Abstract
Background:
Approximately 10% of patients with non-small cell lung cancer (NSCLC) are complicated with comorbid interstitial pneumonia (IP) with a poor prognosis. The pharmacotherapy for advanced lung cancer occasionally induces fatal acute exacerbation of pre-existing IP. Due to the lack of prospective studies, there is an urgent need to establish a safe and effective pharmacotherapy, especially for second-line or later settings. Atezolizumab, an anti-programmed cell death-ligand 1 antibody, is thought to be the safest candidate for second-line therapy among various immune checkpoint inhibitors. Moreover, compared with patients without IP, the patients with comorbid IP may have higher tumor mutation burden (TMB) or microsatellite instability (MSI), which are partly associated with a more favorable response to immune checkpoint inhibitors.
Methods:
The Thoracic Oncology Research Group 1936/AMBITIOUS study is an ongoing, multicenter, single-arm, phase II trial to assess the safety and efficacy of atezolizumab for pretreated advanced/recurrent patients with NSCLC complicated with idiopathic, chronic fibrotic IP with a forced vital capacity of >70%. The patients will receive atezolizumab (1200 mg, day 1) every 3 weeks until the discontinuation criteria are met. The primary end point of this study is the 1-year survival rate, and a sample size of 38 patients is set. As a translational research, we will perform the analysis of TMB, somatic mutations, and MSI for nucleic acids extracted from archival tumor samples.
Discussion:
Since there is no standard second-line or later therapy of advanced NSCLC with IP, the results of this study are expected to have a major impact on clinical practice.
Trial registration:
Japan Registry of Clinical Trials, jRCTs031190084, registered 26 August 2019 - retrospectively registered, https://jrct.niph.go.jp/en-latest-detail/jRCTs031190084
Keywords: acute exacerbation, atezolizumab, interstitial pneumonia, non-small cell lung cancer, pneumonitis
Background
Approximately 10% of patients with non-small cell lung cancer (NSCLC) are complicated with comorbid interstitial pneumonia (IP) with a poor prognosis.1 The pharmacotherapy for advanced lung cancer occasionally induces acute exacerbation of pre-existing IP (5–20%), with a high mortality rate of 30–50%.2 Several drugs are contraindicated in patients with IP, resulting in more limited treatment options than those of the patients without IP.
There have been few prospective studies which target pretreated NSCLC patients that are complicated with IP. In addition, the retrospective study showed that docetaxel, the most commonly used regimen as a second-line therapy, had a high risk of developing acute exacerbation of pre-existing IP, with an incidence of 14.3%.3 Based on these results, there are currently no standard second-line or later therapies of advanced NSCLC with IP.4 Nivolumab, a fully human immunoglobulin (Ig)G4 monoclonal antibody that targets the programmed cell death 1 (PD-1) receptor found on activated T cells, did not induce acute exacerbation of IP in the pilot trial which targeted six pretreated patients with NSCLC complicated with mild idiopathic IP.5 Thus, immune checkpoint inhibitors may be feasible in patients with NSCLC with idiopathic IP. In addition, a single-arm phase II trial of nivolumab showed promising efficacy, with a 6-month progression-free survival (PFS) rate of 56% and an overall response rate (ORR) of 36% in 18 pretreated patients with NSCLC complicated with mild idiopathic IP from four centers.6 One possible explanation is that IP is associated with smoking and microsatellite instability (MSI), which are factors partly associated with higher tumor mutation burden (TMB).7 Therefore, compared with patients without IP, patients with NSCLC complicated with comorbid IP may have higher TMB or MSI. A higher tumor mutation burden is associated with a more favorable response to immune checkpoint inhibitors.8 Therefore, we speculated that, compared with patients without IP, there are higher expectations for the efficacy of immune checkpoint inhibitors in patients with NSCLC complicated with comorbid IP.
