Abstract
Background:
Galcanezumab, along with three other monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) pathway, represents the latest disease-specific and mechanism-based treatment for the prophylaxis of migraine. Galcanezumab shares data also for the prophylaxis of cluster headache.
Objective:
To provide a pooled safety and efficacy analysis of all phase III randomized controlled trials of galcanezumab in the preventive therapy of migraine.
Methods:
A computer-based literature search was conducted on MEDLINE and the US National Institutes of Health Clinical Trials Registry for phase III randomized controlled trials of galcanezumab in migraine prevention. The primary outcome was the mean change in monthly migraine days (MMDs). The proportions of patients who reported at least one adverse event (AE), at least one serious adverse event (SAE) or withdrew from the study were used as safety outcomes.
Results:
Two trials were included in the efficacy meta-analysis and three in the safety meta-analysis. Migraine preventive treatment with subcutaneous galcanezumab, at both 120 mg and 240 mg dosages, was associated with a significantly greater reduction in the mean number of MMDs versus placebo (120 mg, MD = –1.98, 95% CI = –2.33 to –1.63; p < 0.0001) or (240 mg, MD = –1.86, 95% CI = –2.20 to –1.53; p < 0.0001). Galcanezumab was found to be more efficacious in all key secondary outcomes as well. Regarding safety, most of the adverse events were mild to moderate, while drop-out rates and serious adverse events were low.
Conclusions:
Galcanezumab is an efficacious and well-tolerated preventive treatment for migraine. Larger clinical trials with longer follow-up periods need to be conducted in order to provide more safety data of the above-mentioned drug.
Keywords: CGRP, galcanezumab, meta-analysis, migraine, monoclonal antibodies, preventive therapy
Introduction
Migraine is found to be the second leading cause of years lived with disability (YLDs) with an enormous economic burden.1,2 Yet, no disease-specific, nor mechanism-based prophylactic treatment was available until the recent development of four monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) pathway. CGRP is a proinflammatory and vasodilating neuropeptide involved in peripheral and central sensitization,3 with adequate documentation regarding its involvement in migraine pathophysiology. It remarkably increases during migraine attacks,4 while intravenous infusion of CGRP induces migraine-like attacks in migraine patients.5
Galcanezumab is a fully humanized monoclonal antibody, targeting the CGRP ligand, studied and developed for migraine prophylaxis. In addition, it is the only one among the four monoclonal antibodies targeting the CGRP pathway that exerts efficacy in the prevention of cluster headache as well,6 thus attracting even more interest. A number of studies and reviews have been published so far, but none of them is reliable due to poor inclusion criteria. In some cases, phase II trials in which galcanezumab was tested in different doses and most importantly in different treatment periods were included. Consequently, that led to high inconsistency across trials and has probably biased the final outcome. The purpose of this systematic review and meta-analysis is to assess the efficacy of galcanezumab in the pooled phase III data of the randomized trials for episodic migraine prophylaxis and its safety in the pooled phase III data of the randomized trials for episodic or chronic migraine prophylaxis. A separate meta-analysis of galcanezumab efficacy in chronic migraine as well as its efficacy and safety in cluster headache prophylaxis should be performed, when a greater number of phase III trials will be published.
