Abstract
Background:
Cutaneous adverse events (AEs) have been positively associated with immune checkpoint inhibitor (ICI) efficacy in patients with melanoma, but little is known regarding the association between checkpoint inhibitor pneumonitis (CIP) and programmed cell death protein 1/programmed death ligand 1 (PD-1/PD-L1) inhibitor efficacy in non-small cell lung cancer (NSCLC).
Methods:
A single-institution, retrospective medical record review of patients with advanced or recurrent NSCLC who were treated with PD-1/PD-L1 inhibitors between 1 September 2015 and 1 June 2019 was conducted. A total of 276 NSCLC patients with or without immune-related pneumonitis who received at least one dose of ICIs and had at least one follow-up visit were identified. Kaplan–Meier curves of the progression-free survival (PFS) of patients stratified according to immune-related pneumonitis development were evaluated with the log-rank test as a preplanned primary objective. Multivariate analysis of PFS was performed with Cox proportional hazard regression models.
Results:
In the cohort of 276 patients, 42 patients developed CIP attributed to PD-1/PD-L1 inhibitors. Survival analysis showed that the overall response rate was significantly higher in patients with CIP than in those without CIP (61.90% versus 29.91%, respectively, p < 0.01), and that CIP development was significantly associated with increased PFS (45.80 weeks versus 21.15 weeks, respectively, p < 0.01). Additionally, 16-week landmark analysis produced the same results. Similarly, subgroup analysis of PD-1 inhibitor-treated, nivolumab-treated, and pembrolizumab-treated groups also revealed that CIP increased survival in NSCLC patients. Additionally, grade 1–2 pneumonitis showed an association with increased ICI efficacy in NSCLC; however, grade 3–4 pneumonitis did not. In addition, only two of the four pneumonitis radiological subtypes showed associations with increased ICI efficacy in NSCLC.
Conclusion:
CIP is associated with enhanced PD-1/PD-L1 inhibitor efficacy in NSCLC patients. Grade 1–2 pneumonitis and the radiological features of hypersensitivity and cryptogenic organizing pneumonia (COP) may be signs of enhanced ICI efficacy. However, further studies with larger numbers of patients and longer follow-up times are needed to validate our findings.
Keywords: efficacy, immune-related pneumonitis, non-small cell lung cancer, PD-1 inhibitors, PD-L1 inhibitors
Introduction
The development of immune checkpoint inhibitors (ICIs) has improved treatment outcomes for various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), and renal cell cancer.1–8 On the other hand, patients treated with ICIs sometimes experience unique adverse events (AEs) called immune-related adverse events (irAEs).9,10 The development of cutaneous AEs has been found to be associated with a survival benefit in melanoma,11–14 indicating that the occurrence of such events might reflect antitumor responses. Similarly, recent studies have suggested a similar association in patients with advanced NSCLC.15–17 However, whether the occurrence of immune-related pneumonitis has predictive value in NSCLC patients treated with programmed cell death protein 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors remains to be confirmed. We performed a retrospective cohort study to investigate immune-related pneumonitis and its association with the clinical activity of PD-1/PD-L1 inhibitors in NSCLC.
Methods
Study design and patients
The Ethics Committee of the Chinese People’s Liberation Army General Hospital approved this retrospective cohort study on patients with advanced (stage IIIB–IV) or recurrent NSCLC who were treated with PD-1/PD-L1 inhibitors at the Cancer Center of the Chinese People’s Liberation Army General Hospital between 1 September 2015 and 1 June 2019. Patients who received at least one cycle of ICI treatment and completed at least one follow-up visit were included. The end of the follow-up period was 31 August 2019. We reviewed patient medical records from 1 September 2015 to 31 August 2019. From this review, we identified 276 patients who received PD-1/PD-L1 inhibitors.
Both monotherapy with PD-1/PD-L1 inhibitors and combined treatment with chemotherapy were included. The monotherapy included PD-1/PD-L1 inhibitors for NSCLC. The combination therapy included PD-1/PD-L1 inhibitors + pemetrexed + carboplatin for non-squamous NSCLC and PD-1/PD-L1 inhibitors + carboplatin + nab-paclitaxel for lung squamous carcinoma.