Atezolizumab, a fully humanized monoclonal antibody of the engineered IgG1 isotype, targets programmed cell death-ligand 1 (PD-L1). In the OAK trial, a randomized phase III study for pretreated patients with NSCLC, atezolizumab, compared with docetaxel, demonstrated an overall survival (OS) benefit across PD-L1 and histological subgroups.9 In addition, the incidence of pneumonitis was 1%, which was lower than the past reports of anti-PD-1 antibody and other cytotoxic agents. Moreover, meta-analysis showed that there was a lower incidence of pneumonitis with the use of PD-L1 inhibitors than with the use of PD-1 inhibitors.10 It is speculated that anti-PD-1 antibody blocks PD-1 on activated T cells; therefore, PD-L2 are prone to bind to additional binding partners, such as repulsive guidance molecule b (RGMb).11 RGMb is highly expressed in alveolar epithelial cells, and the increased PD-L2 availability for binding to RGMb may lead to pneumonitis. Meanwhile, anti-PD-L1 antibodies have minimal influence on the interaction between PD-L2 and PD-1; thus, the risk of pneumonitis may be lower. Therefore, atezolizumab is thought to be the safest candidate for second-line therapy among various immune checkpoint inhibitors.
In terms of acute exacerbation of IP induced by cytotoxic chemotherapy, the most common risk factor is the radiologic appearance of honeycomb lung, suggestive of idiopathic pulmonary fibrosis.2 Meanwhile, risk factors of immune checkpoint inhibitor-induced acute exacerbation of IP remain unclear. In the previously reported studies of nivolumab, the eligibility criteria of IP were limited to “mild” cases, with ⩾80% vital capacity (VC) and no presence of honeycomb lung on high-resolution computed tomography (HRCT).5,6 However, the definition of honeycombing remains quite controversial; there is often disagreement about the identification of honeycomb lung, even among experienced chest radiologists.12 Therefore, more simple and generalizable criteria are required to select patients with lower risk of acute exacerbation. Our past retrospective study suggested that the baseline low forced vital capacity (FVC) was a stronger risk factor for chemotherapy-induced acute exacerbation of IP than for the presence of honeycomb lung.13 Moreover, according to the post-marketing surveillance of pirfenidone and the subgroup analysis of the randomized phase III trials of nintedanib (INPULSIS-1 and -2) in patients with idiopathic pulmonary fibrosis, there is a particularly high risk of acute exacerbation in patients with a baseline FVC (or VC) of <70%.14,15 Based on these results, certain safety measures should be implemented for patients with an FVC of >70%.
With this background, we have now designed a single arm phase II trial to assess the safety and efficacy of atezolizumab for pretreated advanced/recurrent patients with NSCLC complicated with idiopathic IP.
Methods/design
Study design and treatment
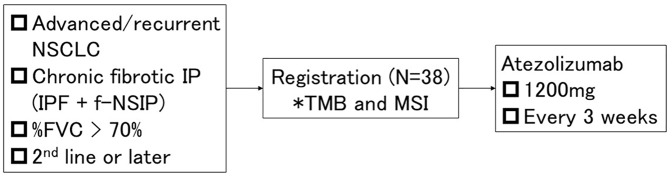
This multicenter, single-arm, phase II trial is conducted by the Thoracic Oncology Research Group (TORG) in accordance with the Declaration of Helsinki and Japan’s Clinical Trials Act (Figure 1). The Niigata University Certified Review Board of Clinical Research approved this protocol on 23 July 2019 (approval number: SP19005). This clinical trial was registered in the Japan Registry of Clinical Trials on 26 August 2019 (registry number: jRCTs031190084).
Figure 1.
Study design.
*As a translational research of this study, we will extract deoxy-nucleic acids from tumor samples and analyze tumor mutation burden, somatic variations on 409 cancer-related genes, and microsatellite instability.
FVC, forced vital capacity; IP, interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; MSI, microsatellite instability; NSCLC, non-small cell lung cancer; NSIP, nonspecific interstitial pneumonia; TMB, tumor mutation burden.