Methods
Search strategy
A computer-based literature search was conducted on MEDLINE (accessed by PubMed) and the US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov) using ‘migraine’, ‘treatment’ and ‘placebo’ as key words and electronic publication date from 1 January 2010 to 1 September 2019, randomized controlled trials and English language as limitations. The search followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.7
Eligibility criteria
The following inclusion criteria were applied to the 220 articles retrieved by the search. Studies were eligible if they were randomized, double-blinded, placebo controlled, parallel group, phase III studies with active and control groups receiving galcanezumab and matched placebo, respectively. Phase II studies were not included, because they tested different dosages of the drug for different treatment periods, thus they could not be synthesized in the meta-analysis. Participants had to meet the following criteria: any sex, any ethnicity, age (⩾18 years), history of episodic migraine for the efficacy meta-analysis and history of episodic or chronic migraine for the safety meta-analysis, with or without aura, according to the criteria of the International Headache Society.8,9
Quality assessment
The Jadad scale was used to assess the quality of the included studies. The Jadad scale scores three parameters: randomization, blindness (of the patients, caregivers, investigators) and adequate documentation of withdrawals and drop-outs, and is considered to be the most reliable.10,11
Outcome measures safety: tolerability
The primary outcome was the overall mean change from baseline in the number of monthly migraine days (MMDs) during the 6-month treatment phase. The key secondary outcome measures were the following: the mean proportion of patients with reduction from baseline of 50%, 75%, and 100% in MMDs during the 6-month double-blind treatment phase; the mean change from baseline in the Role Function–Restrictive (RF-R) domain score of the Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ v2.1),12 as an average of months 4–6; the overall mean change from baseline in the number of monthly acute migraine-specific medication days (MSMDs) during the 6-month double-blind treatment phase; and the mean change from baseline in the Patient Global Impression of Severity (PGI-S) rating (average of months 4–6).13 The safety and tolerability outcomes included the proportion of participants who experienced any adverse event (AE), any serious adverse event (SAE) and who withdrew from treatment for AEs.
Study selection, data extraction, and assessment of the risk of bias
Trials were assessed for inclusion and the following data were extracted from the included studies: main study author; age of publication; methodology and trial design (methods of randomization, allocation concealment and blinding, duration of baseline and treatment periods, dose/s of galcanezumab tested); number, demographics, and clinical characteristics of participants (age, sex, ethnic origin, age at migraine onset, disease duration, previous preventive history, MMDs, and monthly acute MSMDs during the baseline phase); number of participants experiencing each outcome; and changes in baseline frequency for each endpoint per randomized group. In the current systematic review and meta-analysis only data for the dosages of 120 mg and 240 mg were considered.
Statistical analysis
Meta-analysis was carried out using the StatsDirect statistical software (www.statsdirect.com). The heterogeneity within trials was tested by Cochran’s Q-test based on inverse variance weights.14 The I2 statistic was also used to quantify the extent of inconsistency in outcomes across studies. Provided no significant heterogeneity was present (p > 0.05), results were synthesized using a fixed effect model; if the probability value was ⩽0.05, heterogeneity determined the choice of a fixed or random effects model for I2 <40% or ⩾40%, respectively.15,16 The mean difference (MD) and risk ratio (RR) with their 95% confidence intervals (CIs) were the measures of associations between treatment and continuous/dichotomous outcomes.
Results
Literature search and study characteristics
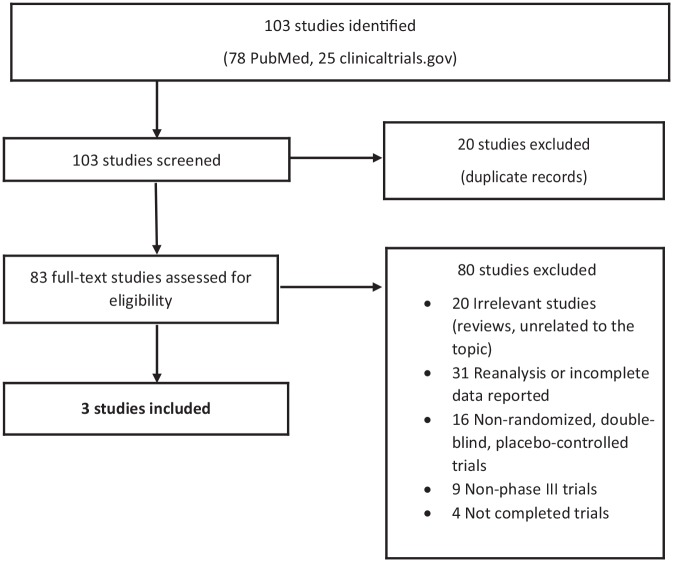
The results of our search are summarized in Figure 1. After repeated filtering, from the 220 articles retrieved, only two were included in the efficacy meta-analysis (two studies for episodic migraine – see Appendix 1) and three studies were included in the safety meta-analysis (two studies for episodic migraine and one study for chronic migraine – see Appendix 1). These studies published in 2018 involved 2886 patients, 1434 of whom were treated with galcanezumab (722 with 120 mg and 712 with 240 mg) and 1452 with placebo. Baseline patient demographics and disease characteristics for both active and placebo-treated groups are summarized in Table 1.