Medical records were reviewed, and data for clinicopathological features and treatment history were extracted. We collected the following data: patient demographics, therapeutic regimen, type of disease, stage of disease, time point of disease progression, pulmonary AEs attributed to ICIs, time point of immune-related pneumonitis, driver gene mutation status, and PD-L1 expression. Tumor response was assessed by computed tomography according to RECIST version 1.1 every 6–8 weeks. Progression-free survival (PFS) was measured from the time of treatment initiation to clinical or radiographic disease progression or death from any cause. Patients without documented clinical or radiographic progression were censored on the date of the last follow up. A checkpoint inhibitor pneumonitis (CIP) diagnosis was determined clinically by the treating oncologist in consultation with pulmonologists and a radiologist. Briefly, using a combination of clinical (history, exam, pulse oximetry, and pulmonary function testing), radiographic (presence or absence of tumor progression and the pattern of parenchymal infiltrates), and biological parameters (sputum cultures and/or bronchoscopy, respiratory viral cultures, and routine labs), we excluded alternative aetiologies such as heart failure, infection, and tumor progression. Pneumonitis was graded based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.10. Disease progression was defined according to immune-related response criteria.18 ICI therapy was discontinued in all cases when a patient was diagnosed with symptomatic CIP (CTCAE grade 2 or higher). All patients diagnosed with CIP were treated with high-dose corticosteroids as per current guidelines.19,20 Following initiation of steroid therapy, clinical improvement was defined as a decrease in oxygen requirement, an increase in exercise capacity, and a decrease in radiographic infiltrates. Conversely, worsening was defined as a lack of improvement in oxygen requirement, exercise capacity, and/or radiographic infiltrates after 72 h of corticosteroid therapy.
Each radiologist described the radiological patterns of pneumonitis according to the study by Naidoo et al.21 The radiological features of CIP were classified into four subtypes: cryptogenic organizing pneumonia (COP)-like, ground-glass opacities (GGO), interstitial, and hypersensitivity.
The study was approved by the institutional review board of People’s Liberation Army General Hospital, Beijing, China (approval number: S2018-092-01). This clinical study was conducted in accordance with the Declaration of Helsinki. Because of the retrospective nature of the study, informed consent was waived by the Ethics Committee of Chinese People’s Liberation Army General Hospital. This paper does not contain any individual person’s data in any form.
Statistical analysis
Statistical analyses were performed using Stata, version 15.1 (StataCorp). To compare groups, we used Fisher’s exact test (categorical variables) or the Wilcoxon rank-sum test (continuous variables). Time to disease progression was calculated from the first ICI cycle to progression. Differences in PFS curves were estimated with the Kaplan–Meier method, according to the absence or presence of CIP, and evaluated with the log-rank test as a preplanned primary objective. Other analyses were performed as secondary objectives. Fisher’s exact test was applied to compare overall response rates. Univariate and multivariate Cox proportional hazard regression models were adopted to determine hazard ratios. Multivariate analysis was performed with adjustments for age, sex, the number of treatment lines, PD-L1 expression, central nervous system (CNS) metastasis, histology, and treatment cycles. Taking into account the lead-time bias due to the time-dependent nature of irAEs, we also performed 16-week landmark analysis of PFS, including only patients manifesting disease control at 112 days after initiation of ICI therapy (that is, patients who manifested disease control with development of pulmonary AEs before day 113 were compared with those who manifested disease control without development of immune-related pneumonitis before day 113 in the 16-week landmark analysis of PFS, whereas patients whose follow-up time was less than 113 days were excluded). In addition, 12-week and 20-week landmark analyses were performed as complementary evaluations. All p values were based on a two-sided hypothesis, and those less than 0.05 were considered statistically significant.
Results
Patient characteristics
We included 276 patients with advanced or recurrent NSCLC who were treated with PD-1/PD-L1 inhibitors in our study (consisting of 175 non-squamous NSCLC patients and 101 squamous carcinoma patients). Of the 276 patients, 205 (74.28%) were males and 71 (25.72%) were females. Their ages ranged from 29 to 86 years, with a median age of 61 years. The baseline patient characteristics are shown in Table 1.
Table 1.