The patients will receive atezolizumab (1200 mg, day 1) every 3 weeks until the discontinuation criteria are met. The key discontinuation criteria include (a) disease progression, (b) occurrence of acute exacerbation of IP, and (c) occurrence of unacceptable immune-related adverse events, including pneumonitis, hepatotoxicity, hepatitis, nervous system disorder, renal disorder, eye disorder, and myocarditis with Common Terminology Criteria for Adverse Events (CTCAE) grade ⩾3; colitis, diarrhea, pancreatitis, pan-hypopituitarism, and skin disorder with CTCAE grade ⩾2; and encephalitis, meningitis, Guillain–Barre syndrome, and myasthenia gravis with CTCAE grade ⩾1.
Eligibility criteria
The key inclusion and exclusion criteria for participation in this study are shown in Table 1.
Table 1.
Key eligibility criteria.
| Inclusion criteria | |
|---|---|
| 1 | Histologically or cytologically proven non-small cell lung cancer |
| 2 | Unresectable stage III/IV or recurrent |
| 3 | Received prior chemotherapy, including platinum doublet |
| 4 | Chronic fibrotic IP (all four items are required) (a) HRCT revealed (i) reticular shadow with basal, peripheral predominance suggestive of UIP pattern or (ii) peribronchovascular shadow suggestive of NSIP pattern(b) Without known etiology (e.g. infection, pneumoconiosis, drug, sarcoidosis, and collagen vascular disease)(c) %FVC > 70%(d) %DLCO > 35% |
| 6 | Age ⩾ 20 years |
| 7 | ECOG performance status 0–1 |
| 8 | With measurable or unmeasurable lesions according to RECIST Version 1.1 |
| 9 | Vital organ functions are preserved within 14 days prior to enrollment |
| 10 | Received sufficient explanations about the name and severity of the illness |
| 11 | Written informed consent |
| Exclusion criteria | |
| 1 | History of acute exacerbation of pre-existing IP |
| 2 | Treatment history with immune checkpoint inhibitor |
| 3 | Systemic treatment with steroids at a daily dose of >10 mg of prednisolone or equivalent immunosuppressant |
| 4 | Active autoimmune disease or history of autoimmune disease requiring treatment |
| 5 | Symptomatic brain metastasis or spinal cord metastases |
| 6 | Active viral hepatitis |
| 7 | Local or systemic active infection requiring treatment |
| 8 | Synchronous or metachronous active double malignancies |
| 9 | Pregnant or breastfeeding |
| 10 | Disapprove of contraception during the protocol treatment period |
| 11 | Treatment history with thoracic radiotherapy |
| 12 | History of serious drug allergies |
| 13 | Other conditions not suitable for the study |
DLCO, diffusing capacity of the lungs for carbon monoxide; ECOG, Eastern Cooperative Oncology Group; FVC, forced vital capacity; HRCT, high-resolution computed tomography; IP, interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; RECIST, Response Evaluation Criteria in Solid Tumors; UIP, usual interstitial pneumonia.
Patient registration
After the eligibility criteria have been confirmed and informed consent has been obtained, the eligible patients will be registered, and planned treatment will be initiated by the investigators. Accrual began in September 2019 and will continue for 3 years.
Evaluation of response
Before treatment initiation, computed tomography (CT) scans of the chest and abdomen, magnetic resonance imaging scans or CT scans of the brain, and positron emission tomography scans or bone scans must be obtained. The patients will undergo tumor assessments at baseline, every 6 weeks during the first 24 weeks and every 9 weeks thereafter. The tumor response will be evaluated in accordance with the Response Evaluation Criteria in Solid Tumors, version 1.1. Adverse events will be recorded using the National Cancer Institute’s CTCAE, version 5.0.