Figure 1.
Article selection flow diagram.
Table 1.
Patient demographics and baseline disease characteristics of studies included in the meta-analysis.
| EVOLVE-1 | EVOLVE-2 | REGAIN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | Placebo | 120 mg | 240 mg | Placebo | 120 mg | 240 mg | Placebo | 120 mg | 240 mg |
| Patient number (n) | 433 | 213 | 212 | 461 | 231 | 223 | 558 | 278 | 277 |
| Age mean, (SD) | 41.3 (11.4) | 40.9 (11.9) | 39.1 (11.5) | 42.3 (11.3) | 40.9 (11.2) | 41.9 (10.8) | 41.6 (12.1) | 39.7 (11.9) | 41.1 (12.4) |
| Female n, (%) | 362 (83.6) | 181 (85.0) | 175 (82.6) | 393 (85.3) | 197 (85.3) | 191 (85.7) | 483 (87) | 237 (85) | 226 (82) |
| White race n, (%) | 356 (82.2) | 169 (79.3) | 165 (77.8) | 325 (70.5) | 166 (71.9) | 152 (68.2) | 432 (77) | 223 (80) | 224 (81) |
| Disease characteristics | /// | /// | /// | /// | /// | /// | /// | /// | /// |
| Disease duration, mean (SD) | 19.9 (12.3) | 21.1 (13) | 19.3 (11.9) | 21.2 (12.8) | 19.93 (11.7) | 20.01 (12.1) | 21.9 (12.9) | 20.4 (12.7) | 20.1 (12.7) |
| MMDs, mean (SD) | 9.1 (3.0) | 9.2 (3.1) | 9.1 (2.9) | 9.2 (3.0) | 9.07 (2.9) | 9.06 (2.9) | 19.6 (4.6) | 19.4 (4.3) | 19.2 (4.6) |
| Monthly acute MSMDs | 7.4 (3.5) | 7.4 (3.7) | 7.3 (3.3) | 7.6 (3.4) | 7.47 (3.3) | 7.47 (3.3) | 15.5 (6.6) | 15.1 (6.3) | 14.5 (6.3) |
| Prior preventive treatment, n (%) | 257 (59.4) | 133 (62.4) | 125 (59.0) | 298 (64.6) | 157 (68.0) | 151 (64.6) | 435 (78.0) | 211 (76.0) | 220 (79.0) |
| MSQ RF-R score, mean (SD) | 52.9 (15.4) | 51.4 (16.2) | 48.8 (16.8) | 51.4 (15.7) | 52.5 (14.8) | 51.7 (16.3) | 38.4 (17.2) | 39.3 (17.3) | 38.9 (17.3) |
| PGI-S, mean (SD) | 4.2 (1.1) | 4.4 (1.1) | 4.5 (1.1) | 4.3 (1.2) | 4.1 (1.2) | 4.2 (1.2) | 4.9 (1.2) | 4.8 (1.2) | 4.9 (1.3) |
| MIDAS total score, mean (SD) | 31.8 (27.3) | 32.9 (28.2) | 36.1 (27.8) | 34.3 (31.0) | 30.9 (27.9) | 32.8 (28.8) | 68.7 (57.4) | 62.5 (49.5) | 69.2 (64.1) |
MIDAS, Migraine Disability Assessment; MMDs, monthly migraine days; MSMDs, migraine-specific medication days; MSQ, Migraine-Specific Quality of Life Questionnaire version 2.1; PGI-S, Patient Global Impression–Severity of Illness; RF-R, Role Function–Restrictive.