Patient characteristics.
| No. of patients (%) | ||||
|---|---|---|---|---|
| Characteristic | All patients (n = 276) |
Pneumonitis (n = 42) | Non pneumonitis (n = 234) | p value |
| Median age (range), years | 61 (29–86) | 61 (39–86) | 60.5 (29–79) | 0.720 |
| Sex | 0.181 | |||
| Male | 205 (74.28) | 35 (83.33) | 170 (72.65) | |
| Female | 71 (25.72) | 7 (2.38) | 64 (27.35) | |
| ECOG performance status | 0.075 | |||
| 0–1 | 228 (82.61) | 39 (92.86) | 189 (80.77) | |
| ⩾2 | 48 (17.39) | 3 (7.14) | 45 (19.23) | |
| Smoking history | 0.171 | |||
| Current or former | 170 (61.59) | 30 (71.43) | 140 (59.83) | |
| Never | 106 (38.41) | 12 (28.57) | 94 (40.17) | |
| Stage | 0.622 | |||
| Recurrence | 37 (13.41) | 4 (9.52) | 33 (14.10) | |
| IIIB–IV | 239 (86.59) | 38 (90.48) | 201 (85.90) | |
| Metastasis | ||||
| CNS versus no CNS | 63 (22.83) versus 213 (77.17) | 9 (21.43) versus 33 (78.57) | 54 (23.08) versus 180 (76.92) | 1.000 |
| Intrathoracic only versus
no intrathoracic |
132 (47.83) versus 144 (52.17) | 16 (38.10) versus 26 (61.90) | 116 (49.57) versus 118 (50.43) | 0.183 |
| Histology | 0.387 | |||
| Squamous | 101 (36.59) | 18 (42.86) | 83 (35.47) | |
| Non-squamous | 175 (63.41) | 24 (57.14) | 151 (64.53) | |
| EGFR mutation status | 0.406 | |||
| Positive | 39 (14.13) | 5 (11.90) | 34 (14.53) | |
| Negative | 145 (52.54) | 19 (45.24) | 126 (53.85) | |
| Not examined | 92 (33.33) | 18 (42.86) | 74 (31.62) | |
| ALK fusion status | 0.035 | |||
| Positive | 4 (1.45) | 0 (0) | 4 (1.71) | |
| Negative | 204 (73.91) | 25 (59.52) | 179 (76.50) | |
| Not examined | 68 (24.64) | 17 (40.48) | 51 (21.79) | |
| PD-L1 expression | 0.142 | |||
| <1 | 16 (5.80) | 0 (0) | 16 (6.84) | |
| 1–49 | 32 (11.59) | 3 (7.14) | 29 (12.39) | |
| ⩾50 | 40 (14.49) | 9 (21.43) | 31 (13.25) | |
| Not examined | 188 (68.12) | 30 (71.43) | 158 (67.52) | |
| Treatment lines | 0.293 | |||
| 1 | 97 (35.14) | 18 (42.86) | 79 (33.76) | |
| ⩾2 | 179 (64.86) | 24 (57.14) | 155 (66.24) | |
| Previous target therapy | 0.700 | |||
| No | 206 (74.64) | 33 (78.57) | 173 (73.93) | |
| Yes | 70 (25.36) | 9 (21.43) | 61 (26.07) | |
| Prior thoracic radiotherapy | 0.848 | |||
| No | 207 (75.00) | 31 (73.81) | 176 (75.21) | |
| Yes | 69 (25.00) | 11 (26.19) | 58 (24.79) | |
| Combined with chemotherapy | 0.089 | |||
| No | 160 (57.97) | 19 (45.24) | 141 (60.26) | |
| Yes | 116 (42.03) | 23 (54.76) | 93 (39.74) | |
| Agent | 0.337 | |||
| PD-1 inhibitors | 255 (92.39) | 37 (88.10) | 218 (93.16) | |
| PD-L1 inhibitors | 21 (7.61) | 5 (11.90) | 16 (6.84) | |
| Agent | 0.098 | |||
| Nivolumab | 109 (39.49) | 13 (30.95) | 96 (41.03) | |
| Pembrolizumab | 146 (52.90) | 24 (57.14) | 122 (52.14) | |
| Atezolizumab | 15 (5.43) | 2 (4.76) | 13 (5.56) | |
| Durvalumab | 6 (2.17) | 3 (7.14) | 3 (1.28) | |
| Cycles of treatment, median (range), No. | 5 (1–38) | 8 (1–38) | 5 (1–31) | 0.005 |
| Follow up time, (range), days | 256.5 (13–1288) | 350 (31–1288) | 232 (13–1286) | 0.005 |
| Overall response rate | 96 (34.78) | 26 (61.90) | 70 (29.91) | 0.000 |
ALK, anaplastic lymphoma kinase gene; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor gene; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; No., number.