Evaluation of pneumonitis and acute exacerbation of IP
A chest HRCT scan is mandatory within 28 days prior to enrollment. Laboratory testing, including Krebs von den Lungen-6 and blood gas analysis, is also mandatory within 14 days prior to enrollment. Respiratory symptoms will be assessed at baseline, on the day of atezolizumab administration, and at every visit after completion or discontinuation of atezolizumab. When development of pneumonitis or acute exacerbation of IP is suspected by the investigator, patients will be recommended to complete a chest CT, laboratory testing (e.g. brain natriuretic protein, Krebs von den Lungen-6, β-D glucan, cytomegalovirus antigen), blood gas analysis, and echocardiogram.
A central review committee will adjudicate all “investigator-reported” pneumonitis events to determine whether acute exacerbation of pre-existing IP has developed based on the diagnostic criteria defined in the protocol: (a) acute worsening or development of dyspnea within 1 month, (b) new bilateral ground glass opacity and/or consolidation on HRCT, (c) no obvious clinical cause, such as cardiac failure or fluid overload, and (d) no obvious lymphangitic carcinomatosis.
Statistical design
The primary end point of this study is the 1-year survival rate. The key secondary end points are the incidence of acute exacerbation of IP within 1 year after treatment initiation, OS, PFS, ORR, time to treatment failure, mortality rate due to acute exacerbation of IP or pneumonitis during the observation period, and safety.
The retrospective study administering docetaxel as a second-line therapy in patients with NSCLC complicated with idiopathic IP showed a 1-year survival rate of 10%,3 which was considerably lower than the docetaxel group in the OAK and EAST-LC trials. Since there may be a higher risk of pneumonitis and/or acute exacerbation in patients with IP, a larger treatment effect is expected. Therefore, we considered the lower limit of interest to be 15%. According to the exact binomial test, this study requires 36 patients, assuming a ⩾25% clinically meaningful increase in historical data of 1-year survival rates (two-sided α = 0.05; 1-β = 0.9). Considering patient ineligibility, a sample size of 38 patients was set.
Translational research
To address the translational research aspect of this study, we will extract deoxy-nucleic acids from archival tumor samples and analyze TMB and somatic variations on 409 cancer-related genes by using the Oncomine Tumor Mutation Load Assay (Thermo Fisher Scientific, US) and also analyze MSI on a panel of the so-called Bethesda markers (BAT25, BAT26, NR21, NR24, MONO27). This will aid in exploring markers that predict the efficacy of atezolizumab. We will not perform analyses of hereditary diseases arising from germline mutations.
Discussion
Due to the lack of prospective studies on advanced NSCLC with comorbid IP, there is an urgent need to establish a safe and effective pharmacotherapy, especially for second-line or later settings. Atezolizumab is thought to be the safest and most promising candidate for second-line therapy among various immune checkpoint inhibitors. The results of this study are expected to have a major impact on clinical practice.
Acknowledgments
The authors would like to thank the patients, their families, TORG data center staff, and all the investigators who are participating in the present study.
Footnotes
Authors’ contributions: SI, TK, HK, TO, SI, YM, TY, and HO were involved in study conception and design. SI, TK, HK, TO, SI, TI, RK, YM, TM, TY, and HO will be involved in the analysis and interpretation of the data; SI, TK, HK, YM, and TM were involved in drafting the manuscript; and SI, TK, HK, TO, SI, TI, RK, YM, TM, TY, and HO were involved in revising the manuscript. All authors have read and approved the final manuscript.
Availability of data and material: All data generated or analyzed during this study are included in this published article
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan).
Conflict of interest statement: S Ikeda, T Kato, H Kenmotsu, S Iwasawa, and T Yamanaka received honoraria from Chugai Pharmaceutical. T Kato, H Kenmotsu, S Iwasawa, T Yamanaka, and H Okamoto received research funding from Chugai Pharmaceutical. T Ogura, T Iwasawa, R Kasajima, Y Miyagi, and T Misumi declare no potential conflicts of interest with any companies or organizations whose products or services might be discussed in this article.
Consent for publication: Consent for publication must be obtained from all patients.
Ethics approval and consent to participate: The Niigata University Certified Review Board of Clinical Research approved this protocol on July 23, 2019 (approval number: SP19005). This clinical trial was registered in the Japan Registry of Clinical Trials on August 26, 2019 (registry number: jRCTs031190084). Written informed consent must be obtained from all patients.
