10.1016/j.ntt.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Stratmann G., Lee J., Sall J.W. Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology. 2014;39:2275–2287. doi: 10.1038/npp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilder R.T., Flick R.P., Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joksimovic S.M., Izumi Y., Joksimovic S.L. Novel neurosteroid hypnotic blocks T-type calcium channel-dependent rebound burst firing and suppresses long-term potentiation in the rat subiculum. Br J Anaesth. 2019;122:643–651. doi: 10.1016/j.bja.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atluri N., Joksimovic S.M., Oklopcic A. A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br J Anaesth. 2018;120:768–778. doi: 10.1016/j.bja.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brambrink A.M., Back S.A., Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creeley C., Dikranian K., Dissen G., Martin L., Olney J., Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110:i29–i38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yon J.-H., Daniel-Johnson J., Carter L.B., Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 11.Milanovic D., Popic J., Pesic V. Regional and temporal profiles of calpain and caspase-3 activities in postnatal rat brain following repeated propofol administration. Dev Neurosci. 2010;32:288–301. doi: 10.1159/000316970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks N.P., Lieb W.R. Which molecular targets are most relevant to general anaesthesia? Toxicol Lett. 1998;100–101:1–8. doi: 10.1016/s0378-4274(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 13.Gredell J.A., Turnquist P.A., Maciver M.B., Pearce R.A. Determination of diffusion and partition coefficients of propofol in rat brain tissue: implications for studies of drug action in vitro. Br J Anaesth. 2004;93:810–827. doi: 10.1093/bja/aeh272. [DOI] [PubMed] [Google Scholar]
- 14.Cattano D., Young C., Straiko M.M.W., Olney J.W. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 15.Satomoto M., Satoh Y., Terui K. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 16.Stratmann G., Sall J.W., May L.D. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 17.Pearn M.L., Hu Y., Niesman I.R. Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology. 2012;116:352–361. doi: 10.1097/ALN.0b013e318242a48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F., Rainosek S.W., Frisch-Daiello J.L. Potential adverse effects of prolonged sevoflurane exposure on developing monkey brain: from abnormal lipid metabolism to neuronal damage. Toxicol Sci. 2015;147:562–572. doi: 10.1093/toxsci/kfv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jevtovic-Todorovic V., Absalom A.R., Blomgren K. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chidambaran V., Costandi A., D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29:543–563. doi: 10.1007/s40263-015-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanna E., Garau F., Harris R.A. Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol Pharmacol. 1995;47:213–217. [PubMed] [Google Scholar]
- 22.Edwards F.A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa K., Fukuda A. Pathophysiological power of improper tonic GABAA conductances in mature and immature models. Front Neural Circuits. 2013;7:170. doi: 10.3389/fncir.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurek A.A., Yu J., Wang D.S. Sustained increase in α5GABAa receptor function impairs memory after anesthesia. J Clin Invest. 2014;124:5437–5441. doi: 10.1172/JCI76669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottrell G.A., Lambert J.J., Peters J.A. Modulation of GABAA receptor activity by alphaxalone. Br J Pharmacol. 1987;90:491. doi: 10.1111/j.1476-5381.1987.tb11198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M., He Y., Eisenman L.N. 3 Beta-hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haage D., Druzin M., Johansson S. Allopregnanolone modulates spontaneous GABA release via presynaptic Cl- permeability in rat preoptic nerve terminals. Brain Res. 2002;958:405–413. doi: 10.1016/s0006-8993(02)03704-6. [DOI] [PubMed] [Google Scholar]
- 28.Cherubini E., Gaiarsa J.L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 29.Todorovic S.M., Prakriya M., Nakashima Y.M. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks gamma-aminobutyric acid-modulatory activity. Mol Pharmacol. 1998;54:918–927. doi: 10.1124/mol.54.5.918. [DOI] [PubMed] [Google Scholar]
- 30.Hirota K., Kudo M., Kudo T., Matsuki A., Lambert D.G. Inhibitory effects of intravenous anaesthetic agents on K+-evoked norepinephrine and dopamine release from rat striatal slices: possible involvement of P/Q-type voltage-sensitive Ca2+ channels. Br J Anaesth. 2000;85:874–880. doi: 10.1093/bja/85.6.874. [DOI] [PubMed] [Google Scholar]
- 31.Tsintsadze T., Williams C.L., Weingarten D.J., von Gersdorff H., Smith S.M. Distinct actions of voltage-activated Ca2+ channel block on spontaneous release at excitatory and inhibitory central synapses. J Neurosci. 2017;37:4301–4310. doi: 10.1523/JNEUROSCI.3488-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autry A.E., Adachi M., Nosyreva E. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavalali E.T., Chung C., Khvotchev M. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology. 2011;26:45–53. doi: 10.1152/physiol.00040.2010. [DOI] [PubMed] [Google Scholar]
- 34.Pathirathna S., Todorovic S.M., Covey D.F., Jevtovic-Todorovic V. 5α-Reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain. 2005;117:326–339. doi: 10.1016/j.pain.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Pittson S., Himmel A.M., MacIver B. Multiple synaptic and membrane sites of anesthetic action in the CA1 region of rat hippocampal slices. BMC Neurosci. 2004;5:52. doi: 10.1186/1471-2202-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher M., Mattern C., Ghoumari A. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. 2014;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Zou X., Liu F., Zhang X. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011;33:592–597. doi: 10.1016/j.ntt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Zou X., Sadovova N., Patterson T.A. The effects of L-carnitine on the combination of, inhalation anesthetic-induced developmental, neuronal apoptosis in the rat frontal cortex. Neuroscience. 2008;151:1053–1065. doi: 10.1016/j.neuroscience.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Rizzi S., Carter L.B., Ori C., Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal Guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Euston D.R., Gruber A.J., McNaughton B.L. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.