CIP occurred in 42 of the 276 patients with NSCLC (15.22%), 37 of 255 patients treated with PD-1 inhibitors (14.51%), 5 of 21 patients treated with PD-L1 inhibitors (23.81%), 13 of 109 patients treated with nivolumab (11.93%), 24 of 146 patients treated with pembrolizumab (16.44%), 2 of 15 patients treated with atezolizumab (13.33%), and 3 of 6 patients treated with durvalumab (50%). According to the CTCAE, 30 of the 42 cases (71.43%) were grade 1 pneumonitis, 5 of the 42 cases (11.90%) were grade 2, 5 of the 42 cases (11.90%) were grade 3, and 2 of the 42 cases (4.76%) were grade 4. The radiological features of pneumonitis were classified into four subtypes: COP-like [7 of 42 (16.67%)], GGO [6 of 42 (14.29%)], interstitial [13 of 42 (30.95%)], and hypersensitivity [16 of 42 (38.10%)] (Figure S1 in the Supplement). Of the 42 patients with pneumonitis, 12 (28.57%) received systemic steroid therapy. The time to onset of CIP ranged from 20 to 687 days (median, 134) from the start of ICI treatment. The CIP profile is shown in Table 2.
Table 2.
Pneumonitis in patients treated with PD-1/PD-L1 inhibitors.
| No. (%) | |||||||
|---|---|---|---|---|---|---|---|
| Total (n = 276) | PD-1 inhibitors (n = 255) | PD-L1 inhibitors (n = 21) | Nivolumab (n = 109) | Pembrolizumab (n = 146) | Atezolizumab (n = 15) | Durvalumab (n = 6) | |
| Pneumonitis | 42 (15.22) | 37 (14.51) | 5 (23.81) | 13 (11.93) | 24 (16.44) | 2 (13.33) | 3 (50%) |
| Grade | |||||||
| 1 | 30 (71.43) | 26 (70.27) | 4 (80) | 9 (69.23) | 17 (70.83) | 1 (50) | 3 (100) |
| 2 | 5 (11.90) | 5 (13.51) | 0 (0) | 3 (23.08) | 2 (8.33) | 0 (0) | 0 (0) |
| 3 | 5 (11.90) | 4 (10.81) | 1 (20) | 0 (0) | 4 (16.67) | 1 (50) | 0 (0) |
| 4 | 2 (4.76) | 2 (5.41) | 0 (0) | 1 (7.69) | 1 (4.17) | 0 (0) | 0 (0) |
| Radiologic subtypes | |||||||
| COP | 7 (16.67) | 7 (18.92) | 0 (0) | 4 (30.77) | 3 (12.5) | 0 (0) | 0 (0) |
| Ground glass opacities | 6 (14.29) | 4 (10.81) | 2 (40) | 1 (7.69) | 3 (12.5) | 0 (0) | 2 (66.67) |
| Interstitial | 13 (30.95) | 12 (32.43) | 1 (20) | 1 (7.69) | 11 (45.83) | 0 (0) | 1 (33.33) |
| Hypersensitivity | 16 (38.10) | 14 (37.84) | 2 (40) | 7 (53.85) | 7 (29.17) | 2 (100) | 0 (0) |
| Systemic steroid therapy | 12 (28.57) | 11 (29.73) | 1(20) | 4 (30.77) | 7 (29.17) | 1 (50) | 0 (0) |
| Days to onset, median (range), days |
134 (20–687) | 134 (20–687) | 134 (20–687) | 148 (21–565) | 107.5 (20–687) | 85 (82–88) | 335 (46–336) |
COP, Cryptogenic organizing pneumonia-like; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.