ORCID iD: Satoshi Ikeda  https://orcid.org/0000-0001-5203-7911
https://orcid.org/0000-0001-5203-7911
Contributor Information
Satoshi Ikeda, Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, 6-16-1 Tomioka-Higashi, Kanazawa-ku, Yokohama-city, Kanagawa Prefecture, 236-0051, Japan.
Terufumi Kato, Department of Thoracic Oncology, Kanagawa Cancer Center, Yokohama-city, Kanagawa Prefecture, Japan.
Hirotsugu Kenmotsu, Division of Thoracic Oncology, Shizuoka Cancer Center, Nagaizumi-cho, Sunto-gun, Shizuoka Prefecture, Japan.
Takashi Ogura, Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, Yokohama-city, Kanagawa Prefecture, Japan.
Shunichiro Iwasawa, Department of Respirology, Chiba University Graduate School of Medicine, Chiba-city, Chiba Prefecture, Japan.
Tae Iwasawa, Department of Radiology, Kanagawa Cardiovascular and Respiratory Center, Yokohama-city, Kanagawa Prefecture, Japan.
Rika Kasajima, Molecular Pathology and Genetics Division, Kanagawa Cancer Center Research Institute, Yokohama-city, Kanagawa Prefecture, Japan.
Yohei Miyagi, Molecular Pathology and Genetics Division, Kanagawa Cancer Center Research Institute, Yokohama-city, Kanagawa Prefecture, Japan.
Toshihiro Misumi, Department of Biostatistics, Yokohama City University School of Medicine, Yokohama-city, Kanagawa Prefecture, Japan.
Takeharu Yamanaka, Department of Biostatistics, Yokohama City University School of Medicine, Yokohama-city, Kanagawa Prefecture, Japan.
Hiroaki Okamoto, Department of Respiratory Medicine and Medical Oncology, Yokohama Municipal Citizen's Hospital, Yokohama-city, Kanagawa Prefecture, Japan.
References
- 1. Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004; 91: S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011; 6: 1242–1246. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe N, Niho S, Kirita K, et al. Second-line docetaxel for patients with platinum-refractory advanced non-small cell lung cancer and interstitial pneumonia. Cancer Chemother Pharmacol 2015; 76: 69–74. [DOI] [PubMed] [Google Scholar]
- 4. Ogura T, Takigawa N, Tomii K, et al. ; DLD/TO Assemblies of JRS. Summary of the Japanese Respiratory Society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir Investig 2019; 57: 512–533. [DOI] [PubMed] [Google Scholar]
- 5. Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017; 111: 1–5. [DOI] [PubMed] [Google Scholar]
- 6. Fujimoto D, Yomota M, Sekine A, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer 2019; 134: 274–278. [DOI] [PubMed] [Google Scholar]
- 7. Demopoulos K, Arvanitis DA, Vassilakis DA, et al. MYCL1, FHIT, SPARC, p16(INK4) and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J Cell Mol Med 2002; 6: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest 2017; 152: 271–281. [DOI] [PubMed] [Google Scholar]
- 11. Xiao Y, Yu S, Zhu B, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med 2014; 211: 943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watadani T, Sakai F, Johkoh T, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology 2013; 266: 936–944. [DOI] [PubMed] [Google Scholar]
- 13. Enomoto Y, Inui N, Kato T, et al. Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer 2016; 96: 63–67. [DOI] [PubMed] [Google Scholar]
- 14. Ogura T, Azuma A, Inoue Y, et al. All-case post-marketing surveillance of 1371 patients treated with pirfenidone for idiopathic pulmonary fibrosis. Respir Investig 2015; 53: 232–241. [DOI] [PubMed] [Google Scholar]
- 15. Costabel U, Inoue Y, Richeldi L, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med 2016; 193: 178–185. [DOI] [PubMed] [Google Scholar]