Monthly migraine days and monthly acute migraine-specific medication days
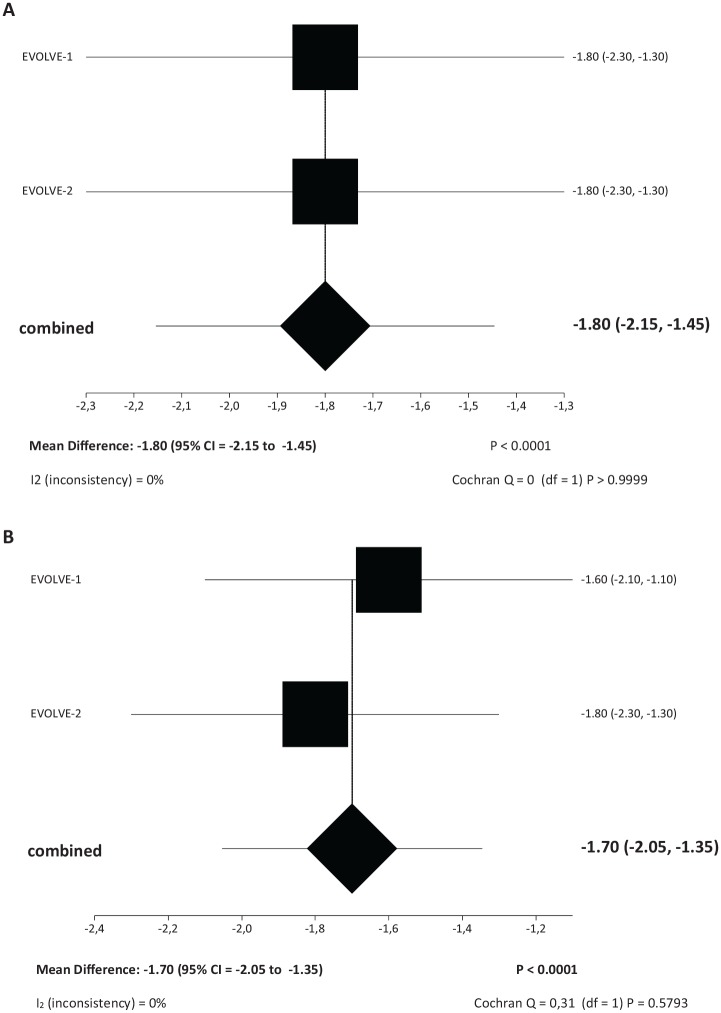
The reduction in baseline monthly migraine days (MMDs) was significantly greater among patients treated with galcanezumab at either the monthly dose of 120 mg [Mean difference (MD): –1.95 (95% CI = –2.34 to –1.56), p < 0.0001] or 240 mg [MD: –1.85 (95% CI = –2.22 to –1.48), p < 0.0001] than with placebo (Figure 2A, B). Both dosing regimens of galcanezumab were superior to placebo in the reduction of monthly acute migraine specific medication days (MSMDs) [galcanezumab 120 mg: MD: –1.80 (95% CI = –2.15 to –1.45), p < 0.0001; galcanezumab 240 mg: MD: –1.70 (95% CI = –2.05 to –1.35), p < 0.0001] (Figure 3A, B).
Figure 2.
Meta-analysis forest plots. (A) Meta-analysis of mean difference of change in monthly migraine days of galcanezumab 120 mg versus placebo and (B) meta-analysis of mean difference of change in monthly migraine days of galcanezumab 240 mg versus placebo.
(A) Change in monthly migraine days – mean difference of galcanezumab 120 mg versus placebo.
(B) Change in monthly migraine days – mean difference of galcanezumab 240 mg versus placebo.
Figure 3.
Meta-analysis forest plots. (A) Meta-analysis of mean difference of change in baseline monthly acute migraine-specific medication days of galcanezumab 120 mg versus placebo and (B) meta-analysis of mean difference of change in baseline monthly acute migraine-specific medication days of galcanezumab 240 mg versus placebo.
(A) Change in baseline monthly acute migraine-specific medication days – Mean difference of galcanezumab 120 mg versus placebo.
(B) Change in baseline monthly acute migraine-specific medication days – Mean difference of galcanezumab 240 mg versus placebo.