For 16-week landmark analysis, 101 patients were excluded from the NSCLC group, 67 patients were excluded from the non-squamous NSCLC group, and 34 patients were excluded from the squamous carcinoma group because of disease progression before day 113 of ICI treatment or because their follow-up time was less than 113 days for the PFS analysis (Table S1 in the Supplement).
Association of CIP with PD-1/PD-L1 inhibitor efficacy
Baseline characteristics, except for treatment cycles and follow-up time, did not differ between patients who developed CIP and those who did not (Table 1 and Table S1 in the Supplement). Survival analysis showed that the overall response rate was significantly higher in the patients with CIP than in those without CIP [26 of 42 patients (61.90%) versus 70 of 234 patients (29.91%), respectively, p < 0.01] and that the development of CIP was significantly associated with increased PFS (p < 0.01) (Figure 1). Taking into account the lead-time bias due to the time-dependent nature of CIP, we performed a 16-week landmark analysis for PFS (n = 175). This analysis showed that the objective response rate was significantly higher in patients with CIP than in those without CIP (81.82% versus 42.07%, respectively, p = 0.013) and that the development of CIP significantly increased PFS in NSCLC (p = 0.01) (Figure S2 in the Supplement). Similar results were obtained with 12-week and 20-week landmark analyses. When adjusted for age, sex, the number of treatment lines, PD-L1 expression, CNS metastasis, histology, and treatment cycles, which may affect ICI efficacy, multivariate analysis also revealed that CIP was significantly associated with increased PFS (Table S2 in the Supplement).
Figure 1.
Analysis of PFS for patients with or without CIP.
CI, confidence interval; CIP, checkpoint inhibitor pneumonitis; PFS, progression-free survival.
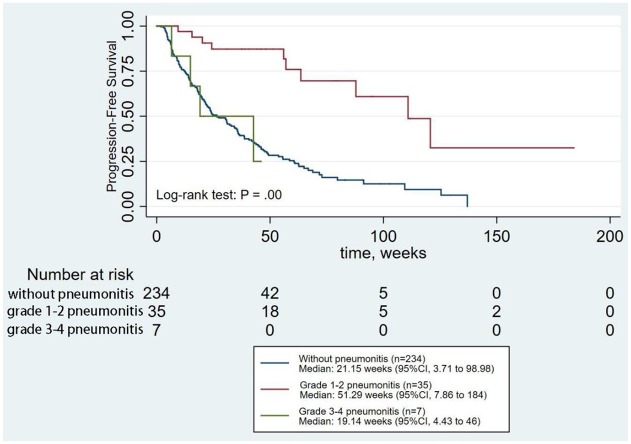
Figure 2.
Analysis of PFS for patients according to the grades of CIP.
CI, confidence interval; CIP, checkpoint inhibitor pneumonitis; PFS, progression-free survival.
Association of CIP with efficacy by subgroup analysis
Subgroup analysis of NSCLC patients was conducted. In the PD-1 inhibitor group, survival analysis showed that the development of CIP was significantly associated with increased PFS (p < 0.01) (Figure S3 in the Supplement). Similar results were obtained in the nivolumab and pembrolizumab groups (Figures S4 and S5 in the Supplement), whereas the PD-L1 inhibitor group did not achieve similar efficacy, which may be due to the limited number of patients included in the subgroup.
We also explored the associations between the grades or radiological subtypes of CIP and ICI efficacy. This analysis showed that grade 1–2 CIP was significantly associated with increased PFS (p < 0.01), whereas grade 3–4 CIP was not (Figure 2). Hypersensitivity pneumonitis and COP-like pneumonitis increased PFS significantly (p < 0.01) (Figure 3). Multivariate analysis also revealed the same results (Table S2 in the Supplement).
Figure 3.
Analysis of PFS for patients according to the radiological subtypes of CIP.
CI, confidence interval; CIP, checkpoint inhibitor pneumonitis; COP, cryptogenic organizing pneumonia; GGO, ground-glass opacities; PFS, progression-free survival.
Discussion
In this study, we described the relationship between the development of pneumonitis and survival in ICI-treated NSCLC patients. The results of this retrospective cohort study describe the incidence of CIP in a cohort of 276 patients treated with ICIs. A total of 42 patients (15.22%) developed CIP that was attributed to ICIs, and CIP was positively associated with ICI efficacy in patients with NSCLC. Additionally, our landmark analyses minimized the lead-time bias potentially associated with time-dependent factors such as irAEs. This result was further supported by our multivariate analysis. Subgroup analysis showed that CIP was also positively associated with efficacy in patients treated with PD-1 inhibitors, including nivolumab and pembrolizumab. CIP was not associated with efficacy in the PD-L1 inhibitor group; however, this result needs to be further confirmed by studies including a large number of patients. This study also revealed that the development of low-grade CIP (grade 1–2) increased survival in NSCLC patients treated with ICIs, whereas higher grade CIP did not. Four subtypes of radiological features were described in our study, and the interstitial and hypersensitivity types were more common than the other two types, which was not in accordance with the results of Naidoo et al.21 And our study further analyzed the association between the radiological features and the efficiency of ICIs, which suggested that hypersensitivity and COP-like pneumonitis were associated with increased ICI efficacy in NSCLC.
The effect of irAEs on disease progression has been studied in other malignancies, and the development of irAEs has been associated with improved responses to therapy and improved survival in some cases.15,22–25 Most studies have suggested that the development of irAEs improves overall survival (OS) and PFS. However, Suresh et al. described the relationship between the development of pneumonitis and survival in ICI-treated NSCLC patients using a multistate model of illness and death, and suggested that the development of CIP decreased survival in NSCLC.26 In contrast, our data suggest that the development of CIP after the initiation of ICI therapy is associated with increased survival. Several reasons may explain this difference: Firstly, Suresh’s study used a continuous time Markov multistate model to describe the clinical transitions of those alive with and without CIP to death.26 This model can describe the transitory states of CIP; thus, it may be more appropriate in the analysis of a time-varying event such as CIPs than the landmark analysis. However, the Markov assumption of the models specified that an individual’s transition was based solely on the current state and not dependent on the time that was spent in a prior state. Whereas patients without CIP transited from patients with CIP may have a higher probability of the development of CIP again. So this model should be used with caution when analyzing CIP. Secondly, the majority of patients in Suresh’s study received nivolumab. However, pembrolizumab has a larger proportion than nivolumab in our study. Furthermore, ICI combination with chemotherapy was included in our study, but not in that of Suresh. Thirdly, the endpoint of Suresh’s study was OS, which was more convincing than PFS in our study.
Few studies have explored the association between CIP and responses to ICI therapy in NSCLC.26 However, our study is the first, to our knowledge, to suggest that the grades and radiological features of CIP may also affect the efficacy of ICIs in NSCLC. The mechanisms underlying the association of irAEs with the outcome of PD-1 inhibitor treatment are unknown. Previous studies have suggested that antigens shared between melanoma cells and normal melanocytes may contribute to this association.11,14 Suresh et al. observed several dysregulated immune cell subpopulations in CIP.27 However, whether lung cancer cells share antigens with the tissues affected by CIP in patients with NSCLC remains to be determined.
There are several limitations to our results. First, we performed only landmark analysis of the primary results, which may affect the interpretation of subgroup analysis; however, potential confounding factors, including treatment cycles, were included in our multivariate analysis. Second, the follow-up time was not long enough to allow us to fully address long-term survival outcomes, and OS was not analyzed in this study because of the high loss rate to follow up. Third, this was a single-center retrospective study of NSCLC patients, and information bias, including other irAEs which may affect the efficiency of ICIs, could not be excluded; thus, the association between CIP and ICI efficacy warrants further study with larger cohorts.
Conclusion
Our findings indicate that CIP is positively associated with PD-1/PD-L1 inhibitor efficacy in patients with NSCLC. Specifically, grade 1–2 pneumonitis, hypersensitivity pneumonitis and COP-like pneumonitis increased the survival of NSCLC patients treated with PD-1/PD-L1 inhibitors. These findings may suggest that the lower grades of pneumonitis and the radiological features of hypersensitivity and COP may be signs of enhanced ICI efficacy. However, future studies with larger numbers of patients and longer follow-up times are needed to validate our findings.