Other key secondary outcomes
Galcanezumab treated patients were more likely to achieve at least 50% reduction in baseline MMDs either at the dose of 120 mg [RR: 1.55 (95% CI = 1.39–1.73) p < 0.0001 or 240 mg RR: 1.50 (95% CI = 1.34–1.68) p < 0.0001] (Table 2A). Also, patients treated with galcanezumab were more likely to have at least 75% reduction in baseline MMDs in both dose regimens [(120 mg RR: 1.89 (95% CI = 1.57–2.27) p < 0.0001, 240 mg RR: 1.91 (95% CI = 1.59–2.30) p < 0.0001] (Table 2B). Furthermore, galcanezumab-treated patients were more likely to achieve a 100% reduction in MMDs than placebo-treated patients [120 mg RR: 2.28 (95% CI = 1.60–3.23) p < 0.0001, 240 mg RR: 2.37 (95% CI = 1.67–3.36) p < 0.0001] (Table 3C). Galcanezumab treatment significantly improved MSQ RF-R scores compared to placebo during treatment [120 mg MD: 8.26 (95% CI = 6.48–10.05), p < 0.0001 and 240 mg MD: 7.35 (95% CI = 5.53–9.17), p < 0.0001]. Both galcanezumab groups showed a greater improvement in patients’ global impression of severity of their disease as assessed by PGI-S rating than placebo [120 mg MD: –0.30 (95% CI = –0.44 to –0.16) p < 0.0001 and 240 mg MD: –0.24 (95% CI = –0.38 to –0.11) p < 0.0001].
Table 2.
(A) 50% or greater reduction in baseline monthly migraine days for galcanezumab versus placebo; (B) 75% or greater reduction in baseline monthly migraine days for galcanezumab versus placebo; and (C) 100% reduction in baseline monthly migraine days for galcanezumab versus placebo.
| Subgroup | Number of pooled events/participants |
I 2 | Risk ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Galcanezumb | Placebo | ||||
| A | |||||
| Galcanezumab 120 mg | 268/441 | 347/886 | 0% | 1.55 (95% CI = 1.39–1.73) | <0.0001 |
| Galcanezumab 240 mg | 253/431 | 347/886 | 0% | 1.50 (95% CI = 1.34–1.68) | <0.0001 |
| B | |||||
| Galcanezumab 120 mg | 158/441 | 168/886 | 0% | 1.89 (95% CI = 1.57–2.27) | <0.0001 |
| Galcanezumab 240 mg | 156/431 | 168/886 | 0% | 1.91 (95% CI = 1.59–2.3) | <0.0001 |
| C | |||||
| Galcanezumab 120 mg | 60/441 | 53/886 | 0% | 2.28 (95% CI = 1.6 to 3.23) | <0.0001 |
| Galcanezumab 240 mg | 61/431 | 53/886 | 0% | 2.37 (95% CI = 1.67–3.36) | <0.0001 |
CI, confidence interval.
Table 3.
(A) Changes in baseline migraine specific quality of life questionnaire, version 2.1, role-function restrictive (MSQ R-FR) for galcanezumab versus placebo and (B) changes in baseline patient global impression-severity (PGI-S) for galcanezumab versus placebo.
| Subgroup | Galcanezumab versus placebo mean difference (95% CI) | I 2 | p value |
|---|---|---|---|
| A MSQ R-FR | |||
| Galcanezumab 120 mg | 8.26 (95% CI = 6.48–10.05) | 0% | <0.0001 |
| Galcanezumab 240 mg | 7.35 (95% CI = 5.53–9.17) | 0% | <0.0001 |
| B PGI-S | |||
| Galcanezumab 120 mg | −0.30 (95% CI = –0.44 to –0.16) | 0% | <0.0001 |
| Galcanezumab 240 mg | −0.24 (95% CI = –0.38 to –0.11) | 0% | =0.0003 |
CI, confidence interval.