Supplemental Material
Supplemental material, eFigures for Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer by Pengfei Cui, Di Huang, Zhaozhen Wu, Haitao Tao, Sujie Zhang, Junxun Ma, Zhefeng Liu, Jinliang Wang, Ziwei Huang, Shixue Chen, Xuan Zheng and Yi Hu in Therapeutic Advances in Medical Oncology
Supplemental material, eTables for Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer by Pengfei Cui, Di Huang, Zhaozhen Wu, Haitao Tao, Sujie Zhang, Junxun Ma, Zhefeng Liu, Jinliang Wang, Ziwei Huang, Shixue Chen, Xuan Zheng and Yi Hu in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Professor Hu for managing the review process and language assistance.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key R&D Program of China (grant number 2017YFC0907904 to YH).
ORCID iDs: Zhaozhen Wu  https://orcid.org/0000-0001-8962-5741
https://orcid.org/0000-0001-8962-5741
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Pengfei Cui, Department of Graduate Administration, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, China; Department of Medical Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, China.
Di Huang, School of Medicine, Nankai University, Nankai, Tianjin, China; Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Zhaozhen Wu, School of Medicine, Nankai University, Nankai, Tianjin, China; Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Haitao Tao, Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Sujie Zhang, Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Junxun Ma, Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Zhefeng Liu, Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Jinliang Wang, Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Ziwei Huang, School of Medicine, Nankai University, Nankai, Tianjin, China; Department of Medical Oncology, Chinese PLA General Hospital, Haidian, Beijing, China.
Shixue Chen, Department of Graduate Administration, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, China; Department of Medical Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, China.
Xuan Zheng, Department of Graduate Administration, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, China; Department of Medical Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, China.
Yi Hu, Department of Medical Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Haidian, Beijing, 100853, China; School of Medicine, Nankai University, Nankai, Tianjin, China.
References
- 1. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 3. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 4. Balaji A, Verde F, Suresh K, et al. Pneumonitis from anti-PD-1/PD-L1 therapy. Oncology (Williston Park) 2017; 31: 739–746, 754. [PubMed] [Google Scholar]
- 5. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13: 473–486. [DOI] [PubMed] [Google Scholar]
- 10. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016; 2: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 11. Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015; 151: 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 2017; 44: 117–122. [DOI] [PubMed] [Google Scholar]
- 13. Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016; 152: 45–51. [DOI] [PubMed] [Google Scholar]
- 14. Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015; 33: 773–781. [DOI] [PubMed] [Google Scholar]
- 15. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018; 115: 71–74. [DOI] [PubMed] [Google Scholar]
- 17. Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 2017; 12: 1798–1805. [DOI] [PubMed] [Google Scholar]
- 18. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15: 7412–7420. [DOI] [PubMed] [Google Scholar]
- 19. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017; 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018; 36: 1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017; 35: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD anderson cancer center experience. Invest New Drugs 2018; 36: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martini DJ, Hamieh L, McKay RR, et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD-1/PD-L1 therapy for immune-related adverse events. Cancer Immunol Res 2018; 6: 402–408. [DOI] [PubMed] [Google Scholar]
- 24. Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am Soc Clin Oncol Educ Book 2012: 174–177. [DOI] [PubMed] [Google Scholar]
- 25. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017; 35: 785–792. [DOI] [PubMed] [Google Scholar]
- 26. Suresh K, Psoter KJ, Voong KR, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint Immunotherapy. J Thorac Oncol 2019; 14: 494–502. [DOI] [PubMed] [Google Scholar]
- 27. Suresh K, Naidoo J, Zhong Q, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest 2019; 130: 4305–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, eFigures for Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer by Pengfei Cui, Di Huang, Zhaozhen Wu, Haitao Tao, Sujie Zhang, Junxun Ma, Zhefeng Liu, Jinliang Wang, Ziwei Huang, Shixue Chen, Xuan Zheng and Yi Hu in Therapeutic Advances in Medical Oncology
Supplemental material, eTables for Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer by Pengfei Cui, Di Huang, Zhaozhen Wu, Haitao Tao, Sujie Zhang, Junxun Ma, Zhefeng Liu, Jinliang Wang, Ziwei Huang, Shixue Chen, Xuan Zheng and Yi Hu in Therapeutic Advances in Medical Oncology