Adverse events and treatment withdrawals
Across the trials, AEs were reported by 913 (64%) and 827 (57%) patients treated with galcanezumab and placebo, respectively; the overall RR to develop any AE during galcanezumab treatment was 1.12 (95% CI = 1.05–1.18) p = 0.0002. SAEs occurred in 24 (1.7%) and 14 (1%) patients treated with galcanezumab and placebo, respectively; RR: 1.74 (95% CI = 0.90–3.34) p = 0.0989. Treatment was discontinued in 36 (2.5%) and 23 (1.6%) cases in the galcanezumab and placebo groups, respectively; the corresponding RR was 1.59 (95% CI = 0.95–2.66) p = 0.0808. The incidence rates of the most common AEs in the galcanezumab versus placebo-treated patients were as follows: injection site pain 10.9% versus 9.5% (p = 0.2157); injection site erythema 3.4% versus 1.4% (p = 0.0005); injection site pruritus 2.7% versus 0.1% (p < 0.0001); injection site reaction (localized skin reactions that occur at the injection site besides pain, erythema and pruritus) 4.7% versus 1% (p = 0.0159); nasopharyngitis 5.8% versus 6.5% (p = 0.4402); upper respiratory tract infections 3.7% versus 2.4 (p = 0.0465). Safety results are summarized in Table 4. No statistically significant and clinically meaningful differences were observed between galcanezumab and placebo in laboratory values, vital signs, weight, or quantitative or qualitative electrocardiograms (ECGs).
Table 4.
Adverse events for galcanezumab versus placebo.
| Subgroup | Number of pooled events/participants |
I 2 | Risk ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Galcanezumb | Placebo | ||||
| Any AE | 913/1435 | 827/1451 | 0% | 1.12 (95% CI = 1.05–1.18) | 0.0002 |
| Any SAE | 24/1435 | 14/1451 | 0% | 1.74 (95% CI = 0.9–3.34) | 0.0989 |
| Discontinuation due to AEs | 36/1435 | 23/1451 | 0% | 1.59 (95% CI = 0.95–2.66) | 0.0808 |
| Injection site pain | 156/1435 | 138/1451 | 0% | 1.14 (95% CI = 0.92–1.42) | 0.2157 |
| Injection site erythema | 49/1435 | 20/1451 | 0% | 2.48 (95% CI = 1.48–4.15) | 0.0005 |
| Injection site pruritus | 39/1435 | 2/1451 | 0% | 15.99 (95% CI = 4.47–57.3) | <00001 |
| Injection site reaction | 67/1435 | 14/1451 | 68.9% | 4.98 (95% CI = 1.35–18.38) | 0.0159 |
| Nasopharyngitis | 83/1435 | 94/1451 | 0% | 0.89 (95% CI = 0.67–1.19) | 0.4402 |
| Upper respiratory tract infection | 53/1435 | 35/1451 | 0% | 1.53 (95% CI = 1.01 –2.33) | 0.0465 |
| Back pain | 23/1435 | 20/1451 | 54,6% | 1.21 (95% CI = 0.48–3.03) | 0.686 |
| Urinary tract infection | 10/1435 | 7/1451 | 0% | 1.43 (95% CI = 0.83–2.44) | 0.1965 |
| Abdominal pain | 10/1435 | 9/1451 | 0% | 1.12 (95% CI = 0.46–2.73) | 0.8079 |
| Fatigue | 23/1435 | 22/1451 | 0% | 1.06 (95% CI = 0.59–1.88) | 0.8523 |
| Diarrhea | 19/1435 | 20/1451 | 0% | 0.96 (95% CI = 0.52–1.79) | 0.8982 |
| Dizziness | 26/1435 | 21/1451 | 0% | 1.26 (95% CI = 0.71–2.22) | 0.4305 |
| Migraine | 16/1435 | 9/1451 | 0% | 1.79 (95% CI = 0.8–4.04) | 0.1582 |
| Influenza | 31/1435 | 22/1451 | 24,8% | 1.43 (95% CI = 0.83–2.45 | 0.1973 |
| Injection site bruising | 6/1435 | 6/1451 | 0% | 1.01 (95% CI = 0.33–3.12) | 0.9805 |
| Neck pain | 14/1435 | 12/1451 | 0% | 1.18 (95% CI = 0.55–2.53) | 0.3849 |
| Nausea | 13/1435 | 15/1451 | 0% | 0.88 (95% CI = 0.42–1.82) | 0.729 |
AE, adverse event; CI, confidence interval; SAE, serious adverse event.
Discussion
Galcanezumab is a fully humanized monoclonal antibody specifically developed and studied to prevent migraine by targeting the CGRP pathway and trigeminal pain system along with erenumab, eptinezumab, and fremanezumab. It is also the only one found to be effective in cluster headache prophylaxis.6 Unfortunately, a meta-analysis of galcanezumab efficacy in chronic migraine prophylaxis as well as its efficacy and safety in cluster headache prophylaxis cannot be performed at this time due to the small number of published studies.
In the two studies analyzed for episodic migraine (EVOLVE-1, EVOLVE-2), preventive treatment with subcutaneous galcanezumab, at both 120 mg and 240 mg dosages, was associated with a significantly greater reduction in the mean number of MMDs versus placebo. Moreover, a greater reduction in the number of monthly acute MSMDs during the 6-month double-blind treatment phase was shown, further supporting the efficacy of galcanezumab as migraine preventive therapy. This meta-analysis also shows that patients treated with galcanezumab 120 mg or 240 mg had a greater improvement in quality of life than did placebo-treated patients, as measured in the MSQ role-function restrictive domain at month 6. Similarly, a significant improvement in patients’ global impression of severity of their disease measured by the Patient Global Impression of Severity (PGI-S) score was detected in comparison with placebo at the end of the 6-month double-blind treatment phase. Furthermore, the clinical benefit of galcanezumab is supported by the statistically greater proportion of galcanezumab-treated patients versus placebo-treated patients that achieved 50% or greater, 75% or greater and 100% reduction from baseline in the mean number of MMDs at both dose regimens. However, it has to be mentioned that in EVOLVE-2 the authors stated that: ‘response rate was defined as the percentages of patients meeting predefined thresholds (i.e. 50%, 75%, and 100%) in the reduction from baseline in the number of MMDs for each month, and the overall percentages of patients meeting these thresholds averaged over months 1 through 6 were analyzed’. Thus, the proportion reported in these trials refers to the mean proportion of patients who achieved the thresholds for 1-month treatment and not within the entire treatment phase (6 months). This approach may have increased the presented response rates.
Regarding the safety profile of the drug, galcanezumab seems to be well tolerated as indicated by the comparable rates of AEs between the active and placebo-treated groups of the trials analyzed (EVOLVE-1, EVOLVE-2, REGAIN). Most of the AEs were mild to moderate, while the incidence of patients who withdrew from the studies due to AEs was not significant, especially when compared to other non-specific preventive migraine therapies such as topiramate.17,18 Moreover, injection site reactions were low and SAEs due to galcanezumab were below 2%.
A probable explanation for the low drop-out rates of galcanezumab could be the greater specificity of action and the greater half-life time (25–30 days), which allows the drug to be administered once a month. Monthly dosing instead of daily intake of oral preventive drugs seems to be more convenient for patients and may improve treatment adherence.19 Therefore, the effectiveness of the treatment may increase. In addition, the high efficacy of galcanezumab should lead to lower indirect costs (visits to emergency departments, missing work due to migraine or children missing school due to parent’s migraine). Finally, the cardiovascular safety of galcanezumab is crucial and needs to be determined through randomized controlled trials (RCTs). The CGRP and its receptors are distributed not only in the central and peripheral nervous system but also in the cardiovascular system, both in blood vessels and in the heart. Under normal conditions, CGRP has important vasodilating effects and is thought to protect organs from ischemia.20 Existing data from a double-blind clinical trial for erenumab showed that it did not impair the total exercise time in a treadmill test in high-risk cardiovascular patients, indicating that CGRP pathway inhibition does not increase the risk of myocardial ischemia.21
This study has a number of limitations that should be acknowledged while interpreting the findings. Firstly, only two studies in the efficacy meta-analysis and only three in the safety meta-analysis met the inclusion criteria. All of them were funded by the manufacturing pharmaceutical companies. Moreover, the long-term safety profile of galcanezumab should be studied through real-world evidence, trials with larger numbers of patients and longer follow-up periods. Finally, it must be recognized that our findings have been calculated in phase III clinical trials and cannot be readily generalized to clinical practice.
Conclusion
Galcanezumab is an efficacious and well-tolerated preventive treatment for migraine in adults. The low drop-out rate during the clinical trials may be a good indicator of better treatment adherence, thus more effective therapy for migraine patients. The monthly subcutaneous dosing instead of daily oral intake seems to be preferred by migraine patients while injection site reactions do not differ significantly between galcanezumab and placebo. Studies with longer follow-up periods and real-world data need to be conducted.
Article highlights
Galcanezumab is an efficacious and well-tolerated preventive treatment for migraine.
The low drop-out rate during the clinical trials may be a good indicator to better treatment adherence, thus more effective therapy for migraine patients.
Larger clinical trials with longer follow-up periods need to be conducted in order to provide more safety data of the above-mentioned drug.
Appendix 1: Studies included in the meta-analysis
Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 2018; 75: 1080–1088.
Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia 2018; 38: 1442–1454.
Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018; 91: e2211–e2221.
Footnotes
Author contributions: PG contributed to study design, data collection, statistical analysis, data interpretation and drafted the manuscript.
DDM conceived the study, participated in the design, data collection and interpretation and drafted the manuscript.
Both authors discussed the results and approved the final manuscript.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest: P. Gklinos declares that there is no conflict of interest. D.D. Mitsikostas has received honoraria, research and travel grants from Allergan, Amgen, Biogen, Cefaly, Eli Lilly, Electrocore, Mertz, Novartis, Roche, Sanofi, Specifar and Teva.
ORCID iD: Panagiotis Gklinos  https://orcid.org/0000-0001-5022-6460
https://orcid.org/0000-0001-5022-6460
Contributor Information
Panagiotis Gklinos, Neurology Department, KAT General Hospital, Athens, Greece.
Dimos D. Mitsikostas, First Neurology Department, Aeginition Hospital, Medical School, National and Kapodistrian University of Athens, 74 V. Sofia’s Avenue, Athens 11528, Greece.
References
- 1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017; 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol 2012; 19: 703–711. [DOI] [PubMed] [Google Scholar]
- 3. Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010; 6: 573–582. [DOI] [PubMed] [Google Scholar]
- 4. Lassen LH, Jacobsen VB, Haderslev PA, et al. Involvement of calcitonin gene-related peptide in migraine: regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain 2008; 9: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010; 30: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 6. Goadsby PJ, Dodick DW, Leone M, et al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med 2019; 381: 132–141. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 8. Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia 2004; 24 (Suppl. 1): 9–160. [DOI] [PubMed] [Google Scholar]
- 9. Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 10. Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008; 88: 156–175. [DOI] [PubMed] [Google Scholar]
- 11. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 12. Cole JC, Lin P, Rupnow MF. Validation of the migraine-specific quality of life questionnaire version 2.1 (MSQ v. 2.1) for patients undergoing prophylactic migraine treatment. Qual Life Res 2007; 16: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 13. Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville: National Institute of Mental Health, Psychopharmacology Research Branch, 1976, pp. 217–222. [Google Scholar]
- 14. Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M, Smith GD, Altman DG. (eds) Systematic reviews in health care: meta-analysis in context. London: BMJ Books, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S. (eds). Cochrane handbook for systematic reviews of interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://handbook-5-1.cochrane.org (2011; accessed 26 March 2020).
- 16. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 17. Silberstein SD, Neto W, Schmitt J, et al. ; MIGR-001 Study Group. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol 2004; 61: 490–495. [DOI] [PubMed] [Google Scholar]
- 18. Brandes JL, Saper JR, Diamond M, et al. ; MIGR-002 Study Group. Topiramate for migraine prevention: a randomized controlled trial. JAMA 2004; 291: 965–973. [DOI] [PubMed] [Google Scholar]
- 19. Seng EK, Rains JA, Nicholson RA, et al. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015; 19: 24. [DOI] [PubMed] [Google Scholar]
- 20. Favoni V, Giani L, Al-Hasany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain 2019; 20: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Depre C, Antalik L, Starling A, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache 2018; 58: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]